Abstract
We recently established an effective immune T-cell–mediated graft-versus-leukemia (GVL) murine model system in which complete tumor remissions were achievable even in advanced metastasized cancer. We now describe that this T-cell–mediated therapy is dependent on host macrophages expressing the lymphocyte adhesion molecule sialoadhesin (Sn). Depletion of Kupffer cells in tumor-bearing mice during adoptive immunotherapy (ADI) or the treatment of these animals with anti-Sn monoclonal antibodies led to complete or partial inhibition of the immune T-cell–mediated therapeutic effect. Furthermore, Sn+ host macrophages in livers formed clusters during ADI with donor CD8 T cells. To test for a possible antigen presentation function of these macrophages, we used as an in vitro model the antigen β-galactosidase for which a dominant major histocompatibility complex (MHC) class I Ld-restricted peptide epitope is known to be recognized by specific CD8 cytotoxic T lymphocytes (CTL). We demonstrate that purified Sn+ macrophages can process exogenous β-galactosidase and stimulate MHC class I peptide-restricted CTL responses. Thus, Sn+ macrophages, which are significantly increased in the liver after ADI, may process tumor-derived proteins via the MHC class I pathway as well as via the MHC class II pathway, as shown previously, and present respective peptide epitopes to CD8 as well as to CD4 immune T cells, respectively. The synergistic interactions observed before between immune CD4 and CD8 T cells during ADI could thus occur in the observed clusters with Sn+ host macrophages.
SOME MOUSE TISSUE macrophages, located in bone marrow, spleen, liver, and lymph nodes, express a lectin-like receptor, sialoadhesin (Sn), that mediates divalent cation-independent binding but not phagocytosis of sheep erythrocytes.1-3 Sn has been shown also to function as an adhesion molecule for lymphocytes.4
We previously described a highly effective adoptive cellular immunotherapy (ADI) protocol in the well-defined ESb T-lymphoma model of DBA/2 (H-2d, Mlsa) mice.5,6 The adoptive transfer of in situ-activated tumor immune spleen lymphocytes from ESb tumor-resistant, major histocompatibility complex (MHC) identical but superantigen different B10.D2 (H-2d, Mlsb) mice into tumor-bearing DBA/2 mice led to a rejection of primary tumors (1.5 cm in diameter) from the skin and to eradication of liver metastases. This therapy requires only relatively few specific T cells and involves synergistic interactions between CD4 and CD8 tumor-immune T cells.5 B10.D2 anti-ESb cytotoxic T lymphocyte (CTL) responses were made up of a strong response to DBA/2 minor histocompatibility (H) antigens and a weaker one to ESb-specific MHC class I (Kd)-restricted tumor-associated antigen (TAA).7 The frequency of B10.D2 CTL precursors for H antigen was about 1:3,700, whereas the frequency of CTL precursors for TAA was about 1:17,000.8
After immunohistological staining of livers from ADI-treated mice with a monoclonal antibody (MoAb) against Sn, we observed (1) an increase in Sn+ host macrophages and (2) a close physical association between the latter and CD4 T lymphocytes.9 Sn+macrophages that were isolated from the liver of ADI-treated animals could directly restimulate in vitro CD8 T lymphocytes primed against the tumor cells, suggesting that they could process and present exogenous TAA to T lymphocytes.9 Furthermore, we demonstrated that Sn+ macrophages were capable of processing and presenting human C-reactive protein via MHC class II to a CD4 T-helper clone specific for an identified MHC II-associated C-reactive protein-derived peptide.10
The classical pathways of antigen presentation to T cells involve (1) endogenous proteins that are presented via MHC class I molecules and (2) exogenous proteins that are presented via class II molecules.11,12 However, a number of recent publications demonstrate that this classification is neither absolute nor exclusive. Thus, CD8 T-cell responses were observed in vivo in the absence of de novo antigen synthesis within host antigen-presenting cells (APC) after immunization with purified proteins13 or in cross-priming experiments in which CD8-mediated responses to donor cell antigens were restricted to MHC molecules expressed only on recipient cells.14 The APC involved in this type of antigen presentation were identified as macrophages or dendritic cells.15-19 Recent studies have suggested different mechanisms for presentation of exogenous antigens via MHC class I molecules in macrophages.20-23 It is at present not clear whether some macrophage subpopulations can process and present exogenous soluble antigen (eg, TAA) via MHC class I molecules to CD8 T lymphocytes and thus be a part of host antitumor response.
In this report, we provide evidence that a subset of macrophages bearing the lymphocyte adhesion molecule Sn can stimulate in vitro a CD8 CTL response against an MHC class I-restricted model antigen. Furthermore, Sn+ Kupffer cells will be shown to form clusters with donor CD8 T cells in the livers of tumor bearing mice upon adoptive cellular immunotherapy. The depletion of Kupffer cells or treatment with anti-Sn MoAbs in vivo after ADI decreased the efficiency of this therapy. We therefore suggest that Sn+ macrophages are important for processing exogenous TAA from metastases and presenting it, not only via MHC class II to CD4 cells, but also via class I to CD8 immune donor T lymphocytes, and contribute thereby to the eradication of lymphoma liver metastases during ADI.
MATERIALS AND METHODS
Animals and tumor cell lines.
DBA/2 mice and Balb/c nude mice (nu/nu) were obtained from IFFA Credo (Lyon, France) and B10.D2 mice were obtained from Olac (Bicester, UK). All animals were used at 6 to 12 weeks of age. ESb cells represent a spontaneous highly metastatic variant of the chemically induced T-lymphoma L5178Y (Eb) of DBA/2 mice. ESbL cells transduced with the bacterial lacZ gene (clone L-CI.5s) were cultured as described.24 The ESb-MP subline is a plastic adherent variant of ESb lymphoma cells that have reduced malignancy and altered metastatic capacity in vivo.25 P815 mastocytoma cells of DBA/2 mice and P13.1 cells, a lacZ-transduced variant of the P815 mastocytoma,26 were used in the cytotoxicity assay. All media used for tumor cell culture and inoculation as well as for cell isolation were free of endotoxins. DBA/2 mice were anesthesized with Rompun (0.1%):Ketanest (0.25%):phosphate-buffered saline (PBS) at 1:1:3 (vol) and 2 × 105 ESb-MP cells in 100 μL PBS were injected intradermally (ID) into the dermis of the shaved flank.
Adoptive cellular immunotherapy.
To generate allogeneic immune effector cells, ESb-MP lymphoma cells were inoculated intravenously (IV) at a dose of 105 cells into B10.D2 mice.5 Seven days later, spleen cells were isolated and transferred IV (2 × 107 cells/200 μL RPMI-1640 medium; GIBCO BRL, Eggenstein, Germany) into 5 Gy (60Co source Gammatron F 80S; Siemens, Braunschweig, Germany) sublethally irradiated DBA/2 mice that carried tumors (>1 cm in diameter). This adoptive transfer of antitumor immune spleen cells (ISPL) was made 3 weeks after ID tumor cell inoculation. Sublethally irradiated tumor-bearing DBA/2 mice of the control group remained untreated. In some experiments, 2 × 107 ISPL were injected IV into nu/nu mice 3 weeks after ID inoculation of ESb-MP cells.
To generate syngeneic antitumor immune cells, DBA/2 mice were primed by injection into the ear pinna of 5 × 104 ESb lymphoma cells and challenged 7 days later intraperitoneally with 1.5 × 107 100 Gy irradiated ESb cells.27Three days after challenge, 5 × 106 in situ activated lymphocytes from peritoneal exudate (PEC) were transferred IV into 5 Gy irradiated DBA/2 mice that were IV injected 1 day before with 104 ESb lymphoma cells.
Antibodies and other reagents.
The following rat MoAbs were used as culture supernatants: antimouse Thy-1.2 (clone AT83A),28 anti-CD8 (clone 53-6-72),29 specific for mouse B cells (clone B220),30 for mature mouse macrophages (F4/80),31 and for Sn (SER-4 and 1C2).4 In addition, SER-4 MoAb was labeled with digoxigenin. Hamster-antimouse B7.1 MoAb (clone 16-10A)32 was used as culture supernatant. Rat-antimouse B7.2 MoAb (clone GL1) was purchased from PharMingen (Hamburg, Germany). Rat-antimouse MHC class II MoAb (clone AMS32-1; PharMingen) was biotinylated. Hamster-antimouse dendritic cell marker N418 MoAb was labeled with digoxigenin and kindly provided by Dr B. Kyewski (German Cancer Research Center, Heidelberg, Germany). R-Phycoerythrin–conjugated streptavidine (GIBCO BRL) and antidigoxigenin-fluorescein F(ab′)2 antibody (Boehringer Mannheim, Mannheim, Germany) were used as second reagents. The mouse MoAbs T19.191 and K9-18,33 hybridoma supernatants directed against H-2Ld and H-2Kd MHC class I, respectively, were kindly provided by Dr B. Arnold (German Cancer Research Center) and used in blocking experiments in vitro. The rat MoAbs were visualized by using polyclonal donkey antirat IgG (H+L) antibodies linked to horseradish peroxidase (PO) and to alkaline phosphatase (AP; both from Dianova, Hamburg, Germany). β-gal protein (coded by the lacZ gene) and its derived MHC class I-restricted peptide (TPHPARIGL), which were kindly provided by Dr H.J. Schild (Eberhard-Karls-University, Tübingen, Germany), were used in functional assays. Dichloromethylenebisphosphonate (chlodronate, Cl2MBP) was kindly provided by Boehringer Mannheim.
Isolation of Sn+ macrophages from the spleen.
Single-cell suspensions from spleens of normal DBA/2 mice were prepared by mechanical dissociation and macrophages isolated by adherence to the plastic culture dishes were resuspended in PBS without divalent cations (Biochrom KG, Berlin, Germany). The Sn+ macrophage subpopulation was isolated by means of a rosetting technique using unopsonized sheep erythrocytes, as described previously.1Briefly, a 0.5% erythrocyte suspension was added to the macrophage suspension (at a ratio of 100:1). After thorough mixing, centrifugation at 200g for 15 minutes, and subsequent incubation on ice for 30 minutes, the percentage of macrophages binding greater than 4 erythrocytes was assessed by phase-contrast microscopy. Rosetted cells were separated from unbound erythrocytes and nonrosetted macrophages using Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation at 400g for 20 minutes. Then unbound erythrocytes were removed by lysis with NH4Cl hypotonic solution.
Isolation of dendritic cells (DCs) from the spleen.
Spleen DCs were isolated according to van Voorhis et al.34Briefly, after erythrocyte lysis and digestion with collagenase IV (Boehringer Mannheim) and dispase (Interchemie, Munich, Germany), spleen cells were depleted of T lymphocytes by treatment with MoAb AT83A, rabbit complement (Camon, Wiesbaden, Germany), and DNase (Sigma Chemical Co, Munich, Germany), followed by dBSA (Biomex, Mannheim, Germany) density gradient centrifugation and depletion of B lymphocytes using MoAb B220 and MACS column (Miltenyi, Bergisch Gladbach, Germany).
Depletion of Kupffer cells.
Depletion of Kupffer cells was performed using the liposome-mediated intracellular delivery of Cl2MBP that was entrapped in liposomes and kindly provided by Dr N. van Roojien (Vrije Universiteit, Amsterdam, The Netherlands).35 DBA/2 mice (10 animals per experimental group) were injected ID with ESb-MP cells and 3 weeks later were treated IV with immune spleen cells as described above. Two hundred microliters of Cl2MBP-liposomes was injected IV into tumor-bearing DBA/2 mice twice at 2 days before and 4 days after transfer of immune spleen cells. Another group of tumor-bearing mice was treated with PBS at the same time points. It has been described that, after treatment with Cl2MBP-liposomes, the liver remains selectively depleted of macrophages for about 5 to 6 days, before repopulation starts, which is completed within 10 to 14 days.35 Other liver cell populations (eg, DCs, lymphocytes, endothelial cells, etc) were shown to be unaffected by chlodronate-containing liposomes.35-37
Treatment with MoAbs against Sn.
To block the function of Sn+ macrophages in host liver after ADI, we used anti-Sn MoAbs (1C2 and SER-4 clones directed against different epitopes of Sn). Tumor-bearing mice (6 animals per experimental group) were treated IV twice (1 and 3 days after ADI) with the mixture of IC2 and SER-4 MoAbs containing 0.5 mg of ammonium sulfate-precipitated protein. Control tumor-bearing ADI-treated mice were injected with normal rat Ig (0.5 mg of protein) on the same days as anti-Sn MoAbs. Data were expressed in terms of animal survival.
Tissue preparation and staining.
Livers were removed and snap-frozen in liquid nitrogen. Five-micrometer–thick consecutive cryostat sections were mounted on uncovered glass slides. After drying overnight at room temperature, the sections were fixed in acetone for 10 minutes at room temperature and air-dried. After the fixation, the slides were washed in PBS three times for 5 minutes. To avoid nonspecific binding, the sections were incubated for 30 minutes with 1% normal rat serum followed by rat MoAb treatment for 45 minutes. After washing with PBS, the sections were incubated for another 45 minutes with the second antibody, washed again, and treated with the substrate for PO or AP. The sections were then washed with water, counterstained with haemalaun (Merck, Darmstadt, Germany), and mounted with glycerylgelatine (Merck). Negative controls were incubated as described above, but either with omission of the first antibody or treated with rat MoAbs directed to irrelevant antigens. All steps were performed at room temperature.
The double-staining procedure represented a combination of consecutive single stainings for Sn and CD8 molecules, as described above. PO-linked secondary antibody and the corresponding enzyme substrate was used in the first reaction, followed by an AP-mediated reaction for the second staining. No counterstaining with haemalaun was performed. PO activity was shown by immersing the sections in a solution containing 6 mg 3-amino-9-ethylcarbasole dissolved in 1.5 mL N,N-dimethylformamide (Merck), 15 μL 30% hydrogen peroxide, and 28.5 mL of 0.1 mol/L acetate buffer, pH 5.0.38 The substrate for the development of AP consisted of 6.3 μL 5% Neufucsin (Sigma) or 2 mg Fast Blue (Sigma), 16 μL 4% sodium nitrite (Fluka, Buchs, Switzerland), 2 mg naphthol As-Bi-Phosphate (Sigma), 20 μL N,N-dimethylformamide, and 3 mL of 0.05 mol/L Tris-HCl buffer, pH 8.7. The freshly prepared solution was filtered into the staining jar containing the sections. Development lasted approximately 3 to 5 minutes, with regular checking of the reaction intensity by microscopy. Immunohistochemical results were evaluated by counting positively stained cells and relating them to the liver lobuli. The means and standard deviations of the data obtained from the number of mice and experiments indicated were then calculated and presented in graphics.
Antibody staining of Sn+ macrophages.
Spleen Sn+ macrophages (1 × 106) were washed in PBS supplemented with 5% fetal calf serum (FCS), stained with digoxigenin-labeled SER-4 first antibody and antidigoxigenin-fluorescein F(ab′)2 second antibody, and analyzed by flow cytometry using a FACScan analyzer with CELLQuest software (Becton Dickinson, Heidelberg, Germany). Control cells were incubated with PBS/FCS instead of the first-step antibody before staining with the second-step reagent. To study the possible contamination of these macrophages with DCs, we stained them with MoAbs for DC marker N418 and rat-antimouse MHC class II. Double-positive cells were considered as DCs.
Functional assay for Sn+ macrophage-mediated antigen processing and presentation through MHC class I.
To generate CD8+ CTLs specific for lacZ-transduced ESbL cells, a subtumorigenic dose of live ESbL-lacZ cells (5 × 104 cells/50 μL PBS) was injected into the ear pinna of DBA/2 mice. Nine days later, immune spleen lymphocytes were isolated and depleted of macrophages and DCs by plastic adherence.34 These lymphocytes containing β-gal–specific CTL precursors were then incubated for 5 days with the following stimulator cells (2 × 107 responder and 2 × 106 stimulatory cells): (1) Sn+ spleen macrophages isolated from normal DBA/2 mice; and (2) spleen cells (SPL) containing all APC from normal DBA/2 mice. Before use, stimulator cells were γ-irradiated with 100 Gy (Gammacell 1000, Ottawa, Ontario, Canada). The incubation mixture contained also protein antigen (2.2 μg/mL β-gal protein) or a derived peptide (0.1 μg/mL of class I-restricted peptide of the β-gal–TPHPARIGL) that represents a major epitope of antigen-specific CTLs. To exclude the possible contamination of the β-gal solution with peptides, we purified this solution by ultrafiltration through low adsorption hydrophilic membrane using the manufacturer’s instructions (Amicon, Beverly, MA). This purification results in the removal of all peptides with a molecular weight less than 10 kD. In some experiments, anti–H-2Ld (6 μg/mL) and anti–H-2Kd (6 μg/mL) MHC class I MoAbs, normal rat IgG (6 μg/mL), or the mixture of 1C2 and SER-4 MoAbs (12 μg/mL) directed against different epitopes of Sn were also added to the culture to interfere with the generation of cytotoxic CD8 T cells. Negative controls included responder and stimulatory cells without the antigen or responder cells and antigen without APC.
To test for cytotoxic activity of generated effector T lymphocytes in vitro, the following target cell lines were used: P13.1, alacZ-transduced variant of the P815 (H-2d) mastocytoma cell line (as a positive control), and the parental P815 cells (as a negative control). For labeling, 1 × 106target cells were incubated for 90 minutes at 37°C with 0.2 μCi51Cr sodium chromate (Amersham, Braunschweig, Germany) in RPMI-1640 medium with 30% FCS. After extensive washing with the medium, cells were resuspended at a final concentration of 5 × 104/mL. The restimulated effector cells containing β-gal specific CTLs were resuspended at a concentration of 2.5 × 106/mL RPMI-1640 medium. One hundred microliters of effector (E) cells was mixed with 100 μL of target (T) cells (E:T cell ratio = 50:1, 25:1, 12:1, or 6:1) in 96-well round-bottom plates (Renner, Dannstadt, Germany). After centrifugation to promote cell contact, the plates were incubated for 4 hours at 37°C in 5% CO2. To assess target cell death, supernatants (100 μL) were harvested, the radioactivity released was measured in a gamma counter (LKB-Wallac, Turku, Finland), and the percentage of specific lysis was calculated from the mean of triplicate cultures according to the following formula: percentage of specific lysis = 100 × (experimental release − spontaneous release)/(maximal release − spontaneous release).
RESULTS
Depletion of host Kupffer cells in vivo results in breakdown of the ADI therapy effect.
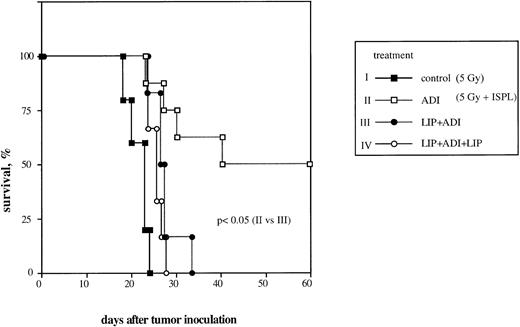
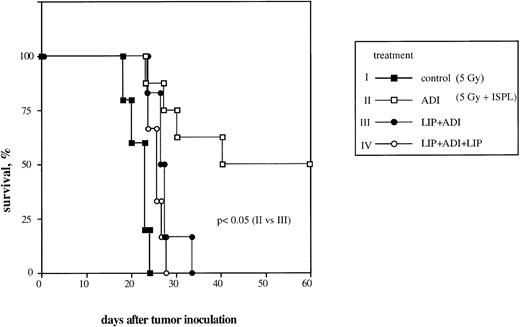
We previously reported that adoptive transfer of antitumor immune T lymphocytes from B10.D2 mice into ESb-MP tumor-bearing animals led to complete regression of liver metastases and survival in 50% to 100% of treated mice.5,6 To investigate the significance of host liver macrophages in this ADI therapy, we now performed the selective Kupffer cell depletion in vivo by liposomes containing chlodronate according to a protocol that caused a selective elimination of liver macrophages for about 5 to 6 days without any effect on other liver cell populations.35-37 Preliminary experiments showed that chlodronate entrapped in liposomes did not induce any toxicity both in normal mice and in ESb-MP tumor-bearing animals (data not shown). Adoptive transfer of tumor-immune spleen cells on day 21 into tumor-bearing mice resulted in complete tumor regression and survival in 50% of treated mice (Fig 1, ADI). A single injection of liposomes containing chlodronate 2 days before ADI led to the death of all animals, despite the transfer of immune spleen cells (Fig 1, LIP+ADI; P < .05). A similar result was obtained in a group of animals treated twice with liposomes: 2 days before and 4 days after ADI (Fig 1, LIP+ADI+LIP; P < .05).
Effect of host liver macrophage depletion in vivo on the efficiency of ADI therapy in tumor-bearing DBA/2 mice. To generate antitumor immune effector cells, ESb-MP lymphoma cells were inoculated IV at a dose of 105 cells into B10.D2 mice. One week later, spleen cells were isolated and transferred IV into sublethally irradiated ESb-MP tumor-bearing DBA/2 hosts (ADI, d0). Kupffer cells were depleted from the host by IV injection of Cl2MBP-liposomes. Each experimental group contained 10 mice. Chlodronate entrapped in liposomes did not induce any toxicity both in normal mice and in ESb-MP tumor-bearing animals (data not shown). (▪) Control (nontreated tumor-bearing mice); (□) ADI-treated tumor-bearing mice; (•) tumor-bearing mice injected with Cl2MBP-liposomes 2 days before ADI; (○) tumor-bearing mice injected twice with Cl2MBP-liposomes 2 days before and 4 days after ADI. One representative experiment of three is shown.
Effect of host liver macrophage depletion in vivo on the efficiency of ADI therapy in tumor-bearing DBA/2 mice. To generate antitumor immune effector cells, ESb-MP lymphoma cells were inoculated IV at a dose of 105 cells into B10.D2 mice. One week later, spleen cells were isolated and transferred IV into sublethally irradiated ESb-MP tumor-bearing DBA/2 hosts (ADI, d0). Kupffer cells were depleted from the host by IV injection of Cl2MBP-liposomes. Each experimental group contained 10 mice. Chlodronate entrapped in liposomes did not induce any toxicity both in normal mice and in ESb-MP tumor-bearing animals (data not shown). (▪) Control (nontreated tumor-bearing mice); (□) ADI-treated tumor-bearing mice; (•) tumor-bearing mice injected with Cl2MBP-liposomes 2 days before ADI; (○) tumor-bearing mice injected twice with Cl2MBP-liposomes 2 days before and 4 days after ADI. One representative experiment of three is shown.
The number of Sn+ Kupffer cells is elevated during immunotherapy of liver lymphoma metastasis.
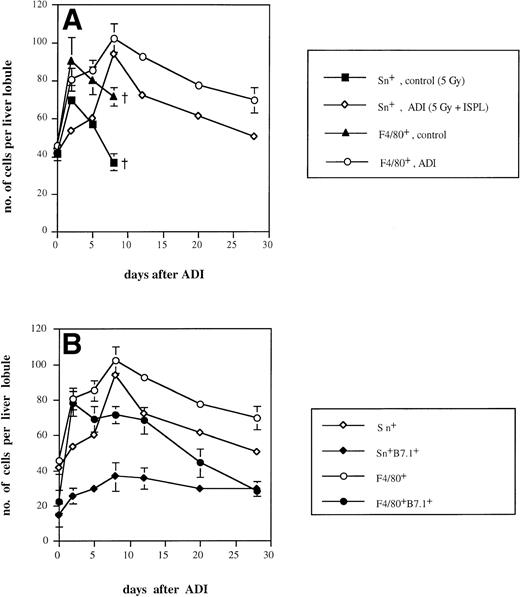
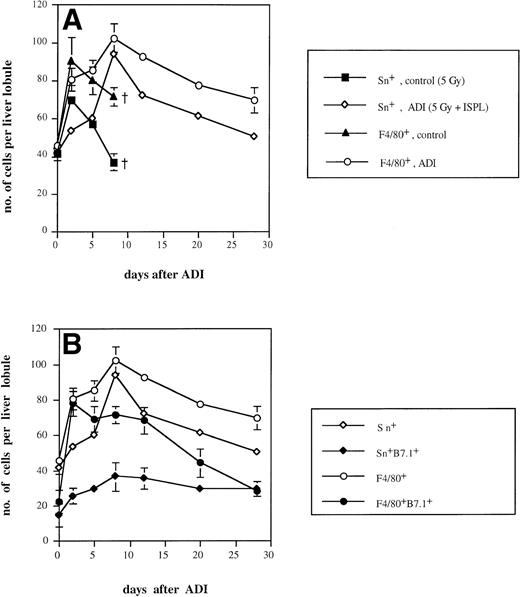
It is known that approximately 50% of normal liver macrophages are Sn+.1 With the help of the MoAb SER-4,2 these macrophages could be mainly detected in liver periportal areas. We found that the intensity of cell staining was clearly stronger in ADI-treated mice in comparison either with normal mice or with tumor-bearing animals receiving irradiation (which had no antitumor effect) or without irradiation. As shown in Fig 2A, the number of Sn+Kupffer cells in irradiated tumor-bearing animals showed an early increase (with a peak of 57 cells per liver lobule 2 days after irradiation) and then sharply decreased throughout further development of liver metastases. The number reached 37 cells per liver lobule 8 days after irradiation, which corresponds to 30 days after tumor cell inoculation. At this time point, tumor-bearing animals started to die; therefore, further observations in this group were not possible. Interestingly, nonirradiated tumor-bearing mice also showed an increase in the number of Sn+ Kupffer cells (with a maximum of 42 cells per lobule). In contrast, in ADI-treated mice, Sn+liver macrophages reached a maximum of 94 cells per liver lobule at day 8 (P < .05; Fig 2A). Thereafter, their number slowly decreased (to 51 at day 28 after ADI; P < .05). These data confirmed our previous observations.9 Although we observed an elevation in the total number of Kupffer cells (stained with MoAbs against the general tissue macrophage marker F4/80; Fig 2) upon ADI treatment, most of these (up to 92% at day 8) were Sn+macrophages. Livers of untreated tumor-bearing mice contained only around 50% of Sn+ macrophages among Kupffer cells.
Kinetics of Sn+ macrophage accumulation in livers of tumor-bearing DBA/2 mice under ADI. Frozen tissue sections were single-stained for total Kupffer cells (F4/80+) and Sn+ Kupffer cells (A) or double-stained for F4/80+/B7.1+ and for Sn+/B7.1+ (B) with corresponding MoAbs. Six lobules per liver were analyzed under the microscope. (A) The number of Sn+ (▪) and F4/80+ (▴) Kupffer cells in ESb-MP tumor-bearing untreated (d −1, 5 Gy; d0, no immune cells) mice and the number of Sn+ (◊) and F4/80+(○) Kupffer cells in ADI-treated (d −1, 5 Gy; d0, 2 × 107 B10.D2 immune spleen cells) animals are shown. (B) The number of Sn+ (◊), Sn+/B7.1+ (⧫), F4/80+(○), and F4/80+/B7.1+ (•) in ADI-treated mice are shown. Data present the means and standard deviations from two experiments with 2 to 3 animals per time point. Data points without SD represent mean values for which the SD was smaller than 1.
Kinetics of Sn+ macrophage accumulation in livers of tumor-bearing DBA/2 mice under ADI. Frozen tissue sections were single-stained for total Kupffer cells (F4/80+) and Sn+ Kupffer cells (A) or double-stained for F4/80+/B7.1+ and for Sn+/B7.1+ (B) with corresponding MoAbs. Six lobules per liver were analyzed under the microscope. (A) The number of Sn+ (▪) and F4/80+ (▴) Kupffer cells in ESb-MP tumor-bearing untreated (d −1, 5 Gy; d0, no immune cells) mice and the number of Sn+ (◊) and F4/80+(○) Kupffer cells in ADI-treated (d −1, 5 Gy; d0, 2 × 107 B10.D2 immune spleen cells) animals are shown. (B) The number of Sn+ (◊), Sn+/B7.1+ (⧫), F4/80+(○), and F4/80+/B7.1+ (•) in ADI-treated mice are shown. Data present the means and standard deviations from two experiments with 2 to 3 animals per time point. Data points without SD represent mean values for which the SD was smaller than 1.
We next studied whether Sn+ Kupffer cells expressed costimulatory molecules for T-cell activation such as B7.1 (CD80) and B7.2 (CD86). As shown in Fig 2B, the maximum of B7.1-expressing Sn+ macrophages occurred at days 8 and 12 after ADI (36 and 35 cells per liver lobule, respectively). At day 12, 49% of host Sn+ liver macrophages were B7.1+. These results were in agreement with our previous findings.39 There was also a clear-cut increase in the number of F4/80+B7.1+ Kupffer cells at day 2 after ADI, but then this population of double-positive cells gradually decreased, and at day 28, all B7.1+ liver macrophages were Sn+. In untreated tumor-bearing mice, the numbers of Sn+B7.1+ and F4/80+B7.1+ liver macrophages were significantly lower than in treated animals (not more than 15 and 27 cells per liver lobule, respectively; P < .05; data not shown). In contrast, the expression of B7.2 molecule on Sn+and F4/80+ macrophages was very low both in untreated and ADI-treated tumor bearing mice (data not shown).
The accumulation of Sn+ Kupffer cells was also observed after other types of immunotherapy. Thus, adoptive transfer of syngeneic immune PEC from DBA/2 mice into irradiated ESb lymphoma-bearing DBA/2 mice resulted in an increase in the number of Sn+ liver macrophages from 42 at day 0 to 63 at day 5 after immunotherapy (P < .05). Similar findings were observed after injection of allogeneic immune spleen cells from B10.D2 mice into ESb-MP tumor-bearing nu/nu mice (from 40 cell per liver lobule at day 0 to 82 cells at day 5; P < .05). An increase in the number of Sn+ Kupffer cells correlated in both cases with graft-versus-leukemia (GVL)-mediated effects that led to complete prevention of liver metastases and survival of treated mice.
Sn+ liver macrophages form clusters with CD8 T lymphocytes after ADI.
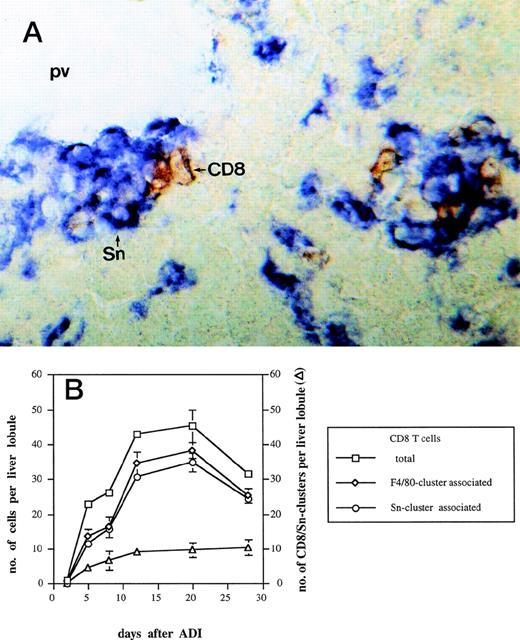
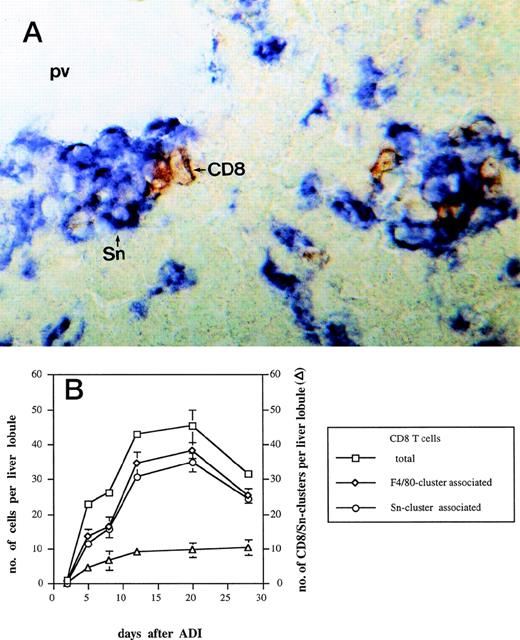
Complete tumor regression under ADI therapy, which consists of sublethal (5 Gy) host irradiation followed by B10.D2 antitumor immune spleen cell (ISPL) transfer, was shown before to depend on synergistic interactions between transfered immune CD4 and immune CD8 T cells.5 In addition, donor CD4 T lymphocytes were detected close to host liver Sn+ macrophages after ADI.9We investigated here the localization of CD8 T lymphocytes in the livers in relation to the position of this subset of macrophages. Immunohistochemical double-stainings showed a close physical association between CD8 T lymphocytes and host Sn+ Kupffer cells. Such cell clusters (defined as containing 1 or more cells of each cell type) were mainly located in periportal areas (Fig 3A). High magnification showed direct contacts between the membranes of the two interacting cell types. Figure 3B shows the kinetics of the formation of such cell clusters at different time points after ADI. The number of clusters steadily increased in a time-dependent manner and reached the maximum of 10 per liver lobule (at day 28 after treatment). The total number of CD8 T cells infiltrating the liver of tumor-bearing mice after ADI showed an increase with a peak of 45 cells per lobule at day 20 (P < .05). Already at day 5, 50% of all CD8 T lymphocytes detected in the liver were in close contact with macrophages that expressed the general marker F4/80 and Sn. The comparison of double-stainings for CD8 and F4/80 as well as for CD8 and Sn markers showed that almost all CD8 T cells were associated with Sn+but not with Sn− macrophages (Fig 3B). Four weeks after treatment, there were still 84% of CD8 T cells in clusters closely associated with Sn+ host macrophages.
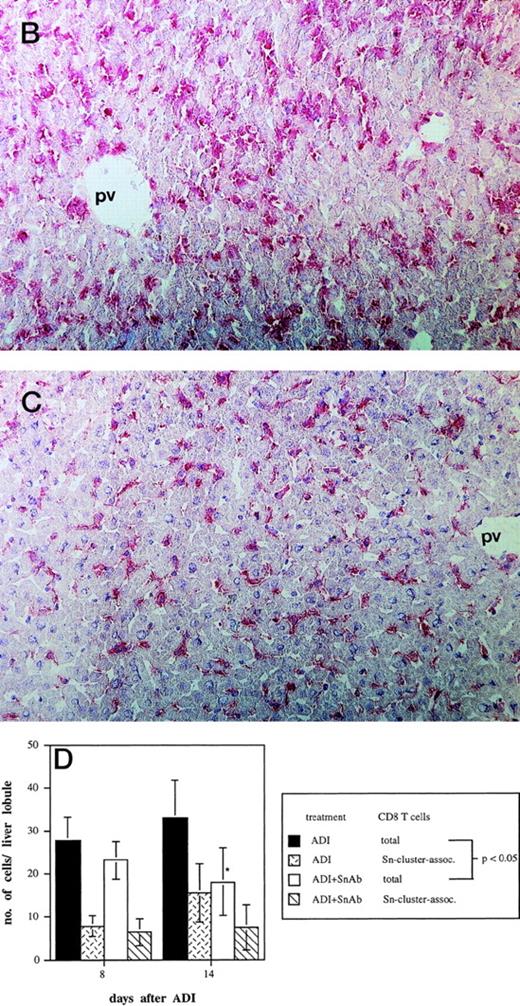
Kinetics of CD8-Sn+ cluster formation in livers of tumor-bearing DBA/2 mice under ADI. Frozen tissue sections were stained for CD8 T lymphocytes and Sn+ Kupffer cells with corresponding MoAbs. Six lobules per liver were analyzed under the microscope. (A) Immunohistochemical picture of sections, double-stained for CD8 (brown) and Sn (blue) in livers of tumor-bearing mice 12 days after ADI treatment. Show is the periportal area in which CD8 T lymphocytes and Sn+ Kupffer cells are mainly located and form cell clusters (original magnification × 400). pv, portal vein. (B) Double-staining of CD8 T lymphocytes and Sn+macrophages in mouse livers after ADI therapy. (□) Total number of CD8 T cells; (◊) the number of CD8 cells associated with F4/80+ cells in clusters; (○) the number of CD8 cells associated with Sn+ cells in clusters; (▵) the number of CD8 cell-Sn+ cell clusters. Data show the means and standard deviations from two experiments with 2 to 3 animals per time point. Data points without SD represent mean values for which the SD was smaller than 1.
Kinetics of CD8-Sn+ cluster formation in livers of tumor-bearing DBA/2 mice under ADI. Frozen tissue sections were stained for CD8 T lymphocytes and Sn+ Kupffer cells with corresponding MoAbs. Six lobules per liver were analyzed under the microscope. (A) Immunohistochemical picture of sections, double-stained for CD8 (brown) and Sn (blue) in livers of tumor-bearing mice 12 days after ADI treatment. Show is the periportal area in which CD8 T lymphocytes and Sn+ Kupffer cells are mainly located and form cell clusters (original magnification × 400). pv, portal vein. (B) Double-staining of CD8 T lymphocytes and Sn+macrophages in mouse livers after ADI therapy. (□) Total number of CD8 T cells; (◊) the number of CD8 cells associated with F4/80+ cells in clusters; (○) the number of CD8 cells associated with Sn+ cells in clusters; (▵) the number of CD8 cell-Sn+ cell clusters. Data show the means and standard deviations from two experiments with 2 to 3 animals per time point. Data points without SD represent mean values for which the SD was smaller than 1.
Interestingly, the immunotherapy of ESb lymphoma liver metastasis in DBA/2 mice with syngeneic antitumor immune PEC from DBA/2 mice also led to the formation of clusters between CD8 T cells and Sn+macrophages in the liver. Similar to the ADI with allogeneic immune spleen cells, 48% of all CD8 T cells detected in the liver at day 5 after treatment were associated with Sn+ macrophages, and 3 cell clusters per liver lobule were found at this time point.
Thus, the transfer of antitumor immune spleen cells caused an increase in host Sn+ macrophages and in donor CD8 T lymphocytes in the liver of tumor-bearing mice. The kinetics of liver infiltration by these two cell types was quite similar, and there was a remarkable physical interaction between them as shown by specifically double-stained cell clusters. The formation of cell clusters was shown not only during immunotherapy with allogeneic immune lymphocytes, but also with syngeneic ones.
Inhibition of the ADI therapy effect caused by anti-sialoadhesin MoAbs.
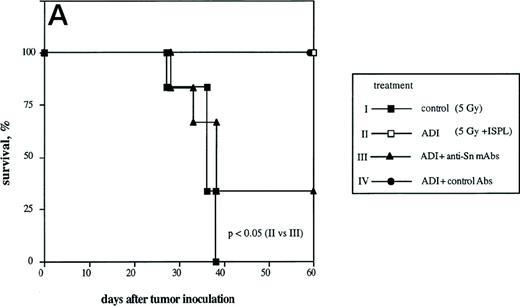
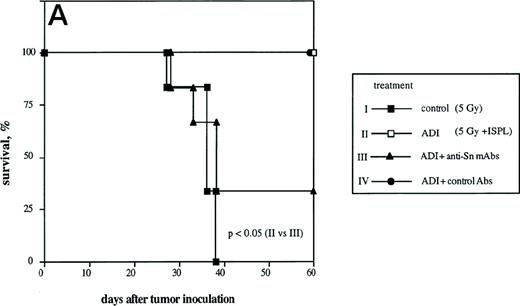
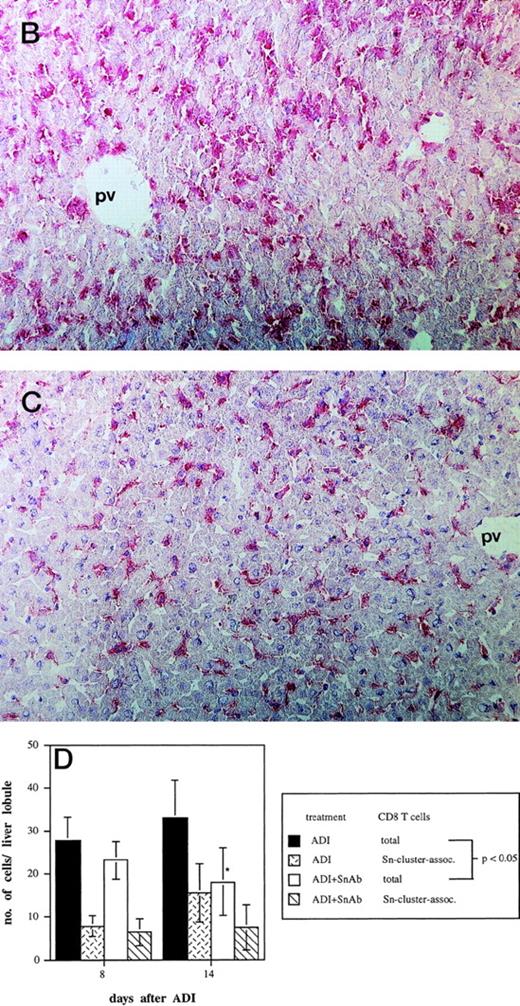
To determine the role of host Sn+ Kupffer cells during ADI, we performed antibody blocking experiments in vivo. Mice were treated twice (1 and 3 days after ADI) with a mixture of 1C2 and SER-4 rat-antimouse MoAbs directed against different epitopes of Sn to block the function of Sn+ macrophages in host livers. Whereas in the positive control (Fig 4A, ADI) 100% of ADI-treated tumor bearing mice were alive at the end of the experiment (at day 60 after tumor cell inoculation), only 33% survived in the ADI-group treated with antisialoadhesin MoAbs (Fig 4A, ADI+anti-Sn MoAbs; P < .05). Injection of normal rat IgG containing Ig with the same isotype as the anti-Sn MoAbs did not affect the immunotherapeutic effect of transferred immune spleen cells (Fig4A, ADI+control Abs). We next tested whether 1C2 and SER-4 MoAbs were able to bind to Sn+ macrophages in the liver or even to deplete these cells from the organ. In mice treated only with ADI, Sn+ cells could be detected predominantly in liver periportal areas with the help of the MoAb SER-42 (Fig 4B). Livers from corresponding mice treated also with rat-antimouse Sn MoAbs were immunohistochemically stained with AP-linked goat-antirat second antibody. The IV inoculated anti-Sn MoAbs were detectable in these livers at the sites of Sn+ Kuppfer cells 8 days after ADI and 3 days after the last injection of MoAbs (Fig 4C). These MoAbs thus were able to target the macrophages in vivo. Targeting was not associated with depletion, because the number of Sn+ Kupffer cells in this group and in mice treated only with ADI were similar (82 and 79 cells per liver lobule, respectively; P > .05). The MoAb-mediated decrease in protective immunity could be interpreted as a blocking effect. Immunohistological stainings under these conditions showed a significant decrease in the numbers of CD8 T lymphocytes infiltrating the livers (Fig 4D). At day 14 after ADI and at day 9 after the second injection of the blocking anti-Sn MoAbs, the total number of CD8 T cells was 1.8 times lower than in mice treated only with immune spleen cells (18 and 33 cells per liver lobule respectively; P < .05). At this time point, the number of CD8 T lymphocytes associated with Sn+ macrophages in clusters was also reduced (Fig 4D). In addition, we found a decrease in the number of these CD8 T cell-Sn+ macrophage clusters (from 7 to 3 clusters per liver lobule at day 14 after ADI; P < .05).
Effect of the treatment with anti-Sn MoAbs in vivo on the efficiency of ADI therapy in tumor-bearing DBA/2 mice. (A) Tumor-bearing mice (6 animals per experimental group) were treated IV with the mixture of anti-Sn MoAbs (1C2 and SER-4 directed against different epitopes of Sn) 1 and 3 days after ADI. (▪) Control (nontreated tumor-bearing mice); (□) ADI-treated tumor-bearing mice; (▴) tumor-bearing mice injected with anti-Sn MoAbs 1 and 3 days after ADI; (•) tumor-bearing mice injected with normal rat IgG (control Abs). One representative experiment of three is shown. (B and C) Immunohistochemical pictures of frozen liver sections from tumor-bearing DBA/2 mice (at day 8 after ADI) treated with immune cells only (B) or in combination with the mixture of anti-Sn MoAbs (1C2 and SER-4) (C). Shown are periportal areas in which most of the Sn+ macrophages are located. (B) shows the staining for Sn (pink) and (C) shows the staining for anti-Sn MoAbs (pink) using goat antirat second antibody (original magnification × 200). pv, portal vein. (D) Presence of CD8 T lymphocytes in the livers of mice treated with ADI and anti-Sn MoAbs. Total number of CD8 T cells in ADI-treated mice (▪) and in animals injected with anti-Sn MoAbs 1 and 3 days after ADI (□). Number of CD8 T cells associated with Sn+ Kupffer cells in clusters in mice treated with ADI only () or with ADI in combination with anti-Sn MoAbs (▧). Data represent the means and standard deviations from two experiments with 2 to 3 animals per time point. *Statistically significant difference when compared with the respective control.
Effect of the treatment with anti-Sn MoAbs in vivo on the efficiency of ADI therapy in tumor-bearing DBA/2 mice. (A) Tumor-bearing mice (6 animals per experimental group) were treated IV with the mixture of anti-Sn MoAbs (1C2 and SER-4 directed against different epitopes of Sn) 1 and 3 days after ADI. (▪) Control (nontreated tumor-bearing mice); (□) ADI-treated tumor-bearing mice; (▴) tumor-bearing mice injected with anti-Sn MoAbs 1 and 3 days after ADI; (•) tumor-bearing mice injected with normal rat IgG (control Abs). One representative experiment of three is shown. (B and C) Immunohistochemical pictures of frozen liver sections from tumor-bearing DBA/2 mice (at day 8 after ADI) treated with immune cells only (B) or in combination with the mixture of anti-Sn MoAbs (1C2 and SER-4) (C). Shown are periportal areas in which most of the Sn+ macrophages are located. (B) shows the staining for Sn (pink) and (C) shows the staining for anti-Sn MoAbs (pink) using goat antirat second antibody (original magnification × 200). pv, portal vein. (D) Presence of CD8 T lymphocytes in the livers of mice treated with ADI and anti-Sn MoAbs. Total number of CD8 T cells in ADI-treated mice (▪) and in animals injected with anti-Sn MoAbs 1 and 3 days after ADI (□). Number of CD8 T cells associated with Sn+ Kupffer cells in clusters in mice treated with ADI only () or with ADI in combination with anti-Sn MoAbs (▧). Data represent the means and standard deviations from two experiments with 2 to 3 animals per time point. *Statistically significant difference when compared with the respective control.
Host Sn+ liver macrophages thus appear to be of real importance in the GVL effect mediated by donor immune T cells.
Sn+ macrophages process and present exogenous antigen via MHC class I to CD8 T cells.
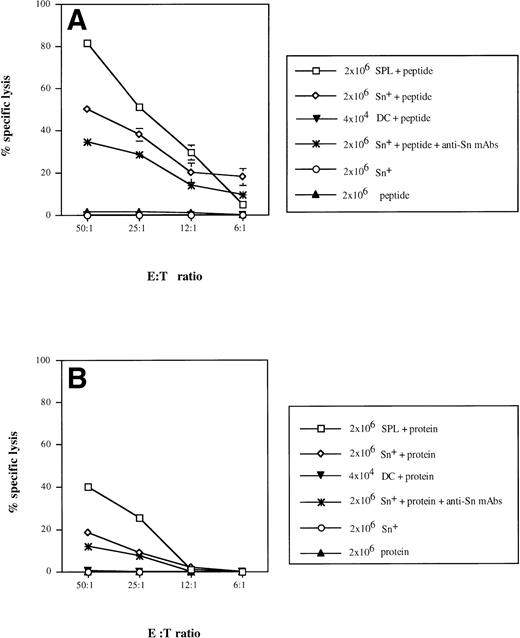
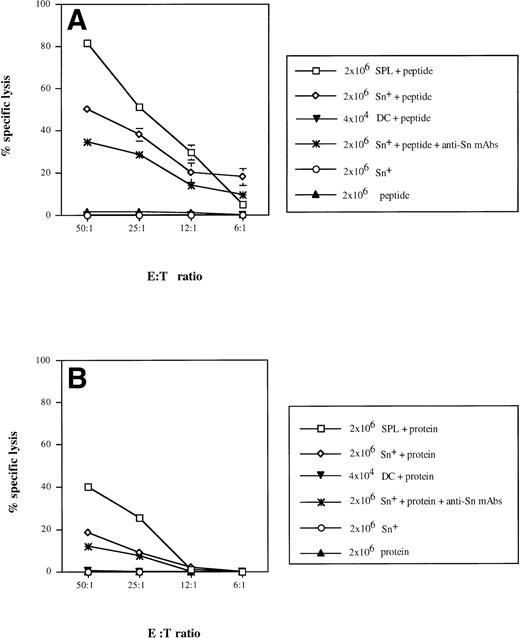
We have previously reported that Sn+ macrophages can process and present MHC class II-restricted antigen to CD4 cells.9 To test for a possible function of Sn+macrophages as APC for CD8 lymphocytes, we used a well-defined antigen, bacterial β-gal, as a model in which a dominant peptide epitope is known to be recognized by anti–β-gal CD8 CTL.40 T lymphocytes were enriched from APC-depleted β-gal immune spleen cells. Specific CTL responses were generated in cultures to which either unprocessed β-gal protein or derived dominant peptide together with either purified Sn+ macrophages or total SPL from normal mice were added. The purity of spleen Sn+macrophages isolated via a rosetting procedure was more than 95% and the contamination by DCs in this cell preparation was 1.8%, as shown by double-staining with MoAbs for DC marker N418 and MHC class II (data not shown). The CTL activity generated was determined in a 4-hour51Cr release assay using lacZ gene-transfected P13.1 mastocytoma as target cells. In positive controls, target cell lysis mediated by CD8 T lymphocytes stimulated with SPL (containing all APC) and β-gal–derived peptide TPHPARIGL ranged from 82% (E:T cell ratio = 50:1) to 5% (6:1), whereas effector cells generated by β-gal peptide without APCs caused only background lysis of 2% (Fig 5A). The addition of syngeneic Sn+ macrophages as APC and the peptide to the cultures resulted in the generation of specific CTL activity with 50% (50:1) down to 19% (6:1) target cell lysis (Fig 5A). When effector cells were generated with SPL as APC and unprocessed whole β-gal protein (extensively dialysed to remove any peptides as potential antigens), target cell killing was significantly lower as compared with stimulation with APC plus peptide (Fig 5B). When Sn+macrophages were used as APC for β-gal protein, a low but significant CD8 CTL response of 18% (50:1) and 10% (25:1) target cell lysis was generated (Fig 5B).
Sn+ macrophage-mediated presentation of MHC class I-restricted peptide of β-gal (A) and processing of β-gal protein and presentation of MHC class I-restricted peptide (B) to CD8 T lymphocytes. Immune spleen lymphocytes (responder cells) from DBA/2 mice primed 9 days before by intrapinna injection of a subtumorigenic dose of lac Z gene-transfected ESb-L cells were depleted of macrophages and DCs by plastic adherence and incubated with SPL (□), Sn+ spleen macrophages (◊; 2 × 107responder and 2 × 106 stimulatory cells), or DCs (▾; 2 × 107 responder and 2 × 104 stimulatory cells). The incubation mixture also contained β-gal whole antigen (B) or its derived MHC class I-restricted peptide (TPHPARIGL) (A). In some cultures, anti-Sn blocking MoAbs (1C2 and SER-4) were added (★). Negative controls included responder cells and Sn+ cells without the antigen (○) or antigen with responder cells only (▴). Five days later, the stimulation cultures were harvested and the cytotoxic activity of generated effector cells (E) was tested in a 4-hour51Cr release assay using as target cells (T) the lac Z-transduced P815 mastocytoma cell line, P13.1. Means and standard deviations from 4 independent experiments are shown. Data points without SD represent values for which the SD was smaller than 1.
Sn+ macrophage-mediated presentation of MHC class I-restricted peptide of β-gal (A) and processing of β-gal protein and presentation of MHC class I-restricted peptide (B) to CD8 T lymphocytes. Immune spleen lymphocytes (responder cells) from DBA/2 mice primed 9 days before by intrapinna injection of a subtumorigenic dose of lac Z gene-transfected ESb-L cells were depleted of macrophages and DCs by plastic adherence and incubated with SPL (□), Sn+ spleen macrophages (◊; 2 × 107responder and 2 × 106 stimulatory cells), or DCs (▾; 2 × 107 responder and 2 × 104 stimulatory cells). The incubation mixture also contained β-gal whole antigen (B) or its derived MHC class I-restricted peptide (TPHPARIGL) (A). In some cultures, anti-Sn blocking MoAbs (1C2 and SER-4) were added (★). Negative controls included responder cells and Sn+ cells without the antigen (○) or antigen with responder cells only (▴). Five days later, the stimulation cultures were harvested and the cytotoxic activity of generated effector cells (E) was tested in a 4-hour51Cr release assay using as target cells (T) the lac Z-transduced P815 mastocytoma cell line, P13.1. Means and standard deviations from 4 independent experiments are shown. Data points without SD represent values for which the SD was smaller than 1.
We next investigated whether the CTL response could be blocked by anti-Sn MoAbs (SER-4 and IC2) when added to the stimulation cultures containing Sn+ macrophages, T cells, and whole β-gal protein or its derived peptide. Treatment with these rat-antimouse MoAbs resulted in a diminished CTL response against β-gal–derived peptide (Fig 5A; P < .01) and against the whole protein (P < .008 at E:T cell ratio 50:1; Fig 5B). Addition of normal rat IgG containing Ig with the same isotype as the anti-Sn MoAbs caused no inhibition of CD8 CTL response (data not shown).
Taking into account that the isolated Sn+ macrophages contained about 2% DCs with the potential to present MHC class I-restricted antigens to CD8 T cells,16,18 19 we purified spleen DCs and studied their capacity to process and present β-gal and/or derived peptides under our above-mentioned conditions. When 4 × 104 DCs (which correspond to 2% from 2 × 106 Sn+ macrophages) were added into stimulatory cultures containing either β-gal protein or peptide, almost no CTL activity against P13.1 target cells could be generated (Fig 5).
We can thus conclude that Sn+ macrophages can principally function as APCs capable of processing and presenting exogenous protein via the MHC class I pathway towards MHC class I/peptide-specific CD8 CTL precursors.
Requirement for restricting MHC class I molecules for antigen presentation by Sn+ macrophages.
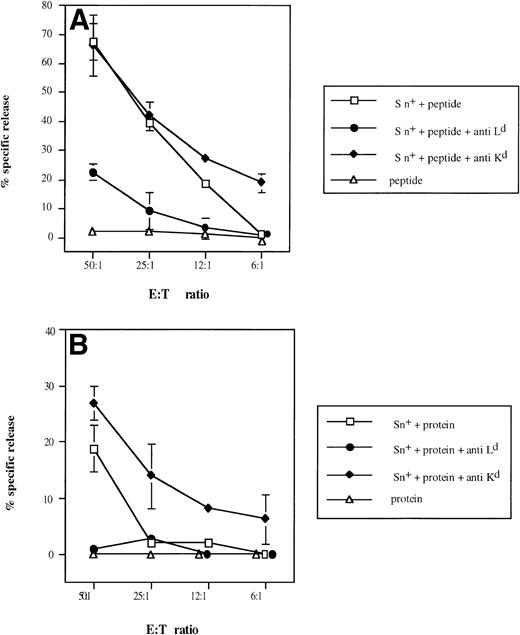
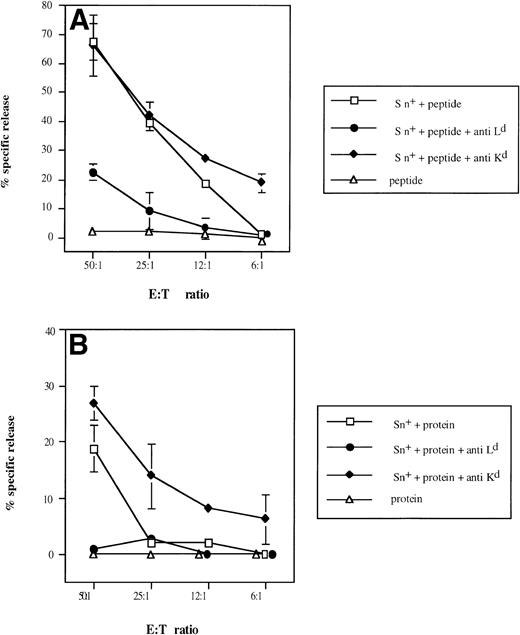
To demonstrate selective involvement of restricting MHC class I molecules on Sn+ macrophages in the presentation of β-gal–derived antigenic epitopes to CTL, we used MHC class I-Ld blocking MoAbs, because it was previously shown that CTL specific for the dominant epitope are restricted by H-2Ld molecules.40Figure 6 shows that anti-LdMoAb exerted strong inhibitory effects on the generation of β-gal peptide-specific CTL activity via either whole β-gal protein or β-gal peptide (95% and 67% of inhibition of kill, respectively, at E:T cell ratio of 50:1). No suppression of CTL activity was observed with an anti-Kd MHC class I antibody used as control (Fig6A and B). If anything, there was an augmentation of generated CTL response. When SPL were used instead as a source of APCs, the lysis of tumor cells was also inhibited by anti-Ld but not by anti-Kd antibody (90% inhibition in the presence of whole β-gal and 69% with the peptide; E:T cell ratio of 50:1).
Selective inhibitory effect of anti-Ldantibodies on Sn+ macrophage-mediated presentation of β-gal–derived MHC class I-restricted peptide (A) and on processing of β-gal protein and peptide presentation (B) to CD8 T lymphocytes. Responder cell priming, restimulation, and kill assays were performed as described in Fig 4. Cultures for responder lymphocyte restimulation contained Sn+ spleen macrophages and antigen (□). In some cultures, anti–H-2Ld (•) or control anti–H-2Kd (⧫) MoAbs were added. Negative controls included antigen (β-gal or its peptide) and responder cells only (▵). Means and standard deviations from 3 independent assays are shown. Data points without SD represent values for which the SD was smaller than 1.
Selective inhibitory effect of anti-Ldantibodies on Sn+ macrophage-mediated presentation of β-gal–derived MHC class I-restricted peptide (A) and on processing of β-gal protein and peptide presentation (B) to CD8 T lymphocytes. Responder cell priming, restimulation, and kill assays were performed as described in Fig 4. Cultures for responder lymphocyte restimulation contained Sn+ spleen macrophages and antigen (□). In some cultures, anti–H-2Ld (•) or control anti–H-2Kd (⧫) MoAbs were added. Negative controls included antigen (β-gal or its peptide) and responder cells only (▵). Means and standard deviations from 3 independent assays are shown. Data points without SD represent values for which the SD was smaller than 1.
The difference between the effects obtained with antibodies against the restricting and a nonrestricting MHC class I molecule in this system clearly demonstrates that restricting MHC class I Ldmolecules on the surface of Sn+ macrophages function as β-gal peptide-binding molecules and are responsible for the stimulatory capacity detected in this model of MHC class I-restricted antigen processing and presentation.
DISCUSSION
In the present study, we investigated the involvement of a well-characterized subpopulation of host tissue macrophages bearing the sialic acid-receptor Sn1-4 in a GVL reactivity and in the presentation of exogenous antigen via MHC class I molecules. To address this question, we used a well-defined protocol of adoptive cellular immunotherapy of ESb lymphoma liver metastasis in DBA/2 mice (H-2d, Mlsa).5 This therapy requires only relatively few tumor-immune CD4 and CD8 T cells, works even in late-stage disease, and results in the rejection of primary tumors (1.5 cm in diameter) from the skin and in eradication of established liver metastases.
We found that the depletion of Kupffer cells with liposomes containing chlodronate in tumor-bearing mice during immunotherapy led to complete prevention of the immunotherapeutic effect. It has been previously reported by us and others that the treatment with chlodronate entrapped in liposomes selectively affects liver macrophages but not other liver cells (eg, lymphocytes, DCs, endothelial cells, etc).35-37Furthermore, the depletion of macrophages with liposome-encapsulated chlodronate did not result in cytokine release.37
It is known that approximately 50% of Kupffer cells in normal livers express Sn.1 We found a dramatic increase in the number and staining intensity of these cells in ADI-treated mice. In addition, Sn+ Kupffer cells expressed also B7.1 and, to a lesser extent, B7.2, costimulatory molecules involved in T-cell activation.32 The observed increase in absolute numbers and staining intensity is probably due to recruitment of monocytes from the blood followed by differentiation into Sn+ macrophages in the environment of the metastatic liver undergoing immunotherapy. Their recruitment may be caused by chemotactic factors released by immune cells. These factors can also be produced by tumor cells, because we observed an increase in the number of Sn+ Kupffer cells in nonirradiated tumor-bearing mice. Proliferative expansion from a liver-derived precursor population is unlikely, because the cells do not express proliferation-associated markers.39Interestingly, irradiation itself can to some extent induce the accumulation of Sn+ macrophages in the liver. However, transferred antitumor immune lymphocytes are the main stimulators of this process, because we observed a much higher increase in Sn+ Kupffer cells both in a syngeneic system (injection of immune PEC from DBA/2 mice into irradiated ESb tumor-bearing DBA/2 mice) or in an allogeneic system without irradiation (transfer of immune spleen cells from B10.D2 mice into ESb-MP tumor-bearing nu/nu mice). These types of immunotherapy also caused complete regression of liver metastases and survival of treated animals.
We previously reported that these macrophages are of host origin, are located in close association with metastatic lymphoma cells, and form clusters with CD4 donor T lymphocytes in the livers.5,9Using a well-established model of the proliferative response of a T-helper clone recognizing a dominant MHC class II-associated peptide of human C-reactive protein, we found that Sn+ macrophages could both process and present this class II-restricted antigen.10 Finally, ex vivo isolated Sn+Kupffer cells from ADI-treated tumor-bearing mice were found to directly activate (without addition of TAA) CD8 T lymphocytes primed against the ESb tumor cells,9 suggesting that they presented TAA in association with MHC class I.
After ADI, an increase in the quantity of Sn+ host cells was correlated with the elevation in the number of donor CD8 T lymphocytes in metastatic livers. Most of the CD8 cells were localized in close association with Sn+ host liver macrophages in the form of clusters, the number of which was increased with the time after immunotherapy. The presence of direct contacts between the membranes of the two interacting cell types was confirmed by transmission electron microscopy.39 Because Sn− Kupffer cells hardly formed clusters with CD8 T lymphocytes, we conclude that this macrophage-lymphocyte interaction is a special property of Sn-expressing macrophages.
We have recently shown that the endogenous viral superantigen 7 (vSAG-7; Mlsa) encoded by mouse mammary tumor virus (MMTV) provirus Mtv-7 is expressed in DBA/2 but not in B10.D2 mice.41 In DBA/2 mice, vSAG7 causes deletion of SAG-reactive T cells with certain Vβ chains (eg, Vβ6) from their repertoire,42 whereas in B10.D2 tumor-resistant mice, Vβ6+ T lymphocytes are present. We showed previously that these cells can infiltrate ESb liver metastasis in DBA/2 mice after ADI with immune spleen cells from B10.D2 mice.39 To exclude the influence of the vSAG7 on the observed clustering of donor CD8 T cells with host Sn+ Kupffer cells, we injected antitumor immune PEC from DBA/2 mice (which contained no Vβ6+ T lymphocytes) into ESb lymphoma-bearing DBA/2 mice. Also under these experimental conditions, which led to complete protection, CD8 T-cell–Sn+ macrophage clusters were detected in the livers. These data suggest that CD8 T-cell clustering with Sn+ macrophages involves cognitive interactions of T-cell receptors with MHC class I-associated peptides from processed tumor antigens. Moreover, upon transfer of both allogeneic or syngeneic antitumor immune cells, we found a significant increase in the number and size of clusters formed by three cell types: donor CD4 and CD8 T lymphocytes and host Sn+ macrophages (data not shown). To clarify directly the role of host Sn+ Kupffer cells during immunotherapy in vivo, ADI-treated mice were injected with a mixture of MoAbs directed against different epitopes of Sn on macrophage surfaces (SER-4 and 1C2). We observed a significant inhibition of the immunotherapeutic effect. Interestingly, anti-Sn MoAbs were found to target to liver macrophages but did not delete the cells. Their inhibitory effect in vivo may be due to a blocking activity. As a consequence, we observed (1) a decrease in the number of CD8 T lymphocytes infiltrating the liver after ADI and (2) a decrease in the quantity of clusters formed between CD8 T cells and host Sn+ Kupffer cells.
Taking all of these observations with Sn+ macrophages into consideration, we suggest that this subpopulation of host tissue macrophages plays a role as APC in the liver during ADI and performs cognitive interactions with both CD4 and CD8 antitumor immune T cells.
To address the question of the functional significance of Sn+ macrophage-CD8 T-cell clusters in MHC class I-mediated antigen presentation, we used a well-defined model antigen (β-gal) to stimulate MHC class I peptide-specific CTL.26 40 The fact that we showed approximately 20% of specific lysis by CD8 T lymphocytes stimulated via Sn+ macrophages and β-gal whole protein suggests that Sn+ cells can process this exogenous protein and present derived peptide via MHC class I to β-gal–primed CTL precursors. The CTL response generated by Sn+ macrophages in vitro could be partially affected (but not totally blocked) by anti-Sn MoAbs added into the stimulation cultures containing Sn+ macrophages, T lymphocytes, and whole β-gal protein or its derived peptide. This inhibitory effect was specific, because the treatment by control antibodies (normal rat IgG with the same phenotype as anti-Sn MoAbs) did not negatively affect the CTL response. We suggest that the lymphocyte adhesion molecule Sn may facilitate Sn+ macrophage-CD8 T-lymphocyte interactions.
It is known that the liver not only contains macrophages, but also DCs with potential APC function for CD4 and CD8 T cells.16,19,20 Using immunohistological staining with MoAb N418 specific for DCs, we observed that the latter can also form clusters with donor CD8 T lymphocytes in livers of ADI-treated mice (unpublished observations). However, it has been recently reported that mouse DCs are negative for Sn, as shown by immunofluorescence and flow cytometry with MoAb SER-4.43Thus, DCs cannot bind sheep erythrocytes, as do Sn+macrophages, a property that is used for their isolation. Nevertheless, we found that the preparation of Sn+ macrophages contains a small number of DCs (<2%), so we had to exclude the possiblity that these were responsible for the observed APC effect. When highly enriched DCs were added as APC to stimulation cultures in numbers corresponding to this contamination (2%), almost no CTL activity against β-gal target cells could be generated. Therefore, the contaminating DCs in the purified Sn+ macrophage population could not be responsible for the observed MHC class I-restricted antigen stimulatory capacity. Interestingly, the inhibitory effect of anti-Sn MoAbs in vivo was significant but not 100% (Fig 1B). This leaves the possibility for other APC (including DCs) to participate in the observed GVL effect.
Our data raise the question about the mechanism(s) of Sn+cell-mediated presentation of exogenous soluble antigens in association with MHC class I molecules. It has recently been reported that macrophages take up bead-bound or soluble ovalbumin by phagocytosis or macropinocytosis and transfer the intact protein to the cytosol.21,44 Another mechanism includes the uptake and digestion of particulate antigens in phagolysosomes followed by binding of produced peptides to MHC class I molecules directly on the cell surface or inside the phagosome.22,45 The third mechanism involves the capture of chaperones such as gp96 that may carry proteasome-derived peptides to MHC class I molecules.23 46We are currently investigating which mechanism(s) among those listed above is operative in the case of MHC class I-restricted antigen presentation mediated by Sn+ cells.
In conclusion, we demonstrate that Sn+ host macrophages play an important role in a highly effective T-cell–mediated GVL immunotherapy of advanced metastasized cancer. We suggest that Sn+ macrophages expressing costimulatory molecules for T-cell activation (such as B7.1 and B7.2) may represent a new type of professional APC in the liver and perhaps in other tissues. They can process and present exogenous TAA via MHC class I to immune CD8 cells and via MHC class II to immune CD4 cells10 and form clusters with T lymphocytes in target organs of metastasis that may be facilitated by Sn molecules. These characteristics appear to enable them to focus CD4 and CD8 T lymphocytes at their cell surfaces, thus allowing synergistic T–T-cell interactions.
ACKNOWLEDGMENT
The authors thank Dr B. Kyewski for critical reading of the manuscript, Dr A. Benner for help with biostatistics, Dr N. van Roojien for providing the liposomes containing chlodronate, Dr B. Arnold for providing MoAbs directed against H-2Ld and H-2Kd MHC class I molecules, and Dr H.J. Schild for providing the MHC class I-restricted peptide of β-gal.
Supported by a grant from the Dr. Mildred Scheel Stiftung (no. 10-0980-Schi2, V.U. and M.R.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Victor Umansky, PhD, Division of Cellular Immunology, Tumor Immunology Program, German Cancer Research Center, D-69120 Heidelberg, Germany; e-mail: V.Umansky@dkfz-heidelberg.de.