Abstract
Even though more immunoglobulin A (IgA) is produced in humans than all other isotypes combined, relatively little is known about receptors that bind the Fc part of IgA. The myeloid IgA receptor, FcRI (CD89), triggers various effector functions in vitro, but its in vivo role remains unclear. Here, a transgenic mouse model is described in which FcRI is expressed under its own regulatory sequences. Receptor expression and regulation by cytokines was comparable to the human situation and hFcRI can trigger phagocytosis and lysis of tumor cells. To analyze the contribution of the FcR γ chain or the β2 integrin CR3 (CD11b/CD18) in FcRI biological function, FcRI transgenic mice were crossed with either FcR γ chain −/− or CR3 −/− mice. In contrast to in vitro data, FcR γ chain was essential for surface expression of hFcRI in vivo. Functional studies in hFcRI/ γ−/−mice were, therefore, limited. In vitro studies showed FcR γ chain to be necessary for phagocytosis. Neither hFcRI expression nor phagocytosis, triggered via hFcRI, were influenced by CR3. Remarkably, the capacity to lyse tumor targets was ablated in hFcRI transgenic/ CR3−/− mice, although binding of neutrophils to tumor cells was intact. This shows a previously unrecognized importance of CR3 for hFcRI-mediated antibody-dependent cellular cytotoxicity (ADCC).
RECEPTORS FOR THE Fc part of immunoglobulins (FcR) play a crucial coordinating role in host defense. Leukocyte receptors for immunoglobulin (Ig) G, E, and A classes have been characterized, and each bear unique ligand-binding α-chains. Both receptors for IgG (FcγR) and IgE (FcεR) have been extensively studied in vitro and in vivo.1 2 In contrast, knowledge about IgA receptors (FcαR) is limited and no information is available about their role in vivo.
In the early nineties, a human myeloid receptor for IgA (FcαRI, CD89) was isolated and biochemically and genetically characterized.3-5 Human FcαRI (hFcαRI) is constitutively expressed as a 55- to 75-kD protein on neutrophils and monocytes/macrophages or as a 70- to 100-kD glycoprotein on eosinophils due to increased glycosylation. It has a medium affinity (Ka ≈5 × 107mol/L−1) for IgA1, IgA2, and secretory IgA, and receptor expression is regulated by cytokines.6 In vitro studies documented the capacity of hFcαRI to trigger release of inflammatory mediators and phagocytosis of IgA-coated particles.7,8 Moreover, tumor cells are effectively lysed using IgA antitumor antibodies or bispecific antibodies (BsAb) directed to hFcαRI and tumor antigens.9 Recent work identified hFcαRI as a promising target for immunotherapy of malignant and infectious diseases.10 11
At present, neither a FcαRI equivalent is known in mice, nor is an appropriate experimental model available. We, therefore, generated a novel transgenic mouse model expressing human FcαRI using its own promoter and regulatory elements. To study involvement of (co-) signaling molecules in hFcαRI function, transgenic mice were crossed with mice lacking such units, eg, FcR γ chain- or CR3-deficient mice. It has been documented that, like most other leukocyte Fc receptors, hFcαRI complexes with the FcR γ chain signaling molecule.12 Both proximal (eg, calcium release) and distal (eg, cytokine production) signaling events initiated via hFcαRI were shown dependent on association with the FcR γ chain.13The integrity of the ITAM signaling motif within the FcR γ chain was essential for hFcαRI signaling ability.14 Work from several laboratories indicated CR3 (CD11b/CD18) to be involved in FcγR function (see Brown15). Antibodies directed to CR3 were able to inhibit IgG-mediated phagocytosis by monocytes.16 In addition, CD11/CD18 devoid polymorphonuclear leukocytes (PMN) from patients with leukocyte adhesion deficiency failed to amplify phagocytosis of IgG-opsonized particles on inflammatory stimuli.17
In the present study, a FcαRI transgenic mouse is described in which expression, regulation, and function of the receptor mimics the situation in man. Crosses of hFcαRI transgenic mice with mice deficient for (co)-signaling molecules and in vitro studies in transfectant models showed that FcR γ chain was important for expression and phagocytosis, whereas CR3 was selectively involved in FcαRI-mediated antibody-dependent cellular cytotoxicity (ADCC).
MATERIALS AND METHODS
Antibodies and flow cytometry.
Surface expression of hFcαRI or CR3 was determined using fluorescein isothiocyanate (FITC)-conjugated F(ab’)2 fragments of antihuman FcαRI monoclonal antibody (MoAb) (A77-FITC) (Medarex, Annandale, NY) or MoAb M1/70-FITC (Boehringer, Mannheim, Germany), respectively. Neutrophils were defined with Gr-1 (PharMingen, San Diego, CA) and monocytes/macrophages with F4/80 (Serotec, Oxford, UK) and on the basis of light scatter characteristics. CD45R/B220 and anti-T–cell receptor (TCR)αβ served to distinguish lymphocytes. Biotin-conjugated MoAb were detected with phycoerythrin (PE)-labeled Streptavidin (Becton Dickinson, San Jose, CA).
Whole blood of mice was incubated with MoAb (10 μg/mL) for 15 minutes at room temperature (RT) and then subjected to FACS Lysing Solution (Becton Dickinson). Peritoneal cells (2 × 105), either freshly isolated or cultured, were incubated with MoAb for 30 minutes at 4°C. To examine IgA binding, whole blood of mice was incubated with human serum IgA (Cappel, Aurora, OH; 250 μg/mL) for 1 hour at 4°C. After washing, cells were incubated with PE-labeled F(ab’)2 fragments of GαHIgA antibody (Southern Biotechnology, Birmingham, AL). Cells were analyzed on a FACScan (Becton Dickinson).
Rabbit anti-Candida albicans (C. albicans) IgG was obtained from Biodesign (Kennebunk, MA). BsAb A77xαCan, BsAb A77x520C9,10 and A77x ox erythrocyte (A77xOE) were prepared as described.18 The anti-HER-2/neu MoAb 520C9 (Medarex) and TA-1 (Calbiochem, La Jolla, CA) recognize different epitopes on HER-2/neu, a proto-oncogene product overexpressed on human carcinoma cells.
Transgenic mice.
A cosmid clone (R31931, 41 kb) of chromosome 19 carrying the 12-kb human FcαRI gene was used to generate transgenic FVB/N mice. The cosmid clone was kindly provided by Dr L.K. Ashworth (Human Genome Center, Livermore, CA).19 DNA was linearized, isolated by electroelution, and microinjected into fertilized oocytes. Two different transgenic founders were mated with FVB/N mice. Heterozygous transgenic offspring were identified by analyzing peripheral blood neutrophils for hFcαRI expression using anti-hFcαRI MoAb A77.
Southern blots.
Southern blots were performed as described.20 Samples with different amounts of DNA were digested with EcoRI, electrophoresed through 0.8% agarose gels, and blotted onto Qiabrane nylon plus filters. Blot hybridization was performed using a random prime32P-labeled 0.9 kb human FcαRI coding region probe.4 Copy numbers of the transgenes were determined by quantitating intensity of bands using ImageQuant PhosphorImager software (Molecular Dynamics, Inc, Sunnyvale, CA). Human genomic DNA digested with EcoRI served as a reference.
Cell culture.
The breast carcinoma cell line SK-BR-3, overexpressing HER-2/neu was obtained from the American Type Culture Collection (Rockville, MD). Cells were cultured in RPMI 1640 medium (GIBCO BRL, Grand Island, NY), supplemented with 10% fetal calf serum (FCS) and antibiotics, and harvested using trypsin-EDTA (Life Technologies, Paisley, UK). The murine IIA1.6 cell line was transfected with the pCAV vector containing human FcαRI cDNA (generously provided by Dr C. Maliszewski, Immunex, Seattle, WA) and pNUT vector containing either wild-type murine FcR γ chain or mutated Y65F-Y76F cDNA.14 Cells were cultured in RPMI, 10% FCS supplemented with 5 mmol/L methotrexate to allow selection for hFcαRI/γ or hFcαRI/γY65F-Y76F positive cells.
Mouse bone marrow cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4.5 g/L glucose, 10% FCS, and antibiotics, with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) (50 ng/mL) or tumor necrosis factor-α (TNF-α) (50 ng/mL). After 24 hours, nonadherent cells were harvested and stained with A77-FITC and Gr-1-PE. Adherent cells were harvested after 8 days and stained with F4/80 and A77-FITC. Peritoneal macrophages were cultured for 24 hours with 50 ng/mL GM-CSF/TNF-α to induce hFcαRI before functional studies.
To increase blood neutrophil counts, mice were injected subcutaneously with granulocyte colony-stimulating factor (G-CSF) (1.6 μg/mouse/day) for 4 days.21 Whole mouse blood was incubated for 1 minute in 0.2x phosphate-buffered saline (PBS) to lyse erythrocytes. Murine GM-CSF and murine G-CSF were generously provided by Dr J. Andresen (Amgen, Thousand Oaks, CA). Murine TNF-α was a kind gift from Dr W. Buurman (University of Limburg, Limburg, The Netherlands).
Crossing of transgenic mice.
Generation of FcR γ chain-deficient mice22,23 and CR3-deficient mice24 has been described before. The hFcαRI transgenic mouse was crossed either with FcR γ chain-deficient or CR3-deficient mice. Heterozygous offspring was crossed back with FcR γ chain-deficient or CR3-deficient mice, yielding four different genotypes: NTg,+/−, NTg,−/−, Tg,+/−, and Tg,−/−. FcR γ chain genotype was detected by genomic polymerase chain reaction (PCR).22 CR3 phenotypes were determined with flow cytometry and genotypes were confirmed by genomic PCR using sense 5′-TGA GCT ATC CAG AGG TAG AC-3′ and antisense 5′-CAT ACC TGT GAC CAG AAG AGC-3′ primers to detect wild-type CR3 alleles or sense and antisense 5′-ATC GCC TTC TTG ACG AGT TC-3′ primers to detect mutant CR3 alleles. Step program was 94°C, 1 minute; 56°C, 1 minute; 72°C, 2 minutes, 40 cycles. hFcαRI PCR was performed using sense primer 5′-GAG CAC AGT CAG TAG ACT TC-3′ and antisense primer 5′-GAT TCC GAG CGT GAG TCC A-3′ (94°C, 1 minute; 60°C, 1 minute, 72°C, 2 minutes; 30 cycles).
Phagocytosis.
Phagocytic capacity mediated via hFcαRI was investigated using ox erythrocytes (OE) or C. albicans as targets. OE were washed three times with PBS and labeled by incubation with FITC (0.4 mg/mL; Sigma, St Louis, MO) in 0.1 mol/l NaH2PO4/Na2HPO4 buffer, pH 9.6 for 30 minutes at RT. OE were washed three times and coated with BsAb A77 × anti-OE. Peripheral blood neutrophils were incubated with OE (E:T = 1:20) for 25 minutes at 37°C. Nonphagocytosed OE were lysed in 0.2 × PBS for 1 minute before microscopical or flow cytometrical analysis. Phagocytosis of C. albicans,10 or phagocytosis of Staphylococcus Aureus Wood bacteria by hFcαRI-transfected IIA1.6 cells was performed as described previously25 with minor modifications. Bacteria (1 × 108) were stained with the fluorochrome PKH26 (2 × 10−3mmol/L, 15 minutes, RT; Sigma) and opsonized with human serum IgA (Cappel; 1 mg/mL, 30 minutes, 37°C). Cell-surface bound bacteria were detected using F(ab’)2 fragments of GαHIgA-FITC (Southern Biotechnology). Samples were analyzed by flow cytometry.
C. albicans kill.
Killing of C. albicans by peritoneal PMN was analyzed using a colony-forming unit assay.26 Freshly grown yeast particles (1 × 105) were incubated with 1 × 105 PMN in RPMI 1640 medium alone or medium with 10 μg/mL BsAb A77xαCan for 2 hours at 37°C. PMN were lysed by incubation for 30 minutes at -70°C, which did not affect C. albicansviability. Samples (quadruplicate) were spread over Sabouraud 4% glucose agar plates (Merck, Darmstadt, Germany), and colony-forming units were calculated after a 24-hour incubation at 37°C.
ADCC.
A 51Chromium release assay21 was used to evaluate the capacity of mouse blood cells to trigger lysis of tumor cells. A total of 1 × 106 SK-BR-3 tumor cells overexpressing HER-2/neu were incubated with 150 μCi of51Cr (Amersham, Little Chalfont, UK) for 2 hours at 37°C, washed three times, and plated (5 × 103/well) in 96-well round bottom microtiter plates. A total of 50 μL whole blood of G-CSF–treated mice and various concentrations of BsAb A77x520C9 were added. Cells were incubated at 37°C for 4 hours, after which 51Cr-release in supernatants was measured.
Immunoadsorption and Western blots.
To verify physical interaction of hFcαRI with murine FcR γ chain, we performed immunoadsorption experiments.22 Peritoneal exudate neutrophils (1 × 107) were incubated with A77 hybridoma culture supernatant, washed, and lysed in 3-[(3-Cholamidopropyl)dimethylammonio]-1-propane-sulfonate (CHAPS) buffer containing several protease inhibitors. Lysates were centrifuged to remove insoluble material and incubated overnight at 4°C with Protein G-Sepharose Beads (Pharmacia, Uppsala, Sweden) in the presence of GαMIgG1 (Southern Biotechnology). Adsorbed material was separated on 12.5% nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. Membranes were stained for FcR γ chain using a rabbit anti-FcR γ chain antiserum (kindly provided by Dr J.-P Kinet, Harvard, Boston, MA).
Statistics.
Statistical analyses were performed with unpaired two-tailed Student’st-tests. P < .05 was considered significant.
RESULTS
Expression of human FcαRI by myeloid cells of transgenic mice.
A 41-kb cosmid insert containing the hFcαRI gene (Fig 1A) was injected into FVB/N oocytes, to generate transgenic mice. Two transgenic mouse lines, designated 2107 and 2126, were established, and copy numbers of transgenes were estimated to be between 1 to 2, and 2 to 3, respectively, by semiquantitative Southern blots (Fig 1B). Neutrophils showed hFcαRI expression, whereas only a subpopulation of monocytes expressed hFcαRI. Macrophages isolated from the peritoneal cavity and nonmyeloid cells, such as lymphocytes (Fig 1C), platelets, endothelial cells, and hepatocytes (data not shown) exhibited no hFcαRI expression. The transgenic receptor was recognized by CD89 MoAb defining different epitopes on hFcαRI (A77 and A59; Monteiro et al27) and bound human serum IgA (Fig 1D) and IgA-opsonizedS. aureus bacteria (results not shown). Both transgenic lines exhibited identical cell expression patterns. However, due to higher expression level of hFcαRI, only results obtained from experiments with line 2126 are presented in this report.
Generation of transgenic mice expressing human FcRI. (A) Structure of the transgene consisting of a 41-kb cosmid insert carrying the gene encoding FcRI. Exons are represented by closed boxes. S1, S2, signal peptide; S3, putative additional signal exon39; EC1 and EC2, extracellular Ig-like domains; TM/C, transmembrane and cytoplasmic region; E, EcoRI; H, HindIII. (B) Southern blot analysis of hFcRI transgenic mice. Genomic DNA from transgenic (Tg) line 2107 (lane 1), line 2126 (lane 2), a nontransgenic (NTg) mouse (lane 3), and human DNA (lane 4) were digested with EcoRI and hybridized with hFcRI cDNA probe. Sizes of DNA fragments (kb) are indicated on the left. (C) Flow cytometric analysis of FcRI surface expression on mouse blood cells and peritoneal macrophages. Cells of nontransgenic (thin lines) and transgenic (bold lines) mice were stained with anti-FcRI MoAb A77-FITC. Cells were stained with Gr-1-PE or F4/80-biotin/streptavidin-PE to identify granulocytes and monocytes/macrophages, respectively. Anti-CD45/B220 and anti-TCRβ served to distinguish lymphocytes. This experiment was repeated at least five times, yielding essentially identical results. (D) Human IgA binding to transgenic neutrophils. Cells of NTg (thin line) and Tg (bold line) were incubated with human serum IgA and PE-labeled antihuman IgA antibody.
Generation of transgenic mice expressing human FcRI. (A) Structure of the transgene consisting of a 41-kb cosmid insert carrying the gene encoding FcRI. Exons are represented by closed boxes. S1, S2, signal peptide; S3, putative additional signal exon39; EC1 and EC2, extracellular Ig-like domains; TM/C, transmembrane and cytoplasmic region; E, EcoRI; H, HindIII. (B) Southern blot analysis of hFcRI transgenic mice. Genomic DNA from transgenic (Tg) line 2107 (lane 1), line 2126 (lane 2), a nontransgenic (NTg) mouse (lane 3), and human DNA (lane 4) were digested with EcoRI and hybridized with hFcRI cDNA probe. Sizes of DNA fragments (kb) are indicated on the left. (C) Flow cytometric analysis of FcRI surface expression on mouse blood cells and peritoneal macrophages. Cells of nontransgenic (thin lines) and transgenic (bold lines) mice were stained with anti-FcRI MoAb A77-FITC. Cells were stained with Gr-1-PE or F4/80-biotin/streptavidin-PE to identify granulocytes and monocytes/macrophages, respectively. Anti-CD45/B220 and anti-TCRβ served to distinguish lymphocytes. This experiment was repeated at least five times, yielding essentially identical results. (D) Human IgA binding to transgenic neutrophils. Cells of NTg (thin line) and Tg (bold line) were incubated with human serum IgA and PE-labeled antihuman IgA antibody.
Regulation of human FcαRI expression by cytokines.
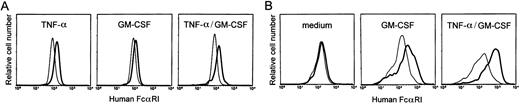
To test whether the proper regulatory elements were present in the transgenic construct, cytokine regulation of the transgene was assessed. Bone marrow cells of transgenic mice were cultured with mouse TNF-α and mouse GM-CSF, as both cytokines were documented to upregulate hFcαRI expression on myeloid cells.6,28,29Both TNF-α and GM-CSF upregulated hFcαRI expression on bone marrow-derived neutrophils (Fig 2A). No additional upregulation was observed when neutrophils were cultured with GM-CSF and TNF-α. Bone marrow-derived macrophages were grown for 8 days in medium alone or with cytokines. GM-CSF induced hFcαRI expression on macrophages and greatly enhanced cell growth, whereas cells cultured without cytokines grew slowly and did not express surface hFcαRI (Fig 2B). Growing cells with TNF-α alone inhibited cell growth, and after 8 days, no cells could be detected. However, when macrophages were cultured with both GM-CSF and TNF-α, an additional upregulation of hFcαRI was found. Similar data were generated using peritoneal macrophages (results not shown). G-CSF, interferon (IFN)-γ or interleukin (IL)-10, which all increase hFcγRI (CD64) expression on phagocytes,20 30 had no effect on hFcαRI expression of either macrophages or neutrophils (n = 3).
Cytokine regulation of hFcRI expression in transgenic mice. (A) Bone marrow-derived neutrophils were cultured overnight with TNF- and/or GM-CSF and stained for surface expression of FcRI. Cells cultured in the presence of cytokines (bold lines) were compared with cells cultured in medium alone (thin lines). Gr-1-PE was used to identify granulocytes. (B) Effect of GM-CSF and TNF- on hFcRI expression on bone marrow-derived macrophages. Cells of nontransgenic (thin lines) and transgenic (bold lines) mice were cultured for 8 days. Cells were stained with anti-hFcRI MoAb A77-FITC and counterstained with F4/80-biotin/streptavidin-PE to define macrophages. This experiment was repeated four times with similar results.
Cytokine regulation of hFcRI expression in transgenic mice. (A) Bone marrow-derived neutrophils were cultured overnight with TNF- and/or GM-CSF and stained for surface expression of FcRI. Cells cultured in the presence of cytokines (bold lines) were compared with cells cultured in medium alone (thin lines). Gr-1-PE was used to identify granulocytes. (B) Effect of GM-CSF and TNF- on hFcRI expression on bone marrow-derived macrophages. Cells of nontransgenic (thin lines) and transgenic (bold lines) mice were cultured for 8 days. Cells were stained with anti-hFcRI MoAb A77-FITC and counterstained with F4/80-biotin/streptavidin-PE to define macrophages. This experiment was repeated four times with similar results.
Phagocytosis mediated by human FcαRI.
Phagocytic capacity of peripheral blood PMN was assessed using OE as targets. Neutrophils were incubated with FITC-labeled and anti-hFcαRI BsAb (A77xOE)-coated OE. Before analysis, nonphagocytosed OE were lysed. Only neutrophils of Tg mice, but not of NTg control mice, phagocytosed BsAb-coated OE (Fig 3A, upper panels), whereas nonopsonized OE were not ingested. Fluorescence of neutrophils after ingestion of FITC-OE was determined with flow cytometry (Fig 3A, lower panels). Phagocytosis experiments were also performed with C. albicans yeast particles as targets. Uptake of C. albicans by Tg PMN was effectively enhanced in the presence of BsAb A77xαCan (Fig 3B, right panel), compared with phagocytosis without BsAb (results not shown). PMN from NTg litter mates were unable to phagocytose C. albicans (Fig 3B, left panel). After phagocytosis, enhanced C. albicans killing by Tg PMN was found in the presence of hFcαRI-targeted BsAb (Fig 3C), but not by control NTg PMN.
Biological functions triggered via FcRI on transgenic neutrophils. (A) Phagocytosis of BsAb-coated OE by granulocytes of nontransgenic (upper left panel) and transgenic (upper right panel) mice analyzed by microscopy. Flow cytometric analysis is shown in lower panels. White blood cells were incubated with nonopsonized OE-FITC (thin lines) or A77xOE BsAb opsonized OE-FITC (bold lines). Nonphagocytosed OE were lysed. FITC-fluorescence of granulocytes reflects phagocytosed OE. Gr-1-PE was used to identify granulocytes. (B) Microscopic analysis of phagocytosis of BsAb-coated C. albicans by granulocytes of nontransgenic (left panel) or transgenic (right panel) mice. (C) C. albicans killing by PMN from transgenic and nontransgenic mice in the presence of medium alone (white bars) or 10 μg/mL BsAb A77xCan (black bars). *P < .05 versus NTg control. (D) Capacity of FcRI to trigger whole blood ADCC. Whole blood of NTg (○) and Tg (▪) mice was incubated with51Cr-labeled SK-BR-3 tumor cells in the presence of hFc RI-directed BsAb. 51Cr release from duplicates was measured. *P < .05 versus values of NTg. The data shown (mean ± standard deviation [SD]) are representative of results obtained in four separate experiments.
Biological functions triggered via FcRI on transgenic neutrophils. (A) Phagocytosis of BsAb-coated OE by granulocytes of nontransgenic (upper left panel) and transgenic (upper right panel) mice analyzed by microscopy. Flow cytometric analysis is shown in lower panels. White blood cells were incubated with nonopsonized OE-FITC (thin lines) or A77xOE BsAb opsonized OE-FITC (bold lines). Nonphagocytosed OE were lysed. FITC-fluorescence of granulocytes reflects phagocytosed OE. Gr-1-PE was used to identify granulocytes. (B) Microscopic analysis of phagocytosis of BsAb-coated C. albicans by granulocytes of nontransgenic (left panel) or transgenic (right panel) mice. (C) C. albicans killing by PMN from transgenic and nontransgenic mice in the presence of medium alone (white bars) or 10 μg/mL BsAb A77xCan (black bars). *P < .05 versus NTg control. (D) Capacity of FcRI to trigger whole blood ADCC. Whole blood of NTg (○) and Tg (▪) mice was incubated with51Cr-labeled SK-BR-3 tumor cells in the presence of hFc RI-directed BsAb. 51Cr release from duplicates was measured. *P < .05 versus values of NTg. The data shown (mean ± standard deviation [SD]) are representative of results obtained in four separate experiments.
Antibody-dependent cell-mediated cytotoxicity triggered via hFcαRI.
The ability of hFcαRI to trigger ADCC in whole blood was tested using HER-2/neu-overexpressing SK-BR-3 tumor cells as targets. Cells of Tg mice were capable of lysing tumor cells even in the presence of low concentrations (0.08 μg/mL) of anti-hFcαRI x anti-HER-2/neu BsAb (A77x520C9). No tumor cell lysis was observed using blood of NTg mice (Fig 3D). In parallel experiments, we verified the capacity of NTg cells to lyse tumor target cells via endogenous mouse FcγR. In the presence of the parental mouse IgG1 anti-HER-2/neu MoAb 520C9 (2 μg/mL), 70.3% ± 12.1% of tumor cells were lysed (n = 3). Forty-five percent of the cells in whole blood of G-CSF–treated animals was hFcαRI-expressing neutrophils, while only few other cells (monocytes) expressed hFcαRI. It was therefore likely that neutrophils were responsible for the observed tumor cell lysis (Fig3D). To test this, experiments were performed with isolated peritoneal neutrophils from mice injected with thioglycollate. Both neutrophils of PBS- or G-CSF–treated Tg mice induced lysis of tumor cells in the presence of anti-hFcαRI × anti-HER-2/neu BsAb (65% lysis with 2 μg/mL A77x520C9; n = 2). As a control, we also tested a human FcγRI (CD64)-directed BsAb, (MDX-H210), which is an anti-FcγRI × anti-HER-2/neu BsAb.21 This BsAb did not exhibit any cytotoxic activity with FcαRI Tg neutrophils as effector cells (n = 3; data not shown).
hFcαRI interaction with the FcR γ chain signaling molecule.
To elucidate the necessity of FcR γ chain for hFcαRI function in vivo, hFcαRI Tg mice were crossed with FcR γ chain-deficient animals, resulting in hFcαRI transgenic, FcR γ chain-deficient mice (Tg,γ−/−). Genotypes were checked by genomic PCR (Fig 4A). hFcαRI PCR showed a 900-bp band. PCR of wild-type or mutant FcR γ chain yielded bands of 300 bp (closed arrowhead), or 950 bp (open arrowhead), respectively. Surface hFcαRI expression was checked on mouse cells (Fig 4B). Remarkably, neutrophils of Tg,γ−/− mice did not express hFcαRI on their membrane. Also, monocytes or GM-CSF/TNF–α cultured macrophages entirely lacked hFcαRI expression (n = 4). Immunoadsorption of hFcαRI from neutrophils showed mouse FcR γ chain, indeed, to be physically associated with hFcαRI in Tg,γ+/−mice (Fig 4C).
The FcR γ chain is essential for both surface expression of and phagocytosis by hFcRI. (A) Genomic DNA from wild-type and FcR γ chain-deficient mice was checked for the presence of hFcRI and FcR γ chain by PCR. Wild-type FcR γ chain PCR products are marked by the closed arrowhead and positions of mutant FcR γ chains are marked by the open arrowhead. (B) Surface expression of hFcRI on granulocytes of Tg,γ⌈+/− (bold line), Tg,γ−/− (thin line), or NTg,γ⌈+/− (filled area) analyzed by flow cytometry. Cells were stained with A77-FITC. (C) Physical interaction between FcR γ chain and hFcRI was shown in Tg mice by FcR γ chain immunoadsorption with MoAb directed to hFcRI. The gel was run under nonreducing conditions and the blot was stained with a rabbit anti-FcR γ chain antiserum. Position of FcR γ chain homodimers is marked by the arrowhead. (D) Phagocytosis of IgA-coated PKH26-labeled bacteria by hFcRI/γ chain transfectants is dependent on a functional ITAM. Human secretory IgA-opsonized bacteria were incubated with hFcRI transfectant cells. FL-2 fluorescence represents bacterial binding to transfectants. After incubation at either 4°C or 37°C, remaining cell-surface bound bacteria was detected using GhIgA-FITC. A decrease in FITC-fluorescence (FL-1) reflects phagocytosis. One representative experiment of five is shown.
The FcR γ chain is essential for both surface expression of and phagocytosis by hFcRI. (A) Genomic DNA from wild-type and FcR γ chain-deficient mice was checked for the presence of hFcRI and FcR γ chain by PCR. Wild-type FcR γ chain PCR products are marked by the closed arrowhead and positions of mutant FcR γ chains are marked by the open arrowhead. (B) Surface expression of hFcRI on granulocytes of Tg,γ⌈+/− (bold line), Tg,γ−/− (thin line), or NTg,γ⌈+/− (filled area) analyzed by flow cytometry. Cells were stained with A77-FITC. (C) Physical interaction between FcR γ chain and hFcRI was shown in Tg mice by FcR γ chain immunoadsorption with MoAb directed to hFcRI. The gel was run under nonreducing conditions and the blot was stained with a rabbit anti-FcR γ chain antiserum. Position of FcR γ chain homodimers is marked by the arrowhead. (D) Phagocytosis of IgA-coated PKH26-labeled bacteria by hFcRI/γ chain transfectants is dependent on a functional ITAM. Human secretory IgA-opsonized bacteria were incubated with hFcRI transfectant cells. FL-2 fluorescence represents bacterial binding to transfectants. After incubation at either 4°C or 37°C, remaining cell-surface bound bacteria was detected using GhIgA-FITC. A decrease in FITC-fluorescence (FL-1) reflects phagocytosis. One representative experiment of five is shown.
The cytoplasmic tail of FcR γ chain contains an ITAM signaling motif that proved crucial for calcium mobilization after hFcαRI cross-linking.14 To test whether this ITAM was important for hFcαRI phagocytosis, both tyrosines (present at amino acid positions 65, and 76 within the FcR γ chain cytoplasmic tail) within this ITAM were changed into phenylalanines (γ:Y65F-Y76F). IIA.1.6 cells expressing this mutant FcR γ chain were evaluated for their ability to bind and ingest human serum IgA-coated S. Aureusbacteria. Cells expressing hFcαRI/γ or hFcαRI/γ:Y65F-Y76F showed comparable FL-2 fluorescence intensities (indicating comparable binding of IgA-opsonized bacteria). Only cells expressing hFcαRI/γ, however, phagocytosed bacteria, represented by a drop in FITC-fluorescence, (shown in FL-1) on incubation at 37°C (Fig 4D, upper right panel).
CR3 (CD11b/CD18) plays a role in hFcαRI-triggered ADCC, but not phagocytosis.
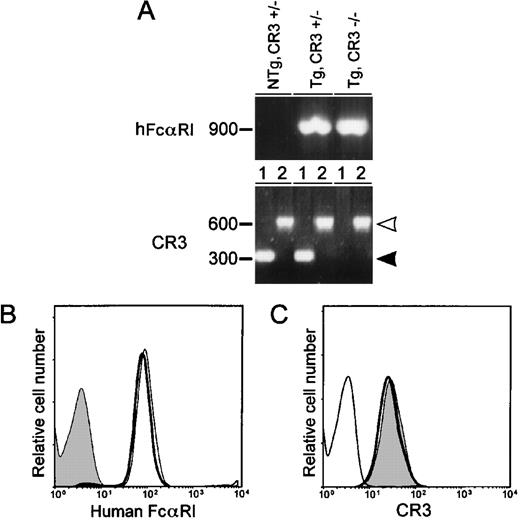
To examine whether the integrin CR3 (CD11b/CD18) was involved in hFcαRI function, CR3-deficient animals were crossed with hFcαRI transgenic mice. Phenotypes of offspring were tested by flow cytometry (Fig 5B and C), and genotypes were confirmed by genomic PCR (Fig 5A). hFcαRI PCR showed a 900-bp band, whereas wild-type CR3 and mutant CR3 yielded bands of 300 bp (closed arrowhead), and 600 bp (open arrowhead), respectively. No differences were observed in hFcαRI expression levels between Tg,CR3+/− and Tg,CR3−/− (Fig 5B).
The β2 integrin CR3 (CD11b/CD18) does not affect surface expression of FcRI in transgenic mice. (A) Detection of FcRI and CR3 in genomic DNA from wild-type and CR3-deficient mice by PCR. Wild-type CR3 (lanes 1) is marked by the closed arrowhead and mutant CR3 (lane 2) by the open arrowhead. (B and C) Flow cytometric analyses of hFcRI and CR3 expression on peripheral blood granulocytes of Tg,CR3⌈+/− (bold lines), Tg,CR3−/− (thin lines), or NTg,CR3⌈+/− (filled area) mice. Cells were stained with A77-FITC to detect hFcRI (B) or M1/70-FITC to assess CR3 expression (C). Experiments were repeated four times, yielding identical results.
The β2 integrin CR3 (CD11b/CD18) does not affect surface expression of FcRI in transgenic mice. (A) Detection of FcRI and CR3 in genomic DNA from wild-type and CR3-deficient mice by PCR. Wild-type CR3 (lanes 1) is marked by the closed arrowhead and mutant CR3 (lane 2) by the open arrowhead. (B and C) Flow cytometric analyses of hFcRI and CR3 expression on peripheral blood granulocytes of Tg,CR3⌈+/− (bold lines), Tg,CR3−/− (thin lines), or NTg,CR3⌈+/− (filled area) mice. Cells were stained with A77-FITC to detect hFcRI (B) or M1/70-FITC to assess CR3 expression (C). Experiments were repeated four times, yielding identical results.
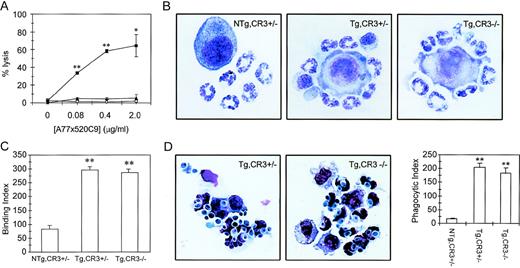
ADCC capacity of Tg,CR3−/− blood cells was determined with a 51Cr-release assay. In the presence of 0.08 μg/mL anti-hFcαRI BsAb (A77x520C9), 37.5% of tumor cells were lysed by Tg,CR3+/−cells, reaching 65% lysis with 2 μg/mL BsAb (Fig 6A). Remarkably, cells of Tg,CR3−/− mice were unable to lyse tumor cells. To test the possibility that neutrophils exhibit defective binding to tumor cells, we studied adherence of PMN to BsAb-coated tumor target cells (Fig 6B and C). No differences in binding capacity were found between Tg,CR3+/− and Tg,CR3−/− (Fig 6C). To test phagocytic capacity in CR3−/− cells, neutrophils were incubated withC. albicans. Both Tg,CR3+/− and Tg, CR3−/− avidly phagocytosed C. albicans in the presence of A77xαCan BsAb (Fig 6D), whereas cells were unable to ingest yeast particles without BsAb (results not shown).
ADCC, but not phagocytosis triggered via hFcRI, depends on the presence of CR3 (CD11b/CD18). (A) hFcRI-mediated ADCC is absent in CR3-deficient mice. Whole blood of NTg,CR3⌈+/−(○), Tg,CR3⌈+/− (▪), or Tg,CR3−/− (◂) mice was incubated with51Cr-labeled SK-BR-3 tumor cells. 51Cr release from duplicates was quantitated as reflection of tumor cell lysis. *P < .05, **P < .001 versus values of NTg. (B) Binding of neutrophils to A77x520C9 BsAb-opsonized tumor cells is unaffected in CR3-deficient mice. (C) Binding of neutrophils to tumor cells was quantitated on cytospin preparations (binding index = number of granulocytes/100 tumor cells). **P < .001 versus NTg controls. (D) Microscopical analysis of hFc RI phagocytosis. Peritoneal granulocytes of Tg,CR3⌈+/− (left panel) or Tg,CR3−/− (middle panel) were incubated with C. albicans in the presence or absence of BsAb A77xCan. Phagocytic index (phagocytosed C. albicans/100 cells) was determined on cytospins (right panel). **P < .001 versus NTg controls.
ADCC, but not phagocytosis triggered via hFcRI, depends on the presence of CR3 (CD11b/CD18). (A) hFcRI-mediated ADCC is absent in CR3-deficient mice. Whole blood of NTg,CR3⌈+/−(○), Tg,CR3⌈+/− (▪), or Tg,CR3−/− (◂) mice was incubated with51Cr-labeled SK-BR-3 tumor cells. 51Cr release from duplicates was quantitated as reflection of tumor cell lysis. *P < .05, **P < .001 versus values of NTg. (B) Binding of neutrophils to A77x520C9 BsAb-opsonized tumor cells is unaffected in CR3-deficient mice. (C) Binding of neutrophils to tumor cells was quantitated on cytospin preparations (binding index = number of granulocytes/100 tumor cells). **P < .001 versus NTg controls. (D) Microscopical analysis of hFc RI phagocytosis. Peritoneal granulocytes of Tg,CR3⌈+/− (left panel) or Tg,CR3−/− (middle panel) were incubated with C. albicans in the presence or absence of BsAb A77xCan. Phagocytic index (phagocytosed C. albicans/100 cells) was determined on cytospins (right panel). **P < .001 versus NTg controls.
DISCUSSION
Although much progress has been made in our understanding of the role Fc receptors play in immunity,1,2 the function of receptors for IgA is poorly understood. Existing data on hFcαRI are based on in vitro experiments, and because no hFcαRI equivalent has been identified in mice, in vivo data are scarce. To overcome this difficulty, two lines of transgenic mice were generated in which hFcαRI expression was restricted to the myeloid lineage. The receptor was recognized by MoAb defining different epitopes on hFcαRI27 and could bind human IgA. Levels of hFcαRI expression were solely regulated by cytokines known to influence FcαRI expression on human cells, eg, GM-CSF28 and TNF-α,29 and not by cytokines that regulate FcγRI expression, eg, G-CSF, IFN-γ, and IL-10.20,30Furthermore, hFcαRI was capable of triggering phagocytosis and ADCC. Taken together, these results indicated hFcαRI to be expressed in a functional way using its own promoter and regulatory elements, thus mimicking the situation in man.6
It has been documented that hFcαRI complexes with the FcR γ chain signaling molecule.12 To assess the role of FcR γ chain in hFcαRI signaling, Tg mice were crossed with FcR γ chain−/− animals. Although the hFcαRI gene was clearly present in Tg,γ−/− mice, no hFcαRI expression was detected on the membrane of neutrophils. This is notably different from earlier in vitro data in IIA1.6 cells that indicated FcR γ chain to be important for hFcαRI function, but not for expression per se.13 Importantly, the present data document FcR γ chain to be essential for hFcαRI expression in vivo on PMN and monocytes/macrophages, which parallels the situations for FcγRI,22 FcγRIII,31 and FcεRI.32 Due to the heterologous nature of our transgenic mouse model, however, we cannot formally exclude subtle differences to exist between transgenic and human phagocytes. Still, our data emphasize the importance of performing studies on phagocytic receptors naturally expressed on cells.
Lack of receptor expression in Tg,γ−/− mice, however, precluded studying FcR γ chain involvement in hFcαRI-function in in vivo derived cells. Phagocytic capacity was therefore tested in vitro, using IIA1.6 cells expressing hFcαRI, and wild-type or mutant FcR γ chains. Although both types of transfectant cells could bind IgA-coated bacteria, only FcαRI/γ cells exhibited phagocytosis. The integrity of the FcR γ chain ITAM was, thus, shown critical for hFcαRI phagocytic capacity. Previous work showed signaling via hFcαRI to require a functional ITAM14 and to involve both Src family and Syk protein tyrosine kinases.33
Several laboratories have documented a requirement for CR3 in both FcγR-mediated ADCC34 and phagocytosis.15 17To study whether CR3 is involved in hFcαRI function, Tg mice were crossed with CR3-deficient mice. No differences were observed in hFcαRI expression or phagocytic capacity between Tg,CR3+/− and Tg,CR3−/−cells. However, the ability of neutrophils of Tg,CR3−/− mice to lyse tumor cells via hFcαRI was absent, indicating CR3 to be essential for ADCC, but not for phagocytosis. Importantly, F(ab’)2 fragments of anti-CR3 MoAb 44a abrogate the capacity of human neutrophils to lyse tumor cells via FcαRI (A.B. van Spriel, unpublished data), as well. These data suggest qualitative differences in the requirement for select signaling molecules between hFcαRI-mediated phagocytosis and ADCC.
The molecular basis underlying the involvement of CR3 in Fc receptor function is incompletely understood. Because immune complexes can block the binding of anti-CR3 Fab fragments to monocytes16 and FcγR were shown to cocap with CR3,35 a close physical contact between these classes of receptors has been suggested. CR3 possibly provides an essential costimulatory signal, which is absent in Tg,CR3−/− cells. FcγRIII and CR3 were reported to cooperate in generation of a neutrophil respiratory burst in normal PMN.36 Work on FcγR-mediated phagocytosis supports a more direct interaction between IgG receptors and CR3: (1) FcγRIIIb-expressing NIH3T3 cells were able to bind, but unable to ingest IgG-coated targets. Cotransfection of CR3 enabled phagocytosis of these particles.37 (2) CR3 could, furthermore, restore phagocytic capacity of a phagocytosis-defective FcγRIIa tail-minus mutant in 3T3 transfectant models.38
In conclusion, the present data uncover a novel level of complexity in IgA receptor function in phagocytes: while FcR γ chain was shown to be crucial for hFcαRI expression and the capacity of the receptor to trigger phagocytosis, CR3 proved indispensable for hFcαRI-triggered ADCC. These results highlight the cooperative nature of different classes of receptors in phagocyte function. This transgenic model provides a valuable tool to further dissect the role of hFcαRI function in vivo. Because hFcαRI was recently identified as a candidate therapeutic target,9-11 these transgenics may provide a suitable model for preclinical evaluation of hFcαRI-directed therapies.
ACKNOWLEDGMENT
We thank Toon Hesp, Els Dorresteijn, and Herma Boere for excellent animal care.
Supported by Grant No. 901-12-214 from Netherlands Organization for Scientific Research (NWO) and Grants No. AI 22816 and DK 51643 from the National Institutes of Health (NIH).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jan G.J. van de Winkel, PhD, Department of Immunology, Immunotherapy Laboratory, KC.02-085.2, University Medical Center Utrecht, Lundlaan 6, 3584 EA Utrecht, The Netherlands; e-mail: J.vandewinkel@lab.azu.nl.


![Fig. 3. Biological functions triggered via FcRI on transgenic neutrophils. (A) Phagocytosis of BsAb-coated OE by granulocytes of nontransgenic (upper left panel) and transgenic (upper right panel) mice analyzed by microscopy. Flow cytometric analysis is shown in lower panels. White blood cells were incubated with nonopsonized OE-FITC (thin lines) or A77xOE BsAb opsonized OE-FITC (bold lines). Nonphagocytosed OE were lysed. FITC-fluorescence of granulocytes reflects phagocytosed OE. Gr-1-PE was used to identify granulocytes. (B) Microscopic analysis of phagocytosis of BsAb-coated C. albicans by granulocytes of nontransgenic (left panel) or transgenic (right panel) mice. (C) C. albicans killing by PMN from transgenic and nontransgenic mice in the presence of medium alone (white bars) or 10 μg/mL BsAb A77xCan (black bars). *P < .05 versus NTg control. (D) Capacity of FcRI to trigger whole blood ADCC. Whole blood of NTg (○) and Tg (▪) mice was incubated with51Cr-labeled SK-BR-3 tumor cells in the presence of hFc RI-directed BsAb. 51Cr release from duplicates was measured. *P < .05 versus values of NTg. The data shown (mean ± standard deviation [SD]) are representative of results obtained in four separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4387/4/m_blod412van03z.jpeg?Expires=1768012472&Signature=z-e6A03QmpQOXno6pyhFiNF0yDjwajGSdDwJ8gcoX1ZtiiNoLY6NyhgtBawWBMhZpqk3nrrbHASo6CMqCSfQd7HmFHaz9xCZkm4eJpoCa~F4jUNaN2oe3vFxen0Tk3qlAUFsTAJovfMNXdhe4lNTkvQNUf8Fn5YFfAcb-u4uI6s3xley3GHZEh2MeWNM~AHWrx~oeqepJuaD5doTEGOnYwa0UNB-FhIKc331NHpTXpe0ozW0mPN6X81r4VlvAODCnW4O26tNkAlT43oC72EV8PUZ~SShpIO-AOLBHzxyeX4WGSWWNMQ9f3Gz97oI73usnlFx4T33pP4WYiwEpoYxKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)