Abstract
A major problem with treating patients with cancer by traditional chemotherapeutic regimes is that their tumors often develop a multidrug resistant (MDR) phenotype and subsequently become insensitive to a range of different chemotoxic drugs. One cause of MDR is overexpression of the drug-effluxing protein, P-glycoprotein.It is now apparent that P-glycoprotein may also possess a more generic antiapoptotic function that protects P-glycoprotein–expressing cancer cells and normal cells from cell death. Herein we show that cells induced to express P-glycoprotein either by drug selection or by retroviral gene transduction with MDR1 cDNA are resistant to cell death induced by a wide range of death stimuli, such as FasL, tumor necrosis factor (TNF), and ultraviolet (UV) irradiation, that activate the caspase apoptotic cascade.However, P-glycoprotein–expressing cells were not resistant to caspase-independent cell death mediated by pore-forming proteins and granzyme B.MDR P-glycoprotein–expressing cells were made sensitive to caspase-dependent apoptosis by the addition of anti–P-glycoprotein antibodies or verapamil, a pharmacological inhibitor of P-glycoprotein function. Clonogenic assays showed that P-glycoprotein confers long-term resistance to caspase-dependent apoptotic stimuli but not to caspase-independent cell death stimuli. This study has confirmed a potential novel physiological function for P-glycoprotein and it now remains to dissect the molecular mechanisms involved in the inhibition of capsase-dependent cell death by P-glycoprotein.
MULTIDRUG RESISTANCE (MDR) is a well-defined phenomenon of cross-resistance of mammalian cells to a number of anticancer agents following exposure to one such drug.1 A diverse range of agents involved in MDR include alkaloid compounds and bacterial and fungal antibiotics, such as anthracyclines and etoposide. An accepted mechanism of MDR is a reduced cellular accumulation and an altered subcellular distribution of cytotoxic drugs. In many instances, this is mediated by increased expression at the cell surface of the MDR1 gene product, P-glycoprotein (P-gp), a 170-kD energy-dependent efflux pump.2,3Transfection studies have clearly shown that P-gp is responsible for MDR.4 MDR-mediated by P-gp is thought to be primarily due to reduced intracellular concentrations of drug and failure of the drug to reach its target.
A number of models have been proposed to explain the mechanism of action of P-gp in MDR. The traditional model for P-gp function was one where P-gp acts as a “drug pump” to export drugs out of a cell against a concentration gradient. This has been further expanded to the “flippase” model, which attempts to explain how P-gp can remove a range of structurally diverse drugs without an apparent substrate specificity.5,6 However, given that there is not always a linear correlation between extent of drug efflux and resistance to cell death, it appears that P-gp may also prevent cell death by some additional mechanism or that other cellular proteins also influence resistance to certain death stimuli. In addition to its drug efflux activity, P-gp has been suggested to function as an ion channel,7 and expression of P-gp may result in alkalinization of the cytosol due to an increase in pHi and altered membrane potential (Vm).8
P-gp is normally expressed in the epithelial cells of kidney, liver, pancreas, and intestine; and in capillary endothelia in brain and testes, consistent with its role in the export of xenotoxins out of exposed epithelial cells; or across the blood-testes or blood-brain barrier.9-11 In addition, P-gp is expressed on CD34+ hematopoietic progenitor cells and on normal human natural killer (NK) cells and CD8+ T cells.12The functional significance of the regulated expression of P-gp on specific hematopoietic cells has yet to be established. However, immunological or developmental defects in mice with functional deletion of the Mdr1a and Mdr1b genes have not been reported.13 14
The molecular events leading to programmed cell death or apoptosis involve an increasingly well-defined cascade of proteolytic cleavage events. The key cell death proteins are the cysteine-aspases (caspases), which are initially expressed as inactive zymogens and are activated by proteolytic processing to produce the active enzyme.15,16 It is now clear that cell death initiated by a range of stimuli, including FasL,17 tumor necrosis factor (TNF),18 ultraviolet (UV) irradiation,19,20granzyme B (GzB),21,22 and chemotherapeutic drugs,20,23 function by activating this central death cascade. Cytotoxic T lymphocytes (CTL) can kill target cells by two independent mechanisms: the granule exocytosis mechanism using the granule proteins such as perforin (pfp) and GzB, and the FasL-Fas pathway.21,22 Cell death due to membrane and cytosolic pertubations by pfp/GzB occurs in the absence of activation of the caspase pathway, whereas nuclear damage requires caspase activation.24 Thus, while most apoptotic stimuli have been shown to activate caspases, pfp/GzB have been shown to additionally induce cell death in a caspase-independent manner.
Chinese hamster ovary cells expressing P-gp are more resistant to apoptosis induced by serum deprivation,23 and expression of P-gp confers resistance to Fas-mediated apoptosis. This resistance to Fas-mediated apoptosis can be reversed by using antibodies that bind the extracellular domains of P-gp or verapamil, a pharmacological inhibitor of P-gp function.25 Expression of P-gp results in decreased production of active caspase 3, a key effector caspase in the apoptosis cascade upon Fas ligation. Inhibition of P-gp function restores caspase 3 activation upon crosslinking of cell-surface Fas.25 These preliminary studies were performed in only one pair of drug-selected cell lines but suggested P-gp could inhibit cell death mediated strictly via the caspase pathway. Herein we have extended this work by using P-gp expressing cells produced by retrovirus transduction of MDR1 cDNA and several drug-selected cell lines. In addition, we have tested a wide variety of caspase-dependent or -independent death stimuli to determine the protective effect of functional P-gp on the short-term and long-term survival of cells. P-gp conferred protection against all forms of caspase-dependent apoptosis studied, but cells were sensitive to caspase-independent cell death stimuli regardless of their P-gp expression.
MATERIALS AND METHODS
Cell culture.
The acute T-cell leukemia cell line, CEM-CCRF, its doxorubicin (DOX)-selected and resistant P-gp expressing line CEM-A7+, and various hybrids of CCRF and A7+expressing high (IC10) or negligible (2H6) levels of P-gp have been previously described.26 The T-cell line 12D7 and production of 12D7-MDR1 cells by transduction with a retrovirus containing the MDR1 gene was described previously.27 All cells were grown in RPMI medium supplemented with 10% (vol/vol) fetal calf serum (FCS), 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO, Grand Island, NY).
Cytotoxicity assays.
Effector cells and various soluble death stimuli were assessed by51Cr release or [125] iododeoxyuridine (125IUdR) release assays as described.25 DOX, etoposide (VP16), and vincristine (VIN) were obtained from Dr Phillip Kantharidis, Peter MacCallum Cancer Institute, East Mebourne, Australia. Dexamethasone (Sigma, St Louis, MO) and CH-11 (anti-human Fas IgM, Upstate Biotech Inc, Lake Placid, NY) were purchased. Recombinant soluble human TNF was kindly provided (Peprotech, Rocky Hill, NJ). Rat pfp and human GzB were purified as previously described28 and were used in combination as described.25 The spontaneous release of 51Cr or125IUdR was determined by incubating the target cells with medium alone (or in the presence of anti–P-gp monoclonal antibody [MoAb], verapamil, or caspase inhibitor where applicable). It should be noted at the concentrations used, inhibitors alone did not cause release, nor did they affect the long-term survival of cell lines. The maximum release was determined by adding sodium dodecyl sulfate (SDS) to a final concentration of 5%. The percent specific lysis was calculated as follows: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. To inhibit P-gp function, the labeled targets were preincubated for 30 minutes with MRK-16 (IgG2a MoAb, final 1 to 100 μg/mL; Kamiya Biochemical Company, Thousand Oaks, CA), UIC2 (IgG2a MoAb, final 0.1 to 5 μg/mL; Coulter, Miami, FL), or verapamil (0.5 to 10 μmol/L; Knoll Australia, Lane Cove, Australia) before the cytotoxicity assay. Isotype control antibodies were included where applicable. To inhibit caspase activity, labeled target cells were preincubated for a further 30 minutes with peptidyl fluoromethyl ketones (fmk; ZFA-fmk, ZVAD-fmk; Enzyme System Products, Dublin, CA; final 0 to 50 μmol/L).
UV irradiation.
Cells were plated at 1 × 106 cells/mL in flat-bottom 24-well plates and irradiated with UV light by placing the plate, without a lid, directly under a UV transilluminator. Cells were removed at appropriate times and allowed to incubate at 37°C for 24 hours before apoptosis was assessed. Apoptotic cells were identified by two separate assays. Trypan blue exclusion assays were performed by mixing an equal volume of cells with 0.3% Trypan blue, and cells from four fields of vision were counted. The percentage of apoptotic cells was also determined by treating cells with propidium iodide (PI) as described,29 and cells in the sub G0/G1 population were detected using a FACScan (Becton Dickinson, San Jose, CA).
Clonogenic assays.
Cells (5 μL from a stock of 4 × 104 cells/mL) treated with various apoptotic stimuli were plated out in triplicate on soft agar. Cells were diluted in 5 mL RPMI containing 20% (vol/vol) FCS, the supplements listed above, and 0.3% noble agar (Difco, Detroit, MI) and plated in 60-mm dishes. Once set, the dishes were overlaid with 2.5 mL of media, incubated at 37°C for 12 days, and the total number of colonies per plate were counted.
Anti–P-gp immunoblotting.
CEM and 12D7 subclones (2 × 105) were lysed in 50 μL of ice-cold Nonidet P-40 lysis buffer (25 mmol/L HEPES, pH 7/250 mmol/L NaCl/2.5 mmol/L EDTA/0.1% Nonidet P-40/0.5 mmol/L DTT/2 mmol/L Pefabloc) at 4°C for 30 minutes. Insoluble material was removed by centrifugation at 4°C. Proteins (25 μg) were separated on SDS-10% polyacrylamide gels and electroblotted onto nylon membranes. Blots were probed with anti–P-gp MoAb C219 (Sapphire Bioscience, Sydney, Australia) and visualized by enhanced chemiluminescence.
RESULTS
Drug-selected P-gp+ve cell lines are resistant to caspase-dependent apoptosis induced by chemotherapeutic drugs.
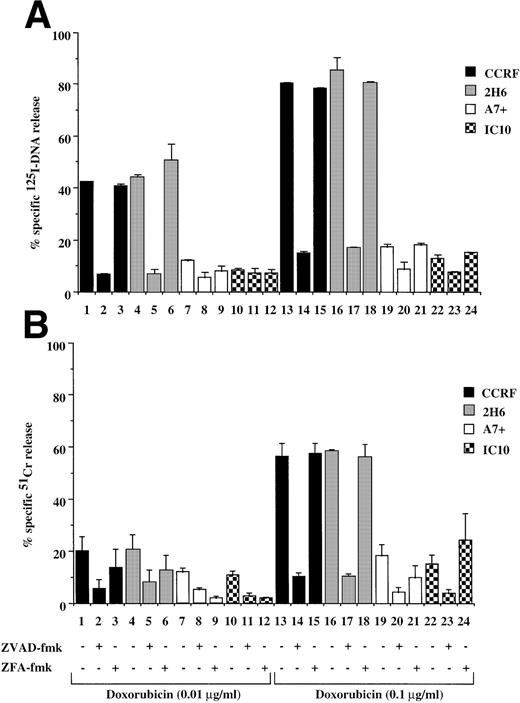
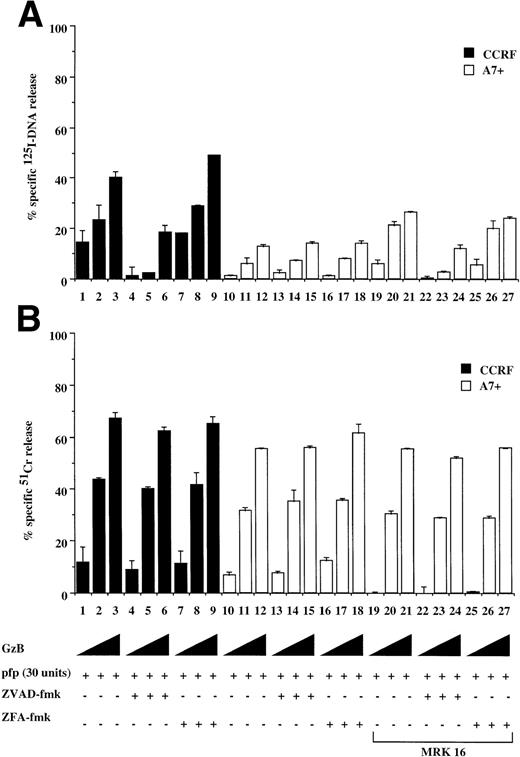
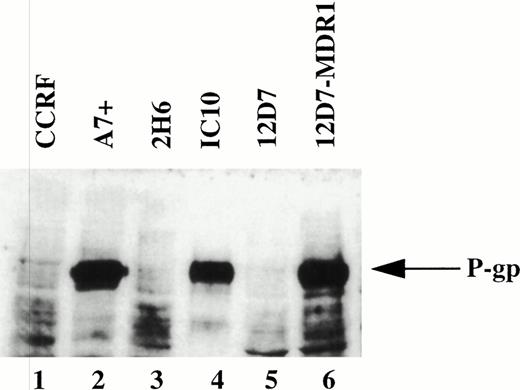
It has been reported that chemotherapeutic drugs induce cell death by activating the caspase apoptotic pathway.20 23 To confirm this finding and to determine the effect of P-gp expression on caspase-mediated apoptosis, we analyzed the cytotoxicity of different drugs on matched P-gp+ve and P-gp−ve cell lines. We initially determined P-gp expression on CEM-CCRF, its DOX-selected P-gp+ve–derived line CEM-A7+, and two hybrids of CCRF and A7+ by Western analysis (Fig 1, lanes 1 through 4). A7+and IC10 express high levels of P-gp (Fig 1, lanes 2 and 4), whereas CCRF and 2H6 are P-gp−ve (Fig 1, lanes 1 and 3). These analyses were confirmed by flow cytometry using two separate MoAbs, MRK-16 and UIC2, which bind to extracellular domains on P-gp (data not shown). Exposure P-gp−ve cell lines to the anthracycline, DOX, resulted in DNA fragmentation (Fig 2A, lanes 1, 4, 13, and 16) and membrane lysis (Fig 2B, lanes 1, 4, 13, and 16), as shown by125I-DNA and 51Cr release, respectively. By contrast, cell death in P-gp+ve cell lines was significantly decreased (Fig 2A and B, lanes 7, 10, 19, and 22). The cell death events induced by DOX in P-gp−ve cells were blocked with the generic caspase inhibitor ZVAD-fmk (Fig 2A and B, lanes 2, 5, 14, and 17), but not with the control ZFA-fmk (Fig 2B, lanes 3, 6, 15, and 18). Similar cell death assays using the vinca alkaloid, VIN; the glucocorticoid, DEX; and the topoisomerase II inhibitor, VP16, revealed that these different classes of drugs used at multiple doses all induced both nuclear and cell membrane damage in the P-gp−ve cell lines but had far reduced effects on P-gp+ve cell lines (Table 1, one drug dose shown). The cell death induced by these different agents was inhibited by ZVAD-fmk but was not affected by ZFA-fmk (Table 1). These data indicate that the two different P-gp−vecell lines tested were sensitive to different classes of chemotherapeutic drugs, whereas P-gp+ve cell lines were truly MDR. Despite their different modes of action, all of the drugs were inhibited by ZVAD-fmk, indicating that they all induce apoptosis in a caspase-dependent manner.
Expression of P-gp on drug-selected and retrovirus transduced cell lines. Western blot of cell lysates from drug-selected CEM cell lines CCRF, A7+, 2H6, IC10; the T-cell line 12D7 and 12D7 transduced with a retrovirus containing full-length human MDR1 cDNA (12D7-MDR1) were probed with the anti–P-gp MoAb, C219. The position of 170 kD P-gp is indicated by the arrow on the right.
Expression of P-gp on drug-selected and retrovirus transduced cell lines. Western blot of cell lysates from drug-selected CEM cell lines CCRF, A7+, 2H6, IC10; the T-cell line 12D7 and 12D7 transduced with a retrovirus containing full-length human MDR1 cDNA (12D7-MDR1) were probed with the anti–P-gp MoAb, C219. The position of 170 kD P-gp is indicated by the arrow on the right.
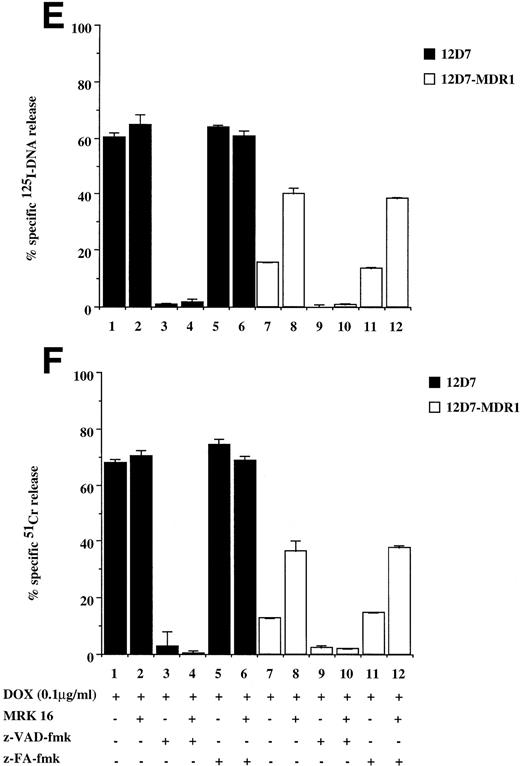
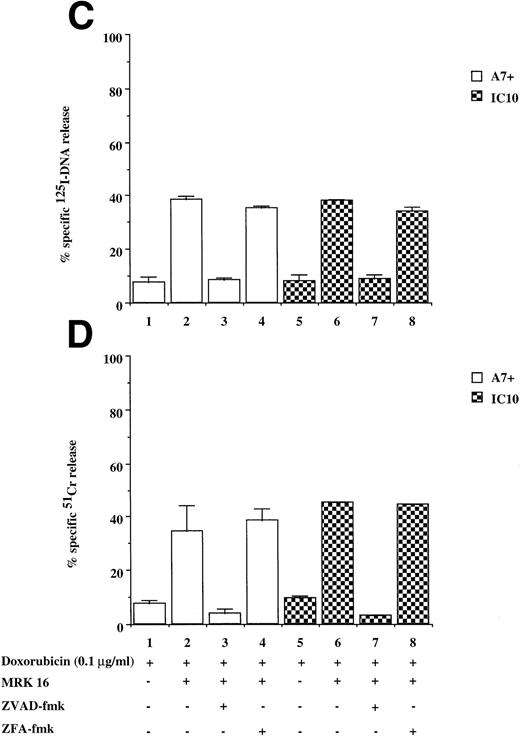
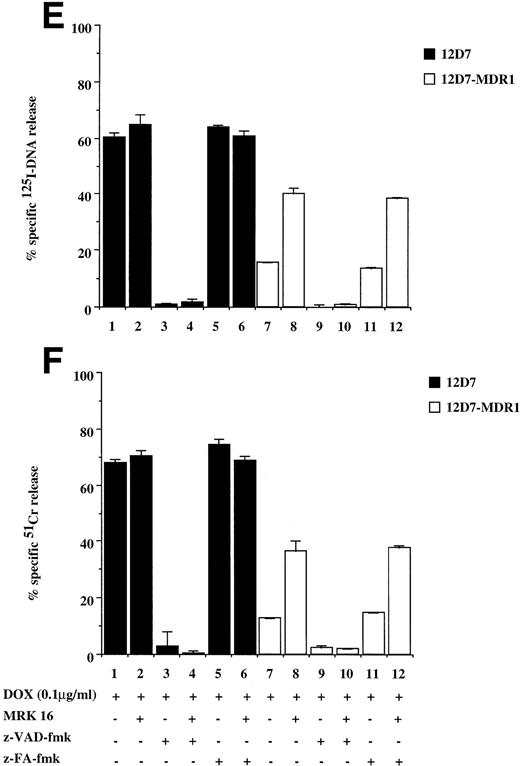
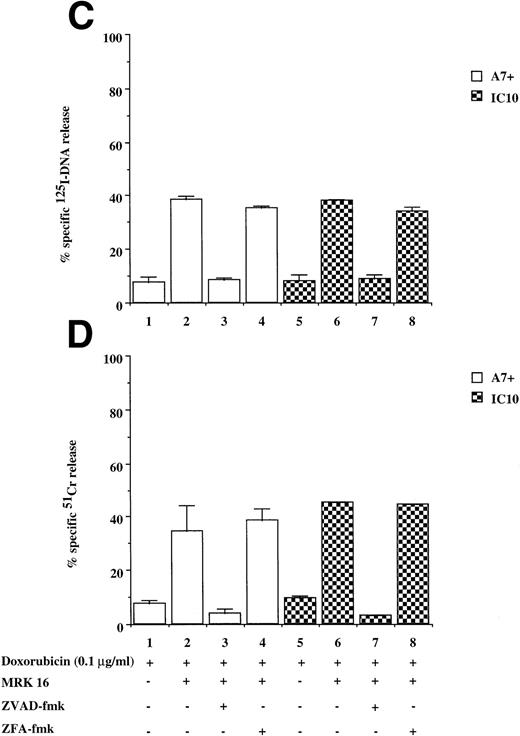
Drug-selected and retroviral transduced P-gp+ve cells are resistant to DOX-mediated, caspase-dependent DNA fragmentation and membrane lysis. Drug-selected CEM cell lines (P-gp−ve CCRF, 2H6; P-gp+veA7+, IC10) (A, B) and parental 12D7 (P-gp−ve) and retrovirus transduced 12D7-MDR1 (P-gp+ve) cells (E, F) were labeled with125IUdR and 51Cr for 1 hour, washed in growth media, and incubated for 48 hours in 96-well plates (2 × 104 cells/well) with DOX. In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor as indicated in Table 1. P-gp+ve cells were made sensitive to DOX-mediated DNA fragmentation (C) and membrane lysis (D) by preincubation with anti–P-gp MoAb, MRK 16 (50 μg/mL). DNA fragmentation and membrane lysis were reflected by125I-DNA release (A, C, E) and 51Cr release (B, D, F), respectively. Data are calculated as the mean ± SE of triplicate samples and are representative of a least two different experiments.
Drug-selected and retroviral transduced P-gp+ve cells are resistant to DOX-mediated, caspase-dependent DNA fragmentation and membrane lysis. Drug-selected CEM cell lines (P-gp−ve CCRF, 2H6; P-gp+veA7+, IC10) (A, B) and parental 12D7 (P-gp−ve) and retrovirus transduced 12D7-MDR1 (P-gp+ve) cells (E, F) were labeled with125IUdR and 51Cr for 1 hour, washed in growth media, and incubated for 48 hours in 96-well plates (2 × 104 cells/well) with DOX. In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor as indicated in Table 1. P-gp+ve cells were made sensitive to DOX-mediated DNA fragmentation (C) and membrane lysis (D) by preincubation with anti–P-gp MoAb, MRK 16 (50 μg/mL). DNA fragmentation and membrane lysis were reflected by125I-DNA release (A, C, E) and 51Cr release (B, D, F), respectively. Data are calculated as the mean ± SE of triplicate samples and are representative of a least two different experiments.
DOX-mediated DNA fragmentation and membrane lysis was induced in A7+ and IC10 by inhibiting the function of P-gp with a specific MoAb directed to an extracellular domain of P-gp (Fig 2C and D, lanes 2 and 6). Cell death in these lines was inhibited by the addition of ZVAD-fmk (Fig 2C and D, lanes 3 and 7), but not by ZFA-fmk (Fig 2C and D, lanes 4 and 8), showing that reversal of resistance to chemotherapeutic drugs by P-gp rendered these cells sensitive to caspase-dependent cell death. Similar results were observed in A7+ and IC10 cells treated with VP16, MRK 16, ZVAD-fmk, and ZFA-fmk (data not shown).
Cells expressing P-gp by retroviral transfer of the MDR1 gene are resistant to caspase-dependent chemotherapeutic drugs.
To show that the protection by P-gp against caspase-dependent apoptosis induced by different chemotherapeutic drugs is not restricted to DOX-selected CEM cells, two other matched P-gp+ve and P-gp−ve cell lines were used. The cell lines used were the human T-cell lines, 12D7 (P-gp−ve), and 12D7-MDR1 (P-gp+ve; Fig 1, lanes 5 and 6). 12D7-MDR1 is a stable cell line produced by transduction of 12D7 cells with a retroviral construct containing the wild-type MDR1 cDNA.27Treatment of 12D7 with DOX resulted in DNA fragmentation (Fig 2E, lane 1) and membrane lysis (Fig 2F, lane 1) that was inhibited with ZVAD-fmk (lane 3), but not ZFA-fmk (lane 5). By contrast, 12D7-MDR1 cells were relatively resistant to DOX-mediated cell death (Fig 2E and F, lane 7), but were rendered sensitive to DNA and membrane damage by DOX upon addition of the anti–P-gp MoAb (Fig 2E and F, lane 8). As with the other P-gp+ve cell lines used above, cell death induced in 12D7-MDR1 cells by drug and anti–P-gp MoAb was caspase-dependent (Fig2E and F, lanes 10 and 12). Similar data were obtained from 12D7 and 12D7-MDR1 cells treated with VP16 (data not shown).
Cells expressing P-gp are resistant to Fas-mediated apoptosis.
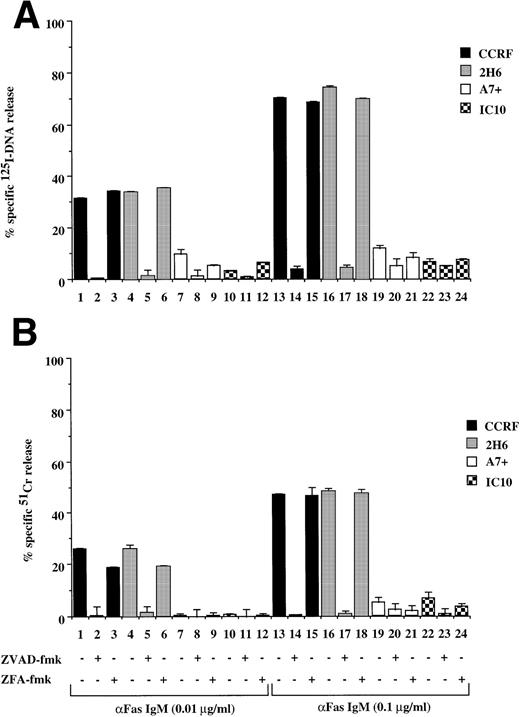
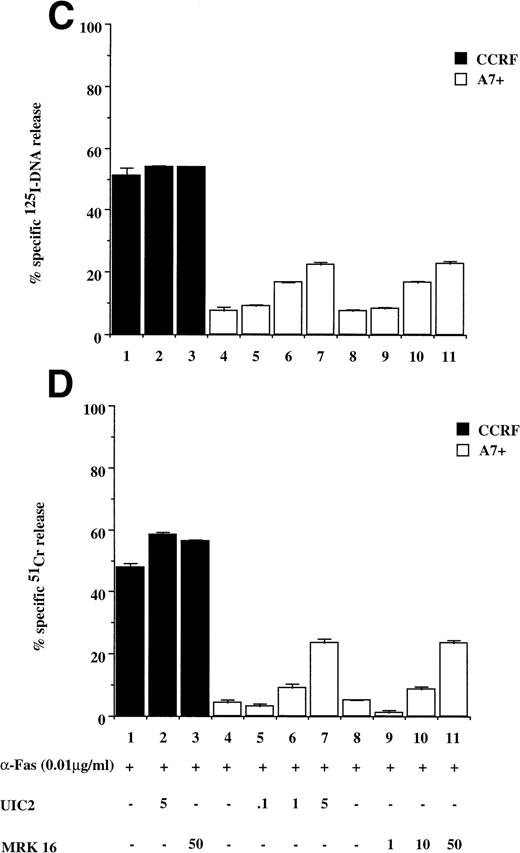
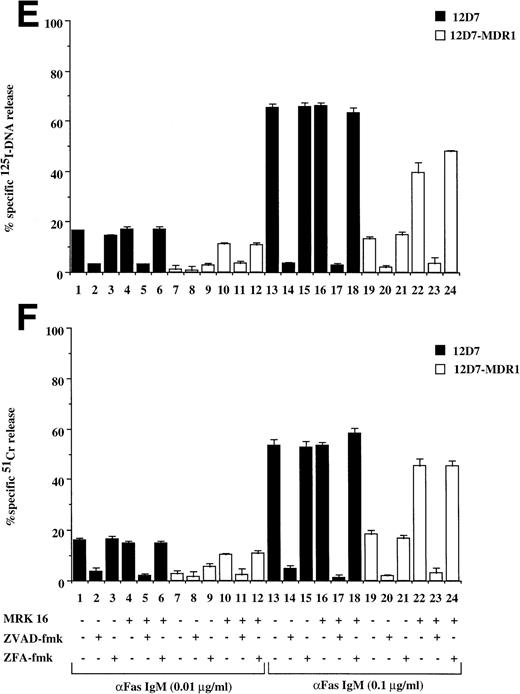
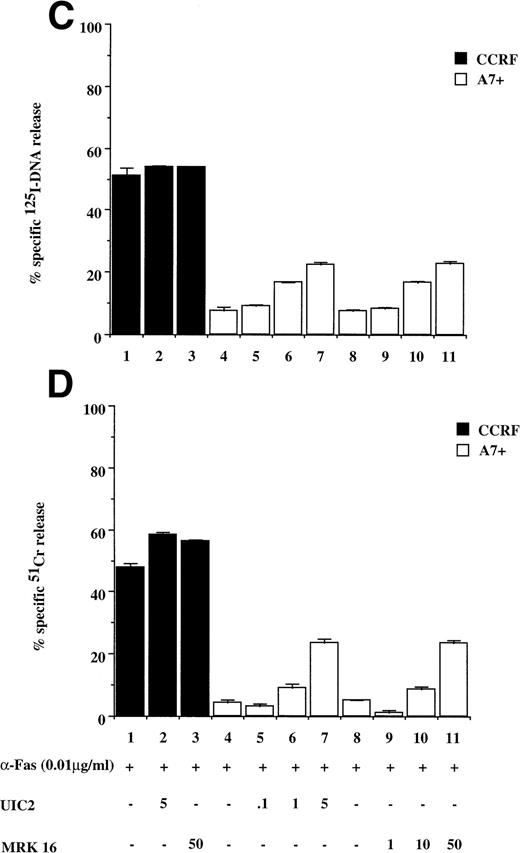
Recently, we have shown that A7+ cells were resistant to cell death induced by the Fas pathway.25 To expand these studies on P-gp+ve cells, Fas-mediated apoptosis was directly induced by three different methods. Cell surface Fas expression on these cells has been assessed previously and has been shown to be equivalent25 (and data not shown). Treatment of P-gp−ve CCRF and 2H6 cells with anti-Fas MoAb to cross-link Fas on the cell surface resulted in significant, dose-dependent DNA fragmentation (Fig 3A lanes 1 through 6, 13 through 18) and membrane lysis (Fig 3B lanes 1 through 6, 13 through 18) that was blocked by pretreatment of cells with ZVAD-fmk (Fig 3A and B, lanes 2, 5, 14, and 17) but not ZFA-fmk (Fig 3A and B, lanes 3, 6, 15, and 18). By contrast, P-gp+ve cells were relatively resistant to Fas-mediated apoptosis (Fig 3A and B, lanes 7 through 12, 19 through 24). Preincubation of A7+ with two different anti–P-gp MoAbs (MRK 16 and UIC2) rendered them sensitive to apoptosis by anti-Fas MoAb (3C and D, lanes 5 through 11). P-gp+ve 12D7-MDR1 cells were also resistant to Fas-mediated DNA fragmentation (Fig 3E, lanes 7 through 9, 19 through 21) and membrane lysis (Fig 3F, lanes 7 thorugh 9, 19 thorugh 21), and this resistance could be reversed by the addition of MRK 16 (Fig 3E and F, lanes 10 and 22) or UIC2 (data not shown). Fas-mediated cell death was caspase-dependent in 12D7-MDR1 P-gp+ve cells treated with MRK 16 (Fig 3E and F, lanes 11 and 23) or UIC2 (data not shown). Treatment of P-gp+ve and P-gp−ve cells with soluble, recombinant FasL also resulted in caspase-dependent apoptosis of Pgp−vecells, whereas P-gp+ve cells were relatively resistant (data not shown). To use a more physiological stimulator of Fas-mediated cell killing, we employed the rat/mouse hybrid cell line d12S, which expresses FasL on the cell surface and kilsl target cells in a FasL-dependent manner. Incubation of cells with d12S resulted in caspase-dependent apoptosis of P-gp−ve target cells (data not shown), but did not induce similar membrane lysis or DNA fragmentation in P-gp+ve cells.
Drug-selected and retroviral-transduced P-gp+ve cells are resistant to Fas-mediated, caspase-dependent DNA fragmentation and membrane lysis. CEM cell lines (A, B) (P-gp−ve CCRF, 2H6; P-gp+veA7+, IC10), parental 12D7 (P-gp−ve), and retrovirus-transduced 12D7-MDR1 (P-gp+ve) cells (E, F) were labeled with 125IUdR and 51Cr for 1 hour, washed in growth media, and incubated for 16 hours in 96-well plates (2 × 104 cells/well) with anti-human Fas IgM MoAb, CH-11 (0.01 μg/mL). In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. CCRF and A7+ cells (C), (D) were preincubated with the given concentrations of anti–P-gp MRK 16 MoAb (1 to 50 μg/mL) or UIC2 MoAb (0.1 to 5 μg/mL), then treated with 0.01 μg/mL anti-Fas MoAb for 16 hours. Data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments.
Drug-selected and retroviral-transduced P-gp+ve cells are resistant to Fas-mediated, caspase-dependent DNA fragmentation and membrane lysis. CEM cell lines (A, B) (P-gp−ve CCRF, 2H6; P-gp+veA7+, IC10), parental 12D7 (P-gp−ve), and retrovirus-transduced 12D7-MDR1 (P-gp+ve) cells (E, F) were labeled with 125IUdR and 51Cr for 1 hour, washed in growth media, and incubated for 16 hours in 96-well plates (2 × 104 cells/well) with anti-human Fas IgM MoAb, CH-11 (0.01 μg/mL). In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. CCRF and A7+ cells (C), (D) were preincubated with the given concentrations of anti–P-gp MRK 16 MoAb (1 to 50 μg/mL) or UIC2 MoAb (0.1 to 5 μg/mL), then treated with 0.01 μg/mL anti-Fas MoAb for 16 hours. Data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments.
P-gp confers resistance to cell death induced by UV irradiation.
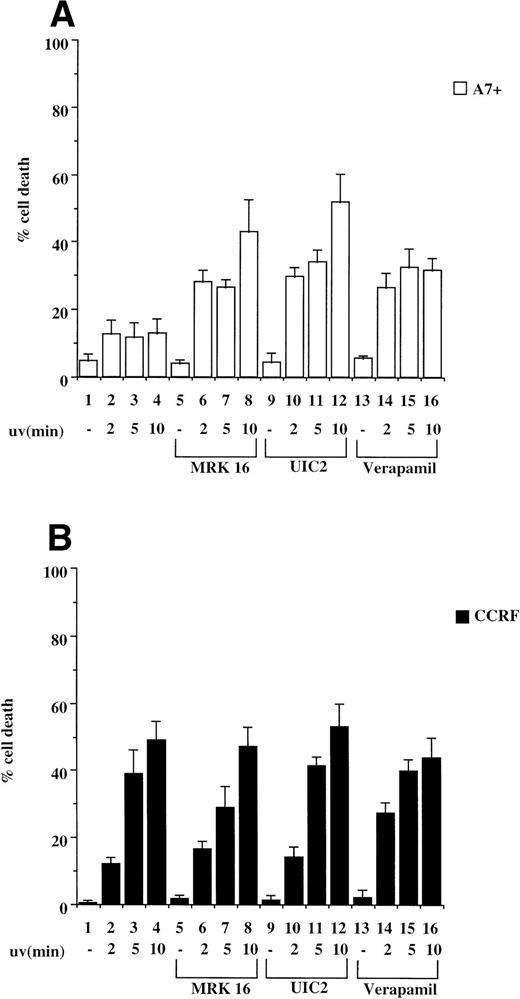
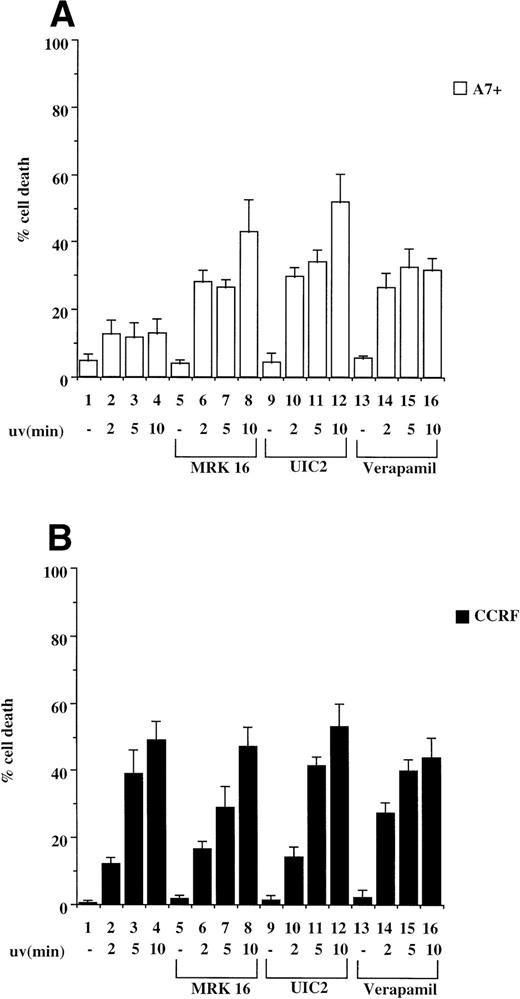
Exposure of cells to UV light has been shown to induce caspase-dependent apoptosis due to cross-linking of cell surface Fas and subsequent activation of the Fas/caspase cell death pathway.19 We therefore tested CCRF/A7+ and 12D7/12D7-MDR1 cells for their relative sensitivity to UV irradiation. P-gp+ve cells A7+ and 12D7-MDR1 were less sensitive to UV irradiation–induced apoptosis than the parental P-gp−ve cell lines CCRF and 12D7 as determined by Trypan blue exclusion assays and fluorescence analysis following PI staining (data not shown). We subsequently pretreated CCRF and A7+ cells with anti–P-gp MoAbs (MRK 16 or UIC2) or verapamil and then exposed the cells to UV irradiation. Resistance of A7+ to UV irradiation was reversed by all three anti–P-gp reagents (Fig 4A, lanes 5 through 16), whereas treatment of CCRF with the P-gp inhibitors had no effect on sensitivity to UV irradiation (Fig 4B, lanes 5 through 16). Viability of P-gp−ve cells following UV irradiation was increased by preincubation in ZVAD-fmk (data not shown). Similar reversal of resistance to UV-induced cell death was seen in 12D7-MDR1 cells treated with P-gp inhibitory agents (data not shown).
P-gp+ve cells are resistant to cell death induced by UV irradiation. A7+ and CCRF cells, 4 × 104 cells in 3-mL media, were subjected to UV-irradiation (2 to 10 minutes), removed from the UV source, and incubated for 24 hours. Cell viability was determined by Trypan blue exclusion and confirmed by fluorescence analysis following staining with PI (data not shown). In some wells, cells were preincubated with MRK 16 (50 μg/mL), UIC2 (5 μg/mL), or verapamil (5 μmol/L) for 30 minutes. Data are calculated as the mean ± SE of quadruplicate samples and are representative of at least two different experiments.
P-gp+ve cells are resistant to cell death induced by UV irradiation. A7+ and CCRF cells, 4 × 104 cells in 3-mL media, were subjected to UV-irradiation (2 to 10 minutes), removed from the UV source, and incubated for 24 hours. Cell viability was determined by Trypan blue exclusion and confirmed by fluorescence analysis following staining with PI (data not shown). In some wells, cells were preincubated with MRK 16 (50 μg/mL), UIC2 (5 μg/mL), or verapamil (5 μmol/L) for 30 minutes. Data are calculated as the mean ± SE of quadruplicate samples and are representative of at least two different experiments.
Cells expressing P-gp are resistant to TNF-mediated apoptosis.
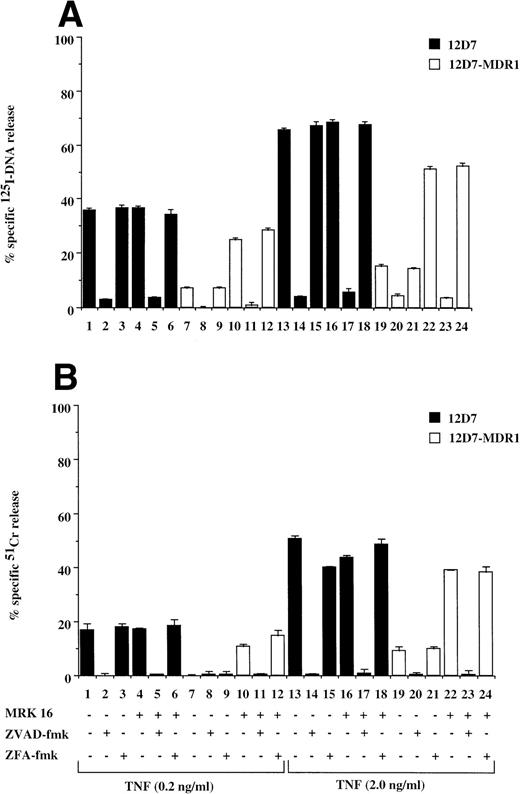
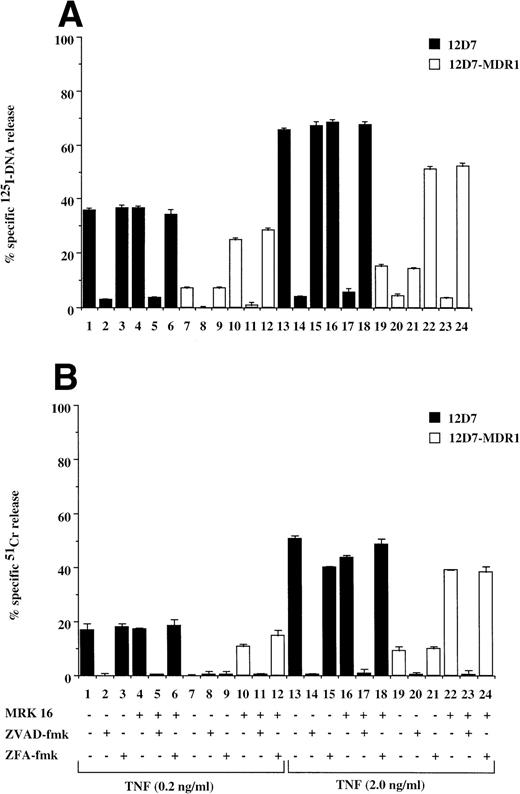
Fas is a member of the TNF receptor (TNFR) superfamily, and a common intracellular caspase pathway is activated leading to apoptosis by Fas or TNFR ligation.16 As TNF can also induce caspase-dependent apoptosis when added to cells expressing TNFR, we tested for the effect of P-gp expression on TNF-mediated cell death. P-gp+ve and P-gp−ve cells expressed equivalent levels of TNFR as determined by binding assays using radiolabeled recombinant TNF (data not shown). Treatment of P-gp−ve CCRF (data not shown) and 12D7 (Fig 5A and B) cells with TNF resulted in DNA fragmentation and membrane lysis, which was inhibited by the addition of ZVAD-fmk (Fig 5A and B, lanes 2, 5, 14, and 17), but not ZFA-fmk (Fig 5A and B, lanes 3, 6, 15, and 18). Both A7+(data not shown) and 12D7-MDR1 (Figs 5A and B) were relatively resistant to TNF, however pretreatment with MRK 16 resulted in sensitivity to caspase-mediated apoptosis (Fig 5A and B, lanes 10 through 12, 22 through 24). Similar reversal of resistance of A7+ and 12D7-MDR1 to TNF was observed using the UIC2 MoAb or verapamil (data not shown).
Retroviral transduced P-gp+ve cells are resistant to TNF-mediated, caspase-dependent DNA fragmentation and membrane lysis. Parental 12D7 (P-gp−ve) and retrovirus-transduced 12D7-MDR1 (P-gp+ve) cells were labeled with 125IUdR (A) and 51Cr (B) for 1 hour, washed in growth media, and incubated for 48 hours in 96-well plates (2 × 104 cells/well) with recombinant soluble TNF (0.2 and 2.0 μg/mL). In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. Data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments.
Retroviral transduced P-gp+ve cells are resistant to TNF-mediated, caspase-dependent DNA fragmentation and membrane lysis. Parental 12D7 (P-gp−ve) and retrovirus-transduced 12D7-MDR1 (P-gp+ve) cells were labeled with 125IUdR (A) and 51Cr (B) for 1 hour, washed in growth media, and incubated for 48 hours in 96-well plates (2 × 104 cells/well) with recombinant soluble TNF (0.2 and 2.0 μg/mL). In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. Data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments.
P-gp+ve cells treated with caspase-dependent apoptotic stimuli retain proliferative and colony-forming ability.
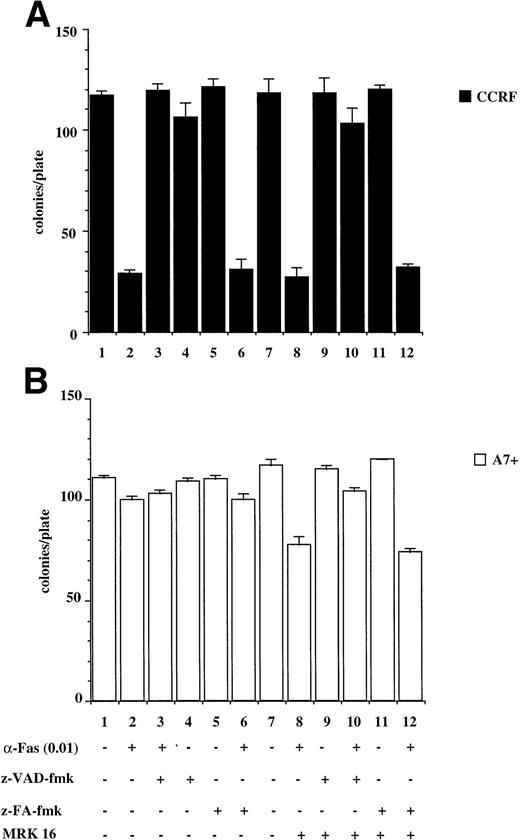
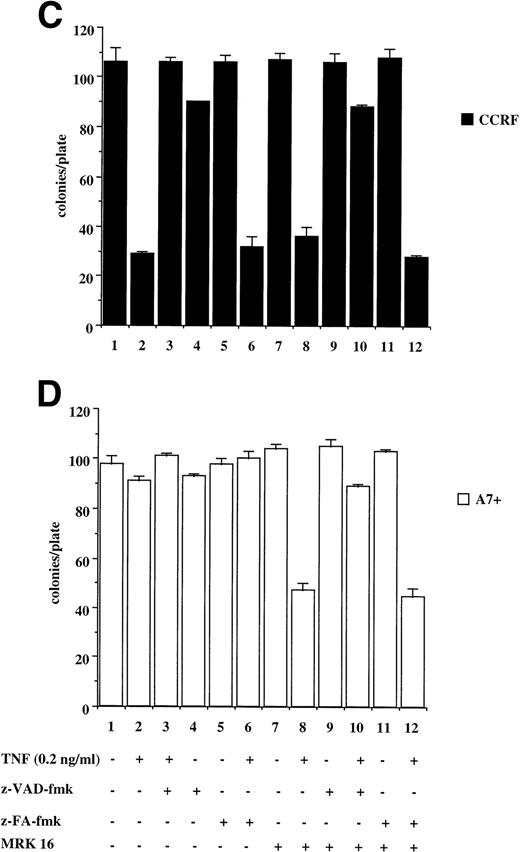
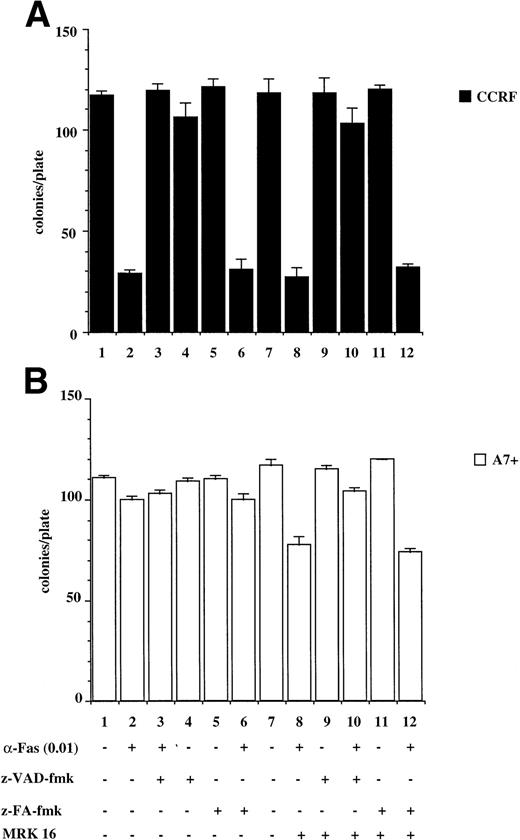
The results presented above are from relatively short-term assays (4 to 48 hours). To determine whether P-gp truly protected cells against caspase-dependent apoptosis or merely delayed the apoptotic events, colony formation assays were performed. This is a stringent in vitro assay because it examines the ability of single cells to proliferate and form colonies. Treatment of CCRF with anti-Fas MoAb (Fig 6A) or purified recombinant TNF (Fig6C) caused a significant reduction in the number of colonies that grow in soft agar (Fig 6A and C, lanes 1 and 2). Pretreatment of CCRF cells with ZVAD-fmk protected them against Fas- or TNF-induced cell death and restored the number of colonies growing per plate. Pretreatment with ZFA-fmk had no effect on Fas- or TNF-mediated cell death as measured by colony formation (Fig 6A and C, lanes 3 through 6). By contrast, A7+ cells were resistant to anti–Fas- and TNF-mediated cell death as treatment of cells with these apoptotic stimuli in the presence or absence of ZVAD-fmk or ZFA-fmk had no effect on colony formation (Fig 6B and D, lanes 1 through 7). However, pretreatment of A7+ cells with MRK 16 MoAb resulted in reversal of resistance to these stimuli, and the resultant cell death was caspase-dependent (Fig 6B and D, lanes 7 through 12).
P-gp+ve cells treated with caspase-dependent apoptotic stimuli retain proliferative and colony-forming ability. CCRF (A, C) and A7+ (B, D) cells (4 × 104 cells/mL) were treated with anti-Fas MoAb (0.01 mg/mL) (A, B) for 16 hours, or with recombinant soluble TNF (0.2 ng/mL) (C, D) for 48 hours. Cells were assayed for colony-forming ability by plating out 5 μL of cells in soft agar and incubating at 37°C for 12 days. In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. Data are calculated as the mean ± SE of triplicate plates and are representative of at least two different experiments.
P-gp+ve cells treated with caspase-dependent apoptotic stimuli retain proliferative and colony-forming ability. CCRF (A, C) and A7+ (B, D) cells (4 × 104 cells/mL) were treated with anti-Fas MoAb (0.01 mg/mL) (A, B) for 16 hours, or with recombinant soluble TNF (0.2 ng/mL) (C, D) for 48 hours. Cells were assayed for colony-forming ability by plating out 5 μL of cells in soft agar and incubating at 37°C for 12 days. In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. Data are calculated as the mean ± SE of triplicate plates and are representative of at least two different experiments.
P-gp does not protect cells against caspase-independent cell death.
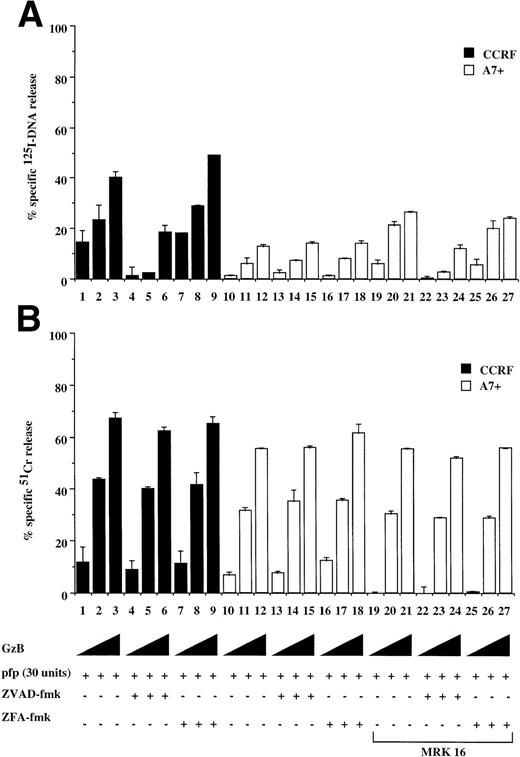
CTL and natural killer (NK) cells destroy target cells via a granule exocytosis mechanism involving pfp and GzB, two key cytolytic molecules present in the cytoplasmic granules of these immune cells. Purified GzB and pfp can induce both caspase-dependent nuclear and caspase-independent cytoplasmic pertubation and membrane lysis of target cells.22 Treatment of P-gp+ve and P-gp−ve cells with sublytic concentrations of pfp in combination with increasing amounts GzB resulted in significant DNA fragmentation in CCRF (Fig 7A, lanes 1 through 3) and 12D7 cells (data not shown) but not of A7+(Fig 7A, lanes 10 through 12) or 12D7-MDR1 cells (data not shown). The DNA fragmentation in CCRF cells was caspase-dependent (Fig7A, lanes 4 through 9), whereas cell membrane lysis of CCRF or A7+ was caspase-independent (Fig 7B, lanes 7 through 9, 16 through 18). Both A7+ (Fig 7A, lanes 19 through 21) and 12D7-MDR1 cells (data not shown) pretreated with MRK 16 were subsequently more sensitive to DNA fragmentation induced by pfp/GzB. There was no significant difference in long-term survival of CCRF or A7+ exposed to GzB and pfp (data not shown). This indicates that the caspase-independent cytoplasmic events induced by GzB/pfp, in the absence of DNA degradation, are sufficient to induce cell death.
Caspase-independent cell lysis is unaffected by P-gp expression. P-gp+ve and P-gp−ve CEM cell lines were labeled with 125IUdR (A, C) and 51Cr (B, D) for 1 hour, washed in growth media, and incubated for 16 hours in 96-well plates (2 × 104 cells/well) with a sublytic concentration of pfp (30 U) and increasing amounts of GzB (0.2, 0.5, 2.0 μg/mg) (A, B) or with pfp alone (300 U) (C, D). In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. Data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments.
Caspase-independent cell lysis is unaffected by P-gp expression. P-gp+ve and P-gp−ve CEM cell lines were labeled with 125IUdR (A, C) and 51Cr (B, D) for 1 hour, washed in growth media, and incubated for 16 hours in 96-well plates (2 × 104 cells/well) with a sublytic concentration of pfp (30 U) and increasing amounts of GzB (0.2, 0.5, 2.0 μg/mg) (A, B) or with pfp alone (300 U) (C, D). In some wells, cells were preincubated for 30 minutes with 20 μmol/L ZVAD-fmk or control ZFA-fmk inhibitor and/or anti–P-gp MoAb MRK 16 (50 μg/mL) as indicated in Table 1. Data are calculated as the mean ± SE of triplicate samples and are representative of at least two different experiments.
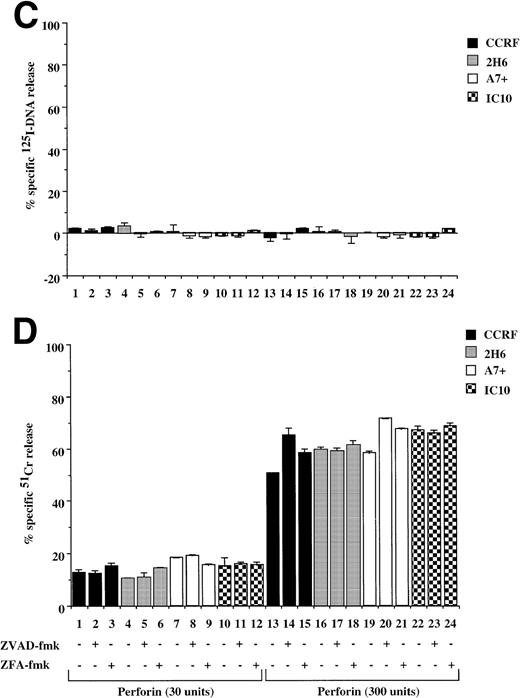
Pfp and the functionally related bacterial toxin, pneumolysin, can lyse cells by forming pores in the target cell membrane to induce cell death.22 Treatment of Pgp+ve and P-gp−ve cells with either of two concentrations of purified pfp (Fig 7C) or pneumolysin (data not shown) resulted in equivalent membrane damage in all cells that were not affected by ZVAD-fmk or ZFA-fmk (Fig 7D). Consistent with previous reports, pfp (Fig 7C) or pneumolysin (data not shown) did not cause significant DNA fragmentation when added to either P-gp+ve or P-gp−ve cells. Similar results were seen in 12D7 and 12D7-MDR1 cells where pfp alone caused membrane lysis but not DNA fragmentation (data not shown).
Thus, P-gp confers resistance to caspase-dependent cell death stimuli such as Fas, TNF, and UV irradiation, but the presence of P-gp on the cell surface does not affect cell death induced by caspase-independent death stimuli.
DISCUSSION
P-gp, responsible for multidrug resistance of tumor cells, has traditionally been thought to function by actively effluxing toxic compounds from cells.1-3 We have recently shown that drug-selected cells expressing P-gp were protected from cell death mediated by cross-linking cell surface Fas,25 and Robinson et al23 have shown that P-gp–expressing cells produced by gene transfer are resistant to apoptosis induced by serum deprivation. Because the outgrowth of cells in the presence of cytotoxic drugs may cause cellular changes other than P-gp expression, we used different paired P-gp+ve and P-gp−ve cell lines, produced by either drug selection or by transfection of the MDR1 cDNA, and inhibitors of P-gp function to show that expression of P-gp confers resistance to apoptosis mediated by a range of cell death stimuli. Protection by P-gp was dependent on the mode of action of the cell death mediator. P-gp protected against all stimuli that function by activating the caspase pathway but did not affect caspase-independent cell death. Significantly, the anti-apoptotic function of P-gp could be inhibited by the use of two MoAbs that bind to different extracellular epitopes or by the pharmacological inhibitor of P-gp function, verapamil. This protection and reversal of resistance of P-gp+ve cells was shown in short-term 51Cr and125I-DNA release assays and in long-term survival or clonogenic assays. The clonogenic assays are important because they show that functional P-gp inhibits apoptosis by caspase-dependent stimuli and does not merely delay death in P-gp+ve cells treated with apoptotic inducers.
Although the mechanism of anti-apoptotic action of P-gp remains to be defined, a number of hypotheses can be put forward. It has been suggested that P-gp may efflux large proteins such as interleukin-2 from a cell30 and thus it is possible that a key caspase or another mediator of apoptosis is being removed from P-gp+vecells. We have shown previously that CCRF and A7+ cells express equivalent amounts of intracellular caspase 3, but that the cleavage and activation of this important molecule was inhibited in A7+ cells when exposed to apoptotic stimuli.25We therefore, have no evidence to suggest that caspases are effluxed in P-gp+ve cells exposed to apoptotic stimuli, but other mediators of apoptosis may be affected. It is possible that P-gp affects apoptosis by altering intracellular adenosine triphosphate (ATP) levels. ATP is a critical component for the activation of caspase 9.31 P-gp is an ATPase, and even when not effluxing specific drugs, has constitutive ATPase activity.1,32,33 Thus, it seems possible that a decrease in the intracellular ATP pool may affect caspase-mediated apoptosis. However, verapamil, which reverses the anti-apoptotic effect of P-gp,1,23,25 increases the ATPase activity of P-gp,33,34 presumably further decreasing intracellular ATP levels. Thus, we do not favor this hypothesis, although careful studies need to be performed to determine the minimum intracellular ATP levels required for caspase 9 activation and the effect P-gp has on these levels. It has been reported that expression of P-gp is associated with intracellular alkalinization,1,35 and an elegant study by Hoffman and Roepe8 showed that P-gp induces a novel Na+- and Cl−-dependent pathway for transmembrane H+ transport to bring about this change in intracellular pH (pHi). It has been shown that apoptosis induced by Fas ligation, UV irradiation, serum starvation, or chemotherapeutic drugs is preceded by intracellular acidification,29,36 and that the induction of apoptotic events such as DNA laddering can be inhibited by increasing pHi.23 Cells that are normally sensitive to apoptosis by Fas cross linking29 or serum starvation23 can be made resistant to these caspase-dependent death stimuli by elevating pHi. Thus, it is possible that expression of P-gp alters pHi placing the cell in a state of caspase-inactivity, and thereby rendering P-gp+ve cells relatively resistant to multiple forms of caspase-dependent cell death stimuli. The addition of verapamil causes a reversal in intracellular alkalinization of P-gp+vecells,23 which correlates with increased sensitivity to a range of apoptotic stimuli23,25 (and this study). We have yet to determine what effect MoAbs MRK 16 and UIC2 have on pHi; however, it has been shown that binding of anti–P-gp MoAbs to extracellular P-gp epitopes causes a change in conformation of the molecule, possibly holding it in an “inactive” state.37
P-gp is expressed normally on epithelial cells of the gut, liver, and kidney,38 which is consistent with its function in removing toxins from cells. However, P-gp is also expressed on some hematopoietic cells, including NK and T lymphocytes,12 that can induce apoptosis of target cells via granule exocytosis (involving pfp and granule serine proteases such as GzB)22 or Fas ligation.39 It is not immediately obvious why NK and T cells require a drug efflux pump, but given our data, it is possible that P-gp expression may protect immune cells against stress-induced or bystander lysis in the hostile environment of sites of inflammation. We have previously shown that bystander lysis by cytotoxic T lymphocytes in vitro occurs predominantly via the Fas pathway,40 and because P-gp expression confers resistance to this form of cell death, it follows that the primary immune cells expressing P-gp could be somewhat protected against Fas-mediated apoptosis in vivo. It has also been shown that bystander lysis may be mediated by TNF,41and our data clearly show that P-gp+ve cells are relatively resistant to TNF-mediated cell death. There has been conflicting reports showing that P-gp expression correlates with either increased42-44 or decreased45 sensitivity of cells to TNF-mediated apoptosis. However, these studies were potentially flawed given that the cells used were selected only on the basis of their drug resistance. We favor the findings that P-gp confers resistance of cells to TNF given that we can reverse resistance of drug-selected P-gp+ve cells to TNF with anti–P-gp antibodies, and that retrovirally transduced P-gp+ve cells are similarly resistant to TNF. P-gp is also expressed on pluripotent CD34+ stem cells that can proliferate and differentiate to give rise to all of the mature hematopoietic cells of the body.12 The physiological function of P-gp on this subset of cells is currently unknown; however, it is conceivable that progenitor cells would have mechanisms in place to generally protect them against apoptotic stimuli, and it is tempting to speculate that P-gp could help serve this purpose.
Different chemotherapeutic agents have been shown to induce the death of susceptible cells by apoptosis,20,23,46 and this can be inhibited by proteins such as Bcl-247 and Bcl-xL.48 The different P-gp+ve and P-gp−ve cell lines used in this study express equivalent amounts of Bcl-2 and the intracellular levels of Bcl-2 to not alter in response to death stimuli such as drug or Fas ligation in the presence or absence of anti–P-gp MoAbs (Johnstone et al, unpublished observation, July 1998). We tested a number of cytotoxic drugs of various classes with specific intracellular targets on different parental P-gp−ve cells and on P-gp+ve, and in all cases P-gp conferred resistance to these stimuli. The mechanisms of chemotherapy-induced, caspase-dependent apoptosis have thus far not been delineated, and there is some controversy as to the role of upregulated FasL on the surface of cells treated with chemotherapeutic agents. It has been shown that treatment of cells with chemotherapeutic drugs or DNA damaging agents such as UV irradiation resulted in upregulated FasL expression, causing Fas-mediated apoptosis.20,49 However, recently it has been shown that the triggering of apoptosis by a broad array of chemotherapeutic agents occurs in a Fas/FasL-independent fashion but the downstream effector proteins (ie, caspases) are the same as those activated by Fas ligation.50 In addition, cells expressing a dominant-negative form of FADD, which is a key adapter between receptors such as Fas and TNFR, and caspase 8 are not protected from cell death induced by a range of apoptotic stimuli including chemotherapeutic drugs.51 It is presently unclear whether the presence of P-gp affects the expression and/or function of FasL. However, we have recently shown that K562 cells, which are resistant to apoptosis mediated by anti-Fas MoAbs, are still sensitive to caspase-dependent cell death mediated by chemotherapeutic agents,25 and KVIN cells (VIN selected K562) that express P-gp can be made sensitive to a range of caspase-dependent apoptotic stimuli by addition of anti–P-gp MoAbs or verapamil.
Although P-gp expression confers resistance to a range of different caspase-dependent apoptotic stimuli, cell death induced by caspase-independent mediators were not affected. Recently, a number of molecules capable of inducing apoptosis in the presence of caspase inhibitors have been reported, including the GD3 ganglioside52 and the adenovirus protein, E4orf4.53 Although the molecular mechanisms leading to cell death by these and other caspase-independent stimuli have yet to be fully delineated, it is apparent that this mode of apoptosis-induction may have implications in both physiological and pathological cell death.
ACKNOWLEDGMENT
We thank Drs M.M. Gottesman and C.G. Lee for supplying the 12D7 and 12D7-MDR1 cell lines and for critical advice and discussions. We thank Drs J.A. Trapani, G.A. Pietersz, and S.M. Russell for helpful discussions, and John Zalcberg and Phillip Kantharidis for reagents.
R.W.J. is a CJ Martin Fellow of the National Health and Medical Research Council of Australia. M.J.S. is currently supported by a Wellcome Trust Australasian Senior Research Fellowship. This work was supported by project grants from the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ricky W. Johnstone, PhD, The Austin Research Institute, Austin Hospital, Studley Rd, Heidelberg 3084, Victoria, Australia; e-mail: r.johnstone@ari.unimelb.edu.au.