Abstract
A recent hypothesis suggests that tumor-specific killing by radiation and chemotherapy agents is due to defects or loss of cell cycle checkpoints. An important component of some checkpoints is p53-dependent induction of p21cip-1/waf-1. Both p53 and p21 have been shown to be required for microtubule damage checkpoints in mitosis and in G1 phase of the cell cycle and they thus help to maintain genetic stability. We present here evidence that p21cip-1/waf-1 deficiency relaxes the G1 phase microtubule checkpoint that is activated by microtubule damage induced with nocodazole. Reduced p21cip-1/waf-1expression also results in gross nuclear abnormalities and centriole overduplication. p53 has already been implicated in centrosome regulation. Our findings further suggest that the p53/p21 axis is involved in a checkpoint pathway that links the centriole/centrosome cycle and microtubule organization to the DNA replication cycle and thus helps to maintain genomic integrity. The inability to efficiently upregulate p21cip-1/waf-1 in p21cip-1/waf-1antisense-expressing cells in response to microtubule damage could uncouple the centrosome cycle from the DNA cycle and lead to nuclear abnormalicies and polyploidy. A centrosome duplication checkpoint could be a new target for novel chemotherapy strategies.
HEMATOPOIESIS depends on accurate duplication and transfer of genetic information during cell proliferation and differentiation, which requires the precise ordering of cell cycle events. Proliferating hematopoietic cells, like most other cells, achieve this coordination by using cell cycle checkpoints.1 2 As the name implies, checkpoints monitor events at critical points in the cycle and stop the progress of some processes until other processes have been completed.
Mutations in some checkpoint molecules, like p53, greatly increase the frequency of gene loss and amplification and contribute significantly to the etiology and progression of human tumors.3,4Checkpoint loss has recently been linked to tumor-specific killing by chemotherapy agents.5
The tumor suppressor, p53, is highly implicated in human tumorigenesis. p53 transactivates the expression of several proteins, including p21cip-1/waf-1 (p21).6,7 p21 is a major inhibitor of several key cell cycle regulating enzymes (cyclin-dependent kinases)7 that are ultimately controlled in hematopoietic cells by numerous exogenous cytokines and growth factors. Together, p53 and p21 are important in several cell cycle arresting checkpoints such as the DNA damage checkpoint,8the mitotic spindle checkpoint,9,10 and microtubule (MT) damage checkpoints.11
Our previous investigations of the molecular mechanisms of hematopoietic growth-factor signaling focused on the process of growth factor synergy.12 It was noted that p21 levels were synergistically elevated when the human growth factor-dependent cell line MO7e was synergistically stimulated to proliferate with the combination of steel factor and granulocyte-macrophage colony-stimulating factor (GM-CSF).12 Also, retroviral-mediated gene transfer of the sense sequence of the p21 gene into myeloid progenitor cells enhanced the proliferative capacity of the progenitors in response to cytokine stimulation.13During follow-up studies in which retroviral-mediated gene transfer of antisense p21 sequence was used to reduce p21 expression in MO7e cells, we observed that cells with antisense reduced p21 expression manifested a remarkable change in nuclear morphology and polyploidy, a loss of MT checkpoints, and centriole abnormalities. These results and their potential implications are reported here.
MATERIALS AND METHODS
Cells, antibodies, and cDNA probes.
Parental MO7e cells used for retroviral infection were provided by Genetics Institute (Cambridge, MA).14 MO7e cells were maintained in RPMI 1640 medium with 20% fetal calf serum (FCS) plus 100 U/mL GM-CSF (Immunex Corp, Seattle, WA) as described.12 For experiments using G0/G1 synchronized cells, factor-starvation was performed in medium without GM-CSF for 18 hours as described previously.14 After initial selection, MO7e cells transduced with retroviral vector were constantly maintained in this medium plus 0.4 mg/mL G418 (Sigma Chemicals Co, St Louis, MO). All cells were routinely visualized microscopically after cytospin (Shandon Southern Products/Miles, Inc, Elkhart, IN) preparation and staining with Wright-Giemsa (Leukostat; Fisher Diagnostics, Pittsburgh, PA). Anti–WAF-1 (p21) monoclonal antibody was obtained from Oncogene Research Products (Cambridge, MA). WAF-1 probe was a gift from G. Adami (University of Illinois at Chicago, Chicago, IL).7 Parental HCT 116 (p21 +/+) cells and one clonal heterozygous (p21+/−) deficient and two different clonal homozygous (p21−/−) deficient cell lines were kind gifts from Drs B. Vogelstein and T. Waldman (The Johns Hopkins Oncology Center, Baltimore, MD).15
Retroviral vectors, transductions, and Northern blot analysis.
The human p21 sequence was generated by reverse transcriptase-polymerase chain reaction (RT-PCR) from HL60 cells7 and subcloned into the retroviral vector, LXSN.16 The LXSN and AS vectors were shuttle packaged into amphotropic packaging cells17 and retroviral supernatant from high-titer clones was used to transduce MO7e cells. Transduced cells were selected in medium containing 0.4 mg/mL G418. Total RNA was isolated, separated by electrophoresis, blotted onto nylon membranes, and probed with radiolabeled p21 sequences.
Transmission election microscopy.
Transmission election microscopy was performed after fixing cells in 2% paraformaldehyde, 1% glutaraldehyde, 50 mmol/L phosphate buffer, pH 7.3, and embedded in Spurrs Resin (Structure Probe, Inc, West Chester, PA), and 100-nm sections were cut on a diatome. The sections were stained with uranyl acetate and lead citrate and viewed on a Zeiss 10B transmission electron microscope (Carl Zeiss, Inc, Thornwood, NY).
Western blot analysis.
Western analysis was performed on polyvinylidene difluoride (PVDF) membranes (Millipore Corp, Bedford, MA) with cellular proteins extracted and electrophoresed and transferred as previously described.12 Anti-p21 antibodies bound to protein bands were visualized with horseradish peroxidase-conjugated goat antimouse IgG secondary antibody and enhanced chemiluminescence photography (Amersham, Arlington Heights, IL), followed by digital image scanning and quantitation.
Cell cycle analysis.
Cell cycle analysis was performed on synchronized cells or cells in log phase growth after treatment with either nocodazole, colcemid (Sigma), or diluent by staining the DNA with propidium iodide (Sigma) and analyzing it with a Becton Dickinson (San Jose, CA) FACscan flow cytometer. Laser light scatter was used to gate out dead or dying cells. Cell cycle proportions were calculated using the modfit (Verity Software House, Topsham, ME) computer program, with the model that makes the fewest mathematical assumptions. Hereafter, G0/G1 proportion refers to the proportion of cells with 2N DNA content and G2/M refers to that with 4N DNA content, with the S phase proportion being intermediate. All recorded events (after gating out dead cells and doublets) greater than the highest 4N channel are considered polyploid events.
Fluorescence in situ hybridization (FISH) and immunofluorescence staining.
Cells were applied to glass slides using cytospin preparations, permeabilized with 0.05% sodium dodecyl sulfate (SDS), and then fixed with ethanol. Cells were then denatured with 70% formamide at 75°C and washed with ethanol. Denatured, digoxigenin-labeled DNA probe specific for the centromeric region of human chromosome 7 or 1 (Oncor, Inc, Gaithersburg, MD) and mouse anti-γ tubulin (Sigma) were added and incubated at 37°C. Sheep anti-digoxigenin-fluorescein isothiocyanate (FITC) and horse antimouse-Texas Red (Oncor, Inc) were then added for 15 minutes. Slides were washed with 0.1% tween 20 and then with water and covered with 4,6-diamidino-2-phenylindole (DAPI) antifade solution and covered with glass slips. They were examined and digitally recorded with a fluorescent microscope equipped with a cooled CCD camera and image acquisition and processing software (Vysis, Inc, Downers Grove, IL). Statistical tests for significance were performed using the Student’s t-test.
RESULTS
To reduce p21 expression in MO7e cells, we generated the rectoviral vector, L(ASp21)SN, containing full-length human antisense p21 sequence and a selectable marker gene (neo). The antisense sequence is transcriptionally regulated by the Moloney murine leukemia virus LTR and the neo gene is regulated by the SV40 early promoter. MO7e cells were transduced with control vector, LXSN, or L(ASp21)SN. Cell lines stably expressing antisense p21 sequence (AS21) or the control vector (LXSN) were obtained by culture in the presence of G418.
Antisense p21 mRNA expression decreases steady-state p21 protein levels.
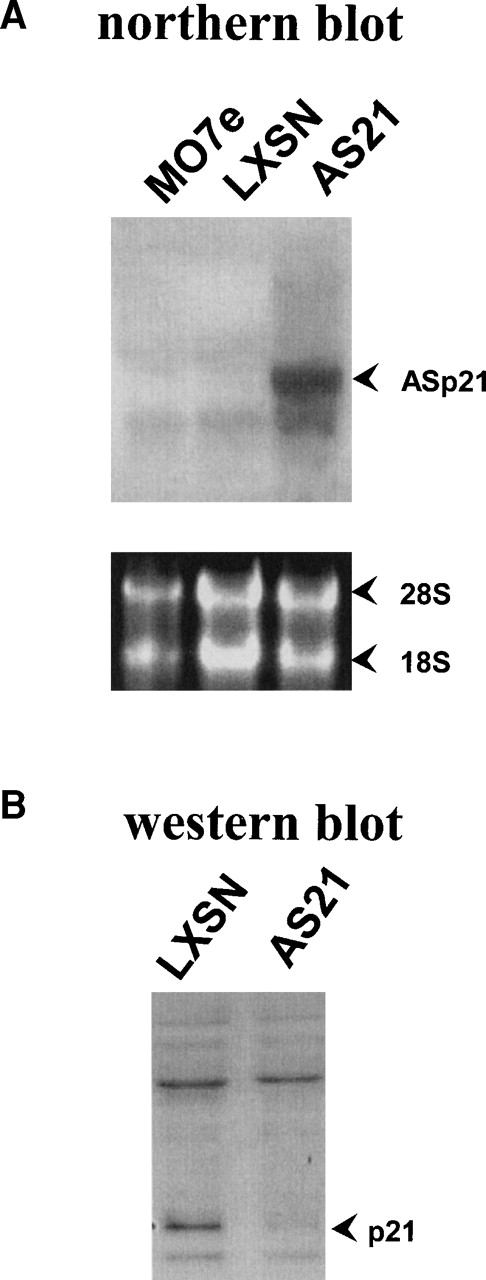
High levels of p21 mRNA expression in AS21 cells are demonstrated by Northern analysis (Fig 1A). Neither parental MO7e cells nor control LXSN cells expressed the antisense p21 message or detectable levels of the endogenous p21 mRNA. Reduced p21 protein levels (Fig 1B) resulted from antisense p21 expression in AS21 cells during log phase growth.
Antisense p21 mRNA expression and its effect on p21 protein levels. (A) The top picure is a Northern blot hybridization of p21 mRNA expression from parental (MO7e), LXSN (vector control), and AS21(antisense p21) cells visualized with probe specific to human p21. The lower picture is ethidium bromide staining of the same blot to show total RNA loading; 18S and 28S rRNA is indicated. (B) Western blot visualization of p21 protein in lysates from LXSN and AS21 cells probed with monoclonal antibody to human p21 (equal amounts of protein were loaded per lane). Data are representative of two experiments.
Antisense p21 mRNA expression and its effect on p21 protein levels. (A) The top picure is a Northern blot hybridization of p21 mRNA expression from parental (MO7e), LXSN (vector control), and AS21(antisense p21) cells visualized with probe specific to human p21. The lower picture is ethidium bromide staining of the same blot to show total RNA loading; 18S and 28S rRNA is indicated. (B) Western blot visualization of p21 protein in lysates from LXSN and AS21 cells probed with monoclonal antibody to human p21 (equal amounts of protein were loaded per lane). Data are representative of two experiments.
Antisense p21 mRNA expression causes deformed nuclear architecture and polyploidy.
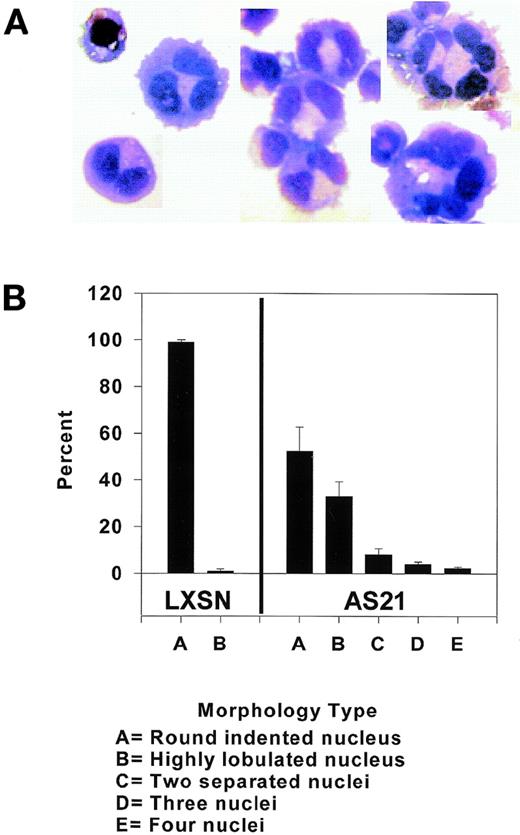
After growth of the transduced cell lines in G418 was stabilized, we noted the presence of cells with differing sizes. We found that some AS21 cells were very large and had multiple apparent nuclei (Fig 2A).
Effect of antisense p21 mRNA expression on nuclear morphology. (A) Wright-Giemsa stain of a mononuclear LXSN cells (upper left). The others are examples of multilobular cells and/or cells with two, three, or more apparent nuclei from AS21 cultures. (B) Quantitation of different nuclear morphologies in transduced cells. Data from three separate cultures were pooled. Two hundred cells per culture were scored. Mean ± SD of each morphology is compared.
Effect of antisense p21 mRNA expression on nuclear morphology. (A) Wright-Giemsa stain of a mononuclear LXSN cells (upper left). The others are examples of multilobular cells and/or cells with two, three, or more apparent nuclei from AS21 cultures. (B) Quantitation of different nuclear morphologies in transduced cells. Data from three separate cultures were pooled. Two hundred cells per culture were scored. Mean ± SD of each morphology is compared.
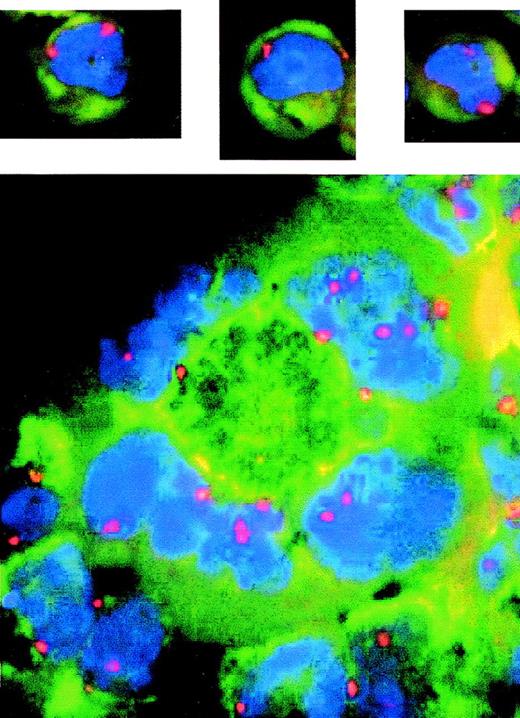
Cells with multiple or deformed nuclei have multiple copies of some chromosomes. Fluorescence in situ hybridization of log phase cells using probe to centromere no. 7. Blue shows DAPI-stained DNA and red shows centromere no. 7 signal indicating the number of copies of chromosome no. 7. The upper three pictures show examples of LXSN cells that are mononuclear and are diploid, as indicated by two copies each of chromosome 7. The AS21 picture (bottom) shows an example of a single giant cell (center) with 4 nuclear lobular structures. Two of these lobes contain several copies of chromosome 7; therefore, this cell is polyploid.
Cells with multiple or deformed nuclei have multiple copies of some chromosomes. Fluorescence in situ hybridization of log phase cells using probe to centromere no. 7. Blue shows DAPI-stained DNA and red shows centromere no. 7 signal indicating the number of copies of chromosome no. 7. The upper three pictures show examples of LXSN cells that are mononuclear and are diploid, as indicated by two copies each of chromosome 7. The AS21 picture (bottom) shows an example of a single giant cell (center) with 4 nuclear lobular structures. Two of these lobes contain several copies of chromosome 7; therefore, this cell is polyploid.
In the AS21 cells, there was a pronounced shift in the percentage of cells with grossly deformed or multiple nuclei compared with control LXSN cells (Fig 2B). Because p21 has been linked to megakaryocyte differentiation,18 we considered whether AS21 cells were beginning a megakaryocytic differentiation program. Electron photomicrographs could not confirm separate multiple nuclei, but did show that many apparent nuclei were connected by a single nuclear membrane (data not shown). These micrographs also showed what appears to be structures similar to α granules, but there was no difference in their frequency between these two cell lines. No evidence of a demarcation membrane system could be seen, and it therefore appears that the change in morphology is not due to megakaryocytic differentiation. This suggestion is supported by flow cytometric analysis of CD61 and CD41 surface expression using anti-CD61 and anti-CD41 antibodies, which also showed no difference between the two cell lines (data not shown).
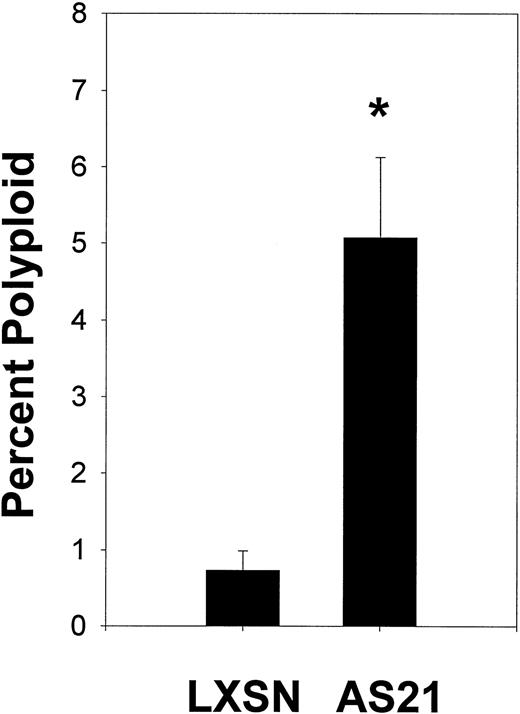
The ploidy of AS21 cells was checked using fluorescence in situ hybridization and centromeric probes to chromosome 1 (not shown) and 7 (Fig 3). All mononuclear cells observed contained two copies of each of these chromosomes indicating that they were diploid. However, some of the giant multinuclear cells found in the AS21 cultures contained multiple copies of the same chromosome per nucleus demonstrating that they were polyploid. This suggests that antisense reduced p21 expression can cause increased polyploidy. This finding is supported by flow cytometric analysis of polyploid cells in LXSN and AS21 cultures that demonstrate the increase in incidence of polyploid cells in AS21 cultures (Fig 4).
Effects of antisense p21 mRNA expression on polyploidy. Cells from log phase cultures of LXSN control cells or AS21 cells were stained with propidium iodide and subjected to cell cycle analysis according to Materials and Methods. After gating out dead cells and doublets, cell events in channel numbers greater than the highest 4N channel were enumerated as a polyploid event. The percentage polyploid cells is shown. *Significantly different from control, P < .05.
Effects of antisense p21 mRNA expression on polyploidy. Cells from log phase cultures of LXSN control cells or AS21 cells were stained with propidium iodide and subjected to cell cycle analysis according to Materials and Methods. After gating out dead cells and doublets, cell events in channel numbers greater than the highest 4N channel were enumerated as a polyploid event. The percentage polyploid cells is shown. *Significantly different from control, P < .05.
Reduced p21 expression causes relaxed MT-dependent checkpoints.
p53 and p21 have both been implicated in checkpoints involving MTs.9-11 Deficiencies in either of these molecules have been linked to genetic instability and polyploidy, in part through defects in these checkpoints. We considered whether a defective MT checkpoint could, in part, be responsible for the polyploidy we observed in AS21 cells. We therefore tested if MT checkpoints were intact in AS21 cells by treatment with the MT poisons, nocodazole and colcemid. These agents disrupt the mitotic spindle and activate the spindle assembly checkpoint and arrest cells in metaphase that can be seen microscopically and can easily be quantitated by flow cytometric cell cycle analysis.
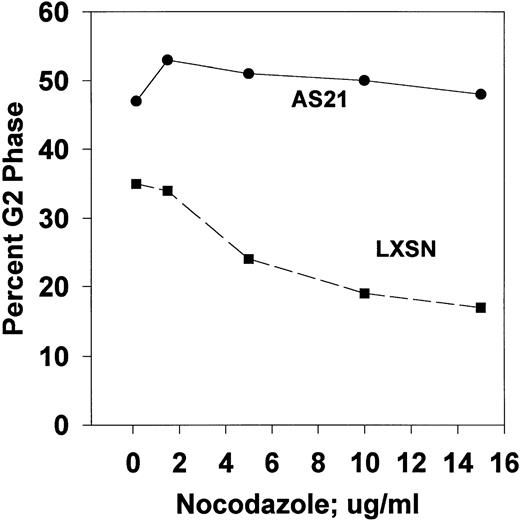
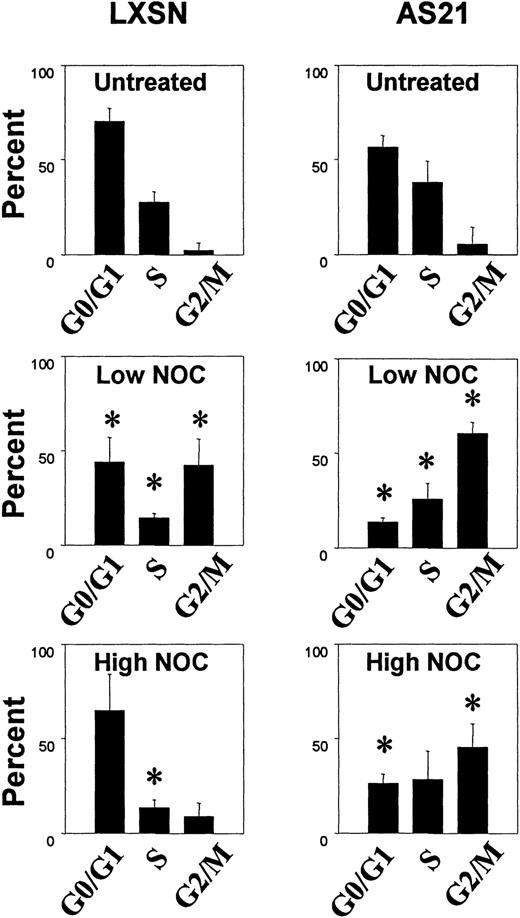
A dose-response experiment (Fig 5) using nocodazole (NOC) showed that low concentrations were more effective than high NOC concentrations in inducing G2 phase (metaphase) arrest in control LXSN cells. This biphasic dose-response was not apparent in AS21 cells. However, NOC induced G2 phase accumulation was greater in AS21 cultures. Similar results were obtained with colcemid (data not shown). Further experiments with high and low NOC concentrations (Fig 6) showed two salient points. (1) High NOC concentrations cause significantly more G0/G1 and less G2/M phase arrest than lower concentrations in control cells. (2) NOC induced more G2/M phase arrest of AS21 cells than control cells at all concentrations tested. Together, these data show that the mitotic spindle assembly checkpoint was activated in both cell lines by NOC. The newly described MT-dependent arrest checkpoint in G0/G110 also appears to have been activated in control cells by high NOC, but is defective in AS21 cells. This leads to greater G2/M accumulation in AS21 cutlures, especially with high NOC concentrations.
Dose-response effect of nocodazole on G2/M phase proportion in transduced cells. Log phase cultures were washed with phosphate buffered saline and put into fresh medium with GM-CSF plus the indicated amount of nocodazole and incubated for 24 hours. Cells were then harvested and cell cycle analysis was performed. This experiment was repeated twice with similar results.
Dose-response effect of nocodazole on G2/M phase proportion in transduced cells. Log phase cultures were washed with phosphate buffered saline and put into fresh medium with GM-CSF plus the indicated amount of nocodazole and incubated for 24 hours. Cells were then harvested and cell cycle analysis was performed. This experiment was repeated twice with similar results.
Effect of nocodazole on cell cycle distribution in transduced cells. Cells were treated as described in Fig 5 except for 48 hours. G0/G1, S, and G2/M DNA contents are shown. Data are the averages ± SD from three separate experiments. *Significant difference from untreated control, P < .05. Low NOC was 0.15 μg/mL and high NOC was 15 μg/mL.
Effect of nocodazole on cell cycle distribution in transduced cells. Cells were treated as described in Fig 5 except for 48 hours. G0/G1, S, and G2/M DNA contents are shown. Data are the averages ± SD from three separate experiments. *Significant difference from untreated control, P < .05. Low NOC was 0.15 μg/mL and high NOC was 15 μg/mL.
Because high NOC concentrations seem to suppress G0/G1 exit, we compared NOC allowable G1 exit in LXSN and AS21 cells as represented by the percentage of decrease of G0/G1 phase induced by NOC treatment compared with control (Table 1). After 24 hours, NOC treatment significantly inhibited G1 exit (negative percentage of decrease). After 48 hours of treatment, G1 exit in LXSN cells occurred in the presence of low NOC, but was still suppressed by high NOC. On the other hand, significant G1 exit of AS21 cells occurred even in the presence of high NOC. This further supports the idea that antisense p21 expression relaxes an MT-dependent cell cycle checkpoint in G1 phase.
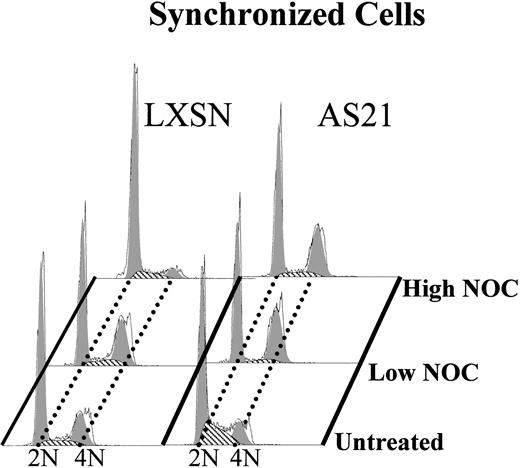
A more definitive confirmation of a relaxed G1 MT checkpoint is shown in Fig7. Cells were synchronized in G0/G1 phase by growth-factor deprivation arrest and then released from this arrest in the presence or absence of NOC. Cell cycle analysis was then performed 24 hours later, which is within the first cycle after release. It is seen that high NOC treatment suppressed G1 exit in control LXSN cells but did not suppress G1 exit in antisense expressing AS21 cells. We therefore suggest that antisense p21 expression relaxes an MT polymerization-dependent cell cycle arresting checkpoint in G1 phase.
Effect of high and low nocodazole treatment on the cell cycle in synchronized LXSN and AS21 cells. Cells were synchronized by growth factor starvation for 18 hours and then released by adding back growth factor (GM-CSF) in the presence or absence of the indicated amount of nocodazole and incubation for 24 hours before cell cycle analysis. Nocodazole concentrations were the same as described in Fig5. The 4N proportion at time zero was negligible. Data are representative of three separate experiments.
Effect of high and low nocodazole treatment on the cell cycle in synchronized LXSN and AS21 cells. Cells were synchronized by growth factor starvation for 18 hours and then released by adding back growth factor (GM-CSF) in the presence or absence of the indicated amount of nocodazole and incubation for 24 hours before cell cycle analysis. Nocodazole concentrations were the same as described in Fig5. The 4N proportion at time zero was negligible. Data are representative of three separate experiments.
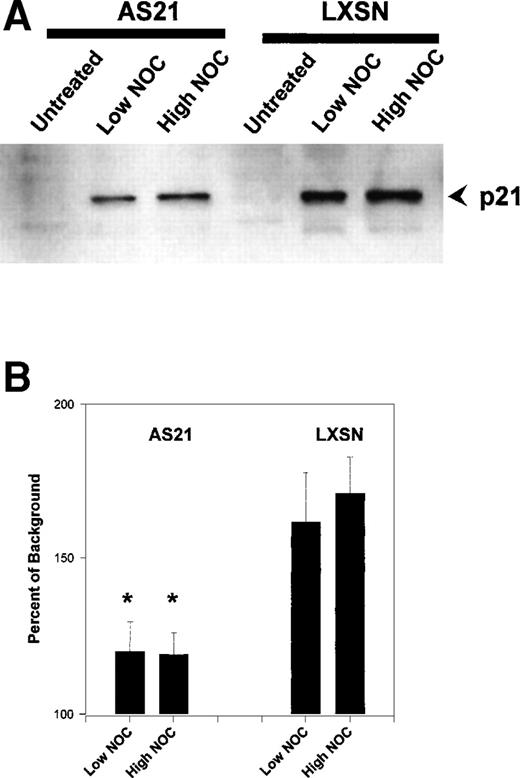
NOC has been shown previously to induce p21.19 We therefore investigated if NOC treatment upregulated p21 in our cell lines and if antisense p21 expression suppressed this induction. Figure 8 shows the results of Western blot analysis of p21 protein levels after NOC treatment. p21 was induced by NOC, and this response was significantly attenuated by antisense p21 expression. This then suggests that reduced p21 protein induction is responsible for the MT checkpoint relaxing effects of antisense p21 sequence expression.
Effect of nocodazole on p21 protein levels in transduced cells. (A) Example of a Western blot of whole cell lysate using anti-p21 antibody. (B) Quantitation and statistical analysis of three experiments like that in (A). Data are expressed as the percentage above background density. The mean ± SD is shown. *Statistically significant difference compared with control LXSN cells (P < .05). NOC concentrations were the same as described in Fig 5.
Effect of nocodazole on p21 protein levels in transduced cells. (A) Example of a Western blot of whole cell lysate using anti-p21 antibody. (B) Quantitation and statistical analysis of three experiments like that in (A). Data are expressed as the percentage above background density. The mean ± SD is shown. *Statistically significant difference compared with control LXSN cells (P < .05). NOC concentrations were the same as described in Fig 5.
The human G0/G1 phase MT checkpoint requires p21.
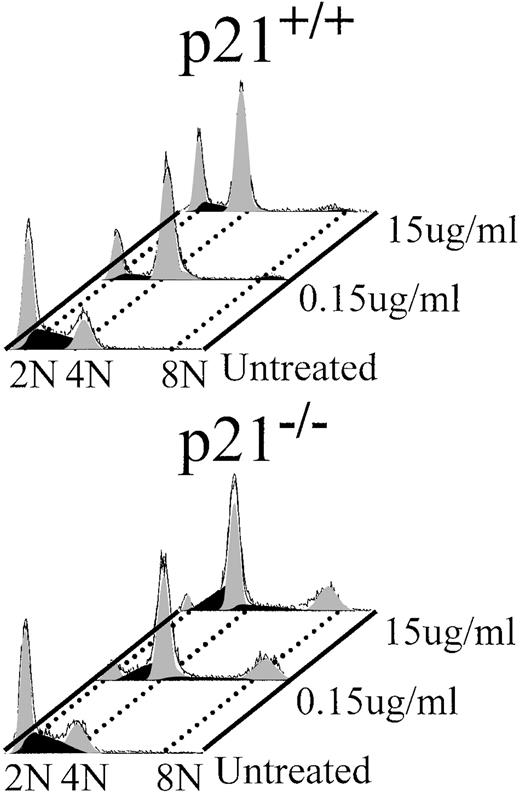
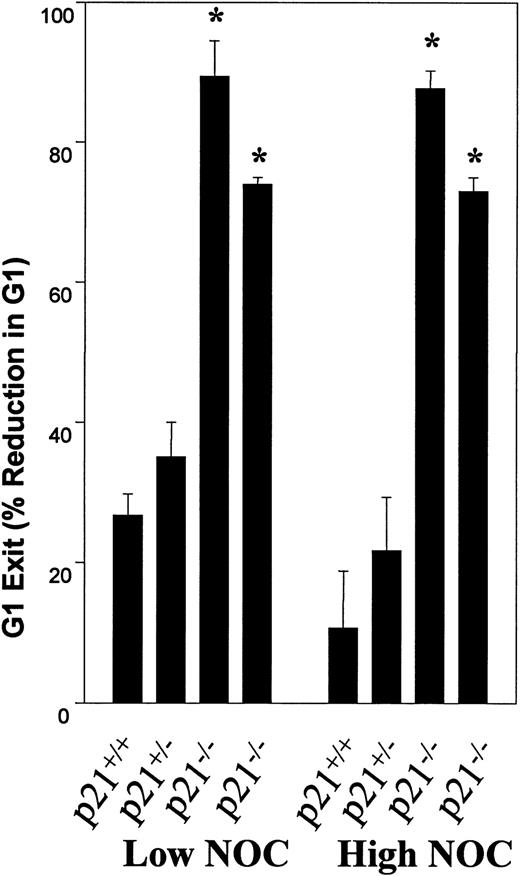
p21 was previously reported to be required for G0/G1 arrest after mitotic slippage after spindle damage11 and also for another MT-dependent G0/G1 arrest,10 both in murine embryonic fibroblasts. The effects of NOC have not been reported in p21 null human cells. Some murine checkpoints are different than the same human checkpoints, and because a model human cell line was used, we wanted to verify the G1 MT checkpoint in human p21−/− cells. Figure 9 shows the effects of NOC on wild-type and p21−/− human colorectal cell lines. Low NOC caused G2/M phase arrest, whereas high NOC treatment resulted in more G0/G1 and less G2/M arrest in wild-type cells, which is similar to the dose-response observed in LXSN cells. In contrast, there was a greater increase in G2/M arrest in p21−/− cells compared with wild-type cells at both NOC concentrations. This was similar to but more pronounced than the same dosing effect found in AS21 cells. In addition, there was an increase in the 8N (polyploid) cells in treated p21−/− cultures. This indicates a loss of the re-replication block in the p21 null cells. Figure 10 is an analysis of G1 phase exit in one heterozygous and two different clonal p21 null human cell lines that was performed in a manner similar to that reported in Table 1. There was no statistically significant difference between the wild-type and the heterozygous cell line, but there was a significant increase in NOC allowable G1 exit in both of the p21 null cell lines. Furthermore, the G1 exit reducing effects of high NOC were observed in wild-type cells, but not in the knock-out cells, a dose-response similar to that observed in AS21 cultures. From these data it is concluded that human p21 null cells have a similar, but more robust, loss of the G1 MT checkpoint as that found in AS21 cells, which is also similar to that reported for murine p21 null cells. Also, p21 is required for the prevention of re-replication in response to mitotic slippage in the presence of NOC in these human cells.
Effect of nocodazole treatment on cell cycle profile in human colorectal cell lines. Log phase culture of wild-type (p21+/+) or p21 null (p21−/−) cells were treated with the indicated amount of nocodazole for 24 hours and then cell cycle analysis was performed. 2N(G0/G1), 4N(G2/M), and 8N(polyploid) DNA contents are shown (data are representative of 3 separate experiments).
Effect of nocodazole treatment on cell cycle profile in human colorectal cell lines. Log phase culture of wild-type (p21+/+) or p21 null (p21−/−) cells were treated with the indicated amount of nocodazole for 24 hours and then cell cycle analysis was performed. 2N(G0/G1), 4N(G2/M), and 8N(polyploid) DNA contents are shown (data are representative of 3 separate experiments).
Effect of nocodazole on p21 deficient human colorectal cell lines. Wild-type, heterozygous (p21+/−), and two different homozygous p21-deficient cell lines were treated as described in Fig 8and then cell cycle analysis was performed. G0/G1 exit was calculated as in Table 1. Mean ± SD from three separate experiments are shown. *Statistically significant difference from wild-type response (P < .05).
Effect of nocodazole on p21 deficient human colorectal cell lines. Wild-type, heterozygous (p21+/−), and two different homozygous p21-deficient cell lines were treated as described in Fig 8and then cell cycle analysis was performed. G0/G1 exit was calculated as in Table 1. Mean ± SD from three separate experiments are shown. *Statistically significant difference from wild-type response (P < .05).
Antisense p21 mRNA expression causes centriole overduplication.
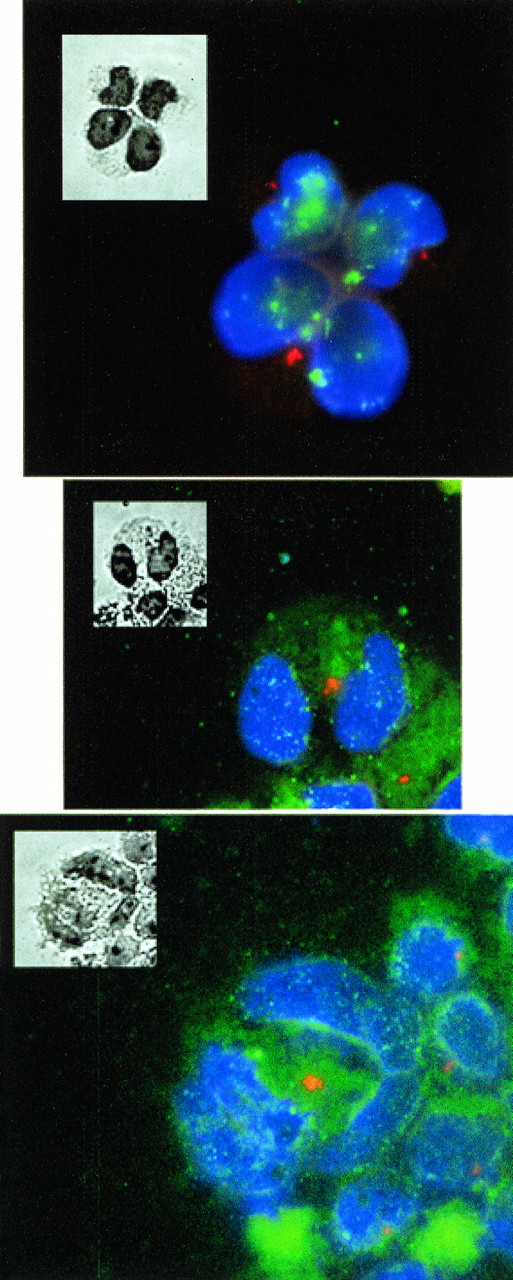
The centrosome is the MT organizing center in mammalian cells. p53 deficiency results in centrosome amplification and nuclear abnormalities similar to that observed in AS21 cells.20 p53 activates p21 expression, especially during G1 phase, when the centrioles duplicate.21 This line of evidence caused us to investigate the status of the centrosome in AS21 cells, especially since this has not been reported in human p21-deficient cells. Figure 11 shows three examples of cells with abnormal nuclei after staining with γ-tubulin. γ-Tubulin localizes to the centrioles. Cells in control LXSN cultures were mononuclear and displayed one or two centriolar/centrosomal signals as exemplified in the two upper cells in the top picture of Fig11. Almost every cell in AS21 cultures that we identified in this fashion that had more than one apparent nucleus displayed supernumerary centriolar signals as exemplified in the top picture and other pictures. Therefore, it is concluded that, similar to p53 deficiency, p21 deficiency results in abnormal centriole replication.
Effects of antisense p21 mRNA expression on centrosome distribution. These three pictures show examples of cells containing nuclei and stained in situ with antibody to γ-tubulin, which localizes the centrosome (red foci). The nuclei are visualized with DAPI (blue). The insets are phase contrast images of the same cells stained with Giemsa to show the cellular boundries. The upper picture shows three cells. The upper two cells are mononuclear cells and demonstrate a single red centrosomal focus (see text for further descriptions). The lower cell contains a deformed nucleus and demonstrates a cluster of several centrosomal foci. This type of cell was found exclusively in AS21 cultures. The other two pictures show further examples of cells with deformed nuclei also containing several centrosomal foci each.
Effects of antisense p21 mRNA expression on centrosome distribution. These three pictures show examples of cells containing nuclei and stained in situ with antibody to γ-tubulin, which localizes the centrosome (red foci). The nuclei are visualized with DAPI (blue). The insets are phase contrast images of the same cells stained with Giemsa to show the cellular boundries. The upper picture shows three cells. The upper two cells are mononuclear cells and demonstrate a single red centrosomal focus (see text for further descriptions). The lower cell contains a deformed nucleus and demonstrates a cluster of several centrosomal foci. This type of cell was found exclusively in AS21 cultures. The other two pictures show further examples of cells with deformed nuclei also containing several centrosomal foci each.
DISCUSSION
During our investigations of the effects of hematopoietic growth factors on the cell cycle and on cell cycle regulating molecules,12,13 we generated a factor-dependent human cell line that has suppressed p21 expression. We subsequently noted a remarkable change in the nuclear morphology of many of these cells. Anitsense expressing cultures contained cells with more than one apparent nucleus, and many cells had strangely shaped, multilobed nuclei. This could not be explained by megakaryocytic differentiation, at least within the context of surface marker expression. However, because the parental MO7e cell line already expresses some megakaryocytic markers, an effect of antisense p21 on megakaryocytic differentiation remains possible. Increased p21 was previously reported to be linked with increased megakaryocytic differentiation.18 Because we reduced p21 expression and found no change in surface markers, our findings do not necessarily contradict this report. The megakaryocyte-like changes in nuclear morphology we observe after antisense p21 mRNA expression could be solely due to miscoordination of mitotic or other cell cycle checkpoints. However, this remains to be shown.
p21 has been previously linked to chromosomal positioning and intranuclear chromatin structure.22 Our data suggest that p21 is also important to gross nuclear architecture. p21 is considered to be important to the maintenance of genetic stability because of its tumor-suppressor function.23 Our findings of increased polyploidy and deformed nuclei in p21-deficient human cells are consistent with this.
p21 is required for MT surveillance cell cycle checkpoints.
The p53/p21 axis has been shown to be involved in several cell cycle checkpoints,3,4 8-11 including the mitotic spindle assembly checkpoint, and a newly described MT damage checkpoint in G1 phase, as well as a G1 checkpoint that blocks DNA re-replication. As a potential cause of the polyploidy observed in AS21 cells, these MT checkpoints were investigated by treatment of cells with nocodazole. Nocodazole activated the spindle assembly checkpoint in both AS21 and vector control cells, as noted by the increased arrest of cells in G2/M phase measured by DNA analysis and as noted by increased metaphase cells observed microscopically (data not shown). Increasing concentrations of nocodazole caused less G2/M arrest and more G0/G1 arrest in control cells, which is consistent with a hightened activation of a G1 MT checkpoint. AS21 cells showed an apparent loss of the G1 MT checkpoint-activating effects of high concentrations nocodazole treatment. A relaxed G1 MT checkpoint in AS21 cells was substantiated using G0/G1 phase synchronized cells. The requirement of p21 for G1 arrest and re-replication blockage in response to high nocodazole was further demonstrated with p21-deficient human colorectal cell lines. Together, these data support the existence of an MT damage checkpoint in G1 phase in human hematopoietic cells and show that p21 is required for the G1 phase arresting effects of this checkpoint. Because suppressed p21 induction in G1 phase in response to MT disruption results in a defective G1 cell cycle arrest checkpoint, it likely contributes to the observed polyploidy found in AS21 cells. This checkpoint therefore contributes to genetic stability during hematopoiesis.
The p53/p21 axis couples centriole replication to DNA replication.
Spatial organization and cell polarity are maintained by the cytoskeletal proteins, including the MTs.24 The centrosome is the focal point for MT organization.25 p53 deficiency has been shown to result in centrosome amplification and grossly deformed nuclear morphologies similar to those observed in AS21 cells.20 However, before now, p21-deficient cells had not been tested for this effect. Our data show that AS21 cells with multiple or deformed nuclei have supernumerary centrioles. p21 overexpression has recently been reported to inhibit centriole replication in amphibian embryonic cells.26 We have now documented the converse of this by showing that reduced p21 expression promotes centriole overduplication in human hematopoietic cells. This strongly suggests that the p53/p21 axis is important to the coordination of the centriole duplication cycle with the DNA replication cycle. Loss of this coordination could account for the deformed nuclei observed in AS21 cells and could therefore contribute to the generation of aneuploidy frequently observed in leukemia and other human cancers.
The following points can now be considered together. (1) The p53/p21 axis is important for MT checkpoints. (2) Centrioles organize interphase MTs and the mitotic spindle. (3) The p53/p21 axis is important to centriole replication. (4) The centrioles duplicate in G1 phase. (5) MTs are important for centriole replication. When considered together, this line of evidence raises the intriguing possibility that the G1 MT checkpoint that depends on the p53/p21 pathway may in fact be a centriole/centrosome duplication checkpoint.
MTs are the target for one of the most widely used and successful class of chemotherapy agents (antimitotics). Tumor-specific killing by chemotherapy agents has been linked to loss of cell cycle checkpoints.5 A centrosome duplication checkpoint could potentially be targeted by novel chemotherapeutic strategies.
ACKNOWLEDGMENT
The authors thank Kent Robertson, MD, and Robert Hromas, MD, for the retroviral vector; Scott Cooper for help with illustrations; Perluigi Porcu, MD, for megakaryocyte assays; Yu Tian for Northern blot analysis; and Cindy Booth for secretarial assistance.
Supported by US Public Health Grants No. R01 HL56416, R01 DK53674, and R01 HL54037 and by a Project in P01 HL53586 from the National Institutes of Health to H.E.B. S.E.B. is a Fellow of the Leukemia Society of America, Inc.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Charlie Mantel, Walther Oncology Center, R4-325, 1044 W Walnut St, Indianapolis, IN 46202-5121; e-mail: cmantel@topaz.iupui.edu.