Abstract
Oncostatin M (OSM) is an interleukin-6 (IL-6) family cytokine known in particular to induce the synthesis of acute-phase proteins by hepatocytes. Because human polymorphonuclear neutrophils (PMN) can secrete numerous cytokines, the potential production of OSM by PMN was investigated. Highly purified PMN were found to contain an intracellular stock of preformed OSM that was rapidly mobilized by degranulating agents such as phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor (GM-CSF). Moreover, PMN produced OSM after a few hours of stimulation by various agonists. The most potent effect was observed with the combination of lipopolysaccharide and GM-CSF, which had a concentration- and time-dependent effect at both the protein and mRNA levels. Actinomycin D strongly reduced OSM mRNA induction, suggesting the involvement of gene transcription. Cycloheximide inhibited OSM protein synthesis but did not affect the release of preformed stores. In addition, OSM production was downregulated by dexamethasone, whereas IL-10 had no effect. The OSM produced by PMN was biologically active, as demonstrated by its ability to induce 1-acid glycoprotein synthesis by HepG2 cells. OSM secretion thus occurs through a two-step mechanism in PMN, consisting of early release of a preformed stock, followed by de novo protein synthesis. This would allow rapid and sustained OSM release to occur at inflammatory sites, and may contribute to the modulation of local inflammation.
POLYMORPHONUCLEAR neutrophils (PMN) are circulating phagocytes that participate in the immune and inflammatory responses to endogenous and exogenous stimuli. In addition to their phagocytic and killing properties, PMN synthesize numerous cytokines such as proinflammatory agents (tumor necrosis factor-α [TNF-α], interleukin-1α [IL-1α], and IL-1β), the antiviral agent interferon-α (IFN-α), chemokines (macrophage inflammatory protein-1α [MIP-1α], MIP-1β, IL-8, and IP-10), growth factors (granulocyte colony-stimulating factor [G-CSF], macrophage colony-stimulating factor [M-CSF], and granulocyte-macrophage colony-stimulating factor [GM-CSF]) and anti-inflammatory mediators (IL-1ra and transforming growth factor-β [TGF-β]).1 2 PMN are far more abundant than other inflammatory cells in the early stages of the inflammatory response, suggesting that some of the cytokines released by PMN may be critical for regulating the inflammatory process.
Oncostatin M (OSM) is a cytokine originally isolated from the supernatants of U937 histiocytic leukemia cells differentiated into macrophage-like cells by treatment with phorbol myristate acetate. It was identified by its ability to inhibit the replication of A375 melanoma cells.3 OSM acts on a wide variety of cells and elicits a multitude of biological responses, including modulation of cell growth and differentiation, low-density lipoprotein (LDL) receptor upregulation, and induction of hematopoietic factors (reviewed in Wallace et al4). OSM belongs to the IL-6 cytokine family, which includes IL-6, leukemia inhibitory factor (LIF), IL-11, ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT-1). These cytokines are mainly produced during inflammatory disorders, in which they induce a systemic acute-phase response marked by the enhancement of acute-phase protein synthesis by hepatocytes.5 Activated T lymphocytes6 and monocytes7 are the only human cells known to produce OSM. Given their involvement in numerous inflammatory processes, we investigated whether purified human PMN could synthesize and secrete OSM and studied the regulation of this production.
We report for the first time that highly purified PMN contain a preformed stock of OSM and that bioactive OSM can be synthesized and released after stimulation with various agonists; these findings suggest that OSM production by PMN constitutes a new element of the acute-phase response.
MATERIALS AND METHODS
Purification of human PMN.
Human PMN were purified as previously described.8 9Briefly, blood PMN from healthy volunteers were isolated in sterile conditions by sedimentation in medium containing 9% Dextran T-500 (Pharmacia, Uppsala, Sweden) and 38% Radioselectan (Schering, Lys-lez-Lannoy, France); the leukocyte suspension was then centrifuged on Ficoll-Paque medium (Pharmacia). The cell pellet was washed with phosphate-buffered saline and erythrocytes were removed by hypotonic lysis. PMN were further purified by 20 minutes of incubation with pan antihuman HLA class II-coated magnetic beads (Dynal, Oslo, Norway) to deplete B lymphocytes and activated T lymphocytes and monocytes, but not resting T cells. PMN were then resuspended in culture medium (see below). Nonspecific esterase staining always showed less than 0.5% of monocytes, and flow cytometry showed the absence of CD14+, CD3+, CD19+ cells, confirming the recovery of highly purified PMN.
T lymphocyte preparation.
After Ficoll-Paque centrifugation, the mononuclear cell ring was treated with magnetic beads as described above to deplete B cells, activated T cells, and monocytes, retaining only purified resting T cells.
Cell culture.
Pure PMN (107/mL) were cultured for up to 48 hours at 37°C with 5% CO2 in 24-well tissue culture plates (Costar, Cambridge, MA). The culture medium was RPMI 1640 (Sigma, St Louis, MO) supplemented with 2 mmol/L glutamine, antibiotics, and 10% heat-inactivated fetal calf serum (Biowittacker, Gagny, France). Stimulating agents were added to the culture medium at the following optimal concentrations determined in preliminary concentration-response experiments: lipopolysaccharide at 100 ng/mL (LPS; from Escherichia coli 055:B5), phorbol myristate acetate at 100 ng/mL (PMA), N-formylmethionyl-leucyl-phenylalanine at 10−5 mol/L (fMLP), TNF-α at 100 U/mL (TNF), IFN-γ at 500 U/mL (IFN), and GM-CSF at 100 U/mL; all reagents were from Sigma, except for recombinant cytokines, which were from Genzyme (Cambridge, MA). The inhibitory effects of IL-10 (kindly provided by Schering-Plough Research Institute, Kenilworth, NJ) and dexamethasone (DEX; Sigma) were studied by adding them for 30 minutes at 37°C before stimulation with LPS plus GM-CSF. In selected experiments, pure resting T lymphocytes (5 × 105/mL) were cultured alone or cocultured with 107 PMN/mL after stimulation with LPS plus GM-CSF to test the role of contaminating resting T cells in OSM production. Cell-free PMN culture supernatants were collected at various times and stored at −20°C until OSM assay. To determine cell-associated OSM, 107 PMN/mL were incubated for 15 minutes at 37°C with or without 100 ng/mL PMA or 100 U/mL GM-CSF; cell-free supernatants were collected and the cell pellets were sonicated for 30 seconds to measure cell-associated OSM. Both supernatants and cell pellets were stored at −20°C until OSM assay. In some experiments, neutrophils (107/mL) were preincubated with or without 10 μg/mL of cycloheximide (CHX) for 30 minutes at 37°C and then further incubated with 100 U/mL of GM-CSF for 15 minutes or 3 or 8 hours.
OSM assay.
OSM was quantified by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Quantikine; R&D Systems, Abingdon, UK) following the manufacturer’s instructions; the detection limit was 2.1 pg/mL.
Immunocytochemical staining of intracellular OSM.
Unstimulated blood smears from healthy controls were air-dried for 24 hours and fixed in cold methanol/acetone. Smears were then incubated with a polyclonal rabbit antihuman-OSM antibody (50 μg/mL; Genzyme), followed by incubation with a biotinylated antibody and then alkaline phosphatase-labeled streptavidin, as recommended by the manufacturer (LSAB kit-AP; Dako, Carpinteria, CA). Staining was completed by incubation with a chromogenic substrate solution; smears were counterstained with hematoxylin and ammonia water. Positive staining developed as a fuschia-colored reaction product. Smears incubated with a control Ig (Sigma) served as negative controls and smears incubated with an anti-CD11b monoclonal antibody (Immunotech, Marseille, France) served as positive controls.
Northern blot analysis.
For RNA analysis, 5 × 107 PMN were incubated for up to 18 hours in 2 mL of standard culture medium containing the appropriate stimuli. In selected experiments, actinomycin D (5 μg/mL; Sigma) was added to the medium to block transcription. Total cellular RNA was isolated from PMN with RNA-B (Bioprobe, Cergy-Pontoise, France) according to the manufacturer’s instructions, and the RNA concentration was determined at 260 nm. Twenty micrograms of total RNA was analyzed by electrophoresis on 1% agarose-formaldehyde gel and transferred to nylon filters (Amersham, Les Ulis, France). The filters were prehybridized in 5× SSC, 25 mmol/L sodium phosphate (pH 6.4), 5× Denhardt’s reagent, 0.1% sodium dodecyl sulfate (SDS), and 0.2 mg/mL denatured salmon sperm DNA for 12 hours at 42°C. Human OSM mRNA was detected by hybridization with32P-labeled oligonucleotide probes (R&D Systems) for 16 hours at 42°C in 5× SSC, 25 mmol/L sodium phosphate (pH 6.4), 5× Denhardt’s reagent, 0.1% SDS, 50% formamide, and 0.2 mg/mL denatured salmon sperm DNA. The membranes were then washed in 2× SSC, 0.1% SDS for 5 minutes at room temperature and then for 30 minutes at 42°C. The oligonucleotide probes were labeled with [γ32P]ATP (specific activity, 3,000 Ci/mmol; Amersham) by using the Ready To Go T4-Polynucleotide Kinase (Pharmacia) and further purified on Micro Bio-Spin columns (Biorad, Ivry, France) following the manufacturers’ instructions. The glyceraldehyde phosphate dehydrogenase (GAPDH) and actin cDNA probes (Clontech, Palo Alto, CA) were labeled with [α32P]dCTP by random priming using the Rediprime DNA labeling sytem (Amersham). The blots were then exposed for autoradiography.
Biological activity of PMN OSM.
The HepG2 hepatoma cell line was cultured in minimum essential medium Eagle (MEM) containing Glutamax, 25 mmol/L HEPES, and Earle’s salts (Life Technologies, Cergy Pontoise, France) plus 10% heat-inactivated fetal calf serum. Cells were plated in 24-well culture plates (Costar) and allowed to grow to confluence at 37°C in humidified air containing 5% CO2. Monolayers were then incubated for 24 hours with 1 mL of RPMI 1640 medium supplemented with 10% fetal calf serum, antibiotics, 2 mmol/L glutamine, pyruvate (Life Technologies), and 10−6 mol/L dexamethasone and were stimulated with either 10 ng/mL recombinant human OSM (rhOSM; Genzyme) or the culture supernatant of PMN stimulated with LPS + GM-CSF (PMN conditioned medium [CMP]). In some cases, before HepG2 stimulation, rhOSM and CMP were preincubated at 37°C for 30 minutes with polyclonal rabbit antibodies against human OSM (20 μg/mL; Genzyme).
The cell-free supernatants of HepG2 cultures were then harvested and stored at −20°C until ELISA measurement of α1-acid glycoprotein.10 Cell total DNA content was quantified11 to express the amount of secreted α1-acid glycoprotein in nanograms per microgram of DNA. All samples were tested in duplicate.
Statistical analysis.
Data are expressed as the means ± SEM; differences were considered statistically significant if P < .05 in Wilcoxon’s paired test.
RESULTS
OSM synthesis can be induced by various stimuli.
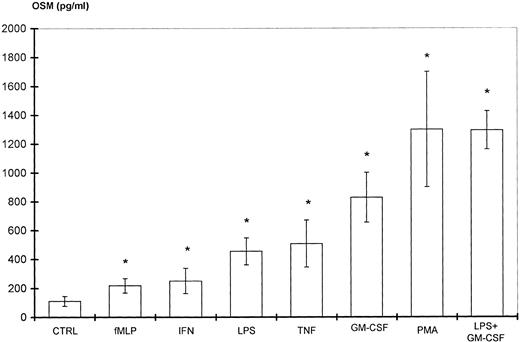
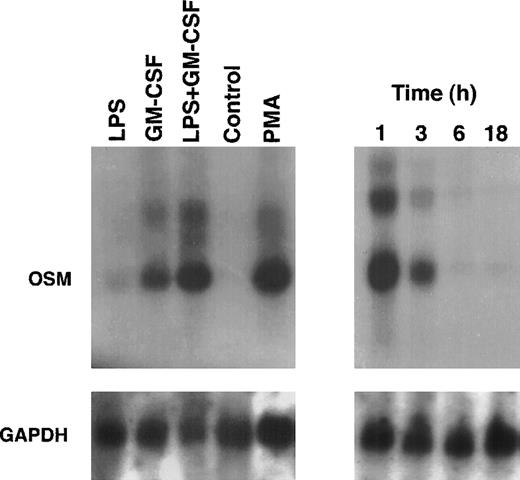
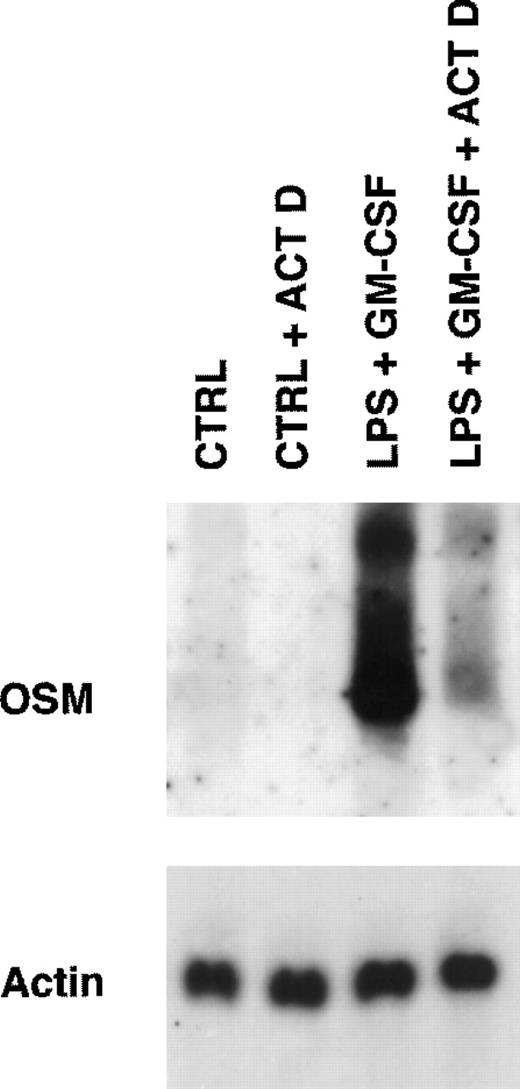
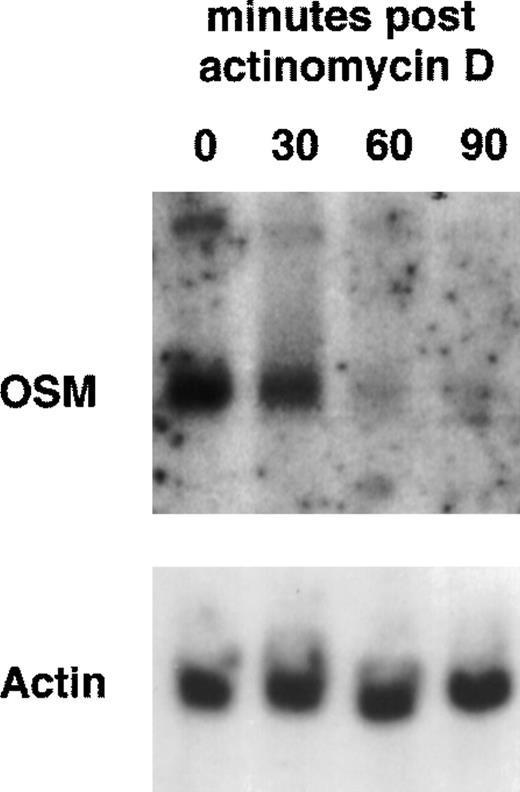
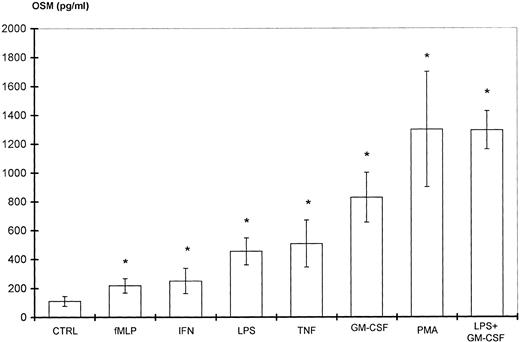
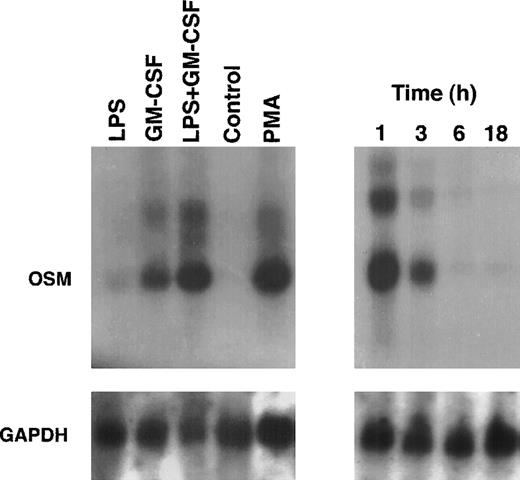
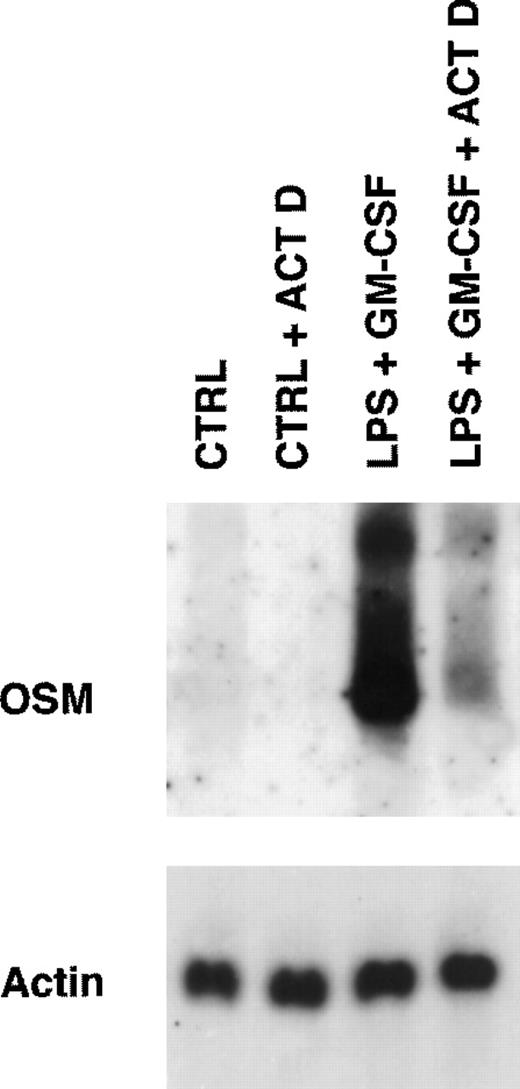
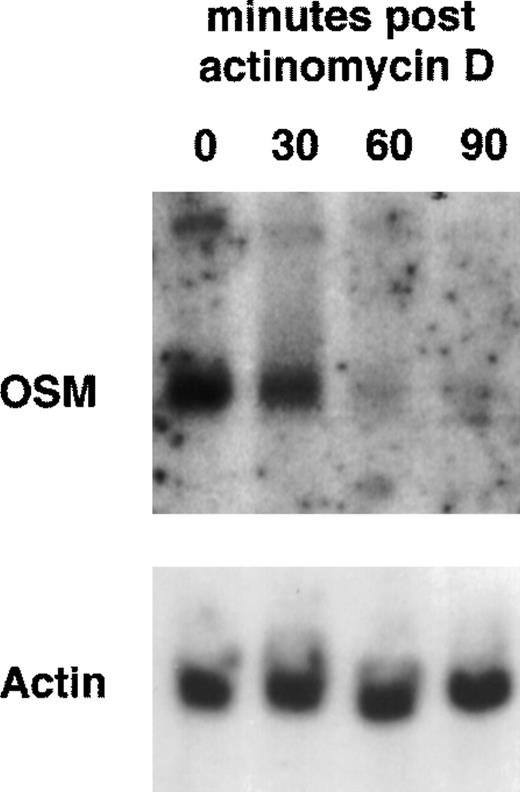
Because PMN have been described to synthesize several cytokines de novo, the ability of PMN to synthesize OSM in various conditions of stimulation for 20 hours of culture was tested. As shown in Fig 1, fMLP, IFN-γ, and LPS alone moderately induced OSM release as compared with unstimulated PMN (respectively, 218 ± 50, 251 ± 88, and 455 ± 93 pg/107 PMN v 126 ± 39 pg/107 PMN;P < .05; n = 6). TNF-α and GM-CSF were potent inducers (respectively, 508 ± 163 and 828 ± 173 pg/107 PMN;P < .05). The strongest OSM production was obtained using PMA alone or LPS plus GM-CSF, giving values of, respectively, 1,300 ± 400 and 1,294 ± 133 pg/107 PMN (P < .05). OSM upregulation by LPS, GM-CSF, LPS plus GM-CSF, and PMA was observed at both the protein and mRNA levels. As shown in Fig 2, OSM mRNA was undetectable after 1 hour in control PMN, whereas stimulated PMN accumulated OSM mRNA. As shown in Fig 3, PMN incubation at the outset of culture with the transcriptional inhibitor actinomycin D strongly reduced the OSM mRNA accumulation induced by LPS plus GM-CSF. After stimulation with these agonists for 1 hour (steady-state mRNA peak in Fig 2), actinomycin D was added to assess OSM transcript stability. In these conditions, OSM mRNA levels decreased rapidly (Fig 4), with a calculated half-life of 45 ± 8 minutes (n = 3, regression analysis). Taken together, our data suggest that the induction of this gene by LPS plus GM-CSF might take place at the transcriptional level.
Stimulation of PMN OSM production. PMN (107/mL) were incubated for 20 hours with various stimulating agents at the concentrations indicated in Materials and Methods. OSM was assayed in the cell-free supernatants. Control cells (CTRL) were incubated with medium alone. Results are expressed as the means ± SEM of six independent experiments. *P < .05 compared with control cells.
Stimulation of PMN OSM production. PMN (107/mL) were incubated for 20 hours with various stimulating agents at the concentrations indicated in Materials and Methods. OSM was assayed in the cell-free supernatants. Control cells (CTRL) were incubated with medium alone. Results are expressed as the means ± SEM of six independent experiments. *P < .05 compared with control cells.
OSM mRNA expression in human PMN. PMN (5 × 107) were cultured for up to 18 hours in the presence of LPS (100 ng/mL) plus GM-CSF (100 U/mL) (right panel). In other experiments, PMN were cultured for 1 hour in the presence of LPS and/or GM-CSF, PMA (100 ng/mL), or in medium alone (control) (left panel). Total RNA was extracted and Northern blots of OSM and GAPDH were run as specified in Materials and Methods.
OSM mRNA expression in human PMN. PMN (5 × 107) were cultured for up to 18 hours in the presence of LPS (100 ng/mL) plus GM-CSF (100 U/mL) (right panel). In other experiments, PMN were cultured for 1 hour in the presence of LPS and/or GM-CSF, PMA (100 ng/mL), or in medium alone (control) (left panel). Total RNA was extracted and Northern blots of OSM and GAPDH were run as specified in Materials and Methods.
Effect of actinomycin D on expression of OSM transcripts induced by LPS plus GM-CSF. PMN (5 × 107) were incubated for 15 minutes in the presence or absence of 5 μg/mL actinomycin D (ACT D) and then stimulated for 1 hour with LPS (100 ng/mL) plus GM-CSF (100 U/mL). Controls cells (CTRL) were incubated in the medium alone. Total RNA was extracted and processed for Northern blot analysis of OSM and actin mRNAs as described in Materials and Methods. Results are from one experiment representative of two.
Effect of actinomycin D on expression of OSM transcripts induced by LPS plus GM-CSF. PMN (5 × 107) were incubated for 15 minutes in the presence or absence of 5 μg/mL actinomycin D (ACT D) and then stimulated for 1 hour with LPS (100 ng/mL) plus GM-CSF (100 U/mL). Controls cells (CTRL) were incubated in the medium alone. Total RNA was extracted and processed for Northern blot analysis of OSM and actin mRNAs as described in Materials and Methods. Results are from one experiment representative of two.
Stability of OSM transcripts in LPS plus GM-CSF–treated PMN. PMN (5 × 107) were incubated with LPS (100 ng/mL) plus GM-CSF (100 U/mL) for 1 hour and then actinomycin D (5 μg/mL) was added for the indicated times. Total RNA was extracted and Northern blot analysis of OSM and actin mRNAs was performed as indicated in Materials and Methods.
Stability of OSM transcripts in LPS plus GM-CSF–treated PMN. PMN (5 × 107) were incubated with LPS (100 ng/mL) plus GM-CSF (100 U/mL) for 1 hour and then actinomycin D (5 μg/mL) was added for the indicated times. Total RNA was extracted and Northern blot analysis of OSM and actin mRNAs was performed as indicated in Materials and Methods.
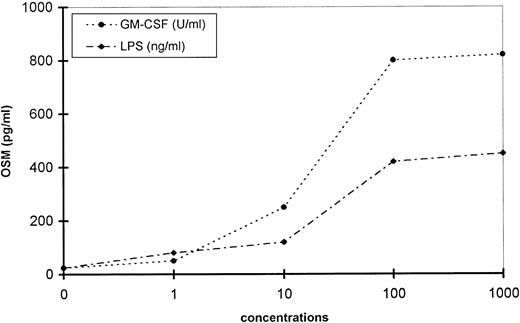
OSM production increased in a concentration-dependent manner and reached a steady level with 100 U/mL GM-CSF and 100 ng/mL LPS (Fig 5). A fixed concentration of GM-CSF (100 U/mL) combined with increasing concentrations of LPS (1 to 1,000 ng/mL) had an additive effect (not shown). Other combinations, including LPS plus IFN-γ or TNF-α, had the same additive effects (not shown).
Concentration-response of GM-CSF– and LPS-induced OSM production by PMN. PMN (107/mL) were stimulated with increasing concentrations of LPS or GM-CSF for 20 hours. OSM was assayed in the cell-free supernatants. These data are representative of one typical experiment of three.
Concentration-response of GM-CSF– and LPS-induced OSM production by PMN. PMN (107/mL) were stimulated with increasing concentrations of LPS or GM-CSF for 20 hours. OSM was assayed in the cell-free supernatants. These data are representative of one typical experiment of three.
Because resting T cells are the only cells potentially not removed by HLA class II-coated magnetic beads, their participation in OSM production was evaluated. T lymphocytes alone (5 × 105) did not produce detectable OSM (<2.1 pg/mL) when stimulated by LPS plus GM-CSF for 20 hours; moreover, PMN alone (107) and combined with 5 × 105autologous T lymphocytes produced similar levels of OSM (data not shown).
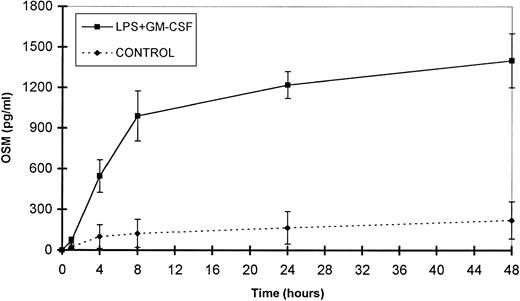
Time course study of OSM release by PMN.
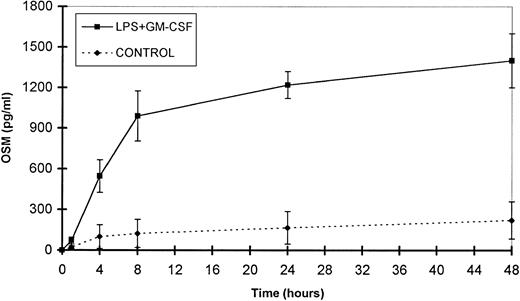
The small amounts of OSM released by control cells were detectable after 4 hours of culture. By contrast, in optimal conditions of stimulation (LPS + GM-CSF), OSM was detectable after 1 hour, reached a plateau by 24 hours, and gradually accumulated for up to 48 hours of culture (n = 3; Fig 6). It is noteworthy that 70% of the total amount of OSM was synthesized within 8 hours. Maximal expression of OSM mRNA by PMN stimulated by LPS plus GM-CSF was observed as early as 1 hour and disappeared after 6 hours (Fig 2).
Time course of OSM release by PMN. PMN (107/mL) were stimulated with GM-CSF (100 U/mL) plus LPS (100 ng/mL). Control cells were incubated with the medium alone. Supernatants were collected at the times indicated and OSM was assayed in an ELISA method. Results are expressed as the mean ± SEM of three independent experiments.
Time course of OSM release by PMN. PMN (107/mL) were stimulated with GM-CSF (100 U/mL) plus LPS (100 ng/mL). Control cells were incubated with the medium alone. Supernatants were collected at the times indicated and OSM was assayed in an ELISA method. Results are expressed as the mean ± SEM of three independent experiments.
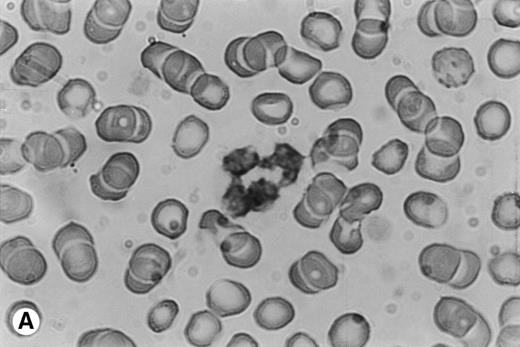
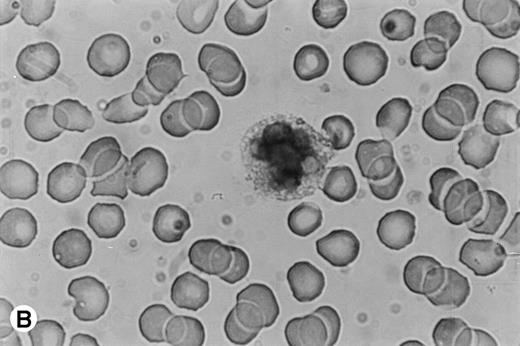
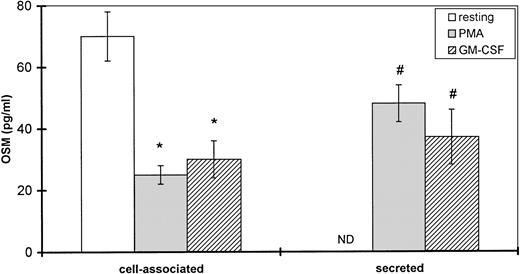
Human PMN contain an intracellular pool of OSM.
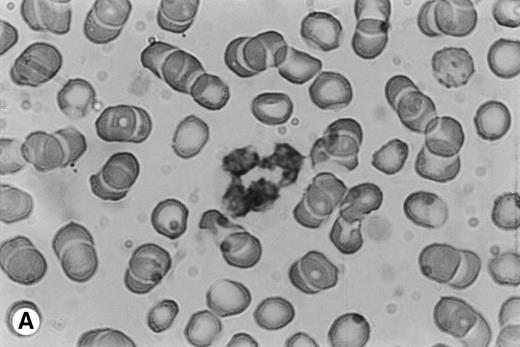
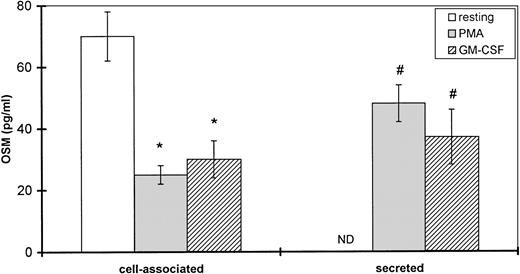
Because time-course studies showed that OSM was released as early as 1 hour after PMN stimulation, we investigated whether PMN contained a preformed stock of OSM in two different ways. First, immunocytochemistry showed the presence of intracellular OSM in unstimulated PMN in whole-blood smears (Fig7). Second, degranulating experiments were conducted with pure isolated PMN incubated with or without PMA or GM-CSF (both potent inducers of neutrophil degranulation8 12) for 15 minutes at 37°C. Released and cell-associated OSM were then measured separately. As shown in Fig 8, the amount of cell-associated OSM was 70 ± 8 pg per 107 PMN, whereas the OSM concentration was below the detection limit in the supernatant of resting PMN maintained for 15 minutes at 37°C in the absence of degranulating agents. PMA or GM-CSF stimulation led to a reduction in cell-associated OSM, matched by a parallel increase in the extracellular OSM level (Fig 8); the total amount of extracellular plus cell-associated OSM was similar to the total amount of cell-associated OSM in resting PMN. These results suggested that a preexisting pool of OSM was released. Similar experiments with incubation times of 5 and 30 minutes gave similar results, in keeping with a maximal degranulating effect of PMA after as little as 5 minutes (data not shown).
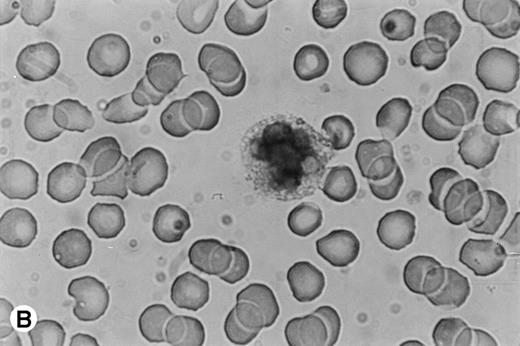
Immunocytochemical staining of PMN in whole-blood smears. (A) Negative control; no staining was seen with a control Ig. (B) Intracellular fuschia staining was observed in PMN with specific polyclonal anti-OSM antibodies; smears were examined by light microscopy at ×1,200.
Immunocytochemical staining of PMN in whole-blood smears. (A) Negative control; no staining was seen with a control Ig. (B) Intracellular fuschia staining was observed in PMN with specific polyclonal anti-OSM antibodies; smears were examined by light microscopy at ×1,200.
Effect of PMA and GM-CSF on OSM secretion by human PMN. PMN (107/mL) were incubated for 15 minutes without (resting) or with 100 ng/mL PMA or 100 U/mL GM-CSF. Secreted and cell-associated OSM were assayed with a specific ELISA. Results are expressed as the mean ± SEM of four independent experiments. #P < .05 compared with OSM secreted without degranulating agent. *P < .05 compared with cell-associated OSM without degranulating agent. ND, not detected (<2.1 pg/mL).
Effect of PMA and GM-CSF on OSM secretion by human PMN. PMN (107/mL) were incubated for 15 minutes without (resting) or with 100 ng/mL PMA or 100 U/mL GM-CSF. Secreted and cell-associated OSM were assayed with a specific ELISA. Results are expressed as the mean ± SEM of four independent experiments. #P < .05 compared with OSM secreted without degranulating agent. *P < .05 compared with cell-associated OSM without degranulating agent. ND, not detected (<2.1 pg/mL).
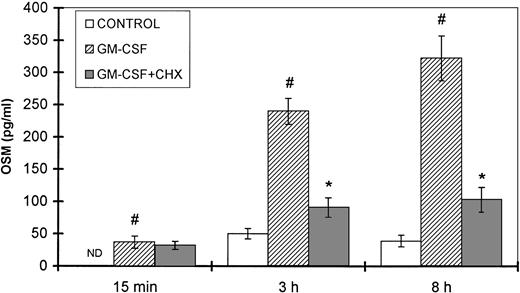
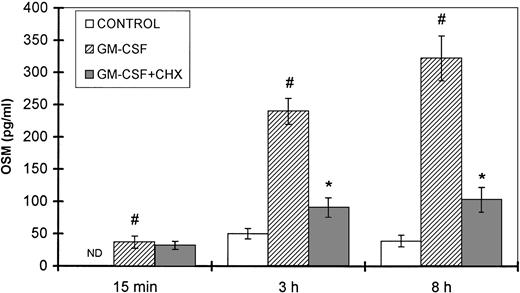
Because GM-CSF is a physiologic inducer of both neutrophil degranulation and OSM synthesis, a kinetic study was performed in the presence and absence of cycloheximide, an inhibitor of protein synthesis. The amounts of OSM detected after 15 minutes of stimulation with GM-CSF were not affected by cycloheximide (Fig 9). In contrast, after 3 and 8 hours of GM-CSF stimulation, a significant reduction in OSM synthesis was observed after CHX treatment (62% and 68% inhibition, respectively) as compared with untreated cells (P < .05, n = 4).
Effect of CHX on GM-CSF–induced OSM secretion. PMN (107/mL) were preincubated for 30 minutes with or without 10 μg/mL CHX and then stimulated with 100 U/mL GM-CSF for the times indicated; control cells were incubated with medium alone. The OSM secreted was assayed in the cell-free supernatants with an ELISA method. Results are expressed as the mean ± SEM of four independent experiments. #P < .05 compared with the control cells. *P < .05 compared with the CHX untreated cells. ND, not detected (<2.1 pg/mL).
Effect of CHX on GM-CSF–induced OSM secretion. PMN (107/mL) were preincubated for 30 minutes with or without 10 μg/mL CHX and then stimulated with 100 U/mL GM-CSF for the times indicated; control cells were incubated with medium alone. The OSM secreted was assayed in the cell-free supernatants with an ELISA method. Results are expressed as the mean ± SEM of four independent experiments. #P < .05 compared with the control cells. *P < .05 compared with the CHX untreated cells. ND, not detected (<2.1 pg/mL).
Taken together, these data strengthen the hypothesis that the observed OSM secretion by PMN is first due to the release of a preexisting intracellular pool, followed by de novo synthesis.
DEX but not IL-10 inhibits OSM production induced by LPS plus GM-CSF.
Because IL-10 and DEX are potent modulators of cytokine production by PMN,13 14 we tested their influence on inducible OSM production. As shown in Fig 10, pretreatment of PMN with DEX resulted in significant concentration-dependent inhibition of OSM production induced by LPS plus GM-CSF (inhibition of 0%, 42%, 61%, and 65% with DEX concentrations of 10−10, 10−8, 10−6, and 10−4 mol/L, respectively; n = 3). By contrast, IL-10 (1 to 1,000 ng/mL) failed to modulate OSM production by PMN in optimal conditions of stimulation (not shown). The same pattern was observed at the OSM mRNA level, as DEX reduced the level of OSM transcripts in stimulated PMN, whereas IL-10 failed to affect it (not shown).
Effect of DEX on OSM production by stimulated PMN. PMN (107/mL) were incubated for 30 minutes with DEX (indicated concentrations) and then stimulated by GM-CSF (100 U/mL) plus LPS (100 ng/mL) for 20 hours; OSM was assayed in the cell-free supernatants by using an ELISA method. The data are expressed as the mean ± SEM of three independent experiments. *P < .05 by comparison with LPS- plus GM-CSF–stimulated PMN without DEX.
Effect of DEX on OSM production by stimulated PMN. PMN (107/mL) were incubated for 30 minutes with DEX (indicated concentrations) and then stimulated by GM-CSF (100 U/mL) plus LPS (100 ng/mL) for 20 hours; OSM was assayed in the cell-free supernatants by using an ELISA method. The data are expressed as the mean ± SEM of three independent experiments. *P < .05 by comparison with LPS- plus GM-CSF–stimulated PMN without DEX.
OSM bioactivity.
OSM is an IL-6–related cytokine that stimulates hepatocytes and induces the expression of acute-phase proteins such as α-1 acid glycoprotein (AGP).5 We investigated the ability of CMP (culture supernatant of PMN stimulated with LPS plus GM-CSF) to stimulate α1-acid glycoprotein synthesis by HepG2 cells. In a preliminary experiment, we found that AGP synthesis was unaffected by direct stimulation with LPS plus GM-CSF. Conditioned medium (500 μL) containing 4 ng of OSM (measured by ELISA) was added to HepG2 cell culture medium for 24 hours. As shown in Fig 11, CMP and rhOSM (10 ng/mL) stimulated α1-acid glycoprotein synthesis as compared with unstimulated HepG2 cells (n = 5; P < .05), and antibodies raised against OSM led to a 35% inhibition of CMP-induced AGP secretion (from 29.3 to 18.9 ng AGP/μg DNA; P < .05 as compared with CMP alone), whereas it completely abolished rhOSM-induced AGP secretion (control).
Biological activity of OSM produced by PMN. HepG2 cells were incubated with the supernatant from LPS- plus GM-CSF–stimulated PMN (PMN conditioned medium [CMP]) or rhOSM (10 ng/mL); control cells (CTRL) were cultured in the medium alone. Anti-OSM antibodies were used as indicated. Secreted 1-acid glycoprotein (AGP) was determined in cell-free supernatants after 24 hours (nanograms per microgram of DNA). Results are expressed as the mean ± SEM of five independent experiments. *P < .05 compared with control cells. #P< .05 compared with AGP synthesis without anti-OSM.
Biological activity of OSM produced by PMN. HepG2 cells were incubated with the supernatant from LPS- plus GM-CSF–stimulated PMN (PMN conditioned medium [CMP]) or rhOSM (10 ng/mL); control cells (CTRL) were cultured in the medium alone. Anti-OSM antibodies were used as indicated. Secreted 1-acid glycoprotein (AGP) was determined in cell-free supernatants after 24 hours (nanograms per microgram of DNA). Results are expressed as the mean ± SEM of five independent experiments. *P < .05 compared with control cells. #P< .05 compared with AGP synthesis without anti-OSM.
DISCUSSION
This study suggests that human PMN contain an intracellular pool of OSM that is rapidly released in degranulating conditions. This release is followed by OSM mRNA accumulation and protein synthesis in PMN stimulated by various agonists. Given the role of OSM in the acute-phase response, these findings support the pivotal role of PMN in the inflammatory process via a newly described two-step mechanism. Indeed, some cytokines are stored in PMN, such as vascular endothelial growth factor (VEGF),8 whereas others can be synthesized de novo, such as TNF-α,2 but we concomitantly explored for the first time both secretion processes for the same cytokine.
The PMN isolation method used here yielded highly purified preparations (>99% PMN). Immunomagnetic depletion of HLA class II-positive cells (B cells, activated T cells, and monocytes) may have left a very small proportion of HLA class II-negative cells (resting T lymphocytes), but these cells were ruled out as a source of the observed OSM release, by means of PMN and T-lymphocyte coculture assays. Furthermore, we failed to detect IL-6 (<10 pg/mL/107cells, ELISA) in our PMN preparations even after LPS stimulation (data not shown), confirming the absence of contaminating monocytes.2 14
Although the intracellular pool of OSM rapidly released during degranulation induced by PMA or GM-CSF seemed small (48 ± 6 pg and 37 ± 9 pg/107 PMN, respectively) as compared with the amount synthesized after 20 hours of culture, this content of pre-existing OSM is of importance, because it allows a rapid and significant secretion of OSM at the inflammatory site and could be an early event in the multistep process of PMN activation. The existence of this intracellular pool was confirmed by using CHX, which failed to affect early OSM release. Other cytokines stocked in PMN granules and rapidly released after exposure to degranulating agents include VEGF in specific granules8 and TGF-α in an undefined granule type.15 PMA and GM-CSF failed to release the entire OSM pool, as shown by a measurable residual level of cell-associated OSM. PMA induces degranulation of specific and azurophilic granules, whereas the effect of GM-CSF is limited to specific granules. OSM may thus also be located in another type of granules or in membrane-bound state.
PMN are relatively short-lived end-stage cells that can synthesize mRNA encoding various cytokines.2 We found that OSM production by PMN was upregulated by a wide variety of stimuli, including chemoattractants and cytokines, corresponding to different mechanisms of cell activation. GM-CSF, TNF-α, and IFN-γ, all of which upregulate the expression of several cytokine genes in PMN,1,16 also induced OSM synthesis; among them, the most potent effect was observed with GM-CSF. Formyl peptide (fMLP), a chemotactic agent for PMN,17 showed weak activity. The protein kinase C activator PMA yielded the most potent induction of OSM synthesis. LPS alone was a relatively moderate inducer of OSM, and its combination with GM-CSF (or IFN-γ or TNF-α) was additive, pointing to the use of different signaling pathways. The low level of OSM produced by unstimulated cells cultured for 20 hours was usually found with other cytokines and could be due to stimulation by cell adherence.16 Interindividual variations were also observed, as commonly described with other cytokines.17 18
Kinetic studies showed that the amount of OSM (70 pg/107PMN) measured after 1 hour of stimulation by LPS plus GM-CSF (the optimal combination of physiological stimuli) was similar to the intracellular stock of OSM, suggesting that the OSM induced by LPS plus GM-CSF is initially cell-associated. OSM production then increased and was strong within 4 hours of stimulation (40% of the total OSM amount), suggesting direct stimulation rather than induction of a second messenger. Similar results were obtained with GM-CSF alone. Moreover, CHX significantly reduced OSM production at 8 hours (by up to 68%), confirming de novo protein synthesis. Northern blotting showed that OSM mRNA expression by stimulated PMN was rapid (as early as 1 hour). In the same way, Cassatella et al17 and Fujishima and Aikawa19 demonstrated that IL-8 mRNA expression was maximal at 1 hour. This maximal expression of OSM mRNA in PMN was transient, with a short half-life. The combination of LPS plus GM-CSF acts at least partly at the transcriptional level, as suggested by Northern blot analysis in the presence of actinomycin D. Taken together, these findings confirm that PMN make an early and sustained contribution to the inflammatory process through two different mechanisms of OSM release: liberation of a preformed stock followed by de novo protein synthesis. It is noteworthy that neither IL-6 (data not shown)2,14 nor LIF (data not shown) was found in PMN culture supernatants. Therefore, in contrast to monocytes,5 20 among the three major cytokines belonging to the IL-6 family, only OSM was released by PMN whatever the conditions of stimulation used.
Some immunoregulatory mediators downregulate cytokine production by PMN. In our study, DEX strongly inhibited OSM production at both the mRNA and protein levels, in keeping with the inhibitory effects of DEX on other cytokines produced by PMN, such as IL-8.13Interestingly, IL-10 preincubation did not inhibit OSM production induced by LPS plus GM-CSF, whereas IL-8 and TNF-α release was downregulated in the same experiments (data not shown). The modulation of cytokine production by IL-10 is complex and dependent on the nature of the stimulus and cell type.20 21
Richards et al22 have shown that OSM has strong hepatocyte-stimulating activity and that α1-acid glycoprotein, among other acute-phase proteins, is potently induced by OSM in the hepatocyte cell line HepG2. In this work, we found that the supernatant of stimulated PMN upregulated α1-acid glycoprotein production by these cells; moreover, α1-acid glycoprotein secretion was, at least in part, inhibited by anti-OSM antibodies, suggesting that OSM present in the conditioned medium was biologically active.
OSM produced by PMN may serve numerous functions, including the stimulation of acute-phase reactant synthesis22 and the modulation of other regulatory cytokines. OSM produced locally by PMN in inflamed tissues may act as an anti-inflammatory mediator. First, OSM inhibits IL-1–induced expression of IL-8 by lung fibroblasts,23 suggesting that PMN may limit their own recruitment to sites of local inflammation. Second, OSM is a potent inducer of several antiproteases in cells of extrahepatic origin. For example, OSM upregulates the synthesis of α1-antitrypsin and α1-antichymotrypsin by epithelial cells originating from the jejunum, lung or skin.24-26 Therefore, OSM released by activated PMN could participate in this local induction of antiproteases, which could be a mechanism against the detrimental effects of PMN proteases (ie, elastase). Most acute-phase proteins induced by OSM possess anti-inflammatory properties in vivo and in vitro.27 For example, α1-antichymotrypsin and α1-antitrypsin inhibit PMN superoxide anion production and may have a role in the modulation of reactive oxygen species-induced tissue damage.28,29 Besides this anti-inflammatory effect, recent data suggest that OSM could also be directly proinflammatory.30 Indeed, Modur et al30 recently showed that OSM induces PMN adhesion and transmigration, as well as chemokine production by endothelial cells. OSM produced by PMN may not only participate in the repair discussed above, but also play a role in initiating the inflammatory response.
In conclusion, our data indicate that PMN at inflammatory sites can rapidly release an intracellular stock of OSM, followed by de novo OSM synthesis. This two-step mechanism of cytokine secretion by PMN would allow rapid and sustained OSM release to occur at inflammatory sites and may contribute to the modulation of local inflammation.
ACKNOWLEDGMENT
The authors are grateful to V. Andrieux-Beautru, P. Soler, C. Poüs, and V. Leçon-Malas for expert technical assistance and to Prof M.A Cassatella (University of Verona, Verona, Italy) and V. Ollivier for helpful discussions and advice.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sylvie Chollet-Martin, PhD, Laboratoire d’Hématologie et d’Immunologie, Hôpital Bichat, 16 rue Henri Huchard, 75018 Paris, France; e-mail:sylvie.martin@bch.ap-hop-paris.fr.



![Fig. 11. Biological activity of OSM produced by PMN. HepG2 cells were incubated with the supernatant from LPS- plus GM-CSF–stimulated PMN (PMN conditioned medium [CMP]) or rhOSM (10 ng/mL); control cells (CTRL) were cultured in the medium alone. Anti-OSM antibodies were used as indicated. Secreted 1-acid glycoprotein (AGP) was determined in cell-free supernatants after 24 hours (nanograms per microgram of DNA). Results are expressed as the mean ± SEM of five independent experiments. *P < .05 compared with control cells. #P< .05 compared with AGP synthesis without anti-OSM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1413/5/m_blod40406011x.jpeg?Expires=1769723184&Signature=xSURCM2Bt5fNbMJvsFk-hT3YtyQgtWu2TFHqteKt6PeLr8-9vG00Ev84CTUgNvSTgU8fpNWkOh1LRlR-uedjIksKoNUjC1ge3aYFXXPdw~R8PnFHdSIzj~yudP7uW5FdzNMY6MoP0GIct8yjQ6MMdJI1SaEnuH8KVKe2uhuinsPWXAcklAI5~dFiL44HD7kh2BWhMu4KAF6g2HBvBvUy7L6XOMh57hFCL5EDW1e0PfSRBFOSIXxiyq~jDm~l4ypfvohbBQkji4unRDlksNBNBggaeO3xvLLrDQtl0kLH5sx61tSsT~a2Suj97rm2gtyAwD3H4DUnCw~vEyiy6D3kmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 11. Biological activity of OSM produced by PMN. HepG2 cells were incubated with the supernatant from LPS- plus GM-CSF–stimulated PMN (PMN conditioned medium [CMP]) or rhOSM (10 ng/mL); control cells (CTRL) were cultured in the medium alone. Anti-OSM antibodies were used as indicated. Secreted 1-acid glycoprotein (AGP) was determined in cell-free supernatants after 24 hours (nanograms per microgram of DNA). Results are expressed as the mean ± SEM of five independent experiments. *P < .05 compared with control cells. #P< .05 compared with AGP synthesis without anti-OSM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1413/5/m_blod40406011x.jpeg?Expires=1769723185&Signature=gVRw1SJTKNKypmA687jePfFNs01pqpPg--uhGEwnLSUc4cz9v0uFkMpwyIyiXKyT2QmifDUK~71ktIrz~SBwtEeaetJwGHkuk2OnjvSGJlp8Ut~tR7EslNfF3lJeWZbLnUAjpHTR1gxUDvQ14SQm~jugreavF9mt0OgKz7dMXPtLr4k6HhqINX47f8k7a-o7~XO~Z4pjNV3Pch2A~jsgTOhbS9jRGChkFrBVddULFX46uw~Q8HAkoNFsN4IqLiuBuk-MfwOnF~ZKOy~zclvjk5Zvy8NEaqGKNY-jxLKRGfd3xTkRc2ddzpkHC69nkU0qive49ZliCZXF-~MZzWQW9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)