We have previously demonstrated that we could separate long-term repopulating stem cells from cells that provided radioprotection (short-term repopulating cells) on the basis of size and suggested that this might be due to the quiescent nature of long-term repopulating cells. To further define the activity of these populations, we used a dye (PKH26), which incorporates into the membrane of cells and is equally distributed to daughter cells when they divide. We developed an assay, which allowed us to retrieve PKH26+ long-term and short-term repopulating cells in the hematopoietic tissues of the recipients posttransplant. We were able to recover the labeled cells and determine their cell cycle activity, as well as their ability to reconstitute secondary lethally irradiated hosts in limiting dilution. The results of our assay suggest that long-term repopulating cells are quiescent in the bone marrow (BM) 48 hours after transplant. We were able to detect only a few labeled cells in the peripheral blood posttransplant and even though cells homed to both the spleen and BM, more long-term repopulating cells homed to the marrow and only these cells, which homed to the marrow, were capable of reconstituting lethally irradiated secondary hosts long-term.
LITTLE IS UNDERSTOOD about the biology of hematopoietic stem cell (HSC) homing after bone marrow (BM) transplant. We have demonstrated that several populations of hematopoietic reconstituting cells exist.1 Cells that provide radioprotection (30-day survival) can be separated on the basis of size from cells that engraft lethally irradiated hosts long-term. Thus, small-sized cells obtained by counter flow-elutriation (flow rate 25, FR25) will long-term reconstitute lethally irradiated mice, but large cells (R/O) only provide for short-term repopulation. By limited dilution FR25 cells, which are further purified by removal of lineage positive cells (Lin−), can reconstitute the mouse for its lifetime.2 Because very few cells are injected (as low as 10 cells per recipient), it suggests that the homing mechanism is exquisitely sensitive. It is thought that homing is mediated by contact of stem cells with cells of the microenvironment through specific receptors.3 The homing receptor has been described as a 110-kD glycoprotein, which requires galactosyl and mannosyl residues to bind hematopoietic progenitors.4 That the process of receptor recognition within hematopoietic environments is a necessity for normal hematopoiesis has been demonstrated by blocking studies. When neoglycoprotein probes have been used that block the binding of the galactosyl or mannose residues to the receptor in long-term marrow culture, the production of colony-forming unit-spleen (CFU-S) is halted.3 Whether there is a difference in homing of progenitors to the spleen versus the BM remains unclear. Neoglycoprotein probes have been shown to block homing of CFU-S to the BM, but do not have a similar effect on homing to the spleen. Whereas treatment of donor cells before transplant with antibodies to very late activation antigen-4 (VLA-4), a β1 integrin that binds to fibronectin and vascular cell adhesion molecule −1 (VCAM-1) causes CFU-S to home more to the spleen and less to the BM.5 These experiments suggest that the mechanism of homing and the receptors involved are likely different for both the spleen and the BM. However, others have shown that the seeding efficiencies from normal donor marrow cells to the spleen and the femur are similar.6
The rarity of stem cells in vivo makes them difficult to track. Hendrikx et al7 have used the dye PKH26 to follow CFU-S localization in mice. PKH26 is a fluorescent marker that stains the cell membrane, the intensity of the stain decreases in linear fashion with each cell division. In this study, we have used PKH26 as a tool to both track and describe the biology of stem cells in vivo. We will demonstrate at 48 hours in the primary recipient we recover a maximum number of PKH26 bright cells from the marrow, which allows us to further study the biology of short-term and long-term repopulating cells.
MATERIALS AND METHODS
Animals.
Male and female C57 BL/6Jx DBA/2 F1 (B6D2F1) mice (National Cancer Institute, Frederick, MD) 6 to 12 weeks of age, were used for all studies. Mice were housed in sterile microisolator cages. They were fed acidified water and sterilized lab chow ad libitum.
Isolating and staining HSC.
For each isolation experiment, 20 male mice were killed by cervical dislocation and the hind legs removed. BM was flushed with medium from the medullary cavities of both the femurs and tibias using a 25-gauge needle. Single cell suspensions were produced by repeated passage through the needle. Approximately 30 million whole bone marrow (WBM) cells were set aside before counterflow centrifugal elutriation (CCE). These served as control cells for recovery and cell cycle analyses (see below). The cells were elutriated as previously described.1,2 Cells were collected at a flow rate of 25 mL/minute (FR25, long-term repopulating cells) and from the rotor off (R/O) fraction (short-term repopulating cells). The FR25 cells were then lineage depleted by placing 107 cells on petri dishes absorbed with a cocktail of 60 μg each rat antimouse AA4.1, B220, CD5, GR-1, MAC-1, and TER119 as described.2 After incubating at 4°C for 90 minutes, the nonadherent cells (Lin−) were removed by gentle rocking and aspiration.
PKH26 staining.
The cells from each group (FR25Lin−, R/O) were washed in alpha-minimal essential media (MEM) without bovine serum albumin (BSA) or serum. Samples were then resuspended in PKH diluent and added to the PKH26 dye at 10-μmol/L concentration. The cells were incubated at room temperature for 2 to 5 minutes with gentle agitation. To stop the reaction, 2 mL of 100% serum was added, and the cells incubated for 1 minute at room temperature with gentle agitation. A total of 4 mL of alpha-MEM with 10% fetal calf serum (FCS) was added. The samples were centrifuged and washed twice with 10 mL alpha-MEM with 10% FCS. Male PKH26-stained cells from each group were then injected into irradiated female mice at a dose of 2.5 million cells per animal. A small number of cells were kept from each group to use as a control for staining efficiency. A single mouse was injected with 2.5 million stained WBM as a control.
Preparation of mice for transplant.
Female primary and secondary recipients received 105 cGy whole body irradiation from a dual-cesium source (Atomic Energy of Canada, Kanata, Ontario, Canada; 8.9 cGy per minute).
Tracking of PKH+ cells.
At 3, 24, 48, and 96 hours posttransplant, primary recipient mice were killed. For the initial studies, peripheral blood was obtained by retro-orbital bleed before recipient animals were killed, but as the yield of PKH26+ cells from these samples was very low, the remainder of experiments was limited to the spleen and BM. The spleen was harvested and ground over wire mesh into media. The spleen samples were then layered over Ficoll Hypaque and centrifuged at 1,200 rpm for 45 minutes. The upper layer was extracted and washed. BM samples were harvested as described above. We obtained 1.43 × 106± 4.2 × 105 cells/hind limb (n = 7 mice, 14 hind limbs) and 8.6 × 106 ± 1.5 × 105 cells/spleen (n = 5 mice) at 48 hours posttransplant. BM cells for transplant were treated with ammonium chloride and then layered over a FCS gradient to remove excess red blood cells and red blood cell ghosts. PKH26 fluorescence intensity was then measured on a Epics 740 flow cytometer (Counter Electronics, Hialeah, FL).
Cell cycle analysis.
BM and spleen samples (PKH26+ cells) collected by cell sorting were washed with phosphate-buffered saline (PBS) containing 0.2% BSA and then resuspended in 100 μL of sucrose (250 mmol/L) trisodium citrate (40 mmol/L) buffer. They were treated with 0.9 mL trypsin (30 μg/mL) and incubated for 10 minutes at room temperature. They were then incubated with 0.75 mL trypsin inhibitor (500 μg/mL) with ribonuclease A (100 μg/mL) for 10 minutes, stained with 0.75 mL propidium iodide (416 μg/mL) with spermine tetra-hydrochloride (1.16 μg/mL, all staining reagents from Sigma, St Louis, MO) at 4°C and analyzed by flow cytometry for percentage of cells in different phases of the cell cycle.
Short-term and long-term reconstitution assays.
Mice that had been injected with PKH26-stained FR25Lin− or R/O cells as described above were killed at 48 hours. The PKH26+ cells from BM or spleen were collected by flow cytometry. The male PKH+ BM or spleen cells from each group were injected at doses to include 101, 102, 103, or 104cells along with 2 × 104 fresh female unstained R/O cells into irradiated secondary female mice. Mice were observed for 30-day survival (short-term reconstitution). In addition, at 6, 12, and 24 weeks, mice receiving 102 PKH+ cells underwent retro-orbital bleeds for donor engraftment. Fluorescent in situ hybridization (FISH) for the Y chromosome was done as previously described2 8 to evaluate over time long-term donor reconstitution.
RESULTS
We first examined the frequency of PKH26 bright cells at various times posttransplant (Fig 1). The highest frequency of labeled cells was found at 48 hours posttransplant in recipient marrow from both FR25Lin− and R/O populations (Fig 1A and B, respectively). This frequency allowed us to obtain sufficient cells for further study of the biology of these cells. Staining FR25Lin− BM with PKH26 at time 0 results in 90.6% PKH+ cells. At 48 hours postprimary transplant of FR25Lin− cells, we can recover 1.02% ± 0.04% labeled cells from the donor within the hind limb BM of the recipient and a reduced number 0.27% ± 0.2% in the spleen. If we label R/O BM cells with PKH26, we can identify 0.01% ± 0.03% PKH+ cells in the hind limb BM and 0.65% ± 0.19 % in the spleen. Because tibia and femur marrow pooled represent approximately 8% of the whole marrow skeleton,9 we extrapolate that FR25Lin− recovery is actually a mean of 12.75% ± 1.0% from the recipient’s whole BM and the R/O recovered cells from recipient’s whole marrow is 10 times less (1.25% ± 0.55%, Table 1; P < .001). Taken together, these results indicate that more donor long-term repopulating cells are found in the marrow than the spleen at 48 hours posttransplant.
Comparison of the number of PKH26+ marrow cells detected in (A) FR25Lin− and (B) R/O fractions at different times posttransplant. These series of dot plots represent the flow diagrams of FR25Lin− (A) and R/O (B) frequencies of PKH-labeled cells present before (0 hours) and various times posttransplant of 2.5 × 106 cells. The number at the top right of each panel represents the percentage frequency of PKH bright cells detected. All plots represent equal numbers of cells counted (100,000).
Comparison of the number of PKH26+ marrow cells detected in (A) FR25Lin− and (B) R/O fractions at different times posttransplant. These series of dot plots represent the flow diagrams of FR25Lin− (A) and R/O (B) frequencies of PKH-labeled cells present before (0 hours) and various times posttransplant of 2.5 × 106 cells. The number at the top right of each panel represents the percentage frequency of PKH bright cells detected. All plots represent equal numbers of cells counted (100,000).
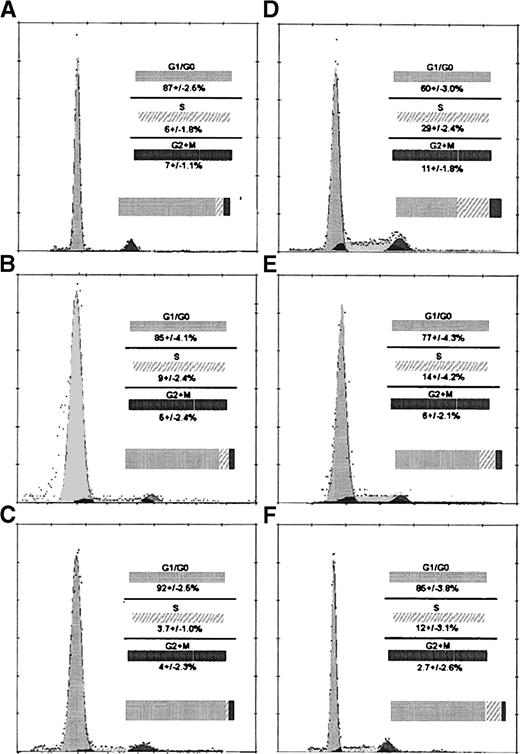
Examination of WBM for cell cycle at time 0 demonstrates 17.0% of cells are in S phase (data not shown). Cell cycle analysis shows that at time 0 FR25Lin− cells are mostly in G1/G0 with very few percent of cells in S phase (Fig 2A), but the fraction of R/O cells in S phase appears to be greater (Fig 2D). At 48 hours posttransplant, there is no increase in the percentage of cells in S phase of FR25Lin− cells recovered in either marrow (Fig 2B) or spleen (Fig 2C) of primary recipients. Recovered PKH+-labeled R/O cells at 48 hours display 12% to 14% cells (Fig 2E and F) in S phase of the cell cycle.
Examination of cell cycle activity from BM and spleen from FR25 Lin− and R/O cells pre- and posttransplant (48 hours). A, B, and C represent FR25Lin−propidium iodide (PI)-labeled cell cycle analyses of preinjection (A), 48 hours in the BM (B), and 48 hours in the spleen (C). D, E, and F are R/O PI cell cycle analyses of preinjection (D), 48 hours in the BM (E), and 48 hours in the spleen (F). Values represent the mean ± standard error of mean (SEM) for five experiments. Comparison of A to B and A to C show no statistically significant differences for S phase. Statistical analysis showed significant difference when comparing D to E (P < .03) and D to F (P < .01) for the S phase of the cell cycle. The histograms and the curve fitting software allowed the determination of the percentage of cells in the phases of the cell cycle (Multi-cycle from Phoenix Flow Systems Inc, San Diego, CA).
Examination of cell cycle activity from BM and spleen from FR25 Lin− and R/O cells pre- and posttransplant (48 hours). A, B, and C represent FR25Lin−propidium iodide (PI)-labeled cell cycle analyses of preinjection (A), 48 hours in the BM (B), and 48 hours in the spleen (C). D, E, and F are R/O PI cell cycle analyses of preinjection (D), 48 hours in the BM (E), and 48 hours in the spleen (F). Values represent the mean ± standard error of mean (SEM) for five experiments. Comparison of A to B and A to C show no statistically significant differences for S phase. Statistical analysis showed significant difference when comparing D to E (P < .03) and D to F (P < .01) for the S phase of the cell cycle. The histograms and the curve fitting software allowed the determination of the percentage of cells in the phases of the cell cycle (Multi-cycle from Phoenix Flow Systems Inc, San Diego, CA).
In a second set of experiments, the PKH26+ cells from the primary recipients’ spleen and BM were obtained by cell sorting. These male PKH26 bright cells were injected into secondary female recipients along with 2 × 104 female R/O cells to provide a source of radioprotection (in preliminary experiments, PKH26 bright cells recovered from the FR25Lin− cell inoculum given alone failed to radioprotect secondary recipients after 48 hours in the primary recipient, data not shown). We observed that survival of secondary recipients at doses from 101 to 103FR25Lin− recovered PKH26 bright cells was possible (Table 2). Interestingly, if the FR25Lin− PKH26 bright cells are harvested from the spleen of primary recipients, the cells fail to support survival and long-term repopulation of the secondary recipients at concentrations as high as 104 cells per recipient (Table 2).
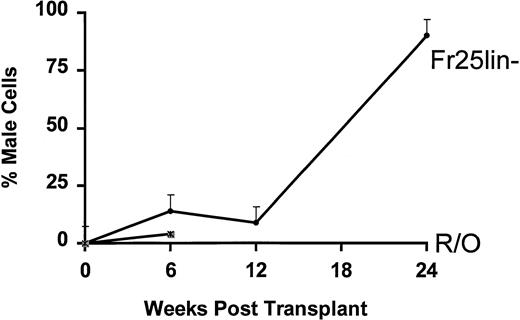
The percentage of male cells present at periods from 6 weeks to 6 months in secondary recipients after injection of only 102FR25Lin− or R/O PKH26 bright cells from the BM is shown in Fig 3. As early as 6 weeks posttransplant, 102 FR25Lin− or R/O cells provide small numbers of donor mature cells in the periphery, but only recipients of FR25Lin− cells continue to demonstrate increases to over 90% donor cells at 6 months posttransplant. Animals that received either 102 (Fig 3) or 103 (data not shown) male R/O PKH26+ cells that had been in the primary recipients for 48 hours failed to long-term repopulate secondary recipients (all died by 12.1 ± 5.2 weeks) when given along with 2 × 104 female R/O cells.
Engraftment of FR25Lin− or R/O cells into secondary recipients of PKH+ cells. One hundred PKH bright BM cells recovered 48 hours posttransplant from male FR25Lin− donors (•) or male R/O donors (∗) were injected into secondary female hosts. Peripheral blood was obtained from these recipients at 6, 12, and 24 weeks posttransplant and analyzed for the presence of male cells. Values represent the mean percent male cells for 4 to 6 recipients. The mean survival for secondary recipients receiving R/O PKH+ cells was 12.1 ± 5.4 weeks.
Engraftment of FR25Lin− or R/O cells into secondary recipients of PKH+ cells. One hundred PKH bright BM cells recovered 48 hours posttransplant from male FR25Lin− donors (•) or male R/O donors (∗) were injected into secondary female hosts. Peripheral blood was obtained from these recipients at 6, 12, and 24 weeks posttransplant and analyzed for the presence of male cells. Values represent the mean percent male cells for 4 to 6 recipients. The mean survival for secondary recipients receiving R/O PKH+ cells was 12.1 ± 5.4 weeks.
DISCUSSION
Our studies demonstrate that early after transplantation cell populations enriched for both short-term radioprotection (R/O) and long-term engraftment (FR25Lin−) home to the hematopoietic organs of lethally irradiated recipient mice. We can recover only a few PKH26-labeled cells in the peripheral blood at 48 hours after transplant, but can find these cells from 3 hours to 96 hours in the BM (Fig 1) and at 48 hours in the spleen of the primary recipients (Table 1). Thus, this assay can be used to determine the site of stem or progenitor cell homing after transplant. Hendrikx et al7 showed that CFU-S cells rapidly undergo proliferation in vivo. CFU-S and long-term repopulating cells have been suggested to be separate populations.1 We show that long-term repopulating cells (FR25Lin−) at 48 hours remain relatively quiescent. Nilsson et al10 recently showed that stem cells can be killed by 5-fluorouracil (5-FU) very shortly posttransplant, suggesting a rapid entering of these cells into cell cycle. However, their study did not directly measure the cell cycle activity shortly after transplant, but relied on engraftment postdrug treatment 6 weeks later (at a time indicative of radioprotection). We believe our assay more directly studies the function of the donor cells by direct harvest 48 hours posttransplant in lethally irradiated recipients. At 48 hours, the stem cell population can still short-term and long-term engraft secondary recipients at low numbers (102 cells, Table 2, Fig 3), whereas up to 104 later progenitors (R/O cells) fail to do so. In the R/O fraction, 29.0% ± 4.4% of the cells are in S phase of the cell cycle before injection into the mice. Of the R/O cells that we collect after 48 hours in the primary recipient, the percent in cycle decreases (14 ± 4.2 in the BM). Many of the cells in this population are no longer PKH bright as evidenced by our significant reduction of the frequency and total number of PKH bright cells that we recovered from R/O-treated mice after 48 hours (Fig 1 and Table 1). These cells home equally well to the marrow and spleen when we account for tissue volumes. The R/O cells that remain PKH bright contain cells that might be expected to offer a short wave of engraftment.11 We have observed (Fig 3) from R/O PKH bright cells limited male reconstitution at 6 weeks. These cells are more mature functionally as evidenced by the fact that engraftment is not sustained and secondary recipients of R/O PKH bright cells were all dead by 12 weeks.
PKH26 has been used to label potential human stem cells, which do not divide (quiescent) in vitro.12 Recent elegant studies13,14 have shown that human CD34 positive cells residing in G0 are more primitive than those in G1 by in vitro assay and their responsiveness to cytokines may be different than cells in cycle. Our observation that our primitive population remains relatively quiescent at 2 days in vivo is consistent with the ability of these cells to continue to long-term repopulate a lethally irradiated host at small numbers. The observation that the cells, which home to the spleen shortly after transplant (FR25Lin− PKH26 bright cells), also fail to repopulate secondary recipients is intriguing. The spleen microenvironment, which also maintains the donor cells in a relatively quiescent state (only 3% in S phase of the cell cycle, Fig 2), contains less recoverable FR25Lin− PKH bright cells at least at 48 hours. The failure of up to 104 of these FR25Lin− recovered PKH+ donor cells from the spleen to long-term engraft secondary recipients may be a reflection of fewer stem cells homing at 48 hours to the spleen. We further find it interesting that Fr25Lin−, but not R/O cells, are recovered in significantly greater number in the BM than the spleen at 48 hours, suggesting that only early and not late precursors favor the marrow environment at this time point. Some evidence exists that more CFU are found in BM compared with spleen after transplant.15 Others, comparing spleen to the femur, showed equal homing of later progenitors,6 but whole marrow was not compared with the spleen. Alternatively, but less likely, our stem cell population (FR25Lin− cells) could be contaminated with donor lymphoid cells, which are preferentially homing to the marrow. The spleen, however, may also contain stem cell inhibitory accessory cells. We have evidence that lymphoid subsets can inhibit both erythroid growth,16 as well as stem cell engraftment.17 It is clear that cells from the same experiment, which home to the BM of primary recipients did have long-term engraftment potential. We favor the hypothesis that long-term repopulating cells early after transplant are located in a microenvironment, which may be favorable for their proliferation and differentiation.
Future potential applications for this assay include its use in further defining the biology of stem cell homing. Papayannopoulou et al5 has demonstrated that cell surface antigens (ie, VLA-4) act as homing receptors for progenitors like CFU-S. Using an antibody to VLA-4, they have shown that lodgement of CFU-S within the BM can be blocked and that long-term reconstituting cells can be mobilized to the peripheral blood using a similar antibody.18 Given that our assay allows identification of quiescent cells shortly after injection it could be used along with antibodies to cell surface adhesion molecules to investigate more closely the interaction of long-term engrafting cells with the microenvironment.
We have described an assay of stem cell homing, which allows us to further study the biology of stem cell growth in vivo and which allows us to identify and isolate cells that provide long-term engraftment.
ACKNOWLEDGMENT
We thank Marie C. Moineau and Chriscinthia Blount for their assistance with manuscript preparation.
Supported in part by Grants No. RO1-HL54330 and T32HL007525 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Saul J. Sharkis, PhD, Johns Hopkins Oncology Center, Room 2-127, 600 North Wolfe St, Baltimore, MD 21287-8967.