Vpr, a 96 amino acid protein, encoded by the human immunodeficiency virus type I (HIV-1), is important for efficient infection of mononuclear phagocytic cells. These cells are abundant in whole bone marrow, which can easily be cultured in vitro to support hematopoiesis. Our experiments indicate that Vpr plays a role in the potent activation of murine and human mononuclear phagocytic cells within a hematopoietic microenvironment. In murine cultures, avid erythrophagocytosis is triggered by transduction of marrow cells with supernatant derived from PA317 cells transfected with a murine retroviral delivery vector bearing a Vpr expression cassette. Supernatants derived from cells transfected with the same vector carrying sequences for the expression of other relevant viral and nonviral proteins do not induce erythrophagocytosis to any marked degree. The effect on human marrow cells is similar, where treatment promotes adhesion of mononuclear phagocytic cells to culture plates in association with other nucleated and nonnucleated cells that undergo subsequent engulfment. The differential effects of Vpr point and deletion mutants in both marrow culture systems fortify the view that the effect is specific to HIV-1 Vpr. Addition of low molar quantities of purified Vpr to marrow cultures is also capable of promoting cell adhesion and phagocytosis, suggesting that extracellular Vpr is the effector of the phenomenon. Accelerated phagocytosis is a hallmark of promonocyte, monocyte, and macrophage activation and its occurrence within a hematopoietic microenvironment may account for critical in vivo pathogenic features of HIV-1 infection. First, activation of mononuclear phagocytes may promote productive viral infection; and second, premature phagocytosis could provide, at least in part, a molecular explanation for the induction of the idiopathic cytopenias that are typical of individuals infected with HIV-1.
HUMAN IMMUNODEFICIENCY virus type I (HIV-1) replication occurs primarily in cells of hematopoietic origin,1,2 and the most severe pathogenic features associated with infection can ultimately be attributed to either malfunction or destruction of subsets of cells derived from this lineage, eg, CD4+ T lymphocytes and monocyte/macrophages. Accordingly, the use of whole bone marrow cultures was pursued as an in vitro model system that might be manipulated easily to obtain a more clear understanding, particularly of early events, that underlie hematologic disorders associated with long-term retroviral infection.3 Specifically with regard to patients with acquired immunodeficiency syndrome (AIDS), these disorders typically include anemia, lymphocytopenia, monocytopenia, and neutropenia. Almost all patients with advanced AIDS exhibit pancytopenia, with greater than 90% suffering from anemia.4 Most current studies indicate that hematopoietic stem cells are refractory to HIV-1 infection and must undergo at least modest differentiation to express critical surface receptors to permit virus entry and subsequent replication.5 Several reports have focused on HIV-1 infection of auxillary cells in bone marrow that are required to support normal hematopoiesis; however, the target cell populations and the ability of virus to replicate in cells susceptible to infection in the bone marrow compartment are conflictory.6-8 In addition, the influence on hematopoiesis and the maturation of stem cells within the marrow compartment in the presence of active viral replication is also relatively obscure. Some studies have demonstrated deficiencies or imbalance in the release of growth factors from HIV-1–infected marrow-derived cells required to support normal hematopoiesis.9 This could account for the onset of the variety of cytopenias that occur in HIV-1–infected individuals. As has previously been reported,10 it also seemed reasonable, however, to begin with the premise that perhaps expression or release of a specific HIV-1 gene product, or perhaps a limited combination of viral proteins, could perturb or alter marrow stem cell function. The effects of direct transduction of marrow cultures with specific expression vectors for various proteins, including the HIV-1 regulatory and accessory proteins such as Tat, Rev, and Vpr, was the focus of this study. The effects of transduction for Vpr expression was of particular interest as this protein has been implicated in the efficient infection and replication of HIV-1 in mononuclear phagocytes. In these experiments, transduction of whole bone marrow propagated in vitro with a Vpr expression vector initiated what appears to be premature or enhanced phagocytosis of cells in the marrow cultures by mononuclear phagocytes. The effect was also observed after addition to marrow cultures of recombinant Vpr either as a fusion to glutathione-S-transferase (GST) or as a free protein released from the GST fusion partner by protease treatment. Accelerated phagocytosis could reasonably account for certain of the cytopenias typical in vivo in persons suffering from advanced stages of AIDS.
MATERIALS AND METHODS
Plasmid construction.
An HIV-1NL4-3 Vpr open reading frame DNA fragment was cloned into an amphotropic murine retroviral delivery vector, SLX-CMV, using compatible 5′ BamHI sites and a filled in 3′HindIII site of the insert into a 3′ Hpa I site of the vector to create the Vpr expression plasmid, SLX-CMV-Vpr. Subsequent sequencing verified the integrity of the construct. HIV-189.6 Vpr point and deletion mutants were obtained by polymerase chain reaction (PCR) amplification from existing plasmids (provided by A.S.), using Vent polymerase and primers bearing 5′BamHI and 3′ Mlu I sites for direct insertion into the vector, SLX-CMV. All plasmids were sequenced through the Vpr insert before use. Construction and use of other vectors such as pLX-Tat, pLX-Rev,11 and SLX-CMV-CAT12 have been described previously.
Bone marrow culture transduction.
PA317 cells, a murine retroviral packaging line, were transfected with 10 to 15 μg of plasmid DNA per 100 × 20 mm dish at 20% confluency. After media change within 12 to 16 hours, supernatant was harvested 48 hours post-transfection, passed through 0.45-μm filters, and stored at −80°C until application to marrow cultures. Murine and human marrow cultures were initiated by seeding aspirates at a density of 2 × 106 nucleated cells per 35 × 10 mm dish. Murine cells were maintained in Dulbecco’s modified Eagle’s medium (D-MEM) with 10% fetal bovine serum (FBS), whereas human cells were grown in D-MEM with 20% FBS. Media was typically replaced 1 to 3 days after plating and 1 mL/dish of supernatant from transfected PA317 cells was applied within 1 week of seeding plates. Adherence and subsequent phagocytosis of resident cells or added latex beads by mononuclear phagocytes occurred typically within 24 to 48 hours after supernatant addition. Cultures were then either photographed directly or washed once with phosphate-buffered saline (PBS), fixed carefully with 100% methanol, and photographed through an inverted light microscope.
Reverse transcriptase assay.
Sixty microliters of PA317 cell supernatant was adjusted to 1% Triton X-100 and added to a final reaction volume of 150 μL containing 40 mmol/L Tris-HCl, pH 7.3, 100 mmol/L KCl, 5 mmol/L MgCl2, 1 μg poly (rA)-oligo (dT), 10 mmol/L dithiothreitol (DTT), and 2.5 μmol/L 3H dTTP. Reactions were incubated for 1.5 hours, spotted onto DE81 filters, dried, and counted with scintillation.
GST-Vpr fusion protein expression, purification, and treatment of marrow cultures.
The construction of the pGEX-GST-Vpr expression plasmid has been described previously.13 Bacterial expression of the fusion protein was initiated by induction of at least 1 L of cells with 0.1 mmol/L isopropyl β-D-thiogalactopyranoside (IPTG) for 1 hour. The induced bacterial cells were harvested by low speed centrifugation and the cells were suspended in 10 mL of PBST (20 mmol/L phosphate buffer, pH 7.3, 150 mmol/L NaCl, 1% Triton-X-100) containing 2 mmol/L EDTA, 0.4 mmol/L phenylmethylsulfonyl flouride, 2 μg/mL leupeptin, 50 μg/mL of 1-chloro-3-tosylamido-7-amino-2-heptone, and 100 μg/mL of L-1-tosylamido-2-phenylethyl-chloromethyl ketone. The cells were disrupted by sonication and the lysate cleared by centrifugation at 10,000g for 10 minutes at 4°C. The supernatant was applied to a 1 mL column of glutathione agarose pre-equilibrated with TBST (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.5% Tween 20). Vpr was released from the GST after the addition of thrombin (Sigma, St Louis, MO) to GST-Vpr at 4 U/mg of fusion protein and incubation for 2 hours at room temperature. The digested sample was applied to 1 mL of glutathione agarose and free Vpr was collected in the column flow-through fraction. The Vpr containing fraction was dialyzed against 4 × 500 mL of PBS at 4°C and sterilized by filtration through a 0.2-μm low protein-binding filter. GST-Vpr or Vpr in PBS was added directly to marrow cultures at a approximate final concentration of 3 nanomolar, which is consistent with the in vivo levels as reported previously by others.14
RESULTS
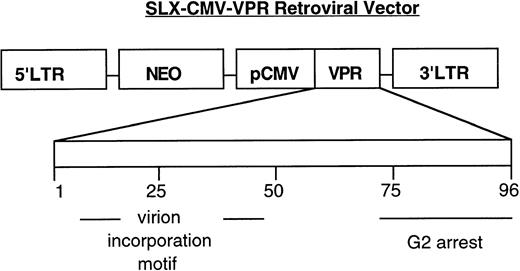
From previous studies, retroviral delivery vectors were available that contained various expression cassettes, including some specific for certain HIV-1 proteins such as viral transcriptional transactivator, Tat, and HIV-1 Rev. As shown in Fig 1, a murine leukemia virus transduction vector, SLX-CMV-Vpr, was subsequently constructed that expresses the 96-amino acid HIV-1 accessory protein, Vpr, independent of other viral products. This protein seemed to be an important candidate for study of its effect on bone marrow cultures, because it has been reported to be capable of cell-cycle arrest15 and differentiation16 and can be found free of virions in the serum of HIV-1–infected individuals.14
Schematic of the HIV-1 Vpr transduction vector. pSLX-CMV-Vpr encodes a Vpr expression cassette from HIV-1 (strain NL4-3) downstream of the cytomegalovirus (CMV) promoter (pCMV). The retroviral packaging cell-line, PA317, provides, in trans, viral proteins necessary for vector transfer.30 Below, the linear map of Vpr, 96 amino acids, denotes the approximate location of domains important for Vpr function.31 32
Schematic of the HIV-1 Vpr transduction vector. pSLX-CMV-Vpr encodes a Vpr expression cassette from HIV-1 (strain NL4-3) downstream of the cytomegalovirus (CMV) promoter (pCMV). The retroviral packaging cell-line, PA317, provides, in trans, viral proteins necessary for vector transfer.30 Below, the linear map of Vpr, 96 amino acids, denotes the approximate location of domains important for Vpr function.31 32
Erythrophagocytosis induced in murine marrow cultures.
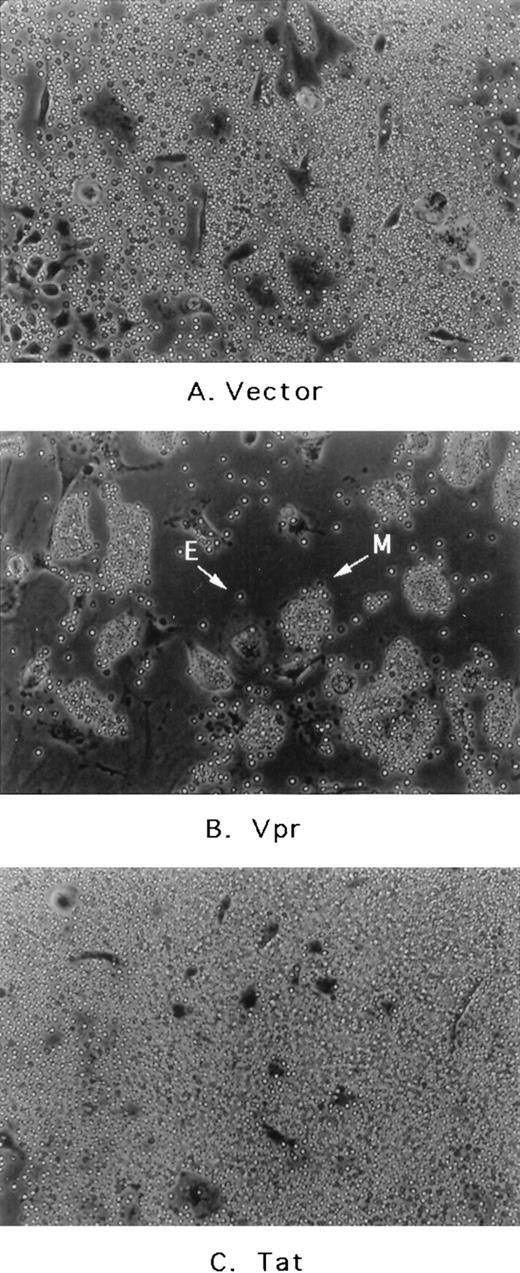
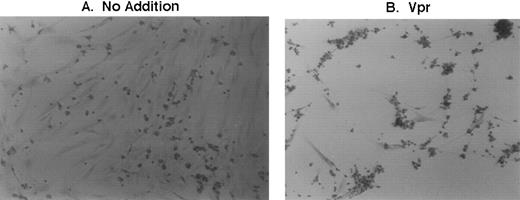
Because mouse bone marrow aspirates are obtained more readily than human samples, the effects of Vpr as well other proteins were initially assessed after transduction of murine whole marrow cultures in vitro. First, the retroviral delivery vectors were introduced into PA317 cells, a murine packaging cell line, by calcium-phosphate–mediated transfection. Forty-eight hours afterwards, supernatants containing Moloney murine leukemia virus (MoMuLV) virions were harvested and applied to murine marrow cells. Supernatants were introduced after the initiation of hematopoietic colony formation, which, under our plating conditions, occurs within a few days to 1 week of seeding the cultures.17 Within 36 to 48 hours after application of supernatant to the murine cultures, an easily detectable gross morphological change was observed. As shown in Fig 2, media derived from cells transfected with a Vpr expression cassette elicited avid erythrophagocytosis that is seen to a significantly lesser extent in cultures transduced with vector alone, SLX-CMV, or vector containing other expressed proteins such as the chloramphenicol acetyl-transferase (CAT) gene (not shown) or even HIV-1 Tat and Rev (not shown). The effect is not observed when supernatants from transfected NIH3T3 cells are added to marrow cultures. NIH3T3 is the parental line for PA317 cells and therefore does not possess the MoMuLV packaging function. This indicates that virion release plays some role in facilitating Vpr action in the system.
Effect of transfected PA317 cell supernatants on murine marrow cultures propagated in vitro. (A) Media from PA317 cells transfected with vector alone or transfected with (B) vector with HIV-1 Vpr expression cassette or (C) vector with HIV-1 Tat expression cassette. E, free erythrocyte; M, erythrocyte-coated mononuclear phagocyte. The results shown are typical of at least three independent experiments.
Effect of transfected PA317 cell supernatants on murine marrow cultures propagated in vitro. (A) Media from PA317 cells transfected with vector alone or transfected with (B) vector with HIV-1 Vpr expression cassette or (C) vector with HIV-1 Tat expression cassette. E, free erythrocyte; M, erythrocyte-coated mononuclear phagocyte. The results shown are typical of at least three independent experiments.
As shown in Fig 2B, erythrophagocytosis is easily visible as adherent cells of the promonocyte/monocyte/macrophage lineage become coated entirely by much smaller, light bright erythrocytes. After attachment, red blood cells (RBCs) are then engulfed completely within the next 12 to 24 hours. Because the attachment and phagocytosis of erythrocytes was so obvious and extensive in these cultures (1) the nature of this effect, (2) whether it was mediated by expression and perhaps release of Vpr either from the packaging cell-line or directly from transduced marrow cells, and (3) finally its biological relevance to HIV-1 infection became obvious issues to be addressed further.
Vpr-associated erythrophagocytosis is similar to that induced by lipopolysaccharide (LPS).
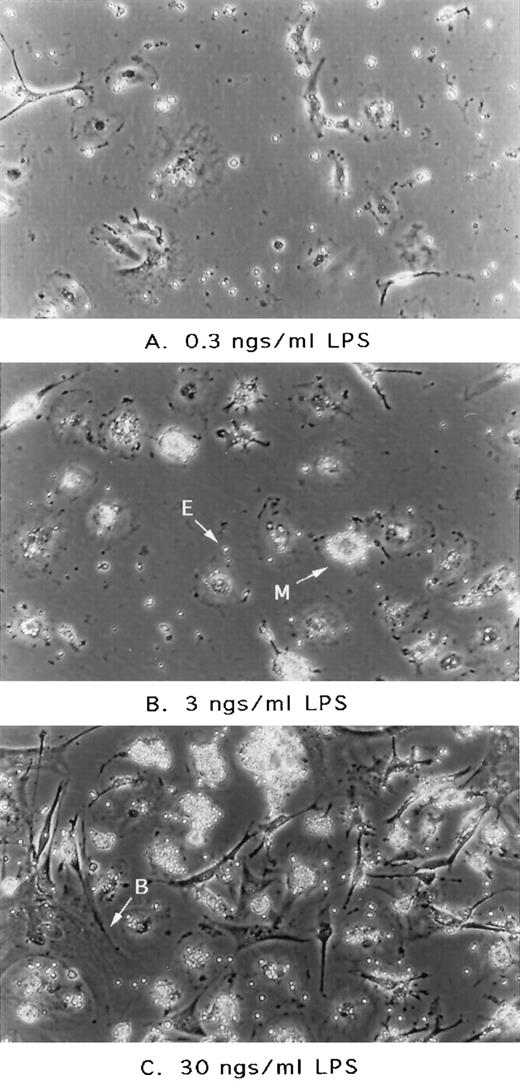
Phagocytosis is known to be a hallmark of promonocyte, monocyte, and macrophage activation.18 19 To assess the nature of the Vpr-associated effect and whether it might be similar to the state of activation triggered by other inducers, marrow cultures were treated with LPS, referred to typically as endotoxin. As shown in Fig 3, treatment of cultures with LPS recapitulated the gross morphological features observed in our supernatant addition experiments. As shown in Fig 3, increasing amounts of LPS added directly to the culture media up to 30 ng/mL lead to greater numbers of mononuclear phagocytes cells displaying active endocytosis. This observation supported our initial hypothesis that the Vpr-associated effect indeed reflected a natural pathway of phagocytic cell activation.
Effect of LPS addition on murine marrow cultures. Cultures initiated as described in previous figure were treated with LPS (Sigma; catalogue no. L-2143) at the concentrations as indicated. After 24 hours, cultures were washed once with PBS and photographed. E, free erythrocyte; M, erythrocyte-coated mononuclear phagocyte; B, blanket cell or stromal cell with large thin cytoplasm and endothelial-like in morphology.
Effect of LPS addition on murine marrow cultures. Cultures initiated as described in previous figure were treated with LPS (Sigma; catalogue no. L-2143) at the concentrations as indicated. After 24 hours, cultures were washed once with PBS and photographed. E, free erythrocyte; M, erythrocyte-coated mononuclear phagocyte; B, blanket cell or stromal cell with large thin cytoplasm and endothelial-like in morphology.
The differential effect of Vpr mutants on the induction of phagocytosis.
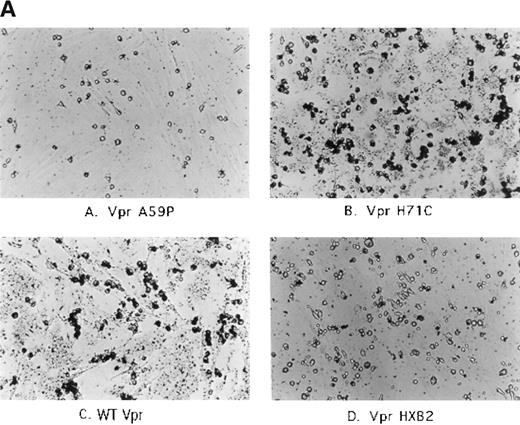
Next, it seemed important to demonstrate convincingly that Vpr was the critical factor required to initiate, either directly or indirectly, activation of adherent, phagocytic cells within the marrow cultures. To that end, a library of Vpr point mutants and HIV-1 Vpr HXB2, a frameshift mutant at residue 72 of the protein, were transferred into the SLX-CMV transduction vector (see Fig 1). As was performed in our initial experiments, the constructs were introduced into PA317 cells and the media derived from the transfections was applied to duplicate cultures of murine marrow cells. After 48 hours, the cultures were washed, fixed, and assessed for the extent of erythrophagocytosis. As shown in Table 1, indeed our collection of mutants displayed a range in their ability to activate phagocytes. H71C induced at least wild-type (WT) levels of activity. R62S, L64S, and C76A appeared intermediate, whereas the HXB2 frameshift and A59P mutants were typically lower in their ability to induce erythrophagocytosis. As shown in Table 1, the supernatants were also assayed for reverse transcriptase (RT) activity to determine whether the sheer number of virions varied drastically among the sample collections. This is a concern, because virions themselves appear capable of inducing activation to some extent. However, RT activity only varied a few fold among the supernatants, thus alleviating the concern that MoMuLV particles were a sole determinant in activating phagocytes. In addition, the effects after direct application of sucrose gradient purified HIV-1 NL4-3 WT and NL4-3 ΔVpr virions, derived from transfection of 293 cells, was assessed. Both were capable of eliciting erythrophagocytosis; however, WT virus showed marked acceleration of the process relative to Vpr deletion mutant virions (data not shown). Thus, collectively, the differential behavior of a series of Vpr mutants fortified our view that this retroviral protein was playing a critical role in triggering both the extent and rate of this process.
Effect of PA317 Supernatants Derived From Transfection With Various Vpr Mutants
| Vpr Mutation . | Supernatant RT Activity (dpm) . | Phagocyte Activation . |
|---|---|---|
| WT | 4,200 | +++ |
| A59P | 5,600 | −/+ |
| R62S | 4,700 | ++ |
| L64S | 3,300 | ++ |
| H71C | 4,400 | +++ |
| C76A | 4,200 | ++ |
| HXB2 | 4,300 | −/+ |
| Control media | 200 | − |
| Vpr Mutation . | Supernatant RT Activity (dpm) . | Phagocyte Activation . |
|---|---|---|
| WT | 4,200 | +++ |
| A59P | 5,600 | −/+ |
| R62S | 4,700 | ++ |
| L64S | 3,300 | ++ |
| H71C | 4,400 | +++ |
| C76A | 4,200 | ++ |
| HXB2 | 4,300 | −/+ |
| Control media | 200 | − |
Murine marrow cultures were treated with supernatants derived from transfections with mutants of Vpr (in HIV-1 strain 86.9) and were also assayed for reverse transcriptase activity. After addition of supernatants, cultures were assessed for their state of activation (erythrophagocytosis).
Abbreviations: +++, wt Vpr activity; −/+, low number of phagocytic islets; −, no phagocytic islets observed.
The induction of Vpr-associated cell adherence and phagocytosis in human marrow cultures.
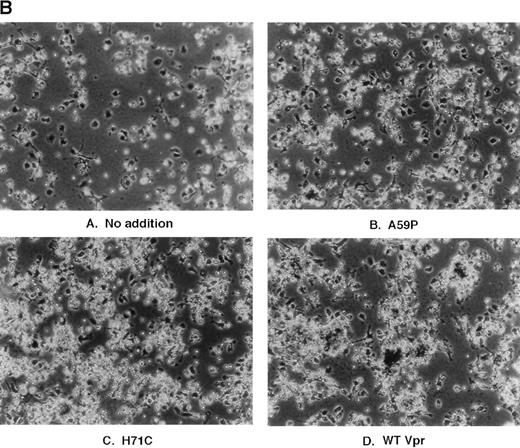
The next series of experiments focused on attempting to demonstrate mononuclear phagocyte cell activation within human bone marrow cultures. The approach was multi-faceted. First, human marrow cells from HIV-1–seronegative individuals were treated with the PA317 supernatants, as was performed for the murine cultures. However, in this case, nonadherent cells were first washed from the plates and activation of phagocytes was assessed by addition of 1-μm deep blue latex beads directly to the culture media. As shown in Fig 4A, cultures treated with Vpr mutants A59P and HXB2 showed low levels of uptake of the colored beads, whereas WT Vpr and H71C supernatants, which triggered erythrophagocytosis in the murine cultures, enhanced markedly the uptake of the latex spheres. Recapitulation of the erythrophagocytic effect was next tested in human marrow cultures seeded densely. As shown in Fig 4B, supernatants derived from WT Vpr and H71C elicited enhanced binding of nonadherent cells relative to other controls, such as no addition or Vpr A59P and HXB2 (data not shown), which were demonstrated previously to be negative or low for erythrophagocytosis when applied to murine cultures. The human cultures behaved somewhat differently from the murine treated cells in that engulfment of the attached cells was not visually apparent, at least in the same time frame.
Effect of transfected PA317 cell supernatants on human marrow cultures propagated in vitro. Approximately 2 × 106 nucleated marrow cells were plated in D-MEM with heat-inactivated 20% FBS. Supernatants (1 mL) from PA317 cells transfected with SLX-CMV bearing expression cassettes for Vpr mutants, as indicated in the figure, were applied. (A) The following day, cells were washed with media and 200 μL of latex beads (Sigma; catalogue no. L-1398) were added directly to cultures replenished with media, and cultures were incubated at 37°C for an additional 30 minutes, washed with PBS, and photographed. Dark rounded cells are phagocytes ingesting deep-blue latex spheres. (B) More densely seeded cultures retaining large numbers of nonadherent cells were treated with PA317 supernatants as described above, except that no further additions were made. After 24 hours, cells were fixed with 100% methanol and photographed. The effects shown are typical of at least three independent experiments.
Effect of transfected PA317 cell supernatants on human marrow cultures propagated in vitro. Approximately 2 × 106 nucleated marrow cells were plated in D-MEM with heat-inactivated 20% FBS. Supernatants (1 mL) from PA317 cells transfected with SLX-CMV bearing expression cassettes for Vpr mutants, as indicated in the figure, were applied. (A) The following day, cells were washed with media and 200 μL of latex beads (Sigma; catalogue no. L-1398) were added directly to cultures replenished with media, and cultures were incubated at 37°C for an additional 30 minutes, washed with PBS, and photographed. Dark rounded cells are phagocytes ingesting deep-blue latex spheres. (B) More densely seeded cultures retaining large numbers of nonadherent cells were treated with PA317 supernatants as described above, except that no further additions were made. After 24 hours, cells were fixed with 100% methanol and photographed. The effects shown are typical of at least three independent experiments.
The effect of HIV-1 infection, with or without Vpr, on human marrow cultures.
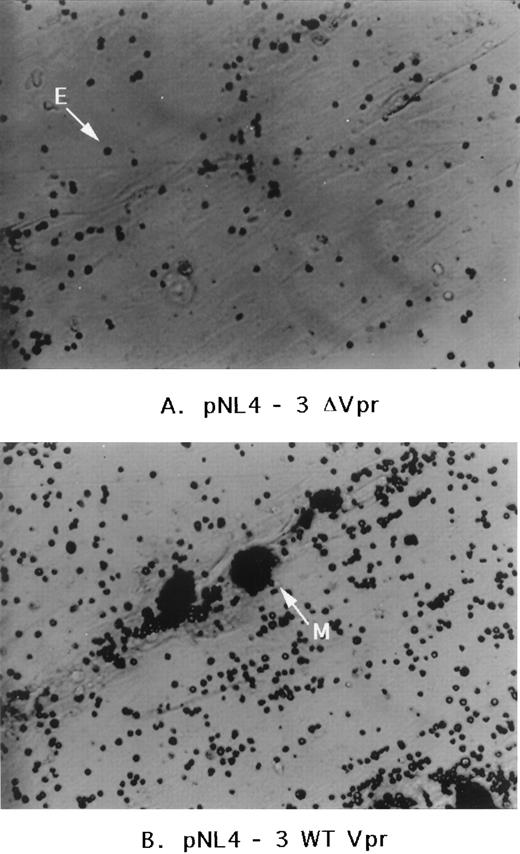
An alternative approach was then taken by infecting human marrow cultures with the HIV-1 clone (NL4-3) that harbors a functional Vpr sequence or with the same clone containing a deletion in Vpr (NL4-3 ΔVpr). Two effects were noted. Within 24 hours after addition of the virus, many more cells infected with the HIV-1 wild-type Vpr clone became adherent relative to the Vpr deletion mutant. This effect appeared to be transient. Nevertheless, within 1 week, both cultures showed obvious signs of infection and were strongly positive for virus production by HIV-1 p24 antigen as assessed by enzyme-linked immunosorbent assay (ELISA-Dupont, Wilmington, DE; data not shown). Visual inspection, after fixation of the cells 10 days later, showed obvious gross morphologic differences between the two cultures. An increase in adherent cells was noted for HIV-1NL4-3 infection relative to the HIV-1NL4-3 Δvpr mutant and those cells, as shown in Fig 5, displayed increased numbers of attached light dense erythrocytes and perhaps granulocytes. Macrophage-tropic virus (Bal 9 strain) with or without Vpr was also tested. Similar but much more modest differences relative to that observed for infection by the T-lymphocyte-tropic HIV-1NL4-3 was observed (data not shown). This appeared to be attributable to a depletion of mononuclear phagocytic cells through syncytia-formation induced by infection with the macrophage-tropic strain in these marrow cultures.
Effect of HIV-1 infection on human marrow cultures. Human marrow cultures were infected with HIV-1 strains NL4-3 or NL4-3▵Vpr (Vpr deletion mutant). The microscope filter was adjusted to project nonadherent cells as light dense. E, free erythrocyte or granulocyte; M, mononuclear phagocyte coated with nonadherent cells.
Effect of HIV-1 infection on human marrow cultures. Human marrow cultures were infected with HIV-1 strains NL4-3 or NL4-3▵Vpr (Vpr deletion mutant). The microscope filter was adjusted to project nonadherent cells as light dense. E, free erythrocyte or granulocyte; M, mononuclear phagocyte coated with nonadherent cells.
The experiments described above may be problematic in the sense that, without careful study, it is unknown how a wild-type Vpr versus a Vpr mutant virus may alter the dynamics of hematopoiesis (in particular, the production of monocyte/macrophages and erythrocytes). Although such did not appear to be the case, the presence of more mononuclear phagocytes and RBCs in one culture versus the other could lead to a similar observation. Although some caution may be warranted, these data are nonetheless entirely consistent with previous results and are important because they relate most directly to events that may occur during natural HIV-1 infection in vivo.
Direct addition of purified, recombinant Vpr induces cell adherence and phagocytosis in marrow cultures.
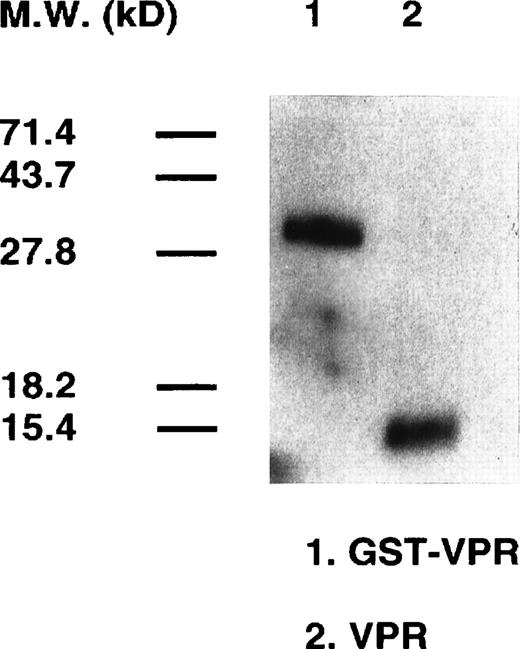
GST-Vpr was expressed in bacterial cells and purified using a single-step procedure by binding and release from glutathione agarose.13 Vpr was removed subsequently from GST by treatment with thrombin, whose cleavage site is between the two fusion partners within the GST-Vpr chimera. Western blotting analysis, shown in Fig 6, indicates that both GST-Vpr and free Vpr obtained after protease treatment are the appropriate molecular weight and intact. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) did not indicate the presence of other contaminating bacterial proteins (data not shown).
Purification of GST-Vpr and Vpr. The HIV-1 (strain NL4-3) Vpr coding sequence was cloned into the GST fusion protein bacterial expression vector, pGEX. The GST-Vpr fusion protein, synthesized after induction of the bacterial cells with 0.1 mmol/L IPTG, was purified (lane 1) by binding to glutathione agarose. Free Vpr was obtained by proteolytic digestion of the GST-Vpr protein and collecting the flow-through fraction from glutathione resin. The purity was assessed by Western blotting analysis using a rabbit polyclonal antisera against Vpr.
Purification of GST-Vpr and Vpr. The HIV-1 (strain NL4-3) Vpr coding sequence was cloned into the GST fusion protein bacterial expression vector, pGEX. The GST-Vpr fusion protein, synthesized after induction of the bacterial cells with 0.1 mmol/L IPTG, was purified (lane 1) by binding to glutathione agarose. Free Vpr was obtained by proteolytic digestion of the GST-Vpr protein and collecting the flow-through fraction from glutathione resin. The purity was assessed by Western blotting analysis using a rabbit polyclonal antisera against Vpr.
GST-Vpr or free Vpr, added directly to murine marrow cultures at 0.25 μg/mL of media, was sufficient to induce avid erythrophagocytosis by mononuclear phagocytes, as described previously in the supernatant (Fig2) or LPS (Fig 3) addition experiments (data not shown). Of note, the effect of adding the recombinant proteins to human bone marrow cultures was of greater interest.
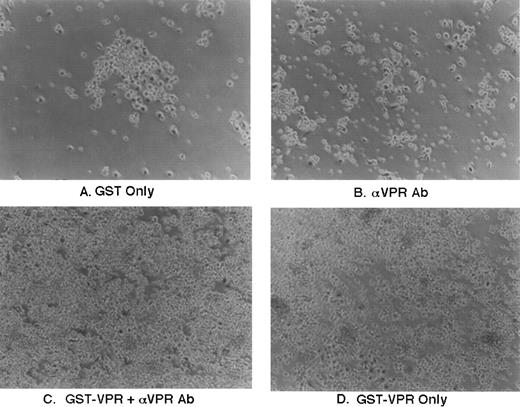
As shown in Fig 7A, the addition of purified GST (0.25 μg/mL culture media) had no effect relative to untreated marrow cultures. As might be expected due to the presence of IgG receptors on phagocytic cells, addition to the marrow culture of 1 μL/mL of rabbit polyclonal antisera, raised against Vpr, resulted in a modest increase in cell adherence to the culture dishes, likely reflecting some level of activation as shown in Fig 7B. The effect was much more pronounced when GST-Vpr was added alone or along with the polyclonal rabbit antisera (Fig 7C and D). There consistently appeared to be a modest additive effect on marrow cell adherence and subsequent phagocytosis when the recombinant GST-Vpr protein was added along with the polyclonal antisera, although this is not evident in the high-power image shown in Fig 7.
Effect of GST-Vpr addition to human marrow cultures. Purified recombinant proteins were added directly to human marrow cultures propogated in vitro as indicated in the figure at a final concentration of approximately 3 nanomolar. Addition was performed in the absence or presence of rabbit polyclonal antisera directed against Vpr, as indicated in the figure, using 1 μL of antisera/mL of media.
Effect of GST-Vpr addition to human marrow cultures. Purified recombinant proteins were added directly to human marrow cultures propogated in vitro as indicated in the figure at a final concentration of approximately 3 nanomolar. Addition was performed in the absence or presence of rabbit polyclonal antisera directed against Vpr, as indicated in the figure, using 1 μL of antisera/mL of media.
The effect of adding free Vpr to the human marrow cultures was next evaluated. As shown in the low-power images of Fig 8, Vpr induced considerable adherence of erythrocytes and likely other cell types to adherent cells on the cultures dishes. The cultures were washed with PBS to remove unbound cells from both the untreated and treated cultures, and cell morphology was enhanced by reaction with iron stain. As shown in Fig 8B, adherent cells are typically coated with deeply stained red blood cells. This effect is minimal in the untreated control culture, as shown in Fig 8A.
Effect of recombinant Vpr addition to human marrow cultures. Purified recombinant Vpr proteolytically cleaved from the GST-Vpr chimera was added directly to cultures at a final concentration of approximately 3 nanomolar. Twenty-four hours later, untreated and treated cultures were washed with PBS and iron stain (Sigma) was applied to enhance cell morphology.
Effect of recombinant Vpr addition to human marrow cultures. Purified recombinant Vpr proteolytically cleaved from the GST-Vpr chimera was added directly to cultures at a final concentration of approximately 3 nanomolar. Twenty-four hours later, untreated and treated cultures were washed with PBS and iron stain (Sigma) was applied to enhance cell morphology.
DISCUSSION
Propagation and manipulation of whole bone marrow cultured in vitro has afforded a novel and tractable system that appears to be well-suited to study the effects of independently expressed HIV-1 proteins after transduction as well as the dynamics of HIV-1 infection. The most important feature of the system specifically with regard to the activities of Vpr is the preponderance of mononuclear phagocytic cells in an environment that reasonably recapitulates natural hematopoiesis and does not require addition of exogenous growth factors.20 This self-supporting microenvironment facilitates the production and maturation of mononuclear phagocytes as well as other cells that may be influenced by the biological properties of Vpr. In this system, Vpr plays a role in the activation of mononuclear phagocytic cells. This was demonstrated in both murine and human marrow cells and the observation is fortified by the differential effects of a series of Vpr mutants on these cultures. It is difficult to correlate the known intracellular activities of Vpr, such as cell-cycle arrest, with the activation of mononuclear phagocytes that is observed in the treated marrow cultures. For instance, Vpr HXB2 and the point-mutants, A59P and H71C, have been reported to be capable of arresting cells.21 The mutants Vpr HXB2 and A59P do not trigger erythrophagocytosis efficiently, but Vpr H71C appears to elicit this effect as well as or perhaps to a greater extent than that of wild-type Vpr. A larger collection of mutants are being tested currently to provide additional information as to whether intracellular functions of Vpr bear any relation to the phenomenon reported herein.
Mononuclear phagocytic cells and CD4+ T lymphocytes represent the major targets of infection by HIV-1 in vivo. Previous data demonstrating a specific requirement of Vpr or its homologues for efficient infection of mononuclear phagocytic cells by HIV-1, HIV-2, and simian immunodeficiency virus (SIV)22-24 are interesting in the light of our results. It seems reasonable to hypothesize that Vpr involvement in the activation of mononuclear phagocytes might likely enhance productive infection by HIV-1, which is perhaps analogous to the requirement that quiescent T lymphocytes undergo exogenous stimulation to initiate active viral replication.25 This hypothesis is currently being tested.
The precise mechanism of action for HIV-1 Vpr-induced activation is also being pursued, and some clues are provided by the fact that the effect is similar morphologically to that triggered by addition of LPS. It seems reasonable to propose that Vpr is released into supernatant of transfected PA317 cells during the process of virion budding and consequently induces activation directly. There is no available evidence that suggests that Vpr can associate with murine virions, although this is under investigation currently. It is also possible that the role of Vpr is secondary, either inducing the expression or allowing the export of another moiety capable of engaging activation. We have shown recently that shuttling of a variety of nonviral and viral proteins into HIV-1 virions is possible when they are fused to a small 23 amino acid Vpr interactor domain.13 Again, it is unknown whether this mechanism of egress for Vpr or its potential interactors can occur from cells during the maturation of murine particles. It is possible that transduction and expression of Vpr in certain cells within marrow cultures may occur. The vector SLX-CMV bears a Neo expression cassette allowing G418 selection after transduction of marrow cultures. Selection leads to the survival of large endothelial-like cells referred to as blanket cells (data not shown). These cells play a critical role in the growth and maturation of a variety of other cells during hematopoeisis.26 Perhaps transduction of this specific population of cells results in a perturbation of the normal cytokine profile in marrow cultures signaling mononuclear phagocytes to initiate erythrophagocytosis.
Of the possibilities listed above, direct activation by cellular binding of Vpr appears most likely, although the others outlined above could occur and contribute to induction of the effect. Nevertheless, the reductionist approach of the protein addition experiments indicates an identical pattern of activation in both murine and human marrow cultures after direct addition of soluble, recombinant Vpr. Interpretation of these experiments must be viewed cautiously due to possible LPS contamination from disrupted bacterial cells as assessed by sensitive assays for the presence of endotoxin. However, the data provided in Fig 3 suggest that rather high levels of LPS (3 to 30 ng/mL) are required to initiate an equivalent level of erythrophagocytosis as that observed by supernatant addition in the marrow cultures. Because cell adherence and accelerated phagocytosis was observed in both murine and human marrow cultures with Vpr introduced to the marrow cells in three different contexts, it seems likely that Vpr is the direct effector of mononuclear phagocyte activation. More interesting is what appears to be accelerated differentiation along the monocyte/macrophage lineage induced by addition of recombinant Vpr to CD34+ stem cells isolated from human cord blood (data not shown). Accelerated differentiation related to Vpr expression has been reported previously for rhabdomyosarcoma cells.16 Experiments described previously suggest it is likely that Vpr is capable of binding to cells extracellularly.27 Such a hormone model of Vpr action is appealing, because release and extracellular uptake of Vpr triggering activation, and perhaps even differentiation of cells in the process of maturation, would likely increase the population of cells susceptible to productive infection of HIV-1. The recent report that extracellular Vpr can perforate the cytoplasmic membranes of neuronal cells and induce ion channel flux leading to cell death28 may be pertinent to our results. Such disturbances in the marrow cultures could lead to enhanced phagocytosis of damaged cells.
Regardless of the underlying mechanism of activation, our experiments further provide some molecular explanation, at least in part, for the induction of cytopenias in HIV-1–infected individuals.29Although studies have not been directed to ascertain whether phagocytosis is enhanced in the marrow compartment of HIV-1–seropositive patients versus uninfected persons, it is quite obvious that this effect occurs in vitro in both the murine and human bone marrow cultures. Premature phagocytosis of erythrocytes would lead to anemia, and the engulfment of nucleated cells during hematopoiesis could result in a variety of other cytopenias typical of long-term HIV-1 infection.29
ACKNOWLEDGMENT
The authors thank Ling-Xun Duan for providing recombinant Vpr and the plasmids containing the VprNL4-3 expression cassette as well as pLXN-Tat and pLXN-Rev and thank Didier Trono (Salk Institute, La Jolla, CA) for kindly providing macrophage-tropic HIV-1Bal 9 infectious clones. Muhammad Amjad kindly provided the protocol and the reagents for RT assays and Dr Omar Bagasra assisted in the photography of some of the cultures. We also appreciate Brenda O. Gordon for formatting of the figures, Rita M. Victor for excellent secretarial assistance, and T.D. Allen (Patterson Institute, Manchester, UK) for kind review of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Roger J. Pomerantz, MD, Thomas Jefferson University, 1020 Locust St, Suite 329, Philadelphia, PA 19107; e-mail:roger.j.pomerantz@mail.tju.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal