Canine -L-iduronidase (-ID) deficiency, a model of the human storage disorder mucopolysaccharidosis type I (MPS I), is an ideal system in which to evaluate the clinical benefit of genetically corrected hematopoietic stem cells. We performed adoptive transfer of genetically corrected autologous hematopoietic cells in dogs with -ID deficiency. Large volume marrow collections were performed on five -ID–deficient dogs. Marrow mononuclear cells in long-term marrow cultures (LTMCs) were exposed on three occasions during 3 weeks of culture to retroviral vectors bearing the normal canine -ID cDNA. Transduced LTMC cells from deficient dogs expressed enzymatically active -ID at 10 to 200 times the levels seen in normal dogs. An average of 32% of LTMC-derived clonogenic hematopoietic cells were provirus positive by polymerase chain reaction and about half of these expressed -ID. Approximately 107autologous gene-modified LTMC cells/kg were infused into nonmyeloablated recipients. Proviral DNA was detected in up to 10% of individual marrow-derived hematopoietic colonies and in 0.01% to 1% of blood and marrow leukocytes at up to 2 to 3 years postinfusion. Despite good evidence for engraftment of provirally marked cells, neither -ID enzyme nor -ID transcripts were detected in any dog. We evaluated immune responses against -ID and transduced cells. Humoral responses to -ID and serum components of the culture media (fetal bovine and horse sera and bovine serum albumin) were identified by enzyme-linked immunosorbent assay. Cellular immune responses to autologous -ID but not neor transduced cells were demonstrated by lymphocyte proliferation assays. To abrogate potential immune phenomena, four affected dogs received posttransplant cyclosporine A. Whereas immune responses were dampened in these dogs, -ID activity remained undetectable. In none of the dogs engrafted with genetically corrected cells was there evidence for clinical improvement. Our data suggest that, whereas the -ID cDNA may be transferred and maintained in approximately 5% of hematopoietic progenitors, the potential of this approach appears limited by the levels of provirally derived enzyme that are expressed in vivo and by the host’s response to cultured and transduced hematopoietic cells expressing foreign proteins.
GENE THERAPIES DELIVERED by hematopoietic stem cells (HSCs) offer several theoretical advantages over other methods of drug delivery. These include long-term, regulated, in vivo production of a wide variety of therapeutic agents derived from DNA permanently integrated into the genomes of cells with life-long capacities for extensive proliferation and multilineage differentiation.1 Recent progress towards this goal has been tenuous, with apparent advances generally followed by the identification of largely unforeseen obstacles. To date there is limited evidence that genetically modified HSCs can actually be of therapeutic benefit in human disease or in large animal models.2-4
Over the last decade, several approaches have been taken to develop HSCs as efficient vehicles for gene delivery. These include the use of HSC-enriched cell target populations5 and methods for selecting gene-modified HSCs.6 One recurring concern is that HSCs, in contrast to their committed progeny, appear to express low levels of the receptor required for successful gene transfer mediated by the retroviral vectors used in most applications.7 Another problem limiting practical applications of HSC gene transfer is that the vast majority of HSCs are likely to be quiescent8 and consequently resistant to stable gene transfer. Furthermore, the likelihood that any given gene-modified HSC would be among the small minority that actually undergo expansion in vivo under steady-state conditions seems remote.9
We focused on gene transfer to candidate HSCs in in vitro hematopoietic microenvironments, because it seemed logical that under the correct culture conditions HSCs may be induced to exit quiescence and enter the cell cycle. Data from our studies10,11 and from others12-14 demonstrated that exposure of hematopoietic cells to retroviral vectors in the presence of marrow-derived stromal cells facilitated reporter gene transfer into a high proportion of committed progenitors. In a canine model system, autologous marrow transduced by multiple exposures to retroviral vectors in long-term marrow cultures (LTMCs) engrafted in the absence of myeloablative conditioning and gave rise to reporter gene-marked committed progeny that were maintained at approximately 5% levels for up to 2 years.11 Similar results were more recently obtained from a human clinical trial of LTMC stem cell gene marking in multiple myeloma.15 These studies suggested that, although the goal of achieving large populations of genetically modified hematopoietic cells in vivo remains elusive, the levels of gene transfer achievable using current technology may alleviate disease symptoms in certain deficiency disorders.
To evaluate the therapeutic benefit of low and sustained levels of genetically modified hematopoietic cells, we attempted gene therapy for canine α-L-iduronidase (α-ID) deficiency. In this autosomal recessive disorder there is a complete deficiency of α-ID due to a single base substitution in the α-ID gene that disrupts intron I splicing and gives rise to a stop codon.16,17 Dogs homozygous for the α-ID mutation do not have any α-ID enzyme as determined by either α-ID activity or immunoprecipitation.18 The α-ID deficiency results in lysosomal storage of large amounts of the glycosaminoglycans (GAGs) heparan and dermatan sulfate in many tissues, including the central nervous system.16,19 α-ID–deficient dogs are clinically similar to human patients with the Hurler/Scheie phenotype of mucopolysaccharidosis type I (MPS I) and exhibit progressive cardiac abnormalities, corneal clouding, stunted growth, and degenerative joint disease, all of which progress to severe states within 2 to 3 years.16 19
We hypothesized that canine α-ID deficiency would be an ideal model system to evaluate the potential clinical benefit of low numbers of genetically corrected hematopoietic cells for the following reasons: the disorder is a single gene defect for which the canine cDNA is cloned; there is a wide range of enzyme levels compatible with a normal or mild phenotype; matched, related bone marrow transplantation is of known clinical benefit in affected dogs20; and enzyme replacement using a human recombinant product is of clinical benefit, despite the development of complement-activating antibodies.21 22
In this study we infused five homozygous affected α-ID–deficient pups with autologous marrow genetically corrected to express high levels of functional α-ID. Our results demonstrate that long-lived hematopoietic progenitor cells, genetically modified ex vivo, engraft and are maintained in vivo at levels thought to be clinically relevant. However, their utility in gene therapy for deficiency states is likely limited by both the low expression level of the transgene and normal host immune responses to foreign proteins and cultured transduced cells. Such responses may be exacerbated when gene-modified autografts contain large numbers of antigen-presenting cells and their precursors, such as cultured marrow cells.
MATERIALS AND METHODS
Dogs and marrow harvests.
The MPS I dogs used in this study were bred and maintained at the University of Guelph’s Central Animal Facility (Guelph, Ontario, Canada) and were not treated with α-ID enzyme before this study. The animal care and bioethics committees of the Universities of Toronto and Guelph approved all protocols. Large-scale marrow harvests were performed on 2- to 12-month-old dogs from either the iliac crest or proximal humeri and femora under general anesthesia. Marrow, equivalent in volume to up to 10% of total blood volume, was collected at each large-scale harvest (range, 100 to 300 mL), whereas approximately 10 mL marrow was collected at each follow-up time point for analysis. Light-density marrow cells (<1.075 g/mL) were recovered after Percoll (Pharmacia, Uppsala, Sweden) density gradient separation and washing, as previously described.10 11
Retroviral vectors.
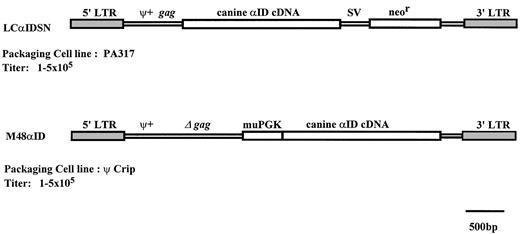
Two Moloney-based retroviral vectors were used in this study (Fig 1). The M48αID vector has extended viral gag sequences23 upstream of an internal murine phosphoglycerate kinase-1 promoter,24 which directs expression of the normal canine α-ID cDNA.25 The LCαIDSN vector, based on LXSN,26 has the normal canine α-ID cDNA25 expressed from the viral LTR and neor from the SV40 promoter and was packaged by PA317.27 The M48αID vector is produced by Ψ CRIP28 and was provided by Drs A. Salvetti and O. Danos (Paris, France).
Illustration of retroviral vectors. Abbreviations: LTR, long terminal repeats; ψ+, packaging signal; gag, gag sequences; SV, SV40 promoter; neor, neomycin phosphotransferase; muPGK, murine phosphoglycerate kinase-1 promoter.
Illustration of retroviral vectors. Abbreviations: LTR, long terminal repeats; ψ+, packaging signal; gag, gag sequences; SV, SV40 promoter; neor, neomycin phosphotransferase; muPGK, murine phosphoglycerate kinase-1 promoter.
The titers of all producer lines were approximately 1 to 5 × 105 colony-forming units (CFU)/mL determined on NIH3T3 as previously described.29 To ensure that stocks were free of replication competent retrovirus (RCR), supernatants from all producer cell lines were routinely assayed by a sensitive marker rescue assay.30
Marrow cultures, transductions, and infusions.
Marrow was set up in LTMCs and transduced as described previously,10,31 with minor modifications. Briefly, 1 × 108 cells were seeded in 150-cm2 tissue culture flasks (Corning, Corning, NY) in LTMC media consisting of 10% fetal bovine serum (FBS) and 15% horse serum (HS) in McCoy’s 5A media supplemented as described.29 Control flasks were initiated and maintained with LTMC media alone and transduced cultures were initiated in a 50:50 mix of fresh LTMC media and retroviral supernatant. Retroviral supernatant was prepared by conditioning LTMC media on confluent retroviral producer cells for 18 to 24 hours at 33°C. Viral supernatants were filtered through a 0.45-μm filter and either used directly or stored at −70°C until use. All cultures were incubated in a humidified environment at 33°C and 5% CO2 in air for 21 days. LTMCs were maintained by removal of half of the nonadherent cells and media at weeks 1 and 2 and replenishment with fresh LTMC media. Twenty-four hours after feeding, half of the nonadherent cells and media were again removed and replaced with retroviral supernatant.
The adherent LTMC cells were harvested by enzymatic digestion with 0.25% trypsin and 1 mmol/L EDTA (GIBCO, Grand Island, NY) and washed twice with Hank’s buffered saline. Aliquots of cells were set aside for molecular assays, histology, and marker rescue assay for RCR. The remaining cells were either cryopreserved until use or infused directly as previously described.32 Where dogs received multiple large-scale infusions, time points postinfusion for polymerase chain reaction (PCR) analysis were calculated from the first infusion. Tissues at postmortem examination were prepared for histopathologic analysis.19
In vitro assays of gene transfer.
Proviral marking in hematopoietic progenitors was evaluated by PCR amplification of proviral specific sequences in individual colony-forming unit–granulocyte-macrophage (CFU-GM) and burst-forming unit-erythroid (BFU-E) colonies. Adherent layer cells were plated in quadruplicate at a density of 1 × 105 cells/mL in complete methylcellulose with either recombinant human or murine cytokines (MethoCult GF M3434 or H4435; Stem Cell Technologies, Vancouver, British Columbia, Canada) and supplemented with 10% canine phytohemagglutinin (PHA)-stimulated leukocyte conditioned media. The colonies were assessed using standard criteria on days 10 to 12 of culture.33 Up to 100 well-isolated colonies and 10 methylcellulose only controls were plucked from each sample. Individual colonies and background methylcellulose controls were placed into 40 μL of lysis buffer (0.5% NP-40, 0.5% tween-20, and 0.9 mg/mL proteinase-K), incubated at 56°C for 1 to 2 hours, and boiled for 10 minutes to inactivate the proteinase-K. Five microliters of each sample was used per PCR amplification.
Proviral α-ID sequences were detected by PCR amplification of a 422-bp amplicon from the α-ID cDNA sequence (the amplicon from α-ID genomic DNA is ∼1 kb). The α-ID sense primer was derived from exon 3 (cID3S; 5′-CAAGGCCTGAGCTACAACTTC-3′) and the antisense primer from exon 6 (cID6AS; 5′-GTGCCGTTGTGACAATGCTCC-3′). Proviral α-ID PCR was performed with denaturation at 94°C for 3 minutes, followed by 40 cycles of amplification: denaturation at 94°C for 25 seconds, annealing at 60°C for 25 seconds, and extension at 72°C for 30 seconds. The neor PCR used in these studies amplifies a 471-bp fragment with a sense primer N1 (5′-GAACAAGATGGATTGCACGCAG-3′) and the anti-sense primer YJR2(5′-GTCCAGATCATCCTGATCGACAAG-3′). The quality of DNA was assessed by PCR amplification of the canine dystrophin gene with the sense primer (5′-ACAGTCCTCTACTTCTTC CCACCA-3′) and the antisense primer (5′-AATTCACAGAGCTTGCCATGC-3′). PCR reactions were performed in Stratagene Buffer 10 (Stratagene, La Jolla, CA), with 10 pmol of each primer in a 25 μL reaction. The cycling conditions for these PCR reactions start with a denaturation at 94°C for 3 minutes, followed by 42 cycles of amplification: denaturation at 94°C for 20 seconds, annealing at 61°C for 25 seconds, and extension at 72°C for 30 seconds.
PCR amplification products of colonies were routinely transferred onto nylon membrane (Hybond N+; Amersham, Arlington Heights, IL), hybridized with an end-labeled radioactive oligonucleotide specific for each PCR product, and analyzed by autoradiography. The sequences of the neor and α-ID probes used are as follows: neor, 5′-CGACCTGTCCGGTGCCCTGAATGAACTGG-3′; and α-ID, 5′-CCAGGCTGACCGCTATGACC-3′.
Assays for proviral α-ID expression.
α-ID activity was assessed in duplicate by the 4-methylumbelliferyl α-iduronide (4MUαID) fluorimetric assay.21 22 In brief, samples were homogenized in 10 mmol/L phosphate buffer pH 5.8, containing 0.1 mmol/L dithiothreitol and 0.01% triton-X 100, and lysed by either 4 cycles of freeze-thawing (for assessment of individual CFU) or sonication (blood, marrow, and LTMC samples). The reaction was performed in 0.2 mol/L formate, pH 3.5, with 25 μmol/L 4MUαID (Sigma, St Louis, MO) at 37°C for 1 to 24 hours. Serial dilutions of 4-methylumbelliferone (4MU) were used to calibrate the fluorimeter. Negative controls included pretreatment samples, tissue blanks, and reagent controls. Positive controls consisted of matched tissues from normal dogs. Liberated 4MU was detected fluorimetrically with 365 nm excitation and 440 emission filters (assay sensitivity ∼1% of normal levels) in either a Turner Diagnostics cuvette reader (Sunnyvale, CA) for tissue samples or the Fluoroskan II 96 well reader (Labsystems, Helsinki, Finland) for CFU. One unit of αID activity was determined to correspond to the release of 1 nmol of 4MUαID substrate per milligram of protein per hour at 37°C. For analysis of α-ID activity from CFU-GM, individual colonies were plucked in 3 to 5 μL methylcellulose and resuspended in 25 μL buffer. The whole colony was used for the assay. Background methylcellulose and reagent controls were included for each sample as negative controls.
Reverse transcriptase-PCR (RT-PCR) was performed to detect proviral α-ID transcripts. RNA was prepared from blood and marrow leukocytes, treated with RNase-free DNase, and phenol-chloroform extracted. α-ID cDNA was synthesized using an antisense primer derived from exon 7 (5′-GGTCCGCCTCGTCGTTGTAA-3′) and Superscript II reverse transcriptase (GIBCO). The mutation in the α-ID gene in MPS I dogs eliminates the splice site in intron 1 and thus endogenous transcripts from the mutant gene contain intron 1 and are 450 bp longer than transcripts from the normal canine α-ID cDNA present in the provirus. PCR amplification of the cDNA with a forward primer from exon 1 (primer CA-1 from Menon et al17; 5′-CGCTGCGGCCCCTGCGG CCCTTCT-3′) and the reverse primer cID6AS described above were used for amplification. This reaction unambiguously distinguishes transcripts derived from the provirus (618 bp) and those from the endogenous mutant gene (1,068 bp). The PCR reaction and cycling conditions were the same described above. The quality of the cDNA samples was assessed by PCR amplification of the exon 3-6 of the α-ID gene that provides the same size fragment from both the normal and the MPS I genomic transcripts. Controls without reverse transcriptase enzyme were included for each sample in all experiments. For RT-PCR amplification of proviral specific transcripts, primers were chosen that span intron 1.
Immunoassays.
Sera from dogs were tested for the presence of IgG antibodies directed against α-ID, HS, FBS, and bovine serum albumin by enzyme-linked immunosorbent assay (ELISA). Serum samples were collected at 1- to 3-month intervals postinfusion. Preinfusion serum was collected from each dog and used as individual baseline controls. ELISA was performed as described21; however, wells were blocked with 0.1% tween-20. For the detection of antibodies against serum or serum components, wells were coated with 100 μL antigen solution containing either 0.2 μg recombinant α-ID (kindly provided by Dr E. Kakkis, University of California San Francisco, San Francisco, CA) or a 2.5% solution of either bovine serum albumin, HS, or FBS in phosphate-buffered saline. The secondary antibody used was alkaline phosphatase-conjugated goat anticanine IgG (Chemicon, Temecula, CA). The optical density was read on a microtiter plate reader at 405 nm (Molecular Devices, Sunnyvale, CA). The titer was reported as the greatest dilution of serum that had an optical density of greater than twice that of the preimmune serum.
A cellular immune response was assessed by evaluating the proliferation of peripheral blood mononuclear cells in response to transduced autologous stroma. Marrow stroma was grown similarly to LTMCs, except that 4 to 24 hours after culture initiation the nonadherent fraction was removed and replaced with fresh LTMC media. The stromal cultures were maintained in LTMC media by twice weekly half volume media changes. At confluence (10 to 14 days), the stroma was trypsinized and replated at a 1:3 dilution. Twenty-four hours later, the stroma was transduced by replacing the media with retroviral supernatant twice daily for 2 days. Control untransduced stroma was manipulated similarly to transduced stroma; however, fresh LTMC media was used instead of retroviral supernatant. Stroma was maintained by 1:3 dilutions once confluent. At passage 3 to 4, the stromal cells were plated in 100 μL of Iscove’s modified Dulbecco’s medium (IMDM) with 15% FBS in 96-well plates. Responder cells were fresh or cryopreserved autologous, ficoll gradient-separated peripheral blood mononuclear cells and were resuspended in IMDM with 15% FBS and either 5% canine or 10% murine leukocyte-conditioned media. One hundred microliters of responder cells was added at a ratio of 1:10 stimulator to responder cells in triplicate to each transduced and control stroma sample. The positive control wells received 2% PHA, whereas blank wells, peripheral blood cells alone, and stroma alone served as negative controls. Cells were incubated at 37°C for 6 days and during the last 18-hour cells were pulsed with [3H]-thymidine. Thymidine incorporation was analyzed on a Wallac 1205 Betaplate counter (Wallac, Gaithersburg, MD) and reported as the mean cpm (±SD) for triplicate measurements.
RESULTS
Autografts.
Large volume marrow harvests were performed on five MPS I-affected dogs. When yields of marrow mononuclear cell were less than 2 × 108 cells/kg, dogs underwent multiple marrow harvests. The time intervals between collections ranged from 1 to 3 months and in all cases subsequent harvests were performed after blood cells counts had normalized. Three dogs (M3, M5, and M6) underwent one marrow harvest, whereas dogs M4 and M2 underwent two and three marrow aspirations, respectively. On average, 1.0 × 108 (standard error [SE]: ±1.6 × 107) mononuclear cells and 5.4 × 104 (SE: ±4.2 × 103) CFU-GM/kg were obtained from each marrow sample after density gradient separation and washing.
LTMCs from MPS I dogs were transduced by three exposures to retroviral vectors bearing the canine α-ID cDNA. During 3 weeks of culture, LTMCs from dogs M3, M4, and M5 were transduced with M48αID (Fig 1). For the remaining two dogs, LTMCs from the first two marrow harvests from dog M2 were transduced with M48αID and the third with LCαIDSN, whereas for dog M6, approximately one half of the LTMCs were transduced with each vector. The cell and progenitor recoveries at the end of the 3-week LTMC and transduction period were 1.5 × 107cells (SE: ±5.2 × 107) and 9.2 × 103 CFU-GM (SE: ±4.8 × 103) per kilogram, corresponding to 15% and 17% recovery of cells and CFU-GM.
Gene transfer and α-ID expression in transduced LTMCs.
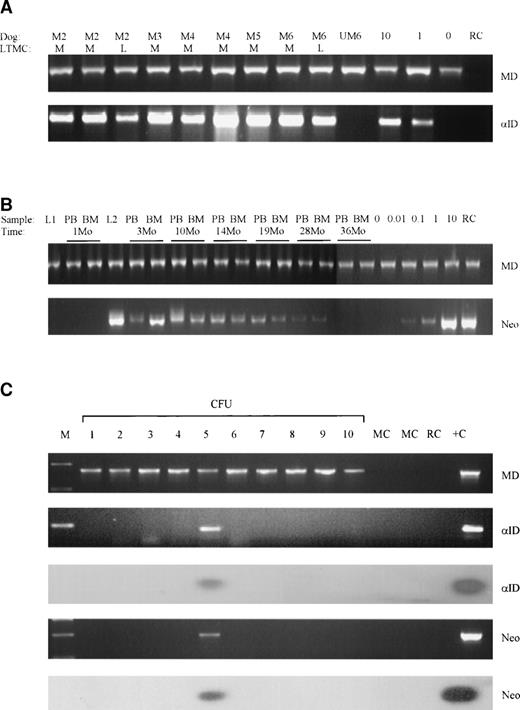
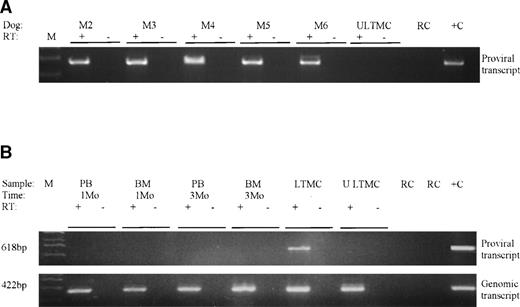
Successful transduction of LTMC cells was confirmed by PCR detection of proviral DNA in cells from all adherent layers (Fig 2A and Table 1). Transduced LTMCs were assayed for proviral α-ID expression. LTMC cells from transduced MPS I cultures had α-ID enzyme activities of between 10 and 198 U (nanomoles per milligram of protein per hour; Table 1), corresponding to approximately 10 to 200 times normal enzyme levels. There was no activity detected in untransduced MPS I control LTMC adherent layer cells. All transduced LTMCs were also positive for proviral α-ID transcripts by RT-PCR (Fig 3A and Table 1).
Detection of proviral sequences in MPS I tissues. (A) Proviral -ID and genomic control dystrophin (MD) PCR on day 21 transduced adherent layer LTMC cells from dogs M2 through M6. The vectors used for each culture were M-M48ID and L-LCIDSN. Positive controls were 10% M48ID producer cell line DNA mixed with 90% untransduced MPS I canine DNA and 1% M48ID producer cell line DNA mixed with 99% untransduced MPS I canine DNA. All samples and control DNA mixes were positive for dystrophin sequences, and all transduced LTMCs were positive for proviral -ID. Untransduced LTMC DNA from dog M6 (UM6) and reagent controls were negative for proviral sequences. (B) Semiquantitative neor and genomic control dystrophin PCR on LTMCs transduced with M48ID (L1) and LCIDSN (L2); postinfusion blood (PB) and marrow (BM) cells from M2 with 0.01%, 0.1%, 1%, and 10% positive control mixed with untransduced canine DNA; and negative untransduced control (0). (C) PCR amplification and Southern blot analysis of 10 CFU from M2 at 1 year postinfusion; dystrophin genomic control (MD), proviral -ID (ID), and neor (neo) PCR analysis. This sample demonstrates 10 CFU positive for genomic DNA (MD) and 1 of 10 CFU positive for both -ID and neor sequences (CFU 5). Abbreviations: M, marker lane; MC, methylcellulose control; RC, reagent control; +C, positive control DNA.
Detection of proviral sequences in MPS I tissues. (A) Proviral -ID and genomic control dystrophin (MD) PCR on day 21 transduced adherent layer LTMC cells from dogs M2 through M6. The vectors used for each culture were M-M48ID and L-LCIDSN. Positive controls were 10% M48ID producer cell line DNA mixed with 90% untransduced MPS I canine DNA and 1% M48ID producer cell line DNA mixed with 99% untransduced MPS I canine DNA. All samples and control DNA mixes were positive for dystrophin sequences, and all transduced LTMCs were positive for proviral -ID. Untransduced LTMC DNA from dog M6 (UM6) and reagent controls were negative for proviral sequences. (B) Semiquantitative neor and genomic control dystrophin PCR on LTMCs transduced with M48ID (L1) and LCIDSN (L2); postinfusion blood (PB) and marrow (BM) cells from M2 with 0.01%, 0.1%, 1%, and 10% positive control mixed with untransduced canine DNA; and negative untransduced control (0). (C) PCR amplification and Southern blot analysis of 10 CFU from M2 at 1 year postinfusion; dystrophin genomic control (MD), proviral -ID (ID), and neor (neo) PCR analysis. This sample demonstrates 10 CFU positive for genomic DNA (MD) and 1 of 10 CFU positive for both -ID and neor sequences (CFU 5). Abbreviations: M, marker lane; MC, methylcellulose control; RC, reagent control; +C, positive control DNA.
Evaluation of Gene Transfer and Proviral Gene Expression Into Canine MPS I LTMC Adherent Layers and Individual CFU-GM Derived From Transduced LTMCs
| Dogs . | Vectors . | Adherent Layer Cells . | Individual LTMC-Derived CFU-GM . | |||
|---|---|---|---|---|---|---|
| Proviral PCR . | α-ID Activity . | RT-PCR . | Proviral PCR* . | α-ID Activity . | ||
| M2 | M48αID | Positive | >10 U† | Positive | 62% | 17% |
| LCαIDSN | (13/21) | (5/30) | ||||
| M3 | M48αID | Positive | 101 U | Positive | 27% | 8% |
| (4/15) | (2/25) | |||||
| M4 | M48αID | Positive | 84 U | Positive | 20% | 16% |
| (3/15) | (5/32) | |||||
| M5 | M48αID | Positive | 198 U | Positive | 24% | 20% |
| (5/21) | (2/10) | |||||
| M6 | LCαIDSN | Positive | 100 U | Positive | 31% | ND |
| M48αID | (13/42) | |||||
| Untransduced control | Negative | 0 U | Negative | 0/20 | 0/12 | |
| Dogs . | Vectors . | Adherent Layer Cells . | Individual LTMC-Derived CFU-GM . | |||
|---|---|---|---|---|---|---|
| Proviral PCR . | α-ID Activity . | RT-PCR . | Proviral PCR* . | α-ID Activity . | ||
| M2 | M48αID | Positive | >10 U† | Positive | 62% | 17% |
| LCαIDSN | (13/21) | (5/30) | ||||
| M3 | M48αID | Positive | 101 U | Positive | 27% | 8% |
| (4/15) | (2/25) | |||||
| M4 | M48αID | Positive | 84 U | Positive | 20% | 16% |
| (3/15) | (5/32) | |||||
| M5 | M48αID | Positive | 198 U | Positive | 24% | 20% |
| (5/21) | (2/10) | |||||
| M6 | LCαIDSN | Positive | 100 U | Positive | 31% | ND |
| M48αID | (13/42) | |||||
| Untransduced control | Negative | 0 U | Negative | 0/20 | 0/12 | |
Abbreviation: ND, sample not done.
CFU positive by PCR amplification of proviral neor or α-ID sequences.
Units are nanomoles per milligram per hour (normal activity ∼1 U).
-ID expression evaluated by RT-PCR. (A) LTMC adherent layers from all dogs and (B) blood (PB) and marrow (BM) leukocytes from M2 at 1 to 3 months postinfusion. PCR amplification of a 618-bp proviral specific -ID transcript (proviral transcript) was performed. PCR amplification of the 422-bp transcript arising from either the normal or mutant genomic -ID cDNA (endogenous transcript) was performed to confirm the presence of amplifiable cDNA in these tissues. A control for each sample was not treated with reverse transcriptase (RT−) to ensure that there was not genomic DNA contamination of samples. Abbreviations: ULTMC, untransduced LTMC control from M2; RC, reagent control; +C, positive control DNA.
-ID expression evaluated by RT-PCR. (A) LTMC adherent layers from all dogs and (B) blood (PB) and marrow (BM) leukocytes from M2 at 1 to 3 months postinfusion. PCR amplification of a 618-bp proviral specific -ID transcript (proviral transcript) was performed. PCR amplification of the 422-bp transcript arising from either the normal or mutant genomic -ID cDNA (endogenous transcript) was performed to confirm the presence of amplifiable cDNA in these tissues. A control for each sample was not treated with reverse transcriptase (RT−) to ensure that there was not genomic DNA contamination of samples. Abbreviations: ULTMC, untransduced LTMC control from M2; RC, reagent control; +C, positive control DNA.
To evaluate the levels of gene transfer and expression in the hematopoietic progenitor subpopulation of LTMCs, individual hematopoietic colonies were assayed. The average percentage of LTMC-derived CFU-GM positive for provirus was 32.8% (range, 20% to 62%; Table 1). Proviral α-ID expression was assayed in CFU-GM from dogs M2 through M5 and on average 15% (range, 8% to 20%) of CFU-GM from transduced LTMCs had proviral α-ID activity. These data indicate that on average half of the progenitors carrying proviral sequences expressed the transgene. The in vitro gene transfer efficiencies obtained in this study were comparable with results obtained in other canine and human gene transfer studies in our laboratory.11 15
Infusion of transduced LTMCs.
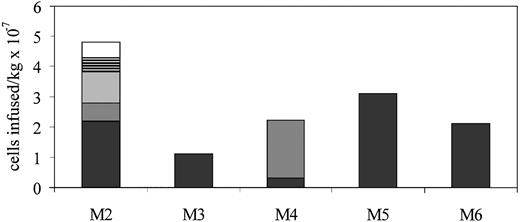
An average of 1.67 × 107 (range, 9.6 × 106 to 3.1 × 107) LTMC cells per kilogram were infused into unconditioned autologous recipient dogs that had not received any type of α-ID enzyme therapy (Fig 4). Dogs were not myeloablated or otherwise preconditioned. Three dogs (M3, M5, and M6) each received one infusion. M4 received two infusions of αID-transduced LTMC cells. After M2 had received three infusions of transduced cells, he was placed on cyclosporine A immunosuppressive therapy and two more infusions of 5 × 106 LTMC cells/kg were administered. Dogs M3 through M6 received immediate posttransplant cyclosporine A and dog M6 received, in addition, methotrexate (0.2 mg/kg) intravenously on days 1, 3, 6, and 11 and weekly thereafter.34 The dose of cyclosporine A used was 10 to 30 mg/kg (cyclosporine A was kindly provided by Sandoz Canada, Dorval, Quebec, Canada) and adjusted to maintain a whole blood cyclosporine A level of 400 to 500 ng/mL. LTMC cells were adminstered intravenously in 50 mL Hank’s buffered saline over 15 to 30 minutes. The injections were well tolerated and there were no late complications.
Summary of LTMC cells infused per kilogram into MPS I dogs. Multiple infusions in M2 and M4 were separated by 3 to 4 weeks. Cell doses administered in multiple infusions are indicated by different shading patterns.
Summary of LTMC cells infused per kilogram into MPS I dogs. Multiple infusions in M2 and M4 were separated by 3 to 4 weeks. Cell doses administered in multiple infusions are indicated by different shading patterns.
Gene transfer into in vivo repopulating cells.
To assess the presence of transduced, long-lived, hematopoietic cells and progenitors, PCR analyses for proviral specific neorand α-ID sequences were performed on samples at up to 36 months postinfusion. Semiquantitative neor PCR analysis was performed on blood and marrow leukocytes from the two dogs (M2 and M6) that received the neor containing vector, LCαIDSN. The proportion of neor-positive cells from both dogs was similar and data from M2 are shown in Fig 2B. The proportion of blood and marrow leukocytes carrying the proviral genome was between 0.1% and 1% at time points up to 26 months postinfusion (Fig 2B). The levels generally decreased to approximately 0.01% at 28 months postinfusion and were undetectable by 36 months.
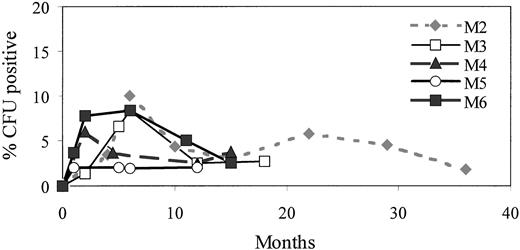
The proportion of hematopoietic progenitors carrying the proviral α-ID cDNA was evaluated by PCR amplification of proviral DNA in marrow-derived hematopoietic colonies (CFU-GM and BFU-E) at time points after adoptive transfer. A representative CFU PCR analysis comparing endogenous control of dystrophin PCR, neor PCR, and α-ID PCR from M2 at 12 months postinfusion is shown in Fig 2C. There was good evidence for the presence of committed progenitors bearing proviral DNA in all dogs, and all had provirus positive CFU at all time points tested (at least 1 year postinfusion). Dog M5, for example, maintained between 2% and 3% provirally marked CFU throughout the 12-month period postinfusion. Other dogs generally had a peak of 5% to 10% positive CFU in the first 6 months postinfusion and then a gradual decrease to 2% to 5% at time points greater than 1 year (Table 2 and Fig 5). Dog M2 was observed the longest and maintained levels of up to 6% provirus positive CFU between 12 and 24 months and 2% to 5% at up to 24 to 36 months postinfusion. Generally, the level of progenitors carrying provirus in the MPS I dogs in this study were similar to those observed in normal dogs used in previous marker gene studies from our center.11 For negative controls, background methylcellulose from each plate and colonies from three marrow samples from control, untreated dogs were harvested, plucked, and analyzed by PCR. A total of 139 colonies from untransduced control dogs and 177 background methylcellulose plucking controls from treated dogs underwent neor and/or α-ID PCR analysis and all were negative.
Detection of Proviral-Specific Sequences in Marrow-Derived Hematopoietic Colonies (CFU-GM and BFU-E) From MPS I Dogs at Time Points Postinfusion
| Dogs . | Time Postinfusion (mo)* . | |||
|---|---|---|---|---|
| 1-4 . | 5-8 . | 9-12 . | 13-24 . | |
| M2 | 3/40 (7.5%) | 2/47 (4.3%) | 1/37 (4.3%) | 3/51 (5.9%) |
| M3 | 1/78 (1.4%) | 5/63 (7.9%) | 1/40 (2.5%) | 2/72 (2.7%) |
| M4 | 3/50 (6.0%) | 1/27 (3.7%) | 1/40 (2.5%) | 2/52 (3.8%) |
| M5 | 2/80 (2.5%) | 2/100 (2.0%) | 1/48 (2.1%) | NA |
| M6 | 9/191 (4.7%) | 2/24 (8.3%) | 7/128 (5.5%) | 3/120 (2.5%) |
| Dogs . | Time Postinfusion (mo)* . | |||
|---|---|---|---|---|
| 1-4 . | 5-8 . | 9-12 . | 13-24 . | |
| M2 | 3/40 (7.5%) | 2/47 (4.3%) | 1/37 (4.3%) | 3/51 (5.9%) |
| M3 | 1/78 (1.4%) | 5/63 (7.9%) | 1/40 (2.5%) | 2/72 (2.7%) |
| M4 | 3/50 (6.0%) | 1/27 (3.7%) | 1/40 (2.5%) | 2/52 (3.8%) |
| M5 | 2/80 (2.5%) | 2/100 (2.0%) | 1/48 (2.1%) | NA |
| M6 | 9/191 (4.7%) | 2/24 (8.3%) | 7/128 (5.5%) | 3/120 (2.5%) |
Where colonies were available for multiple time points during each range of times, the number of colonies were added together.
Abbreviation: NA, sample not available because the dog was killed before this time point.
Time points are calculated from the first infusion.
Percentage of provirus-positive progenitors in five MPSI (M2 through M6) dogs observed for 1 to 2 years postinfusion. At each time point, up to 100 colonies (CFU-GM and/or BFU) and 10 methylcellulose controls were plucked from methylcellulose plates and subject to -ID or neor PCR.
Percentage of provirus-positive progenitors in five MPSI (M2 through M6) dogs observed for 1 to 2 years postinfusion. At each time point, up to 100 colonies (CFU-GM and/or BFU) and 10 methylcellulose controls were plucked from methylcellulose plates and subject to -ID or neor PCR.
Proviral α-ID expression in vivo.
Peripheral blood and marrow leukocytes from all five dogs were assayed for expression by α-ID enzyme activity and RT-PCR on multiple occasions after adoptive transfer. α-ID enzyme activity was not detected in either blood or marrow mononuclear cells from any dog (sensitivity ∼1%). Proviral specific RT-PCR on blood and marrow mononuclear cells collected during the first 3 months postinfusion from all dogs were consistently negative (data from dog M2 shown in Fig 3B). Expression data from two dogs (M2 and M5) are summarized in Table 3. Proviral α-ID expression was also assessed in hematopoietic progenitors, because this population of hematopoietic cells had higher levels of provirus (2% to 10%) than the total blood and marrow leukocytes (<1%). The α-ID activity assay was performed on individual CFU-GM from M2 and M5 at various time points postinfusion. A total of 399 CFU-GM from 1 to 24 months were assayed from M2. One CFU-GM of 155 was positive for α-ID activity at 1 month, whereas 244 CFU-GM from time points up to 24 months were negative. In dog M5, α-ID activity was not detected in any of 193 CFU-GM from 1 to 4 months postinfusion (Table 3). Controls from these experiments included CFU-GM from normal dogs as positive controls and reagent and tissue negative controls.
-ID Activity in Blood and Marrow Leukocytes and Individual CFU-GM Postinfusion
| Dog . | Time Point . | PBL . | BML . | CFU-GM . |
|---|---|---|---|---|
| M2 | 1 mo | Negative | Negative | 1/155 |
| M2 | 8 mo | Negative | Negative | 0/87 |
| M2 | 12 mo | Negative | Negative | 0/85 |
| M2 | 24 mo | Negative | Negative | 0/72 |
| M5 | 1 mo | Negative | Negative | 0/90 |
| M5 | 4 mo | Negative | Negative | 0/103 |
| M2 untransduced control | Negative | Negative | 0/100 | |
| Normal dog control3-150 | Positive | Positive | 22/23 |
| Dog . | Time Point . | PBL . | BML . | CFU-GM . |
|---|---|---|---|---|
| M2 | 1 mo | Negative | Negative | 1/155 |
| M2 | 8 mo | Negative | Negative | 0/87 |
| M2 | 12 mo | Negative | Negative | 0/85 |
| M2 | 24 mo | Negative | Negative | 0/72 |
| M5 | 1 mo | Negative | Negative | 0/90 |
| M5 | 4 mo | Negative | Negative | 0/103 |
| M2 untransduced control | Negative | Negative | 0/100 | |
| Normal dog control3-150 | Positive | Positive | 22/23 |
α-ID activity in BFU-E was not determined, because α-ID activity cannot be detected in BFU-E from normal dogs, likely due to interference of fluorescent measurements by hemoglobin.
CFU-GM from a normal wild-type dog.
Immune response assays.
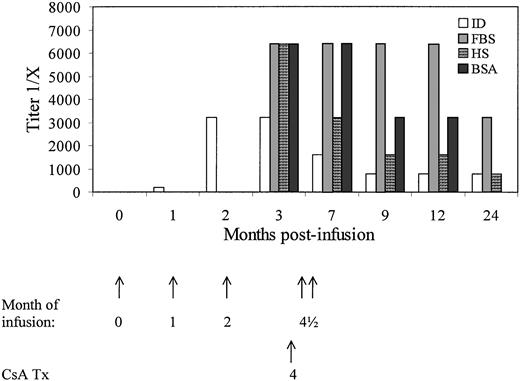
Immune responses against provirally marked cells, proviral gene products, and sera components were evaluated. Specific IgG antibodies against α-ID enzyme were detected by ELISA in sera from dog M2 at all time points postinfusion. The titer peaked after the third infusion (∼2 months postinfusion) at 1:3,200 and decreased to 1:800 to 1:1,600 after initiation of treatment with cyclosporine A at approximately 4 months postinfusion (Table 4). Humoral immune responses against FBS, HS, and bovine serum albumin were assayed after the third infusion and demonstrated IgG titers of 1:3,200 to 1:6,400 (Fig 6). The anti-fetal bovine and anti-horse sera antibodies observed were maintained in the serum of M2 for at least 1 year after the infusion of transduced cells at titers of 1:1,600 to 1:3,200. The highest anti–α-ID titer was observed in M2 after the third infusion of gene modified cells. The other dogs with lower anti–α-ID antibody titers received only one or two infusions of gene-modified cells.
Serum Anti–-ID IgG Titer Detected by ELISA in Serum Samples From MPS I Dogs Postinfusion
| Dogs . | Time Postinfusion (mo) . | ||||||
|---|---|---|---|---|---|---|---|
| 04-150 . | 1 . | 2 . | 3 . | 4-6 . | 7-9 . | 10-12 . | |
| M2 | 0 | 2004-151 | 3,2004-151 | 3,2004-151,‡ | 1,600 | 800-1,600 | 800 |
| M3 | 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| M4 | 0 | 1004-151 | 100 | 100 | 200 | 100 | 100 |
| M5 | 0 | 100 | 400 | ND | 100 | 100 | ND |
| M6 | 0 | <100 | <100 | <100 | <100 | <100 | <100 |
| Dogs . | Time Postinfusion (mo) . | ||||||
|---|---|---|---|---|---|---|---|
| 04-150 . | 1 . | 2 . | 3 . | 4-6 . | 7-9 . | 10-12 . | |
| M2 | 0 | 2004-151 | 3,2004-151 | 3,2004-151,‡ | 1,600 | 800-1,600 | 800 |
| M3 | 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| M4 | 0 | 1004-151 | 100 | 100 | 200 | 100 | 100 |
| M5 | 0 | 100 | 400 | ND | 100 | 100 | ND |
| M6 | 0 | <100 | <100 | <100 | <100 | <100 | <100 |
The titer is recorded as the dilution of serum giving a corrected OD reading of greater than two times each dog’s pretreatment serum OD.
Abbreviation: ND, sample not done.
Preinfusion serum was taken from each dog directly before the first infusion and used an individual background OD for each dog.
Marks the timing of later infusions where dogs received more than 1.
Cyclosporine A treatment was initiated.
Detection of serum IgG specific for -ID (ID), HS, FBS, and bovine serum albumin (BSA) in dog M2 by ELISA. Titers are shown as the dilution of serum that gave a corrected OD reading of greater than two times the dog’s preinfusion serum. Analysis of anti-FBS, -HS, and -BSA antibodies was not evaluated before 3 months. The timing of infusions and initiation of treatment with cyclosporine A are shown with arrows.
Detection of serum IgG specific for -ID (ID), HS, FBS, and bovine serum albumin (BSA) in dog M2 by ELISA. Titers are shown as the dilution of serum that gave a corrected OD reading of greater than two times the dog’s preinfusion serum. Analysis of anti-FBS, -HS, and -BSA antibodies was not evaluated before 3 months. The timing of infusions and initiation of treatment with cyclosporine A are shown with arrows.
Cellular immune responses to autologous transduced cells were evaluated by peripheral blood mononuclear cell proliferation assays. Two experiments were performed with blood mononuclear samples from dog M2 2 and 6 weeks after the third infusion of LTMC cells. In these experiments peripheral blood mononuclear cells were overlaid on transduced and control autologous stroma and the cell proliferation under each condition was measured by incorporation of [3H]-thymidine and reported as mean cpm ± standard deviation. In the first experiment, fresh peripheral blood mononuclear cells from 2 weeks after the third infusion were exposed to LCαIDSN-transduced and untransduced control stroma. There was an approximately threefold increase in [3H]-thymidine incorporation in LCαIDSN-transduced stroma over untransduced controls at blood mononuclear cells to stroma ratios of 10:1 (7,020 ± 719 cpm LCαIDSN transduced stroma v 1,850 ± 383.1 cpm untransduced stroma; P < .005; Fig 7A). In the second experiment, three separate stimulatory autologous stromal cells were used: untransduced, transduced with LNc11 (a neor only containing vector), and M48αID (the vector containing only α-ID). Cryopreserved blood mononuclear cells from 6 weeks after the third infusion of LTMC cells were used as responders. There was approximately twofold higher proliferation when blood mononuclear cells were stimulated with M48αID-transduced autologous stroma versus control untransduced stroma (5,367 ± 1792 cpm M48αID-transduced stroma v3,053 ± 812 cpm untransduced stroma; P < .05) or versus LNc11-transduced stroma (5,367 ± 1,792 cpm M48αID-transduced stroma v 2,635 ± 487 cpm LNc11-transduced stroma; P < .05; Fig 7B). There was no significant difference between stimulation with LNc11-transduced stroma or untransduced stroma (P = .167). The results from these two experiments are consistent with a cellular immune response to α-ID but not to neor-transduced cells. The higher rate of cell proliferation in the second experiment than the first may be due to a dampening of the immune response with time.
Peripheral blood mononuclear cell (MNC) proliferative response to autologous LCIDSN-transduced stroma in dog M2. Blood MNCs from dog M2, which received infusions of both LCIDSN and M48ID transduced LTMC cells, were cocultured in vitro with 1.5 × 103 autologous LCIDSN transduced (□) and untransduced (▪) stroma. MNC proliferation is measured by the incorporation of [3H]-thymidine and reported as the mean counts per minute (cpm) of triplicate wells with standard errors shown. The times after third infusion were (A) 2 weeks and (B) 6 weeks. *P < .005 and ∧P < .05.
Peripheral blood mononuclear cell (MNC) proliferative response to autologous LCIDSN-transduced stroma in dog M2. Blood MNCs from dog M2, which received infusions of both LCIDSN and M48ID transduced LTMC cells, were cocultured in vitro with 1.5 × 103 autologous LCIDSN transduced (□) and untransduced (▪) stroma. MNC proliferation is measured by the incorporation of [3H]-thymidine and reported as the mean counts per minute (cpm) of triplicate wells with standard errors shown. The times after third infusion were (A) 2 weeks and (B) 6 weeks. *P < .005 and ∧P < .05.
The demonstration of immune responses against α-ID, the serum components of the LTMC media, and autologous cells expressing α-ID in dog M2 after multiple infusions led to the treatment of dogs M3 through M6 with fewer infusions of transduced cells and immunosuppressive therapy to abrogate immune responses against α-ID and transduced cells. Three dogs received standard cyclosporine A doses during the immediate posttransplant period. As expected, the titers of α-ID–specific IgG were lower than those for M2, were at 1:100 in M3 and M5 receiving one infusion, and peaked at 1:400 in M4 after a second infusion (Table 4). The humoral immune response was further reduced to background levels in dog M6 treated with one infusion of cells and both cyclosporine A and methotrexate, because titers were less than 1:100.
Pathology.
Three MPS I dogs (M3, M5, and M6) were euthanized at 12 to 24 months postadoptive transfer due to worsening clinical status and increased morbidity, whereas dog M2 died naturally from disease-related causes at 42 months postinfusion. Pathologic examination of all euthanized affected dogs was consistent with normal progression of MPS I disease. Vacuolated cells were prominent in the connective tissues from skin, gastrointestinal tract, liver, pancreas, heart and arteries, respiratory system, reproductive system, and urinary and hemolymphatic systems. Dog M3 is alive at 28 months postinfusion, with advanced but stable disease.
DISCUSSION
Canine α-ID deficiency was first described in 1982 as a new mutation in three Plott hound dogs from Tennessee.16 Through thoughtful breeding programs, colonies were established and arising affected dogs have been the subjects of several important experiments designed to learn more about the comparable human condition, Hurler syndrome or MPS I. Allogeneic bone marrow transplantation in 5-month-old affected pups was shown to result in increased α-ID levels in cerebral cortex, liver, and cerebrospinal fluid and reduced GAG storage in liver, neurons, glial cells, and blood vessels.20 In transplanted pups, urinary GAG excretion decreased to normal levels by 5 months posttransplant. Enzyme replacement studies have also been undertaken in affected dogs using recombinant human α-L-iduronidase.21,22 Intravenous administration of purified enzyme to three affected pups for up to 13 months21 22 resulted in normalization of lysosomal storage in liver, spleen, and kidney glomeruli. There was no improvement in brain tissue GAG storage, and all dogs developed complement-activating antibodies against the human protein.
The promising results of allogeneic bone marrow transplantation20 and evidence for clinical improvement in recombinant enzyme infusion studies, despite the presence of an immune response,21,22 suggested that α-ID–deficient dogs might be ideal for developing and evaluating therapies delivered by genetically corrected HSCs. The need for such model systems was heightened by the generally poor results obtained in several large animal and human studies of HSC gene transfer. Studies in cats,35 dogs,10,36 nonhuman primates,5,37 humans,2,38,39 and surrogate human systems40,41 suggested that only a small proportion of in vivo repopulating HSCs are genetically modified using methods that gave high levels of HSC gene transfer in murine systems. Furthermore, there still is no clear evidence for a therapeutic benefit of genetically modified HSCs in any large animal or human system.3 42 We reasoned that the demonstration of significant disease amelioration in affected α-ID–deficient dogs receiving genetically corrected HSCs would represent a much-needed proof of principle in the arena of HSC gene therapy.
We performed large volume marrow harvests from five affected α-ID–deficient dogs and exposed hematopoietic cells with known long-term in vivo repopulating potential to two retroviral vectors bearing the normal canine α-ID cDNA. One vector, LCαIDSN, expressed α-ID from the LTR and neor from the SV40 promoter/enhancer. In the M48αID vector, α-ID was expressed from the murine PGK promoter. Both vectors were evaluated by us and independently by others and shown to be excellent vectors as determined by in vitro assays of transduction.43 Adoptive transfers of transduced LTMCs to autologous recipients were performed under conditions shown in prior studies to give rise to genetically modified hematopoietic cells with capacities to generate committed progenitors in vivo for up to 3 years and at levels comprising approximately 5% of all committed progenitors.11 Our particular approach to gene transfer has relied heavily on the use of stromal-based culture systems, because data from several sources suggest that such in vitro microenvironments may facilitate both maintenance of and gene transfer into HSCs.10-14 44
There was good evidence that a high proportion of LTMC-derived committed progenitors carried and expressed the proviral α-ID, as well as the bulk LTMC cells that expressed up to 200 times the normal α-ID enzyme levels in vitro. Monitoring of engraftment for up to 2 years by PCR amplification of proviral sequences demonstrated that up to 6% of hematopoietic progenitors and 0.01% to 1% of blood and marrow leukocytes of affected dogs were gene marked. These data confirm prior results from our center.11 The higher level of marking in hematopoietic progenitors than differentiated cells observed in these dogs has also been seen in both our studies of LTMC gene transfer studies in human patients on a stem cell gene marking trial15 and in studies by others.2 45 The reason for this phenomenon is unknown but possibly involves an inability of transduced progenitors to undergo natural processes of proliferation and differentiation or immune responses to differentiated hematopoietic cells presenting foreign antigens.
Despite the documentation of engraftment of up to 1% of blood and marrow leukocytes and 10% of progenitors carrying the provirus, we observed no α-ID activity in blood or marrow leukocytes. At the time of infusion, LTMC cells expressed up to 200 times the normal level of α-ID protein and an average of 15% of individual hematopoietic colonies had proviral α-ID enzyme activity. After infusion, α-ID activity was not detected in blood or marrow leukocytes and only 1 of a total of 592 CFU-GM from dogs M2 and M5 was found to be expressing α-ID, suggesting that proviral gene expression had been silenced in our vectors. Silencing of Moloney murine leukemia virus (MMLV)-based promoters, such as that used in these vectors, has been described in other in vitro and in vivo studies.46-48 In our own previous canine gene transfer studies with the LN vector that has a similar vector backbone to these studies, expression of neor was maintained in approximately 5% of CFU-GM for up to 2 years postinfusion. The lack of α-ID proviral expression in MPS I dogs may be due to the presence of two genes in the LCαIDSN vector, a phenomenon that has been associated with decreased expression of both genes.46 Alternatively silenced proviral α-ID expression in MPS I dogs, but not neor in normal dogs, might be the result of the anti-α-ID immune response noted in MPS I dogs. Inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) induced by immune responses have been shown to inhibit expression of MMLV-based promoters.49 50
We also investigated the role of humoral and cellular immune responses against α-ID, LTMC media components, and α-ID–transduced cells. Strong immune responses to α-ID enzyme, HS, and FBS components and α-ID–transduced autologous cells were detected in dog M2 after three infusions of transduced cells. All subsequently infused dogs (M3 through M6) were treated with posttransplant immunosuppression and received fewer infusions in an attempt to abrogate the immune responses. The humoral anti–α-ID and antiserum immune responses in these dogs, although somewhat lower than those of M2, were still present.
Humoral immune responses have been detected against a variety of nontherapeutic antigens presented by genetically modified cells such as adenoviral vector proteins51 or serum components of culture media,52 whereas cellular responses have been detected against marker genes, such as a thymidine kinase-neorfusion protein.53 However, few gene transfer studies have used animal models of human disease to evaluate gene therapy strategies. This study in dogs with α-ID deficiency demonstrated significant humoral immune responses against the normal canine α-ID and LTMC culture media serum components. As expected, the strongest immune responses in our study were detected after multiple infusions of cells in the absence of immunosuppressive therapy. To our knowledge, this is the first demonstration of both humoral and cellular immune responses against a potentially therapeutic, normal canine enzyme in dogs deficient for this protein. Both the presence of cellular immune responses and lack of detectable α-ID expression in vivo may indicate that only cells that have silenced proviral expression survived. However, further investigation is needed to confirm this.
Two mechanisms may account for the lack of sustained enzyme production: silencing of proviral gene expression and immune responses against α-ID produced in vivo and/or cells expressing proviral antigens. In ongoing studies, transduced LTMC grafts have been infused into preimmune fetal MPS I recipients to evaluate the therapeutic potential of HSC gene transfer for α-ID–deficient dogs in the absence of immune responses.54 Retroviral vectors that have been modified to optimize long-term transgene expression, such as the MND,48 MFG,55 and MSCV56 vectors, will be evaluated in future studies. These and other approaches may enable a definitive evaluation of the therapeutic potential of genetically corrected HSCs in the canine α-ID deficiency model system.
ACKNOWLEDGMENT
The authors express our appreciation to Dr Margaret Hough, Yongjun Zhao, Xiaochen Lu, and the staff of S.D. Laboratories Ltd (Toronto, Ontario, Canada) for their assistance and cooperation. We are grateful to Drs Anna Salvetti and Olivier Danos for providing us with retroviral producer lines and to Dr Emil D. Kakkis for providing the canine α-ID cDNA and purified α-ID enzyme and for his generous assistance with the 4MUαID and ELISA assays.
Supported by grants from the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ian D. Dubé, PhD, Department of Laboratory Medicine, Sunnybrook Health Science Center, 2075 Bayview Ave (Room E346), Toronto, Ontario, Canada M4N 3M5; e-mail:ian.dube@utoronto.ca.

![Fig. 7. Peripheral blood mononuclear cell (MNC) proliferative response to autologous LCIDSN-transduced stroma in dog M2. Blood MNCs from dog M2, which received infusions of both LCIDSN and M48ID transduced LTMC cells, were cocultured in vitro with 1.5 × 103 autologous LCIDSN transduced (□) and untransduced (▪) stroma. MNC proliferation is measured by the incorporation of [3H]-thymidine and reported as the mean counts per minute (cpm) of triplicate wells with standard errors shown. The times after third infusion were (A) 2 weeks and (B) 6 weeks. *P < .005 and ∧P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1895.406k02_1895_1905/5/m_blod40602007ax.jpeg?Expires=1767741613&Signature=WkdUEb1Qjec-KlSwwqKaqvdsZipusHNq~aAExk~1oR58TyAvs5aW1oiEcjPLDEKfWi5WZDrtMM5P3WvjvYHLh60OW8n8H209qCdSN6nEuXkclKiOPUDys2KxPH9icFqGGI~wOYaP6wT2n1U-pO77ox8EBJDmQyfEDyaGSuLDk9QvcQzPNU7qY74-5fEt6libu9c~Nqh5F68njgo1rOSzTOp4nCjMkVjSWSYmZqM5cXkS8iQytp6lY5ikzyOFcxTFfqEJHyLhv~Q3eSncCKxovc2gopNmsEZar4liea~2Qgr-5VDM-561EcNKMbmy6xPFDFX7zCXNu-FWx-0KJDoOUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Peripheral blood mononuclear cell (MNC) proliferative response to autologous LCIDSN-transduced stroma in dog M2. Blood MNCs from dog M2, which received infusions of both LCIDSN and M48ID transduced LTMC cells, were cocultured in vitro with 1.5 × 103 autologous LCIDSN transduced (□) and untransduced (▪) stroma. MNC proliferation is measured by the incorporation of [3H]-thymidine and reported as the mean counts per minute (cpm) of triplicate wells with standard errors shown. The times after third infusion were (A) 2 weeks and (B) 6 weeks. *P < .005 and ∧P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/6/10.1182_blood.v93.6.1895.406k02_1895_1905/5/m_blod40602007bx.jpeg?Expires=1767741613&Signature=X4VaovqrGIzwBA96X6yly0Jsv9ss72YGfm5X4wpg~NZhcF5aNWb-UWp5cyrwN6qivfgTWWtSVOb1Zq7eU4Nr75ERcDAoDai~8geSEROmD6ntpg6VZwIAH3dgzjyXA7Z-f2fccj1Xp7AP4NI8gtz7Jz4FJF857OEyyuL7jtORB5n1ESyNzebNVMAQc9ZxYlWn45J3VK4A34RDR1p43n77AElZDPMWXqzzS8oc2O~HWNpp7-V~NimzthmtXOxgNrYAYXubhKV6iR0Arykp0YPFnXzibbIIKLUvaK362R6P5xc6D9KBLahKpUTk6xvct1Qha1cRts1r4armGEqyOwqgCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal