We evaluated the capacity of adeno-associated virus (AAV) vectors to transduce primitive human myeloid progenitor cells derived from marrow and cord blood in long-term cultures and long-term culture-initiating cell (LTC-IC) assays. Single-colony analyses showed that AAV vectors transduced CD34+ and CD34+38− clonogenic cells in long-term culture. Gene transfer was readily observed in LTC-ICs derived from 5-, 8-, and 10-week cultures. Recombinant AAV (rAAV) transduction was observed in every donor analyzed, although a wide range of gene transfer frequencies (5% to 100%) was noted. AAV transduction of LTC-ICs was stable, with week-8 and -10 LTC-ICs showing comparable or better transduction relative to week-5 LTC-ICs. Fluorescence in situ hybridization (FISH) analyses performed to determine the fate of AAV vectors in transduced cells showed that 9% to 28% of CD34+ and CD34+38− cells showed stable vector integration as evidenced by chromosome-associated signals in metaphase spreads. Comparisons of interphase and metaphase FISH suggested that a fraction of cells also contained episomal vector at early time points after transduction. Despite the apparent loss of the episomal forms with continued culture, the number of metaphases containing integrated vector genomes remained stable long term. Transgene transcription and placental alkaline phosphatase (PLAP) expression was observed in CD34+, CD34+38−LTC-ICs in the absence of selective pressure. These results suggest that primitive myeloid progenitors are amenable to genetic modification with AAV vectors.
EX VIVO GENE TRANSFER into pluripotent hematopoietic stem cells represents an attractive treatment modality for a variety of gene-based pathological processes, including inherited diseases,1,2 oncogenic processes,3,4 and viral infections.5 Despite the widespread use of retroviral vectors in the majority of human gene therapy trials currently underway, several limitations remain. Human trials and nonhuman primate models of hematopoietic progenitor cell transplantation suggest that the efficiency of retroviral gene transfer into hematopoietic cells is low.6 Results of clinical gene therapy trials using transplantation of retroviral vector-transduced CD34 cells derived from either bone marrow,7,8 cord blood,2 or mobilized peripheral blood8 have been informative in determining the potentials of retroviral transduction of human hematopoietic stem cells (HSCs) in vivo. The majority of the results suggest a long-term marking frequency of approximately 1:10,000 to 1:100,000. Reports that marrow- and cord blood-derived human hematopoietic cells capable of multilineage repopulation of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice exclusively present in the CD34+38−fraction were rarely transduced by retroviral vectors9 are in concordance with the results of the human trials.
Although gene transfer with retrovirus vectors10 in lineage-committed colony-forming units (CFU) have been successful, these studies have not been predictive of in vivo results. Attempts to elucidate the reasons underlying poor transduction of HSCs by retroviral vectors suggested that the requirement for cell division11,12 to achieve retroviral integration may impede their use for the genetic modification of quiescent cell populations such as HSCs.13 Therefore, the continued search for other vectors for the safe, stable, and efficient introduction of transgenes into nondividing HSCs is critical. Vectors based on adeno-associated virus (AAV) are emerging as efficient gene transfer vehicles with biological properties quite distinct from retroviruses.
AAV is a single-stranded, replication-defective nonpathogenic human parvovirus with a 4.7-kb DNA genome with palindromic inverted terminal repeats (ITR).14,15 AAV requires coinfection with a helper virus such as adenovirus or herpes simplex virus for productive infection.16,17 In the absence of helper virus coinfection, wild-type AAV integrates site-specifically and in a stable fashion, via the ITRs, into the AAVS1 site of human chromosome 19.18,19However wild-type–free, rep-free AAV vectors integrate into chromosomal sites other than AAVS1. AAV vectors20 have high transduction frequencies in cells of diverse lineages and allow efficient transgene expression from RNA polymerase II-and III-dependent promoters.21 Recent studies from our laboratory and those of others22 have shown that AAV vectors efficiently transduce nondividing cells, including peripheral blood macrophages, a variety of neurons, cardiac cells, skeletal cells, and smooth muscle cells.23-27
We have previously reported the ability of AAV vectors to transduce human marrow and cord blood-derived CD34+ hematopoietic progenitor cells.28 Southern and fluorescence in situ hybridization (FISH) analyses demonstrated the integration of AAV vector sequences into CD34 chromosomal DNA. FISH analyses also showed that integrated vector sequences replicated along with cellular DNA during mitosis, suggesting stable integration. Transgene expression was observed in transduced CD34 cells in suspension cultures and in myeloid colonies differentiating from transduced CD34 cells regardless of selective pressure. These results were in concordance with others who have shown AAV transduction of colony-forming units granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMMs).29-32 In addition, recombinant AAV (rAAV) transduction of murine hematopoietic progenitor cells has also been reported.33 34 Thus, the combined ability of AAV vectors to transduce both nondividing cells and CD34 cells makes them attractive for further evaluation for gene transduction into primitive human hematopoietic progenitor and stem cells. Therefore, in this study, we evaluated the ability of AAV vectors to transduce and integrate into primitive myeloid CD34 and CD34+38− clonogenic progenitor cells from marrow and cord blood. Investigation of AAV transduction of cells with extended clonogenic capacity showed gene transfer into week-5, -8, and -10 long-term culture-initiating cells (LTC-IC) by individual colony analysis. Transduction levels varied from high to low, depending on the donor. FISH analyses of CD34+ and CD34+38− cells showed that, whereas episomal and integrated forms of vector genomes could be detected at early time points, only integrated forms persisted long term and contributed to stable transduction. Sustained expression of vector-encoded antisense RNA was detected in long-term cultures and LTC-ICs in the absence of selective pressure.
MATERIALS AND METHODS
Cells and viruses.
Marrow samples were obtained from healthy donors for allogeneic transplant recipients after obtaining informed consent and using a City of Hope Institutional Review Board approved protocol. Umbilical cord blood was procured from Huntington Memorial Hospital (Pasadena, CA) using a protocol approved by both the COH and HMH Institutional Review Boards. Light-density mononuclear cells were separated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) centrifugation. After three washes with phosphate-buffered saline (PBS), cell and viability counts were performed and cells were resuspended in PBS with 0.5% bovine serum albumin (BSA) and 2 mmol/L EDTA before use.
The CD34+ population of marrow cells was immunomagnetically purified from mononuclear cells using CD34 Isolation kits (Miltenyi Biotech, Auburn, CA) as per the manufacturer’s directions. CD34 cells were passed through two columns sequentially to increase purity and enrich for CD34+++ cells. CD34 purity was 96% to 98% as assessed by flow cytometry after direct immunolabeling with fluorescein isothiocyanate (FITC)-conjugated HPCA-2. CD34+38− cells were cytofluorometrically sorted using a Mo-Flo high speed flow cytometer (Cytomation, Fort Collins, CO) after labeling with FITC-conjugated anti-CD34 and phycoerythrin (PE)-conjugated anti-CD38 (Becton Dickinson, San Jose, CA).
Herpes simplex virus, type 1 (HSV-1), MP17 was used as the helper virus for AAV vector encapsidation and was propagated and titered by plaque assays as previously described.17
AAV vectors.
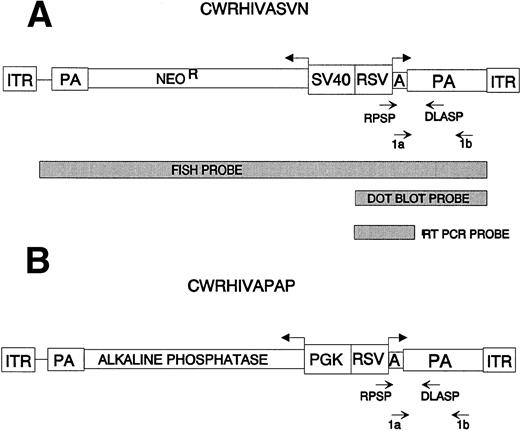
All AAV vectors used in this study were derived from the base vector, CWRSV,5,28 which contains bases 1 through 189 and 4045 through 4680 of wild-type AAV2, including the 5′ and 3′ ITRs and the endogenous AAV2 polyadenylation signal. CWRHIVASVN (Fig 1A) contains two transcriptional cassettes, one encoding the neomycin phosphotransferase (NeoR) gene under the SV40 early promoter control and the other encoding an antisense transcript complementary to human immunodeficiency virus–type 1 (HIV-1) LTR sequences under RSV LTR control.5 CWRHIVAPAP (Fig 1B) is identical to CWRHIVASVN, except for the substitution of a gene cassette encoding the thermostable human placental alkaline phosphatase (PLAP) gene35 36 under the phosphoglycerate kinase (PGK) promoter control for the SVNeo cassette.
Maps of AAV vectors used. Bold arrows denote the direction of transcription of each gene. The promoters, polyadenylation signals (PA), and the AAV ITR are shown. Light arrows denote the location of PCR primers. Primers RPSP and DLASP were used for amplification from DNA templates. Primers 1a and 1b were used for reverse transcription and amplification from RNA templates. Probes used for FISH analyses and Southern hybridization of RT-PCRs and dot blot analyses of vectors are designated. (A) vCWRHIVASVN encodes two transgenes: (1) an antisense RNA (A) complementary to the HIV LTR, including the TAR and the polyadenylation sequences under the transcriptional control of the RSV LTR; and (2) the neomycin phosphotransferase (NeoR) gene under the control of the SV40 early promoter. (B) vCWRHIVAPAP contains the antisense RNA gene cassette described in (B) in addition to the PLAP gene under the control of the PGK promoter.
Maps of AAV vectors used. Bold arrows denote the direction of transcription of each gene. The promoters, polyadenylation signals (PA), and the AAV ITR are shown. Light arrows denote the location of PCR primers. Primers RPSP and DLASP were used for amplification from DNA templates. Primers 1a and 1b were used for reverse transcription and amplification from RNA templates. Probes used for FISH analyses and Southern hybridization of RT-PCRs and dot blot analyses of vectors are designated. (A) vCWRHIVASVN encodes two transgenes: (1) an antisense RNA (A) complementary to the HIV LTR, including the TAR and the polyadenylation sequences under the transcriptional control of the RSV LTR; and (2) the neomycin phosphotransferase (NeoR) gene under the control of the SV40 early promoter. (B) vCWRHIVAPAP contains the antisense RNA gene cassette described in (B) in addition to the PLAP gene under the control of the PGK promoter.
The recombinant viral vectors vCWRHIVASVN, vCWRHIVAPAP, and vCWRAP were encapsidated using HSV-1 MP17 as helper viruses, as described previously.28 37 Briefly, semiconfluent 293 cells were infected with HSV-1 MP17 (multiplicity of infection [MOI], 0.1) and transfected 1 hour postinfection with 20 μg of the vector plasmids by calcium phosphate coprecipitation (CellPhect; Pharmacia Biotech, Uppsala, Sweden). AAV-encoded rep (DNA replication) and cap (capsid proteins) gene functions were provided in trans. Cells were harvested 72 hours posttransfection and were lysed by three cycles of freeze-thawing and sonication. Vector stocks were treated with 400 U DNase I (Boehringer Mannheim, Indianapolis, IN) per 107 cells to digest residual plasmid and cellular DNA. The lysate was digested with 0.25% trypsin and 1% sodium deoxycholate for 30 minutes at 37°C before further purification on two rounds of isopycnic cesium chloride (density, 1.41 g/mL) gradient centrifugation for 64 and 48 hours, respectively, at 40,000 rpm. Fractions of 0.5 to 1 mL were collected and density-measured with a refractometer.
Particle titers were determined by dot blot analysis. DNA was isolated from each fraction using standard procedures38 after proteinase K digestion, phenol and phenol:chloroform extractions, and ethanol precipitation. DNA was dot-blotted onto nitrocellulose and hybridized with a vector-specific probe (BamHI-SnaBI fragment of CWRHIVAPAP; Fig 1). Duplicate blots were hybridized with a wild-type AAV-specific probe (Sac II fragment of pTZAAV). Results were analyzed on a phosphorimager and titers were determined from standard curves. pBluescript DNA served as a negative control. A particle titer of 109/mL was obtained for vCWRHIVAPAP and one of 108/mL was obtained for vCWRHIVASVN.
The functional titers of vCWRHIVAPAP and vCWRHIVASVN stocks were determined by quantitation of specific alkaline phosphatase-expressing cells and neomycin resistant (NeoR) colonies after serial dilutions on 293 cells. Functional titers of 106/mL to 107/mL were obtained for vCWRHIVAPAP and one of 105/mL was obtained for vCWRHIVASVN. The presence of full-length vector genomes and the absence of contaminating wild-type AAV was confirmed by either dot blot or electrophoretic analysis of DNA extracted from vector stocks, followed by Southern hybridization with vector-specific and wild-type–specific probes. All helper virus stocks and cell lines were screened for and found to be free of wild-type AAV contamination.
For most experiments, transductions with vCWRHIVAPAP were performed at functional MOI of 1 to 3 and with vCWRHIVASVN at MOI of 0.1 to 0.2. Specific multiplicities are stated for each set of experiments.
Transductions.
CD34 cells were transduced immediately upon isolation at the time of culture initiation at 37°C in a humidified, CO2incubator. Transductions were performed by the direct addition of vector to cells at functional MOIs of 1 to 10 (corresponding to particles MOIs of 200 to 2,000) and left undisturbed for 24 to 48 hours, after which cells were washed and replated. In separate experiments, we have shown that there is no detectable free vector left in the medium after washes. Two batches of vCWRHIVAPAP of equivalent titer and one batch of vCWRHIVASVN were used for all experiments reported here.
Long-term marrow cultures (LTBMCs).
Heterologous stromal layers were established by plating 5 to 10 × 106 allogeneic marrow mononuclear cells in T75 flasks in RPMI 1640 with 10% fetal bovine serum (FBS) and L-glutamine (cRPMI). Stromal layers developed in 2 to 3 weeks and were used for LTC-IC assays after at least three passages, after which they were harvested by trypsinization, washed, and plated in 12-well plates at 2 to 3 × 104/cm2 in cRPMI. The stroma was irradiated with 15 Gy of 250 KVp x-ray when a confluency of approximately 70% was reached, and media was removed and overlaid with CD34 cells in long-term culture medium.
Purified CD34 cells were suspended in Myelocult (StemCell Technologies, Vancouver, British Columbia, Canada) containing 1 μmol/L of hydrocortisone (Sigma, St Louis, MO) and penicillin-streptomycin and overlaid on irradiated heterologous stromal layers in 12-well plates at a density of 2 to 6 × 104cells/cm2 either with or without the addition of vCWRHIVAPAP at a functional MOI of 2 to 3 (particle MOI, 400 to 600). Suspension cells were washed after 24 to 48 hours and replated on washed stromal layers. Cultures were maintained by demi-depopulation of cells every 7 to 10 days.
LTC-IC assays.
Clonogenic assays to test for LTC-ICs were performed 5 to 10 weeks after culture initiation by inoculation of suspension cells from LTBMCs into methylcellulose media39,40 (StemCell Technologies). Cells were harvested from LTBMCs by trypsinization, washed, and resuspended in 500 μL Iscove’s Modified Dulbecco’s Medium (IMDM) with 30% heat-inactivated FBS (GIBCO, Grand Island, NY) and L-glutamine. The cell suspensions were mixed with a suspension containing 0.8% methylcellulose (MethoCult; StemCell Technologies), 30% fetal calf serum (FCS), 1% BSA, 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, interleukin-3 (IL-3; 10 ng/mL; R&D Systems, Minneapolis, MN), granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL; R&D Systems), and erythropoietin (50 U/mL; Amgen, Thousand Oaks, CA) and placed in 12-well plates at a concentration of 10,000 cells/well in duplicate. Colonies containing greater than 50 cells were scored microscopically approximately 14 days after plating. Individual colonies were plucked and washed. DNA was extracted from colonies by standard methods after RNase treatment, sodium dodecyl sulfate (SDS)-proteinase K digestion, phenol:chloroform extraction, and ethanol precipitation.38DNA extracted from each colony was resuspended in 25 μL TE.
Amplification of rAAV vector sequences.
LTC-IC colony DNA and controls were tested for the presence of vector sequences by polymerase chain reaction (PCR) amplification using specific primers in a Perkin-Elmer Model 9600 Thermal Cycler (Perkin-Elmer, Foster City, CA). Primers (RPSP [sense, 5′-GGTGGAAGTAAGGTGGTACG-3′] and DLASP [antisense, 5′-TGCCGCACTGAAGGTGGTCGAA-3′]) were used to amplify a 418-bp product spanning a region from the Rous Sarcoma virus long terminal repeat (RSV LTR) to the AAV polyadenylation site from RNA of vCWRHIVASVN and vCWRHIVAPAP (Fig 1) transduced cells. Internal controls for DNA concentration and template integrity were provided by amplification of a 268-bp fragment from the ubiquitous human β-globin gene (sense primer, 5′-CAACTTCATCCACGTTCACC-3′; antisense primer, 5′-GAAGAGCCAAGGACAGGTAC-3′). One third of the RPSP primer used was end-labeled with γ [32P]-dATP using T4 polynucleotide kinase (New England Biolabs, Beverly, MA). Amplified products were resolved by electrophoresis in a 5% polyacrylamide gel in Tris borate-EDTA (TBE) buffer, dried, and exposed to Kodak X-omat AR film (Eastman Kodak, Rochester, NY) at −70°C with an intensifying screen. Radioautographic band intensities were quantitated by phosphorimage analysis.
FISH analyses.
CD34 cells were transduced with vCWRHIVAPAP at MOI of 1 to 3 (particle MOI, 200 to 600) immediately upon isolation at the time of culture initiation in IMDM containing 20% FCS, IL-3 (10 ng/mL; R&D Systems), IL-6 (10 ng/mL; R&D Systems), and stem cell factor (1 ng/mL; R&D Systems). FISH analysis was performed as described previously.28 Briefly, at the time of analysis, suspension cells were harvested from cultures, washed, and resuspended in fresh IMDM containing FCS, glutamine, and higher levels of cytokines (50 ng/mL IL-3, 50 ng/mL IL-6, and 5 ng/mL stem cell factor) for 72 hours to boost the mitotic index. To block cells in metaphase, cells were placed in fresh cytokine-containing media containing colcemid (0.025 mg/mL) for 16 to 24 hours. Harvested cells were treated with a hypotonic solution (0.4% KCl) and fixed in 3:1 methanol:acetic acid before dropping on slides to obtain nuclear spreads.41
A 3.6-kb Hpa I-SnaBI fragment from pCWRHIVASVN (Fig 1) was labeled with digoxygenin-deoxyuridine triphosphate by nick translation and used as the probe for FISH. The probe was derived from CWRHIVASVN to avoid inclusion of the human PLAP gene while still providing a large enough fragment for use as an efficient FISH probe. The probe was specific for the RSV LTR, antisense to HIV-1, the polyadenylation region, and the neomycin phosphotransferase gene under the control of the SV40 promoter and was found to hybridize to vCWRHIVAPAP and vCWRHIVASVN transduced cells with equivalent efficiency (Fisher-Adams et al, unpublished data). A BamHI fragment from AAVS118 (kindly provided by R. Kotin, National Institutes of Health, Bethesda, MD) was used for the chromosome 19 AAVS1 site. Hybridization and washes were performed as described.41 Visualization and imaging was achieved using a PSI imaging system (Perceptive Scientific Instruments Inc, League City, TX) housed in the cytogenetics core facility at the City of Hope National Medical Center. At least 100 nuclear spreads were scored for each sample (except for HJ, for which there were fewer suspension cells for analysis; see Table2).
Reverse transcription-PCR (RT-PCR) analyses.
Approximately 0.5 μg of total RNA or 1 μL of oligo-dT purified RNA was used for each reaction. Reverse transcription was performed in 50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, and 5 mmol/L MgCl2with 1 μmol/L antisense primer 1a (5′-ATGCTTCGAAATTACGAGTCAGGTATCTGGTGCCAAT-3′), 0.5U RNase Inhibitor, and 1 mmol/L each of dGTP, dATP, dCTP, and dTTP. Reaction tubes were prepared without RNA, and after RNA was added, each preparation was divided into two separate reactions: (1) +RT, which included 1.75 U Moloney murine leukemia virus (MuLV) reverse transcriptase (Perkin-Elmer), or (2) −RT, which included dH2O. Reverse transcription was performed at 42°C for 45 minutes, followed by denaturing at 99°C for 3 minutes and snap-cooling to 5°C for 5 minutes. The resulting cDNAs were subjected to 30-cycle PCR, after annealing at 55°C, using the sense primer 1b (5′-GATCCTCGAGCCATTTGACCATTCACCACATTGGTGT-3′) and the antisense primer 1a described above to amplify a 526-bp product spanning a region from the RSV LTR transcriptional start site to the AAV poly A region. A total of 2.5 μL of 10× Vent buffer was added to each +/− RT reaction such that the final concentrations were 20 mmol/L KCl, 10 mmol/L (NH4)2SO4, 2 mmol/L MgSO4, 5 mmol/L MgCl2, 0.1% Triton X-100, and 1.8 mmol/L Tris HCl. Primers 1a and 1b were added to a final concentration of 1 μmol/L. Deep Vent (0.25 μL; New England Biolabs) was added immediately before thermal cycling. Amplification conditions were as follows: 94°C for 40 seconds, 55°C for 40 seconds, and 72°C for 1 minute for 30 cycles, followed by a 72°C final extension for 5 minutes. Gel loading buffer was added to each tube and one third to one half of each reaction was electrophoresed on a 1.5% agarose gel and subsequently analyzed by Southern hybridization using a probe containing theBamHI-Xba I fragment isolated from pCWRHIVASVN.
RESULTS
AAV vector transduction of LTBMCs.
CD34 cells were purified from light-density bone marrow or cord blood mononuclear cells and transduced with vCWRHIVAPAP28encoding an antisense RNA complementary to the HIV-1 LTR under the control of the RSV LTR and the gene encoding thermostable human PLAP under the control of the phosphoglycerate kinase promoter (Fig1) at a functional multiplicty of 3 (particle MOI, 600). CD34 cells were transduced immediately after isolation and plated in long-term culture on irradiated heterologous stroma along with untransduced controls. No toxicity or major differences in cellularity were observed in transduced cultures as compared with untransduced controls.
To evaluate transduction of primitive hematopoietic progenitor cells with AAV vectors, we first analyzed gene transfer into clonogenic CD34 cells in long-term cultures. Cells harvested from LTBMCs were assayed for clonogenic potential in LTC-IC assays 5 weeks or later after culture initiation and vector transduction. No reduction in the clonogenic capacity of transduced cells was observed as compared with untransduced cells, suggesting that AAV transduction was not toxic or deleterious to myeloid differentiation from primitive progenitor cells in vitro.
AAV vector sequences in individual colonies derived from LTC-ICs.
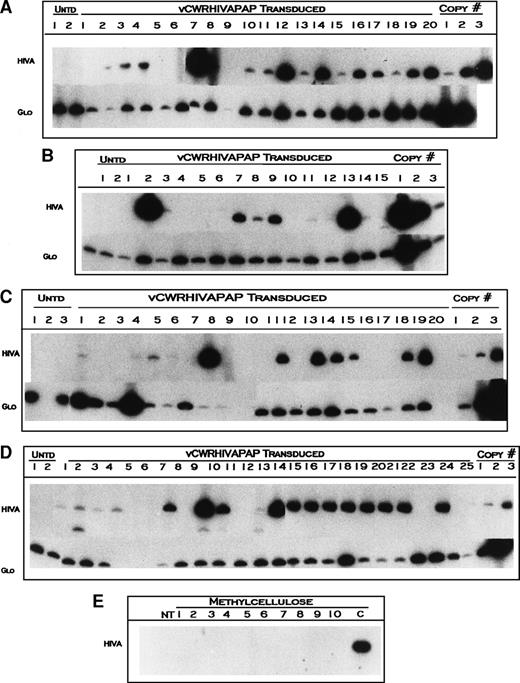
To determine transduction efficiencies of clonogenic cells in long-term cultures, we analyzed individual colonies harvested from LTC-ICs from LTBMCs described above. Entire LTBMC wells were harvested after trypsinization of stromal layers from cultures initiated with vCWRHIVAPAP-transduced and untransduced cells, washed, and placed in LTC-IC assays at 5, 8, and 10 weeks after transduction. Colonies developing from LTC-ICs were primarily CFU-GEMM and colony-forming unit–granulocyte-macrophage (CFU-GM), few colony-forming unit-granulocyte (CFU-G), colony-forming unit-macrophage (CFU-M), and burst-forming unit-erythroid (BFU-E) were also observed. Individual colonies were plucked from methylcellulose in LTC-IC assays, DNA extracted, and analyzed for the presence of vector sequences by amplification of the HIV LTR antisense gene using primers RPSP and DLASP (Fig 1). Figure 2 shows representative PCR amplification analyses of DNA from LTC-ICs from donors TS at weeks 5 and 8 (Fig 2A and B, respectively), donor HJ at weeks 8 and 10 (Fig 2C), and donor FK at weeks 5 and 10 (Fig 2D). Amplification of β globin served as a control for template integrity. The number of colonies showing vector specific bands from the total number containing β globin signals provided the transduction efficiency. Figure 2E shows the amplification of 10 methylcellulose samples harvested between colonies and subjected to DNA isolation procedures. No vector-specific bands were observed from these samples, indicating that free vector DNA was undetectable in these cultures and that the vector signals observed in LTC-ICs originated from transduced cells.
Amplification of vCWRHIVAPAP sequences from individual LTC-IC colonies. CD34 cells were transduced with vCWRHIVAPAP at a functional MOI of 3 (particle MOI, 600) on day 0 and plated in long-term culture as described in Materials and Methods. Cells were harvested from stromal layers at designated time points, washed, and plated in methylcellulose with no G418 selection and colonies were plucked after 2 weeks. DNA was extracted and amplified for either the AAV vector using primers RPSP and DLASP or for β globin. β Globin served as a control for template integrity. The 418 HIVA band denotes the vector signal. The 268-bp Glo band denotes the β globin signal. Representative colonies from untransduced controls (Untd) are shown. Copy number controls are included in each analysis. For HIVA, the copy number corresponds to 12, 120, and 1,200 copies of the genome. For β globin, copy number controls show amplification from 80, 160, and 1,000 cells. (A) Week-5 LTC-ICs from donor TS. All colonies except one had intact DNA, albeit at varying quantities. The absence of a β globin signal from colony 9 indicated that the DNA template was inadequate. (B) Amplification of vCWRHIVAPAP sequences from individual week-8 LTC-IC colonies from donor TS. All colonies analyzed had intact DNA templates and 9 of 15 showed vector-specific signals. The standard curve is reversed in this experiment. (C) Amplification of vCWRHIVAPAP sequences of individual week-8 (transduced lanes 1 through 10) and week-10 (transduced lanes 11 through 20) LTC-IC colonies from donor HJ. Nine of 10 week-8 LTC-ICs had intact templates. Six of these showed vector-specific signals. All 10 week-10 colonies analyzed had intact DNA, and 6 of these were transduced. (D) Amplification of vCWRHIVAPAP sequences of individual week-5 and -10 LTC-IC colonies from donor FK. Colony 4 had intact albeit very low amounts of DNA as evidenced by a faint β globin signal on a prolonged radioautographic exposure. (E) Amplification of methylcellulose samples from between colonies in transduced LTC-IC wells. Ten separate samples of methylcellulose were amplified with primers RPSP and DLASP using PCR conditions identical to above. NT, no template; C, CWRHIVAPAP control (10 copies). This film was exposed for 36 hours longer than exposures in (A) through (D)
Amplification of vCWRHIVAPAP sequences from individual LTC-IC colonies. CD34 cells were transduced with vCWRHIVAPAP at a functional MOI of 3 (particle MOI, 600) on day 0 and plated in long-term culture as described in Materials and Methods. Cells were harvested from stromal layers at designated time points, washed, and plated in methylcellulose with no G418 selection and colonies were plucked after 2 weeks. DNA was extracted and amplified for either the AAV vector using primers RPSP and DLASP or for β globin. β Globin served as a control for template integrity. The 418 HIVA band denotes the vector signal. The 268-bp Glo band denotes the β globin signal. Representative colonies from untransduced controls (Untd) are shown. Copy number controls are included in each analysis. For HIVA, the copy number corresponds to 12, 120, and 1,200 copies of the genome. For β globin, copy number controls show amplification from 80, 160, and 1,000 cells. (A) Week-5 LTC-ICs from donor TS. All colonies except one had intact DNA, albeit at varying quantities. The absence of a β globin signal from colony 9 indicated that the DNA template was inadequate. (B) Amplification of vCWRHIVAPAP sequences from individual week-8 LTC-IC colonies from donor TS. All colonies analyzed had intact DNA templates and 9 of 15 showed vector-specific signals. The standard curve is reversed in this experiment. (C) Amplification of vCWRHIVAPAP sequences of individual week-8 (transduced lanes 1 through 10) and week-10 (transduced lanes 11 through 20) LTC-IC colonies from donor HJ. Nine of 10 week-8 LTC-ICs had intact templates. Six of these showed vector-specific signals. All 10 week-10 colonies analyzed had intact DNA, and 6 of these were transduced. (D) Amplification of vCWRHIVAPAP sequences of individual week-5 and -10 LTC-IC colonies from donor FK. Colony 4 had intact albeit very low amounts of DNA as evidenced by a faint β globin signal on a prolonged radioautographic exposure. (E) Amplification of methylcellulose samples from between colonies in transduced LTC-IC wells. Ten separate samples of methylcellulose were amplified with primers RPSP and DLASP using PCR conditions identical to above. NT, no template; C, CWRHIVAPAP control (10 copies). This film was exposed for 36 hours longer than exposures in (A) through (D)
Table 1 summarizes the transduction efficiencies of individual colonies derived from week-5, -8, and -10 LTC-ICs from seven donors. Week-5 LTC-ICs were consistently observed in each donor analyzed. The presence of week-8 and -10 LTC-ICs representing more primitive progenitors varied from donor to donor. Transduction frequencies varied from 5% to 100% of colonies sampled, depending on the donor. Donors SM and BE showed the lowest level of transduction, ranging from 5% to 7% at 5 weeks to 25% (SM) at 10 weeks. No week-8 or -10 LTC-ICs were present in cells from donor BE. No overall significant decline in transduction frequencies was observed with increasing time in culture except for donor TS, suggesting that the level of AAV vector transduction observed at 5 weeks was stable. In some donors (HJ and SM), the frequency of transduced LTC-ICs was higher in week-8 and -10 LTC-ICs as compared with week-5 LTC-ICs, perhaps due to transduction of more primitive cells. These results suggest that both early (week-5) LTC-ICs and late (week-8 and -10) LTC-ICs can be transduced with AAV vectors in the absence of selective pressure.
vCWRHIVAPAP Sequences in Individual Colonies From LTC-IC Assays
| Donor . | Week-5 LTC-IC . | Week-8 LTC-IC . | Week-10 LTC-IC . |
|---|---|---|---|
| FF* | 42% (5/12) | — | — |
| BE* | 7% (1/15) | — | — |
| CL | 100% (10/10) | — | — |
| TS | 80% (16/20) | 60% (9/15) | — |
| FK | 69% (9/13) | — | 67% (8/12) |
| HJ* | 36% (4/11) | 67% (6/9) | 60% (6/10) |
| SM* | 5% (1/20) | 20% (4/20) | 25% (4/16) |
| Donor . | Week-5 LTC-IC . | Week-8 LTC-IC . | Week-10 LTC-IC . |
|---|---|---|---|
| FF* | 42% (5/12) | — | — |
| BE* | 7% (1/15) | — | — |
| CL | 100% (10/10) | — | — |
| TS | 80% (16/20) | 60% (9/15) | — |
| FK | 69% (9/13) | — | 67% (8/12) |
| HJ* | 36% (4/11) | 67% (6/9) | 60% (6/10) |
| SM* | 5% (1/20) | 20% (4/20) | 25% (4/16) |
Cells were transduced on day 0 at functional MOI of 3 (particle MOI, 600) as described in Materials and Methods. Cells harvested from stromal cultures initiated with marrow-derived CD34 cells were assayed for LTC-ICs at 5, 8, and 10 weeks. Individual colonies were plucked, DNA-isolated, and amplified with primers specific for vector and β globin. The total number of vector positive colonies of all beta globin positive colonies is shown.
Abbreviation: —, no LTC-ICs were detected at those time points from those donors.
Donors with concurrent FISH analyses.
Integration of AAV vector sequences in marrow CD34 cells in long-term cultures.
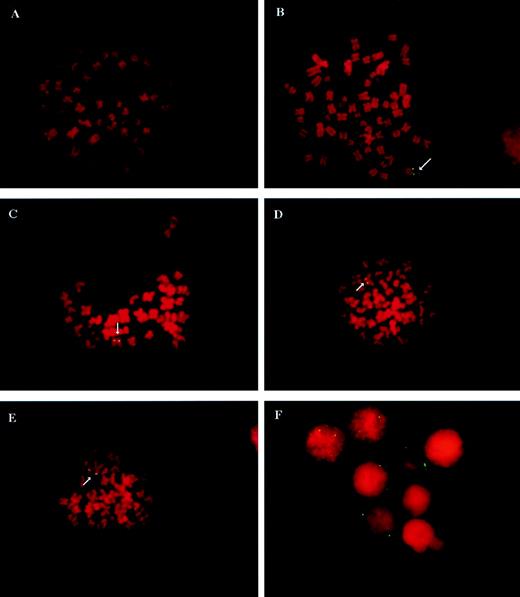
Because AAV vectors can exist intracellularly in either double-stranded episomal or chromosomally integrated forms, we analyzed the frequency of vector integration into chromosomal DNA in CD34 cultures in LTBMCs by FISH analyses. CD34 cells were transduced with vCWRHIVAPAP16 at a functional MOI of 3 (particle MOI, ∼600) immediately after isolation and culture isolation. After boosting the mitotic index with higher concentrations of cytokines, cells were blocked in metaphase and harvested for FISH analyses. Metaphase spreads of transduced and untransduced cells were hybridized with a vector-specific probe representing the RSV LTR, antisense sequences to the HIV LTR, and the polyadenylation region but not including the alkaline phosphatase sequences. Figure 3 shows FISH analyses of integrated vCWRHIVAPAP sequences in transduced cells. Vector-specific signals were never detected in untransduced controls (Fig 3A) from any sample, indicating that there was no nonspecific hybridization of the probe to untransduced cells. The clonal cell line HIIC21, transduced with vCWRHIVASVN and containing one copy of the vector genome per cell, served as the positive control (Fig 3B). Figure 3C, D, and E show representative metaphase spreads from CD34 cells 5 weeks after transduction. FISH signals were not digitally amplified and metaphases were scored as positive only if vector-specific signals were clearly visualized on both sister chromatids. The presence of vector-specific signals on each sister chromatid indicated that the integrated vector sequences had replicated with cellular chromosomal DNA, suggesting stable transmission of the transgene to progeny cells. CD34 cells from 10 donors were analyzed 2 to 9 weeks after transduction and culture initiation. Six percent to 29% of metaphases from different donors were found to have integrated vector signals on both chromatids. By the later time points in culture, the nonadherent progeny of transduced CD34 cells analyzed for FISH were found to differentiate primarily into myeloid cells under these culture conditions as determined by immunophenotyping and flow cytometric analysis. However, because at the time of transductions the population was highly enriched for CD34 cells, vector-positive cells most likely represent the progeny of transduced CD34 cells.
FISH analysis of vCWRHIVAPAP transduced and untransduced cells. CD34 were transduced with vCWRHIVAPAP at a functional MOI of 3 (particle MOI, 600) and placed in culture as described above. Suspension cells were harvested at designated time points for analysis. (A) A metaphase spread from an untransduced CD34 culture showing no hybridization of the vector-specific probe to cellular sequences. (B) Hybridization analysis of HIIC21, a G418-resistant clonal 293-based cell line derived after transduction with vCWRHIVASVN. This clone carries 1 copy of the vector genome per cell (28) and served as a positive control. (C, D, and E) Representative metaphases from vCWRHIVAPAP transduced CD34 cultures at 5 weeks posttransduction. Note that vector-specific signals are observed on both sister chromatids. Signals were not digitally enhanced in this analysis. (F) Representative interphase nuclei from a vCWRHIVAPAP transduced CD34 culture 3 weeks posttransduction. Three nuclei with three or more signals, two nuclei with two signals each, and three nuclei with no signals are seen.
FISH analysis of vCWRHIVAPAP transduced and untransduced cells. CD34 were transduced with vCWRHIVAPAP at a functional MOI of 3 (particle MOI, 600) and placed in culture as described above. Suspension cells were harvested at designated time points for analysis. (A) A metaphase spread from an untransduced CD34 culture showing no hybridization of the vector-specific probe to cellular sequences. (B) Hybridization analysis of HIIC21, a G418-resistant clonal 293-based cell line derived after transduction with vCWRHIVASVN. This clone carries 1 copy of the vector genome per cell (28) and served as a positive control. (C, D, and E) Representative metaphases from vCWRHIVAPAP transduced CD34 cultures at 5 weeks posttransduction. Note that vector-specific signals are observed on both sister chromatids. Signals were not digitally enhanced in this analysis. (F) Representative interphase nuclei from a vCWRHIVAPAP transduced CD34 culture 3 weeks posttransduction. Three nuclei with three or more signals, two nuclei with two signals each, and three nuclei with no signals are seen.
To determine the stability of vector integration, cells from three donors (FF, BE, and MM) were evaluated at early and late time points after transduction (Table 2). Twenty-seven percent and 19% of metaphases from FF were vector-positive at 3 and 5 weeks posttransduction, respectively. Twenty percent and 22% of metaphases from donor BE hybridized with the vector-specific probe at 2 and 4 weeks, respectively. Cells from donor MM showed 27% and 20% vector-positive metaphases at 2 and 8.5 weeks, respectively. These results suggest that the frequency of vector integration was largely stable in CD34 cells and that transduced cells containing integrated AAV vector genomes did not have any growth disadvantage in tissue culture.
FISH Analysis of vCWRHIVAPAP-Transduced Marrow CD34 Cells in Long-Term Culture
| (A) Bone Marrow CD34 Cells . | ||||
|---|---|---|---|---|
| Donor . | Weeks Posttransduction . | Positive Metaphases . | Positive Interphases . | PLAP+ . |
| JD | 3.5 | 18% (8/44) | 6% (11/188) | — |
| KZ | 6 | 9% (8/85) | 11% (11/100) | 5% |
| SM* | 6.5 | 20% (41/208) | 12% (68/558) | — |
| GF | 8 | 22% (8/37) | — | — |
| GA | 8 | 25% (8/32) | — | — |
| HJ*,† | 9 | 20% (1/5) | 8% (1/13) | — |
| LS | 9 | 22% (14/65) | 20% (20/99) | 11% |
| FF | 3 | 27% (26/97) | 29% (42/145) | — |
| FF* | 5 | 19% (12/63) | 13% (39/296) | — |
| BE | 2 | 20% (5/25) | — | — |
| BE* | 4 | 22% (5/23) | 8% (8/105) | — |
| MM | 2 | 27% (21/77) | 24% (30/123) | 27% |
| MM | 8.5 | 20% (12/61) | 17% (18/104) | 8% |
| (B) Bone Marrow CD34+38− Cells | ||||
| Donor | Weeks Posttransduction | Positive Metaphases | Positive Interphases | PLAP+ |
| FC | 4 | 28% (11/40) | 24% (11/45) | 43% |
| KL | 4 | 15% (7/46) | 22% (13/58) | 15% |
| KL | 8 | 11% (8/73) | 12% (12/101) | 10% |
| NT | 4 | 14% (5/37) | 17% (8/46) | 14% |
| NT | 8 | 11% (8/72) | 12% (7/57) | 10% |
| (A) Bone Marrow CD34 Cells . | ||||
|---|---|---|---|---|
| Donor . | Weeks Posttransduction . | Positive Metaphases . | Positive Interphases . | PLAP+ . |
| JD | 3.5 | 18% (8/44) | 6% (11/188) | — |
| KZ | 6 | 9% (8/85) | 11% (11/100) | 5% |
| SM* | 6.5 | 20% (41/208) | 12% (68/558) | — |
| GF | 8 | 22% (8/37) | — | — |
| GA | 8 | 25% (8/32) | — | — |
| HJ*,† | 9 | 20% (1/5) | 8% (1/13) | — |
| LS | 9 | 22% (14/65) | 20% (20/99) | 11% |
| FF | 3 | 27% (26/97) | 29% (42/145) | — |
| FF* | 5 | 19% (12/63) | 13% (39/296) | — |
| BE | 2 | 20% (5/25) | — | — |
| BE* | 4 | 22% (5/23) | 8% (8/105) | — |
| MM | 2 | 27% (21/77) | 24% (30/123) | 27% |
| MM | 8.5 | 20% (12/61) | 17% (18/104) | 8% |
| (B) Bone Marrow CD34+38− Cells | ||||
| Donor | Weeks Posttransduction | Positive Metaphases | Positive Interphases | PLAP+ |
| FC | 4 | 28% (11/40) | 24% (11/45) | 43% |
| KL | 4 | 15% (7/46) | 22% (13/58) | 15% |
| KL | 8 | 11% (8/73) | 12% (12/101) | 10% |
| NT | 4 | 14% (5/37) | 17% (8/46) | 14% |
| NT | 8 | 11% (8/72) | 12% (7/57) | 10% |
(A) CD34 cells were transduced with vCWRHIVAPAP at MOI of 3 (particle MOI, 600) immediately after isolation and placed in culture. At the indicated times suspension cells were harvested and placed in media containing increased concentrations of IL-3, IL-6, and stem cell factor to boost the mitotic index before blocking with colcemid as described in Materials and Methods. Metaphases were identified and scored for the presence of vector-specific signal. Only metaphases showing evidence of vector signal on both sister chromatids were scored as positive. Untransduced controls never hybridized with the probe in every CD34 sample analyzed. PLAP expression from the RSV promoter in vCWRHIVAPAP was analyzed concurrently with FISH where indicated. (B) Flow-sorted CD34+38− cells were transduced with vCWRHIVAPAP at functional MOIs of 1 to 3. Cells were harvested from cultures at the indicated time points and assayed by FISH using a vector-specific probe as described above. Cells donors KL and NT were transduced in parallel.
Abbreviation: —, not done.
LTC-ICs were also analyzed from these donors (Table 1).
In this sample, most CD34 cells had differentiated into adherent macrophages by 9 weeks and few suspension cells were available for analyses.
To evaluate whether cells with episomal copies of vector genomes could be detected in long-term culture, interphase nuclei were scored for vector-specific signals per nucleus and compared with the frequency of metaphase signals from the same time points. Because both episomal and integrated genomes should hybridize with the vector-specific probe in interphase nuclei, we reasoned that the presence of episomal copies would result in the detection of greater than two signals in interphases as compared with metaphases. Figure 3F shows interphase nuclei with several nuclei containing three or more signals and two nuclei with two signals. To date, we have never observed more than a single integration event per metaphase spread in CD34 cells, perhaps due to the relatively low MOI used in these studies. Therefore, we made the assumption that interphase nuclei with three or more signals likely contain episomal genomes either exclusively or in combination with integrated signals. Most CD34 samples analyzed did not show a higher frequency of vector-positive interphase nuclei compared with metaphase spreads (Table 2), suggesting that most signals observed approximately 4 weeks after transduction likely represented integrated signals. However, due to the possibility of differential probe entry into permeabilized interphase nuclei as compared with metaphase spreads, a precise estimation of the frequency of episomal forms is difficult. A comparison of early and late time points after transduction showed some decrease in positive nuclei at the later time points. Whether this was due to the loss of episomal copies or transduced cells is not clear.
We performed two-color hybridization to determine if the rep-negative, wild-type–free vector stocks used in our experiments integrated at sites distinct from AAVS1, the integration site for wild-type AAV. Colocalization of the AAVS1 probe and the vector probe was never observed in any transduced cell (0/100 nuclei) indicating that, in the absence of either rep78 or wild-type AAV, vCWRHIVAPAP did not integrate into AAVS1.
Integration of AAV vector sequences in marrow-derived CD34+38− cells.
To determine if AAV vector genomes could integrate into the more primitive hematopoietic fraction of CD34 cells, we analyzed cultures initiated with marrow CD34+38− cells by FISH. Flow-sorted CD34+38− cells were transduced with vCWRHIVAPAP at a functional MOI of 3 (particle MOI, ∼600) immediately after isolation and placed in culture on irradiated heterologous stroma. Cells from all three donors were harvested at 4 weeks after transduction and culture initiation. Integration levels ranging from 11% to 28% were observed, indicating that this population was also transducible with AAV vectors (Table 2B). Cells from two donors (KL and NT) were also analyzed at 8 weeks posttransduction. The frequency of positive metaphases was comparable at both time points for a given donor, again indicating that the fraction of cells with integrated vector was stable over time. However, for both donors, higher levels of positive interphases were observed at 4 weeks than at 8 weeks, suggesting the presence of episomal copies of the vector at the earlier time point. Interestingly, by 8 weeks, the frequency of positive interphases was comparable with that of metaphases, possibly due to the loss of the episomal forms by this time point and persistence of the integrated forms. These results are consistent with the longer survival of episomal copies of rAAV in the more primitive CD34+38− cells.
Expression of AAV vector-encoded transgenes in CD34 cells.
Because AAV vectors appeared capable of transducing and integrating into primitive CD34+ and CD34+38− hematopoietic progenitor cells, we evaluated expression of vector-encoded transgenes in long-term CD34 cultures transduced with vCWRHIVAPAP at a functional MOI of 3 (particle MOI, 600) under conditions similar to that for FISH analyses. Table 2 shows PLAP expression in four CD34 cultures and three CD34+38− cultures. In most cases, the level of PLAP expression approximated the level of integration seen by FISH, suggesting that most cells containing vector genomes also expressed the transgene. In some cases, notably MM, a decrease in PLAP expression was observed at the 8-week time point as compared with the earlier (week-2 or -4) samples from corresponding donors. This could either be due to expression from double-stranded episomal copies at the early time points that were lost with cell division and/or silencing of transgene expression later in culture.
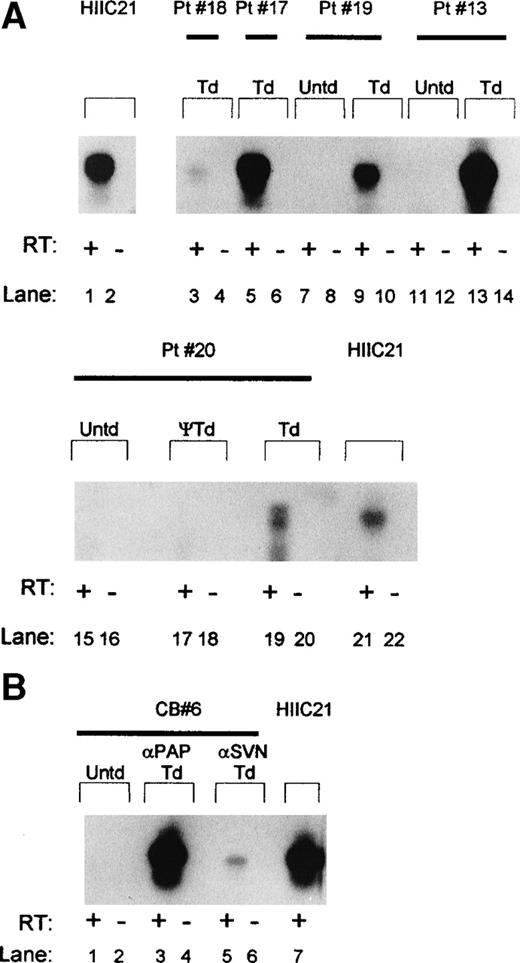
In addition to PLAP, we also analyzed transcription of antisense RNA complementary to the HIV-1 LTR in cells harvested from LTBMCs and LTC-ICs. Transcription of the antisense RNA is under the control of the RSV LTR and represents the clinically relevant transgene present in both vCWRHIVAPAP and vCWRHIVASVN. Cells were transduced at functional MOI of 1 to 2 (particle MOI, ∼500 to 1,000) with vCWRHIVASVN. Figure 4A shows cDNA amplification using primers 1a and 1b (Fig 1) after reverse transcription of RNA extracted from transduced cultures 5 weeks after culture initiation and transduction. A 530-bp product representing the HIV-1 LTR specific antisense RNA transcribed from the vector was detected in transduced cells from each donor tested but not from untransduced cells. The presence of the amplified band only in samples subjected to reverse transcription before amplification confirmed that the product was of RNA origin. All RT-PCR reactions were performed under conditions determined to be in the linear range of the reaction. The levels of HIV LTR antisense transcript detected in cultures from donors 13, 17, and 20 (lanes 5 through 14) were comparable to that observed in the clonal cell line, HIIC21, which contains one copy of vCWRHIVASVN per cell.28 However, donor 18 (lane 3) showed lower levels of transcription, suggesting variability in either transduction efficiency or transgene expression in this donor. To control for artefactual sources of signal, cells from patient no. 20 were exposed to a control sham stock prepared in an identical fashion to vector except for the exclusion of AAV rep and cap genes, thus preventing the generation of encapsidated transducing virus particles. As seen in Fig 4A, lanes 17 and 18, no vector-specific signals were detected in RNA from cells exposed to this control stock, demonstrating that antisense RNA transcription in long-term cultures directly resulted from AAV vector transduction. Overall, these results demonstrated that AAV vector transduction of long-term cultures resulted in readily detectable expression of antisense RNA in the absence of selective pressure.
Southern analysis of HIV antisense transcription from vCWRHIVASVN in transduced marrow and cord blood-derived hematopoietic progenitor cells after RT-PCR amplification. (A) RNA was extracted from week-5 to -7 LTBMCs and antisense sequences were reverse transcribed and amplified using primers 1a and 1b. The amplified products were resolved on a 1.2% agarose gel, transferred to nitrocellulose, and hybridized with an RSV LTR and antisense-specific probe. HIIC21, containing 1 copy of integrated vector per cell, served as the positive control. The 530-bp antisense transcript-specific product is shown. +RT and −RT refer to the presence or absence, respectively, of reverse transcription before amplification. The absence of signals in these lanes indicates that the antisense signals were RNA-specific. ψ, RT-PCR analysis of cells (donor 20) exposed to a sham stock of vector prepared in the absence of AAV rep and cap genes. Untransduced (Untd) cells from each donor tested served as negative controls. Td, transduced cultures. (B) HIV LTR antisense transcription in LTC-ICs initiated with CD34+CD38− cord blood cells 8 weeks after transduction. A representative sample, CB6, was transduced with vCWRHIVAPAP at MOI of 3 (particle MOI, 600) and vCWRHIVASVN at MOI of 0.1 (particle MOI, 50). Cells were harvested from 6 week LTBMCs, washed, and placed in colony-forming assays. RNA was extracted from colonies after 3 weeks, reverse transcribed, and amplified with primers 1a and 1b. The 530-bp antisense product was evident only after reverse transcription.
Southern analysis of HIV antisense transcription from vCWRHIVASVN in transduced marrow and cord blood-derived hematopoietic progenitor cells after RT-PCR amplification. (A) RNA was extracted from week-5 to -7 LTBMCs and antisense sequences were reverse transcribed and amplified using primers 1a and 1b. The amplified products were resolved on a 1.2% agarose gel, transferred to nitrocellulose, and hybridized with an RSV LTR and antisense-specific probe. HIIC21, containing 1 copy of integrated vector per cell, served as the positive control. The 530-bp antisense transcript-specific product is shown. +RT and −RT refer to the presence or absence, respectively, of reverse transcription before amplification. The absence of signals in these lanes indicates that the antisense signals were RNA-specific. ψ, RT-PCR analysis of cells (donor 20) exposed to a sham stock of vector prepared in the absence of AAV rep and cap genes. Untransduced (Untd) cells from each donor tested served as negative controls. Td, transduced cultures. (B) HIV LTR antisense transcription in LTC-ICs initiated with CD34+CD38− cord blood cells 8 weeks after transduction. A representative sample, CB6, was transduced with vCWRHIVAPAP at MOI of 3 (particle MOI, 600) and vCWRHIVASVN at MOI of 0.1 (particle MOI, 50). Cells were harvested from 6 week LTBMCs, washed, and placed in colony-forming assays. RNA was extracted from colonies after 3 weeks, reverse transcribed, and amplified with primers 1a and 1b. The 530-bp antisense product was evident only after reverse transcription.
LTC-IC colonies derived from CD34+38−cells from umbilical cord blood were also tested for AAV vector transduction. Cells were transduced at the time of culture initiation with either vCWRHIVASVN at MOI of 0.1 (particle MOI, 50) or vCWRHIVAPAP at MOI of 3 (particle MOI, 600). As with marrow cells, cord blood cultures were passaged approximately every 10 days. Six weeks after culture initiation, cells were harvested from stroma, washed, and assayed for LTC-ICs. Week 6 LTC-IC colonies were harvested, polyadenylated RNA was extracted from transduced and untransduced cells, and RT-PCR of the HIV LTR antisense RNA was performed as described above. Figure 4B shows the amplified antisense transcript from a representative cord blood sample, CB6. Cultures transduced at a functional MOI of 3 (particle MOI, ∼600) with vCWRHIVAPAP showed levels of antisense transcription comparable to that of the single copy clonal cell line, HIIC21,16 whereas cultures transduced at MOI of 0.1 (particle MOI, ∼50) with vCWRHIVASVN showed lower levels of transcript, consistent with the lower multiplicity of transduction. These results demonstrated that primitive clonogenic CD34+38− cord blood cells transduced with AAV vectors demonstrated transgene expression at 8 weeks posttransduction in the absence of G418 selection. These findings are consistent with both the analyses of transduction of LTC-ICs and FISH analyses of CD34 cells.
DISCUSSION
In this study we evaluated AAV vectors for their capacity to transduce genes into primitive human hematopoietic progenitor cells with replating and differentiative capacity in long-term culture. CD34+ and CD34+38− cells from marrow and cord blood were evaluated for AAV vector transduction by single-colony analysis, FISH, and transgene expression from 22 CD34 samples. rAAV transduction levels varied from donor to donor. Despite variability in transduction efficiencies, every sample analyzed showed evidence of transduction. AAV vector transduction had no effect on colony formation, cobblestone area formation, or cell numbers in long-term cultures.
Amplification of vector sequences from single colonies derived from early and extended LTC-ICs indicated that primitive myelo-erythroid progenitors could be transduced with AAV vectors. The transduction frequency of extended (week-8 and -10) LTC-ICs from most donors was observed to be equal to or higher than week-5 LTC-ICs, suggesting that primitive progenitors also serve as good targets for AAV transduction. Comparable transduction levels in LTC-IC assays initiated at early and late time points after culture initiation suggested that (1) cells with extended clonogenic capacity were transducible with AAV vectors and (2) transduction levels attained by week 5 were largely stable. These results suggest that primitive myelo-erythroid progenitor cells capable of long-term survival in vitro and possessing delayed replating and differentiative capacity are amenable to genetic modification by AAV vectors.
The genomes of both wild-type AAV and AAV vectors are known to exist in either chromosomally integrated or double-stranded episomal forms. Either of these double-stranded forms of the vector genome can direct transgene transcription and serve as templates for DNA amplification. Because DNA amplification from LTC-IC colonies analysis does not distinguish between episomal and integrated states of the vector, we analyzed the fate of vector genomes by FISH after long-term transduction. Metaphase FISH analyses provided an estimate of integration frequencies, because vector signals were exclusively associated with chromosomes. A vector integration frequency of 9% to 28% was observed in marrow CD34+ and CD34+38− cells over the 2- to 9-week period of study in the absence of selective pressure. Similar results were obtained with CD34+ and CD34+38− cells from umbilical cord blood (data not shown). Importantly, the fraction of cells containing integrated vector remained comparable throughout the culture period for a given donor, suggesting that AAV vector integration was stable. Interphase nuclei contained both episomal and integrated vector genomes. Therefore, comparisons of the frequency of vector-positive interphase and metaphase nuclei by FISH allowed a relative approximation of the proportion of nuclei containing episomal vector. At early time points after transduction, a fraction of CD34+38− cells appeared to contain episomal vector, perhaps in addition to cells containing integrated vector. However, the episomal forms appeared to be lost over time in culture, whereas the proportion of cells containing integrated vector remained stable and contributed to long-term transduction. By 8 weeks posttransduction, only the stably integrated population was evident. Interestingly, episomal forms appeared to persist longer in the more primitive and possibly more slowly cycling CD34+38− cells than in CD34+cells. This could be due to the longer persistence of episomal forms of AAV vectors in slowly dividing cells. Thus, there appears to be two populations of cells transduced with rAAV vectors. Stable chromosomal integration is observed in one population, whereas the episomal form of the vector persists in the other and is eventually lost over time.
The factors that determine AAV vector genome integration are not fully defined. The virus-encoded rep68/78 protein has been implicated in site-specific integration of wild-type AAV into the AAVS1 locus on the human chromosome 19. The AAV rep protein recognizes and binds to a consensus sequence present on both the viral ITRs as well as in chromosome 1942-44 and has ATPase, endonuclease, and DNA helicase activities45 that are likely instrumental in site-specific integration of wild-type AAV and rAAV vectors. The complete absence of rAAV integration into AAVS1 observed in our studies is in accordance with previous reports46,47 and provided independent evidence that the vectors used here were free of wild-type AAV. Integration of AAV vectors into chromosomal DNA has been previously demonstrated by us and others. However, wild-type–free rAAV has consistently been found to integrate at sites other than AAVS1,28 46-48 and viral proteins have not been implicated in rAAV integration. It is likely that cellular enzymes, including those involved in DNA repair and replication, play a role in this event. The differences between cell populations exhibiting stable integration of AAV vectors and those containing transient episomal genomes may be useful in further defining factors important for AAV vector integration.
Evaluation of transduction of CD34 cells by both single-colony PCR and FISH analyses showed comparable gene transfer frequencies at similar time points for some donors; however, somewhat higher frequencies were observed by PCR in others. This was attributed to (1) the higher levels of sensitivity of PCR amplification as compared with FISH, especially without digital amplification of hybridization signals; (2) the possible persistence of episomal forms of the vector in some colonies; and (3) differences in the target cell populations being assayed in the two systems. In LTC-IC assays, clonogenic cells capable of giving rise to myelo-erythroid colonies were evaluated, whereas in FISH, all cells capable of undergoing mitosis from 2 to 9 weeks after culture initiation were analyzed.
Each donor evaluated in this study showed evidence of some level of AAV transduction. However, cells from some donors showed significantly lower levels of transduction than others. These results are in concordance with those of Ponnazhagan et al,49who reported donor to donor variability of CD34 transduction by AAV vectors. In keeping with these results, we also observed that LTBMCs processed in parallel and transduced with the same batch of vector showed variability in transduction frequencies. Whether this reflects genetic variability in putative cell surface receptors for AAV, receptor density, second-strand synthesis, or differences in the physiologic status and therefore the intracellular milieu of cells from different donors at the time of transduction is currently unknown.
Antisense RNA to the HIV LTR encoded by AAV vectors used in this study has previously been shown to potently inhibit HIV-1 replication.5 Transcriptional analysis of transduced CD34+, CD34+38−, and LTC-IC colonies showed that cells from each donor tested transcribed antisense RNA to the HIV LTR, with the transcript levels from several donors being comparable to that of a single-copy clone. Importantly, no selective pressure was required to observe high levels of transgene transcription in either marrow or cord blood progenitor cells. The level of antisense RNA transcription observed was proportional to the functional multiplicity of transduction. These results also suggest that the RSV LTR remain transcriptionally active in a significant proportion of hematopoietic progenitor cells over the 5- to 9-week culture period analyzed in this study. Whether these levels of antisense RNA transcription confers resistance to challenge with HIV in the monocyte-macrophages differentiating from CD34 cells in culture is currently being determined.
These results extend our previous studies showing AAV vectors transduction and integration into CD34+ marrow and cord blood-derived lineage-committed progenitor cells to primitive progenitors in long-term culture. The cells evaluated here were functionally more primitive than CD34 cells previously studied in CFU-C assays.28 However, it must be noted that the culture conditions used in this study and the cells assayed here represent myelo-erythroid progenitors and do not address gene transfer into lymphoid progenitor cells. LTC-ICs also most likely do not represent true HSCs capable of long-term engraftment in vivo.9Efficient transduction of LTC-ICs by retroviral vectors, despite inefficient long-term in vivo engraftment with transduced cells as evidenced by several human gene therapy trials, suggests that other studies with in vivo models of hematopoiesis are necessary to determine stem cell transduction. Nevertheless, this is the first systematic report of AAV-mediated gene transfer into primitive clonogenic cells in long-term culture with analysis of gene transfer at the single-colony level and integration at the chromosomal level.
Despite several recent reports of AAV-mediated gene transfer into CD34+ human and murine hematopoietic progenitor cells in vitro and in vivo,28-34,50 some groups have reported difficulty in transducing CD34 cells with AAV vectors. There may be several possible explanations for these differences. (1) The source of CD34 cells may be critical in determining transduction frequencies. Preliminary observations from our group suggest that marrow CD34 cells may have different rAAV transduction frequencies than cytokine-mobilized peripheral blood stem cells (Wong et al, unpublished data). We have previously observed that rapidly dividing cells show poor transduction with AAV vectors possibly due to a quicker loss of episomal vector genomes before integration. (2) Cytokine concentrations used in culture media may also contribute to altered cell cycle time and therefore AAV transduction frequencies. We have found that the use of lower cytokine concentrations correlates with higher levels of AAV transduction of CD34 cells (Fisher-Adams et al, data not shown). (3) Attachment and entry of AAV virions to cells is likely mediated by one or more cell surface receptors.51Heparan sulfate proteoglycan has recently been identified as one AAV binding molecule that may facilitate binding and internalization of virions.52 Thus, the choice of collection media and culture conditions may influence AAV transduction of cells by either facilitating binding or blocking attachment sites. (4) Genetic factors may influence receptor polymorphism and cell surface density affecting virus entry. Genetic polymorphism or the intracellular milieu may also influence postentry processes53 such as second-strand synthesis and genome integration that would ultimately affect the outcome of AAV transduction. (5) Lastly, differences in vector backbones and/or production and purification methods used by different groups may lead to different outcomes. Clarification of these issues is essential for better insight into the potentials of AAV vectors and must await further delineation of AAV vector biology and elucidation of cellular processes associated with AAV transduction.
Recently, numerous studies have been published showing AAV transduction of primary nondividing cells. AAV vector transduction of postmitotic neurons in vivo,54 cochlear, retinal and glial cells of the human central nervous system, nonproliferating respiratory epithelial cells, alveolar stem cells, adult skeletal cells,26,27 and cardiac muscle25 have been documented. Thus, it has become abundantly clear that AAV vectors are capable of transducing nondividing cells. AAV transduction of skeletal muscle and brain accompanied with prolonged gene expression has been demonstrated in vivo28,29 with little or no evidence of AAV-specific T-cell responses. Early reports from a human gene therapy trial testing AAV vectors for cystic fibrosis indicate the lack of any vector-associated toxicity or immune rejection.55 These results, together with our findings of long-term transduction, vector integration, and transgene expression in primitive myeloid progenitor cells, suggest that AAV vectors merit further evaluation as gene transfer vehicles for quiescent HSCs. Further studies with lymphomyeloid progenitors and in vivo models of human hematopoiesis should provide more information about AAV transduction of HSCs. However, ultimately, in vivo human trials testing long-term, multilineage engraftment of AAV-transduced cells will be necessary to evaluate true stem cell transduction by AAV vectors.
ACKNOWLEDGMENT
The authors are indebted to the members of the COH Hematology/BMT unit for providing bone marrow and to the physicians and nurses of the Labor and Delivery Unit and the Department of Obstetrics and Gynecology at Huntington Memorial Hospital for collecting umbilical cord blood samples. We thank Drs Navtej Juty, Robert Krouse, Elizabeth Shaughnessy, Li Jing Li, Edna Rosborough, Deepinder Brar, James Bolen, Marilyn Slovak, and Christine Wright for their assistance and comments. The following COH Cancer Center Core facilities were used in this study: Flow Cytometry, Cytogenetics, Phosphorimaging, and DNA synthesis and sequencing.
Supported in part by Grants No. AI-R0140001 and AI-U1938592 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), and by Grants No. CA-R0171947, CA-P0159308, CA-P0130206, and CA33572 from the National Cancer Institute, NIH.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Saswati Chatterjee, PhD, Division of Pediatrics, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: schatterjee@coh.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal