Proteins containing amino acid repeats are widespread among protozoan parasites. It has been suggested that these repetitive structures act as immunomodulators, but other functional aspects may be of primary importance. We have recently suggested that tandem repeats present in Trypanosoma cruzi trans-sialidase stabilize the catalytic activity in blood. Because the parasite releasestrans-sialidase, this delayed clearance of the enzyme might have implications in vivo. In the present work, the ability of repetitive units from different T. cruzi molecules in stabilizing trans-sialidase activity in blood was evaluated. It is shown that repeats present on T. cruzi shed proteins (antigens 13 and Shed-Acute-Phase-Antigen [SAPA]) increase trans-sialidase half-life in blood from 7 to almost 35 hours. Conversely, those repeats present in intracellular T. cruzi proteins only increase the enzyme half-life in blood up to 15 hours. Despite these results, comparative analysis of structural and catalytic properties of both groups of chimeric enzymes show no substantial differences. Interestingly, antigens 13 and SAPA also increase the persistence in blood of chimeric glutathione S-transferases, thus suggesting that this effect is inherent to these repeats and independent of the carrier protein. Although the molecular basis of this phenomenon is still uncertain, its biotechnological potential can be envisaged.
PROTEINS CONTAINING tandems of amino acid repeats are particularly widespread among several parasitic taxa like the protozoan agents of malaria (Plasmodium spp.), leishmaniasis (Leishmania spp.), and trypanosomiasis(Trypanosoma cruzi and T. brucei).1-5 The meaning of such a particular protein array in these parasites is still a matter of debate. It has been suggested that they are involved in binding to repetitive structures within the parasite,5,6 binding to host-cell receptors and/or polymerization of their associated, nonrepeated domains.7
One relevant feature concerning these repetitive motifs is that they are readily detected by antibodies present in the sera from infected patients, thus suggesting that they are major targets of the immune response.1,3,8 Nevertheless, these humoral responses have not usually been correlated with protection. These observations have led us to speculate that repeated amino acid arrays would act as immunomodulators in protozoan antigens.1,8,9 The precise mechanism of this immunomodulation is not known, but it has been shown, for instance, that repetitive and/or highly organized epitopes could influence both antigen presentation and responsiveness of immune cells.10-13
The trans-sialidase from T. cruzi is a developmentally regulated protein that has been implicated in host-parasite interactions (for review, see reference 14). The enzyme, located on the trypanosome’s surface, is responsible for transferring sialyl residues from host’s glycoconjugates to parasite molecules.15 Once sialylated, these molecules are thought to mediate binding to, and invasion of, mammalian cells.16,17 Thetrans-sialidase displayed by the epimastigote (the parasite form present in the reduviid vector) has a potentialtrans-membrane domain and is not released, even after addition of exogenous phospholipase.18 On the contrary, the enzyme present in the trypomastigote (the infective form of the parasite that circulates in the blood of the vertebrate host) is anchored by a glycosylphosphatidylinositol (GPI) linkage to the T. cruzisurface and is released into the environment.19 Even though the effect of circulating trans-sialidase on T. cruzi infectivity and/or pathogeny is far from obvious, this enzymatic activity has been detected in patient’s blood during acute human infections.20
When compared with the epimastigote trans-sialidase, the trypomastigote enzyme contains an additional C-terminal extension made up essentially of repetitive amino acid sequences termed SAPA (for Shed-Acute-Phase-Antigen). SAPA–domain is not involved in the catalytic activity of trans-sialidase, as shown by using recombinant21 and papain-digested enzymes.7 In a previous work, we suggested that SAPA-domain stabilize thetrans-sialidase activity in blood.22 To further test this hypothesis and to determine the sequences involved in this stabilization, chimeras containing the trans-sialidase coding sequence linked to several T. cruzi tandem repeats have been generated. Some of these tandem repeats are present in intracellularly-located proteins (antigens 1 and 36) whereas others are in GPI-surface anchored proteins (antigen 13).23 It is shown here that the addition of repetitive units present in T. cruzi shed proteins (antigens 13 and SAPA) significantly increase the persistence of the chimeric trans-sialidases in circulation when compared with the enzymes linked to repetitive units present in intracellularly located T. cruzi proteins (antigens 1 and 36). Interestingly, persistence in blood of the gluthathione S-transferase (GST) from Schistosoma japonicum is also increased when linked to antigen 13 or SAPA, thus suggesting that this stabilization in blood is a general property of certain T. cruzi shed amino acid repeats.
MATERIALS AND METHODS
Cloning of chimeric trans-sialidases.
To express the repetitive domain of different T. cruziantigens3 in frame with a functionaltrans-sialidase gene, a so-called “acceptor clone” has been constructed by a polymerase chain reaction (PCR) strategy. This acceptor clone (termed as TSac clone, Table1) is essentially alike TS clone22 but contains a uniqueEcoRI site in its 3′ terminus in the frame of λgt11 phage (Pharmacia Biotech, Uppsala, Sweden) EcoRI cloning site. Inserts recovered from EcoRI-digested λgt11 clones containing repetitive T. cruzi antigens 1, 13, and 363 were ligated with the TSac clone previously digested with EcoRI. Chimeric trans-sialidases were further analyzed by DNA sequencing by the dideoxynucleotide chain-termination method,24 using the Sequenase 2.0 kit (U.S. Biochemical Corp, Cleveland OH). Molecular properties of chimerictrans-sialidases were analyzed with the aid of the LaserGene software (DNASTAR Inc, Madison WI).
Purification of chimeric trans-sialidases.
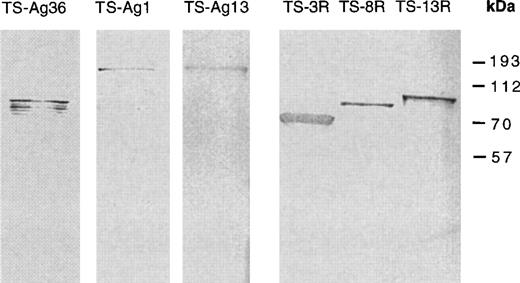
Chimeric trans-sialidases expressed in Escherichia colistrain Novablue (Novagen, Madison WI) were purified using the stretch of histidines present on the N-terminus fusion peptide (Table 1) provided by pTrcHis vector, according to manufacturer’s instructions (Invitrogen, San Diego, CA). Accurate expression of T. cruzirepetitive sequences in frame with trans-sialidase was verified in each case by Western blot analysis using a polyclonal antiserum raised in mice against each repetitive domain used (Fig 1). None of these polyclonal antisera recognize the catalytic domain of trans-sialidase (data not shown).
Identification of chimeric trans-sialidases. Detection of chimeric trans-sialidases in Western blot was performed with antisera raised in mice against each repetitive T. cruzi antigen expressed in pGEX vector. The monoclonal antibody against SAPA-repeats used to probe TS-3R, TS-8R, and TS-13R proteins has been already described.25 The presence of additional immunoreactive bands in TS-Ag 36 protein is due to partial protein degradation. The increase in the apparent molecular weight of TS-Ag 13 protein is attributed to abnormal migration in SDS-PAGE. Molecular weight markers are indicated in kD.
Identification of chimeric trans-sialidases. Detection of chimeric trans-sialidases in Western blot was performed with antisera raised in mice against each repetitive T. cruzi antigen expressed in pGEX vector. The monoclonal antibody against SAPA-repeats used to probe TS-3R, TS-8R, and TS-13R proteins has been already described.25 The presence of additional immunoreactive bands in TS-Ag 36 protein is due to partial protein degradation. The increase in the apparent molecular weight of TS-Ag 13 protein is attributed to abnormal migration in SDS-PAGE. Molecular weight markers are indicated in kD.
Cloning and purification of TS-SAPA proteins containing variable number of tandem repeats.
PCR was performed with the following oligonucleotides: 5′-GGGGCAGAATCAACGGTATCG-3′ and 5′-CGGAATTCTCACCCATTGGCACTGCTGTC-3′, using the TS-SAPA clone22 as template. Because the latter oligonucleotide primes on the repetitive unit, a collection of products containing a randomized number of tandem repeats was obtained. The entire mixture of PCR products was purified from agarose gels and enzymatically digested with KpnI and EcoRI. This latter restriction site is underlined in the repeat-priming oligonucleotide. Ligation with the TSac clone was achieved by using the uniqueEcoRI and KpnI sites.22 The precise number of repeated blocks for each clone was settled by DNA sequencing.24 Expression and purification of three of these recombinant trans-sialidases with a variable number of SAPA-repeats was done as described above. Accurate expression of TS-SAPA deletion proteins was verified by reactivity with a monoclonal anti-SAPA antibody (Fig 1).
Trans-sialidase assay.
Enzymatic activity of purified trans-sialidases was assayed by measuring the amount of sialic acid residue transferred from sialyllactose (Sigma Chemical Co, St Louis MO) to [14C]-lactose (Amersham, Little Chalfont, Buckinghamshire, UK) as previously described.25 One Unit oftrans-sialidase was defined as the amount of enzyme able to transfer 10 nmoles of sialic acid to lactose in 1 minute under standard conditions.
Animals.
C3H/HeN mice 60- to 90-days old (both males and females), bred in our own facilities were used.
Immunization schedules and pharmacokinetics studies with chimerictrans-sialidases.
Animals were injected by the retroorbital sinus with 100 pmoles of chimeric trans-sialidases contained in 150 to 200 uL of sterile saline solution (0.15 mol/L NaCl). Remnant trans-sialidase activity was assayed in blood samples (1 to 5 uL) collected from the tail at different times after inoculation.22
Purification of S. japonicum GST chimeric proteins and determination of GST activities.
Chimeric GST proteins containing different T. cruzi amino acid repeats were generated by gene-fusion to S. japonicum GST encoded by plasmid pGEX-1 (Pharmacia Biotech) using the EcoRI cloning site, expressed in E. coli and purified by affinity on glutathione-agarose columns as described.26 GST activities were measured according to Habig et al.27 Briefly, serial dilutions (0.5 ng/mL to 5 ug/mL) of GST chimeric proteins were incubated in 2 mL of phosphate buffer 0.1 mol/L pH 6.5 containing 2.5 mmol/L glutathione (Sigma) and 0.5 mmol/L 1-Chloro-2, 4 dinitrobenzene (Sigma). Changes in absorbance at 340 nm were recorded.
Pharmacokinetics studies with GST chimeric proteins.
Mice were intravenously injected with 1 nmol of each GST chimeric protein extensively dialyzed against phosphate-buffered saline (PBS). Remnant GST chimeric proteins in circulation were quantitated in blood samples collected at different times after injection both by determination of GST activity (see above) and by a standard enzyme-linked immunosorbent assay (ELISA) capture technique. Briefly, polystyrene ELISA microplates (Maxisorp; Nunc, Roskilde, Denmark) were coated with 75 uL of an 8 ug/mL solution of protein-A purified rabbit immunoglobulins (Igs) to S. japonicum GST in PBS. Blocking of the plates and subsequent dilution of Ig solutions were made in Tris-buffered saline 0.15 mol/L pH 7.6 containing 3% of extensively dialyzed bovine serum albumin (Sigma). Appropriate dilutions of serum samples taken at different times after injection were incubated for 1 hour at room temperature and captured GST was evaluated by the addition of biotin-labeled anti-GST Igs raised in rabbits (2.5 ug/mL). A solution of alkaline phosphatase-conjugated avidin (Pierce, Rockford, IL) was then added to the plate and incubated for an additional hour. Chromogenic reaction with p-nitro phenyl phosphate (Sigma) was allowed to proceed for 30 minutes. Plates were read at 405 nm in a microplate reader (Bio-rad Laboratories, Richmond, CA).
Sensitivity assays of chimeric proteins to blood-proteinases.
GST or GST-SAPA protein solutions (30 ug/mL) in normal mouse serum or normal mouse blood diluted (1:1 vol/vol) in Alsever’s solution (sodium azide 0.02% wt/vol was added in both cases) were incubated at 37°C with occasional agitation. At different times, aliquots (10 uL) were subjected to Western blot analysis using a polyclonal anti-GST antiserum developed in rabbits. Reaction was visualized by the addition of peroxidase-conjugated goat anti-rabbit Igs (DAKO, Ejby, Denmark) followed by the chromogenic substrate 4-Chloro-1-naphtol (Bio-rad).
TSac or TS-SAPA protein diluted to 5 ug/mL in normal mouse serum were incubated at 37°C with occasional agitation. At different times, aliquots (2 uL) were assayed fortrans-sialidase activity as described above.
Statistical analysis.
The stability in blood of the different proteins was compared with that obtained for TS-SAPA (Fig 2 and3) or GST-SAPA values (Fig 4) in every time point of the curve using the Student’s t-test. P < .05 were considered significant.
Pharmacokinetics studies of chimerictrans-sialidases. Mice intravenously inoculated with 100 pmoles (about 10 ug) of the indicated protein, were bled at the indicated days after injection and measured for remnant trans-sialidase activity in serum samples. Data are expressed as the mean ± standard deviation (SD) (n = 3 animals). Values recorded 30 minutes after injection are indicated as day 0 values and were taken as 100%trans-sialidase activity. One out of two experiments with similar results is shown. Asterisks (*) denote significant differences (P < .05) to TS-SAPA protein.
Pharmacokinetics studies of chimerictrans-sialidases. Mice intravenously inoculated with 100 pmoles (about 10 ug) of the indicated protein, were bled at the indicated days after injection and measured for remnant trans-sialidase activity in serum samples. Data are expressed as the mean ± standard deviation (SD) (n = 3 animals). Values recorded 30 minutes after injection are indicated as day 0 values and were taken as 100%trans-sialidase activity. One out of two experiments with similar results is shown. Asterisks (*) denote significant differences (P < .05) to TS-SAPA protein.
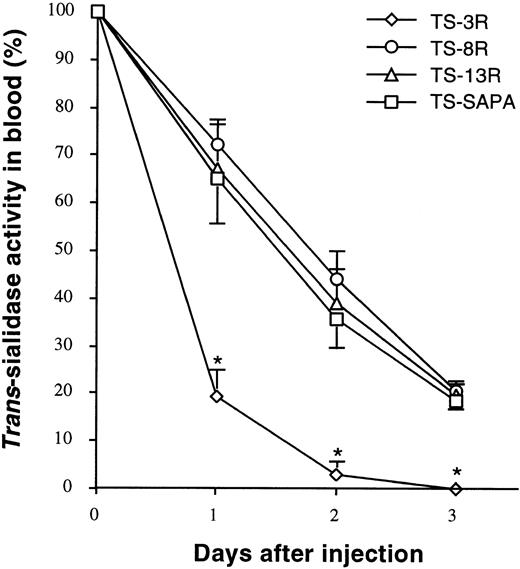
Pharmacokinetics studies of TS-SAPA deletion proteins. Mice intravenously inoculated with 100 pmoles of the indicated protein, were bled at the indicated days after injection and quantitated for remnant trans-sialidase activity in blood as indicated in the legend to Fig 2. Data are expressed as the mean ± SD (n = 3 animals). TS-3R, TS-8R, and TS-13R proteins contain 3, 8, or 13 SAPA-repetitive units, respectively. One out of two experiments with similar results is shown. Asterisks (*) indicate significant differences (P < .05) to TS-SAPA protein.
Pharmacokinetics studies of TS-SAPA deletion proteins. Mice intravenously inoculated with 100 pmoles of the indicated protein, were bled at the indicated days after injection and quantitated for remnant trans-sialidase activity in blood as indicated in the legend to Fig 2. Data are expressed as the mean ± SD (n = 3 animals). TS-3R, TS-8R, and TS-13R proteins contain 3, 8, or 13 SAPA-repetitive units, respectively. One out of two experiments with similar results is shown. Asterisks (*) indicate significant differences (P < .05) to TS-SAPA protein.
Pharmacokinetics studies of GST chimeric proteins. Mice intravenously inoculated with 1 nmol (about 50 ug) of the indicated protein were bled at the indicated days after injection and quantitated for remnant GST in blood using a GST capture assay. Sera from PBS-inoculated mice were used as negative controls. Values recorded 30 minutes after injection are indicated as day 0 values and were taken as 100% circulating GST. Data are expressed as the mean ± SD (n = 4 animals). One out of two experiments with similar results is shown. Asterisks (*) denote significant differences (P < .05) to GST-SAPA protein.
Pharmacokinetics studies of GST chimeric proteins. Mice intravenously inoculated with 1 nmol (about 50 ug) of the indicated protein were bled at the indicated days after injection and quantitated for remnant GST in blood using a GST capture assay. Sera from PBS-inoculated mice were used as negative controls. Values recorded 30 minutes after injection are indicated as day 0 values and were taken as 100% circulating GST. Data are expressed as the mean ± SD (n = 4 animals). One out of two experiments with similar results is shown. Asterisks (*) denote significant differences (P < .05) to GST-SAPA protein.
RESULTS
Chimeric trans-sialidases containing different T. cruzirepetitive units are enzymatically active and display similar specific activities.
Gene fragments coding for repetitive domains present in differentT. cruzi proteins (antigens 1, 13, 36, and SAPA) were placed in cis with a functional trans-sialidase gene and expressed inE. coli (Fig 1). These T. cruzi repeats have been previously shown to be associated to membrane anchored and possibly shed into the milieu proteins (antigens 13 and SAPA) or to intracellularly located proteins (antigens 1 and 36).23,28As shown in Table 1, chimeric trans-sialidases thus obtained have similar structure, containing (from N- to C-terminus) the pTrcHis fusion peptide, the trans-sialidase catalytic domain, and, except for the TSac protein, the different repetitive units. In addition, TS-Ag 13 and TS-SAPA proteins contain a nonrepeated stretch of amino acids in their C-termini that, in the latter case, is compatible with a GPI-anchoring signal.19 Even though theseT. cruzi repeated units are variable in size and amino acid sequence, they all display hydrophilic characteristics and confer a negative net charge at pH 7 to the resultant chimerictrans-sialidase (Table 1).
When assayed for trans-sialidase activity, these chimeric proteins render similar specific activities (Sp. Act.) ranging between 0.48 (for TS-Ag 1 protein) and 1.09 U/nmol (for TS-SAPA protein) (Table1). Furthermore, Sp. Act. of chimeric trans-sialidases are similar to that of the recombinant enzyme expressed by the TSac clone (0.56 U/nmol, Table 1). Thus, as previously shown for thetrans-sialidase molecule containing SAPA-domain,7 21 the presence of any of these T. cruzi repeats in cis does not seem to significantly interfere with the catalytic activity.
Repetitive domains present in T. cruzi shed antigens but not in T. cruzi internal antigens stabilize trans-sialidase activity in blood.
To determine the degree of stabilization of trans-sialidase activity in blood mediated by different T. cruzi repetitive units, 100 pmoles of either TS-Ag 1, TS-Ag 13, or TS-Ag 36 protein were intravenously administered in mice and remnant enzymatic activity monitored in serum samples obtained at different times after inoculation. Equivalent amounts of TSac (lacking any C-terminal extension) and TS-SAPA proteins were used as controls. As shown in Fig2, TS-Ag 13 protein has an estimated half-life in blood of 32 to 34 hours and can be detected in circulation up to 3 days after injection. This persistence in blood is similar to that of TS-SAPA protein (estimated half-life in blood of 37 to 38 hours, Fig 2) and represents a fivefold increase as compared with the half-life in blood of TSac protein (7 hours, Fig 2). On the other hand, trans-sialidase activity of both TS-Ag 1 and TS-Ag 36 proteins is rapidly cleared from mice blood, with estimated half-lives of 16 to 18 hours and 15 to 19 hours, respectively (Fig 2). Thus, it can be concluded that repeated motifs present in T. cruzi shed antigens SAPA and 13 are more efficient in stabilizing trans-sialidase activity in blood than those present in T. cruzi internal antigens 1 and 36.
The repetitive units in the SAPA-domain are required to enhance the half-life in blood of trans-sialidase.
To further characterize the sequences involved in the improved pharmacokinetics of trans-sialidase activity of TS-SAPA protein, a set of deletion clones derived from TS-SAPA gene containing different number of SAPA-repeats was generated (Fig 1). All of them lack the GPI-anchoring signal present in the SAPA-domain (Table 1). Proteins expressed and purified from three of these clones (TS-3R, TS-8R, and TS-13R containing 3, 8, and 13 SAPA-repetitive units, respectively) display similar Sp. Act. (about 0.65 U/nmol) to that reported for the entire TS-SAPA protein (Table 1).
These proteins were intravenously injected in mice andtrans-sialidase activity monitored in blood samples throughout several days. As shown in Fig 3, persistence in blood of TS-8R and TS-13R proteins (both with estimated half-lives in blood of 37 to 39 hours) is almost indistinguishable from that of the entire TS-SAPA molecule, which contains a hydrophobic C-terminal 44-amino acid extension in addition to 13 repetitive units (Table 1). Thus, it can be concluded that the repetitive units present in antigen SAPA, and not its GPI-anchoring signal, are involved in the stabilization of trans-sialidase activity in blood.
On the other hand, TS-3R protein results in a trans-sialidase with shorter half-life in blood (about 12 to 13 hours; Fig 3). This experiment shows that a minimal number of repetitive units is required to stabilize the circulating trans-sialidase activity.
Repetitive shed antigens from T. cruzi could also enhance the stability in blood of a protein unrelated to trans-sialidase.
We next asked if 13- and SAPA-repeats were also able to stabilize in blood a protein unrelated to trans-sialidase. We chose the 28 kD-GST domain from S. japonicum encoded in pGEX vector as a model. GST proteins bearing amino acid repeats from T. cruziinternal antigens (1, 30, and 36) and from proteins spontaneously released by the parasite (antigens 13 and SAPA) on their C-termini were expressed, purified, and analyzed.26
The enzymatic activity of the GST domain present in each chimeric protein was determined as the change in optical density values recorded at 340 nm. These values are about 0.01 Absorbance Units.minute -1 .ug GST-1 in proteins containing or lacking repetitive motifs (data not shown). These results suggest that, as is the case of trans-sialidase, the folding and/or functional activity of GST is not severely modified by the addition in cis of any of these T. cruzi repeats.
Each of these GST chimeric proteins was intravenously administered in mice in equimolar amounts (1 nmol/mouse) and their rate of clearance from blood followed by determination of remnant GST activity in blood samples taken at different times after injection. Values recorded 30 minutes after injection were taken as 100% GST activity in each case. Except for GST-Ag 1, GST-Ag 13, and GST-SAPA–injected mice that retain 8%, 25%, and 21% of their initial GST activities, respectively, the rest of the animals show undetectable GST activity in blood as short as 24-hours after injection (data not shown). Due to the low sensitivity of the enzymatic assay, we decided to try a GST capture procedure to determine the persistence of GST chimeras in blood (see Materials and Methods).
Three groups of GST chimeric proteins can be differentiated according to their persistence in blood using this GST capture assay (Fig 4). The first group includes GST-SAPA and GST-Ag 13 proteins, both with an estimated half-life in blood of 22 to 24 hours. The second group of proteins is characterized by a significant lower half-life in blood (about 10 to 13 hours) and includes GST chimeras containing repeats from internal T. cruzi antigens 1, 30, and 36. The third group is composed just by GST protein lacking repeats (estimated half-life in blood of 3 hours), that is not detected in circulation as short as 24-hours after injection (Fig 4). As is the case with chimerictrans-sialidases, the sole addition of any T. cruzirepetitive domain to the carrier protein seems enough to exert a moderate effect on its stability in blood; but this effect is much more evident when the repetitive motifs added are the ones present in T. cruzi shed antigens 13 or SAPA (Fig 2 and 4).
As shown in Fig 5, a bias in the detection of different GST chimeras can be disregarded. The GST capture assay employed herein is able to detect changes in protein concentration between 0.2 to 50 ng/well for every chimeric GST (Fig 5), thus suggesting that its sensitivity is unaffected by the presence in cis of different repetitive T. cruzi domains.
Standardization of GST capture assay. GST chimeric proteins diluted in normal mouse serum were tested in the GST capture assay described under Materials and Methods. Mean optical density values are shown (SD values did not exceed 10% of mean values in any case; not shown). TS-SAPA and cruzipain protein diluted in normal mouse serum were used as negative controls. Samples were tested in triplicate. One out of three experiments with similar results is shown.
Standardization of GST capture assay. GST chimeric proteins diluted in normal mouse serum were tested in the GST capture assay described under Materials and Methods. Mean optical density values are shown (SD values did not exceed 10% of mean values in any case; not shown). TS-SAPA and cruzipain protein diluted in normal mouse serum were used as negative controls. Samples were tested in triplicate. One out of three experiments with similar results is shown.
Pharmacokinetics data shown in Fig 4 were further confirmed by Western blots of serum samples shown by a polyclonal anti-GST antiserum (data not shown). Taken all together, these results show that the GST domain of S. japonicum circulates in mouse bloodstream for longer periods and in intact form when associated to T. cruzi repeats SAPA and 13.
Evaluation of possible mechanisms involved in the stabilization of carrier proteins mediated by SAPA amino acid repeats.
There are several possible mechanisms through which chimeric proteins containing SAPA-repeats might increase their half-life in blood. Experiments were performed to analyze two likely alternatives: first, whether these T. cruzi tandem repeats prevent or inhibit degradation by blood-proteinases and second, whether SAPA-domain interacts with other proteins in the bloodstream of the vertebrate host.
GST or trans-sialidase molecules containing or not the repetitive extension were incubated at 37°C either with blood or plasma recovered from normal mice. At different times, aliquots were taken and analyzed for proteolytic degradation. As shown in Fig 6, the presence of SAPA-repeats does not significantly affect the course of enzyme inactivation (in the case of trans-sialidase molecules, Fig 6A) or protein degradation (in the case of GST molecules, Fig 6B).
Sensitivity of chimeric proteins to blood-proteinases. (A) TSac and TS-SAPA proteins were incubated with normal mouse serum and assayed for trans-sialidase activity in samples taken at different times. Data are expressed as the mean ± SD. (B) GST or GST-SAPA protein incubated either with mouse serum or mouse blood for the indicated times, were assayed for proteolytic degradation as indicated in Materials and Methods. Controls are indicated as − (mouse serum alone) and + (GST or GST-SAPA–purified protein in PBS). (MK) Molecular weight markers (in kD) are indicated at right.
Sensitivity of chimeric proteins to blood-proteinases. (A) TSac and TS-SAPA proteins were incubated with normal mouse serum and assayed for trans-sialidase activity in samples taken at different times. Data are expressed as the mean ± SD. (B) GST or GST-SAPA protein incubated either with mouse serum or mouse blood for the indicated times, were assayed for proteolytic degradation as indicated in Materials and Methods. Controls are indicated as − (mouse serum alone) and + (GST or GST-SAPA–purified protein in PBS). (MK) Molecular weight markers (in kD) are indicated at right.
Another suitable hypothesis would be that proteins linked to SAPA-repeats increase their persistence in circulation due to the interaction of the repetitive domain with blood protein/s. It has been described, for instance, that certain surface proteins in pathogenic Gram+ cocci allow evasion of immune defense mechanisms by acting as receptors of blood proteins that eventually mask the bacteria.29 30 This seems not to be the case with T. cruzi SAPA-repeats. Immunoprecipitation experiments performed with GST or GST-SAPA protein (in solution or coupled to Sepharose) incubated with normal mouse serum show no additional band/s in SDS-PAGE analyses (data not shown). Altogether, these results suggest that the increase in the circulating half-life of SAPA-repeats carrier proteins could not be attributed to a modification of their sensitivity to blood-proteinases or to the binding to endogenous circulating protein/s.
DISCUSSION
The ability of different T. cruzi repetitive antigens in stabilizing associated polypeptides in blood has been analyzed in two experimental models: T. cruzi trans-sialidase and S. japonicum GST fusion proteins. Three of these antigens (denoted as 1, 30, and 36) have been shown to code for the repetitive units of proteins intracellularly-located in the parasite.6,23 Thus, it might be expected that these repeated structures accomplish other function in vivo (if any at all) than protection against clearance from bloodstream of their nonrepeated carrier domains. This is not the case with antigens SAPA and 13 that are spontaneously released into the environment by the parasite. The latter codes essentially for the repetitive extension of a protein located on the surface and on cell boundaries of both amastigotes (the replicative, intracellular stage ofT. cruzi) and bloodstream trypomastigotes, as shown by electron microscopy studies.23 The overall similarity betweentrans-sialidase and the natural N-domain of antigen 13 is enough to show that they are indeed members of the same superfamily of molecules28; even though, the repetitive unit present in antigen 13 shows no evident similarity to the 12 amino acid-long unit of SAPA-repeats (Table 1).
Despite this lack of amino acid sequence similarity, both antigen SAPA and 13 produce the same effect on trans-sialidase activity when placed in cis: enhancement of its half-life in blood. As shown in Fig2, both TS-SAPA and TS-Ag 13 proteins remain in circulation up to 3 days or longer whereas TS-Ag 1 and TS-Ag 36 proteins are rapidly cleared from the bloodstream after intravenous injection in mice. These results cannot be attributed to thermal stability differences between both groups of chimeric enzymes because, as previously shown,trans-sialidases containing or lacking repetitive extensions show negligible changes in their thermal inactivation when assayed at 37°C.7 22 Furthermore, improved half-life in blood of TS-SAPA and TS-Ag 13 proteins can hardly be attributed to physical modifications introduced by the presence of these repeats. As shown in Table 1, all of chimeric trans-sialidases display similar molecular properties characterized by the prevalence of negatively charged residues and a highly hydrophilic nature. Furthermore, these proteins show almost unaltered catalytic properties (Table 1), probably reflecting the lack of interaction between the enzymatic domain and the repetitive extension. Thus, it might be concluded that the stabilization of chimeric trans-sialidase activity in blood mediated by T. cruzi antigens SAPA and 13 is dependent on the specific amino acid sequence of their repetitive units.
Results obtained in the S. japonicum GST model were in close agreement with those depicted for the trans-sialidase model, suggesting that the persistence in bloodstream is an inherent property to certain T. cruzi amino acid repeats and not dependent on the linked polypeptide. Chimeric GST proteins containing different T. cruzi tandem arrays, though not modified in their enzymatic activities, clearly differ in their half-lives in blood (Fig 4).
Even though the role of T. cruzi circulating molecules is still uncertain, it could be postulated that this novel mechanism might be operating in vivo as part of the strategy displayed by the parasite to establish and/or maintain the infection. In this context, it is noteworthy that the trans-sialidase expressed by the epimastigote (the parasite form present in the midgut of the reduviid bug host) shares many features with trypomastigotetrans-sialidase but lacks SAPA-repeats.18
Whether the mechanism mediated by antigens SAPA and 13 to stabilize carrier proteins in blood depends on preventing the catabolism of carrier protein in certain tissues and/or other alternative/s is currently under investigation. In the case of the 28 kD-GST protein, its rapid clearance might be explained in part by kidney filtration (Fig 4). However, this mechanism seems rather unlikely to explain the rapid loss of the 76 kD TSac protein from circulation (Fig 2) and to justify the differences in half-lives in blood recorded for proteins almost identical in their molecular size (about 60 kD) like GST-Ag 30, GST-Ag 36, and GST-SAPA (Fig 4).26 On the other hand, the possibility of an increased protection against blood-proteinases or binding to endogenous circulating protein/s mediated by T. cruzi SAPA-repeats might be excluded (Fig 6 and data not shown).
The potential implications of these findings could be readily envisaged. Considerable efforts are currently under way to increase the circulating half-life of certain therapeutic proteins. Among the several strategies that are being analyzed, randomized mutagenesis,31 gene fusions to endogenous proteins,32-34 and conjugation to inert polymers like polyethylene glycol35 36 can be mentioned. In the case of intravenously administered hormones, these potentially long-acting analogs seem a promising alternative to daily injections for treatment. The work described herein provides a reasonable alternative for the improvement of protein half-life in blood: the exploitation of natural occurring sequences. As shown herein, both T. cruzi repeats SAPA and 13 promote the persistence of nondegraded, enzymatically active, proteins in blood when linked in cis (Fig 2 and 4 and data not shown). Thus, it seems reasonable to predict that other repetitive sequences present in blood-released T. cruziproteins (or even in other eukaryotic parasites) could be mediating similar stabilization effects.
ACKNOWLEDGMENT
We thank Dr J.J. Cazzulo for critical reading of the manuscript, F. Fraga for excellent care and maintenance of the animals, E. Spinedi (from CITECA, INTI) for the ELISA determinations, and C. Labriola for kindly providing the purified cruzipain.
Supported by the United Nations Development Programme/World Bank/World Health Organization (WHO) Special Program for Research and Training in Tropical Diseases (TDR), the Department for Research Cooperation (SAREC) from the Swedish International Development Cooperation Agency (SIDA), the Agencia Nacional de Promoción Cientı́fica y Tecnológica and the Consejo Nacional de Investigaciones Cientı́ficas y Técnicas (CONICET), Argentina. The research of A.C.C.F. was supported in part by an International Research Scholars grant from the Howard Hughes Medical Institute, Washington, DC. C.A.B. is a fellow and O.C. and A.C.C.F. are researchers from the CONICET. J.A. is a student fellow from the Comisión de Investigaciones Cientı́ficas de la Provincia de Buenos Aires, Argentina.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.