To The Editor:
We read with interest the article by Scott et al1 on contact system activation during platelet concentrate filtration. Several groups endeavoring to find a causative role for negatively charged filters in hypotensive/anaphylactoid reactions have reported activation of the coagulation cascade intrinsic pathway (contact phase) by measuring changes in bradykinin and/or kallikrein activity before and after filtration.2 Although a clear cause and effect relationship between clinical reactions and the use of negatively charged bedside filters has not been unequivocally demonstrated, there have been several case reports that support such a hypothesis.3,4 The observations of Scott et al of minimal high molecular weight kininogen and total kininogen adsorption to negatively charged filters led them to conclude and generalize that there is minimal, if any, contact phase activation during platelet filtration using negatively charged filters. Others that have reported significant activation of the contact system also observed a wide variation in the levels of activation between platelet concentrates.5 We believe these groups are missing contributions from two critical components of this system: the pH of the platelet or plasma being filtered and enhancement from dilution of the products with crystalloids.
Hypotensive/anaphylactoid type or hypersensitivity reactions (HSR) were reported during hemodialysis in the early 1990s with the use of ACE inhibitor therapy (ACEI) and were found to be more frequent in ACEI-treated patients dialyzed using AN69 (HOSPAL, Lyon, France), a negatively charged dialysis membrane.6 In response to these reports, our HOSPAL group has been investigating the role of contact phase activation in extracorporeal circuits, including determination of key factors that affect this phenomenon. Potential similarities between HSR in hemodialysis, transfusion medicine, and therapeutic apheresis such as heterogeneity of occurrence, ACEI, and exposure to negatively charged medical devices have been pointed out by Owen and Brecher.7
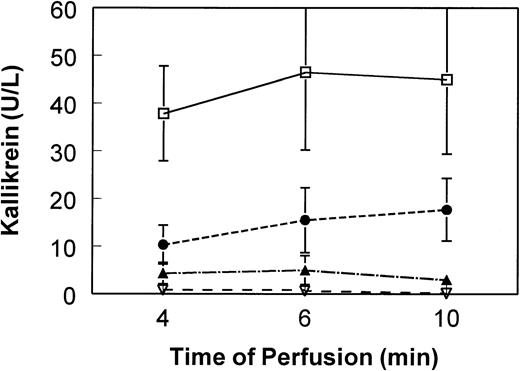
We have seen a significant effect of perfusate pH (eg, platelet concentrate pH) on contact phase activation. For in vitro activation, we see maximum kallikrein and bradykinin activation at or below pH37°C of 7.1 (Fig1). We believe this pH effect may have significantly affected the results observed by Scott et al. Apheresis platelet concentrates are known to have very large excursions in pH over the course of storage, depending on storage bag type and the number of platelets in the bag. An initial increase in pH is typically seen during the first 2 days of storage, coinciding with the products tested by Scott et al.
Influence of pH on contact phase activation induced by negatively charged dialysis membrane. Platelet-poor plasma pools were diluted 1:20 in 0.9% saline (5% final plasma content). Pool pHs (measured at 37°C) were adjusted as indicated and perfused (single pass) through mini-hemodialysers constructed of AN69 membrane (250 cm2). At 4, 6, and 10 minutes of perfusion, aliquots of effluent plasma were immediately frozen in methanol/dry ice bath. Plasma kallikrein was determined by chromogenic assay using substrate S2302 (Biogenic, Maurin, France) after modification of a method described by De La Cadena et al.9 Means and standard deviations (error bars) of six experiments are shown. Kallikrein in the nonperfused pool remained at the baseline of less than 2 U/L over the course of the experiment. Kallikrein for plasma perfused over nonelectronegatively charged membranes (eg, cellulosic) remains at baseline of less than 2 U/L (data not shown). (□) pH 7.1; (•) pH 7.4; (▴) pH 7.6; (▿) pH 7.8.
Influence of pH on contact phase activation induced by negatively charged dialysis membrane. Platelet-poor plasma pools were diluted 1:20 in 0.9% saline (5% final plasma content). Pool pHs (measured at 37°C) were adjusted as indicated and perfused (single pass) through mini-hemodialysers constructed of AN69 membrane (250 cm2). At 4, 6, and 10 minutes of perfusion, aliquots of effluent plasma were immediately frozen in methanol/dry ice bath. Plasma kallikrein was determined by chromogenic assay using substrate S2302 (Biogenic, Maurin, France) after modification of a method described by De La Cadena et al.9 Means and standard deviations (error bars) of six experiments are shown. Kallikrein in the nonperfused pool remained at the baseline of less than 2 U/L over the course of the experiment. Kallikrein for plasma perfused over nonelectronegatively charged membranes (eg, cellulosic) remains at baseline of less than 2 U/L (data not shown). (□) pH 7.1; (•) pH 7.4; (▴) pH 7.6; (▿) pH 7.8.
We have also observed a systematic increase in contact phase activation when plasma is diluted with normal saline (0.9% NaCl, pH 5.5). These observations were recently confirmed by Shimizu et al.8They reported that bradykinin generation increases 30 times in platelet concentrates diluted 85% with a storage solution (Seto sol; 115 mmol/L NaCl, 4 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L Na3PO4, 15 mmol/L acetate, 3 mmol/L Na3citrate, 10 mmol/L glucose, pH 7.1) or saline.8 We currently use 5% plasma in saline at a pH37°C of 7.1 to maximize the activation signal as we investigate various devices and components. Tests are conducted at 37°C, and we have found no differences at room temperature.
Blood interaction with an active foreign surface is multifaceted with many interacting variables such as dilution, pH (which may be 6.0 to 7.6 for platelet concentrates), and physical properties of materials. Effects on patients are potentiated not only by the blood/material interactions, but also by predisposing factors in the patient (bradykinin metabolism, acid/base status, and perhaps genetic factors). Therefore, we would encourage investigators in this area to pay special heed to ex vivo factors such as plasma content, pH, and time effects. Understanding these effects may help to clarify a phenomenon that occurs at an inconsistent frequency in the laboratory and an elusive clinical complication rate when using leukocyte reduction filters.