Abstract
Leukemic patients receiving marrow from HLA-identical sibling donors were randomized to treatment with either busulfan 16 mg/kg (n = 88) or total body irradiation ([TBI] n = 79) in addition to cyclophosphamide 120 mg/kg. The patients were observed for a period of 5 to 9 years. Busulfan-treated patients had an increased risk of veno-occlusive disease (VOD) of the liver (12% v 1%,P = .01) and hemorrhagic cystitis (32% v 10%,P = .003). Acute graft-versus-host disease (GVHD) was similar in the two groups, but the 7-year cumulative incidence of chronic GVHD was 59% in the busulfan-treated group versus 47% in the TBI group (P = .05). Death from GVHD was more common in the busulfan group (22% v 3%, P < .001). Obstructive bronchiolitis occurred in 26% of the busulfan patients but in only 5% of the TBI patients (P < .01). Complete alopecia developed in 8 busulfan patients and partial alopecia in 17, versus five with partial alopecia in the TBI group (P < .001). Cataracts occurred in 5 busulfan-treated patients and 16 TBI patients (P = .02). The incidence of relapse after 7 years was 29% in both groups. Seven-year transplant-related mortality (TRM) in patients with early disease was 21% in the busulfan group and 12% in the TBI group. In patients with more advanced disease, the corresponding figures were 64% and 22%, respectively (P = .004). Leukemia-free survival (LFS) in patients with early disease was 68% in busulfan-treated patients and 66% in TBI patients. However, 7-year LFS in patients with more advanced disease was 17% in the busulfan group versus 49% in the TBI group (P < .01). In patients with chronic myeloid leukemia (CML) in first chronic phase, 7-year LFS was 72% and 83% in the two groups, respectively.
BONE MARROW TRANSPLANTATION (BMT) is the treatment of choice for high-risk acute leukemia and chronic myeloid leukemia (CML) if an HLA-identical sibling donor is available.1-6 Total body irradiation (TBI) combined with cyclophosphamide has for many years been the standard myeloablative therapy for patients undergoing allogeneic BMT. As an alternative to TBI, Santos et al7 introduced busulfan in patients with acute myeloid leukemia (AML).
Since then, five randomized trials have compared busulfan conditioning with TBI in patients with leukemia and those undergoing BMT with an HLA-identical sibling as donor.8-12 We now report the 5 to 9-year outcome in BMT recipients with leukemia or lymphoma receiving marrow from HLA-identical related donors and randomized to busulfan or TBI.
SUBJECTS AND METHODS
Patients.
Patients with hematologic malignancies were randomized at each center after stratification for diagnosis, disease status, and age (children ≤ 17 years v adults). Patients were divided into groups with early disease, ie, acute leukemia or lymphoma in first remission, or CML in first chronic phase. High-risk disease included all patients beyond first remission or beyond first chronic phase. The study was approved by the ethics committees of the Karolinska Institute, Mejlands Hospital, Helsinki, and the Universities of Turku, Copenhagen, Lund, and Gothenburg. Consent was obtained from all patients or legal guardians. From October 1988 to December 1992, 167 patients were randomized to receive busulfan (n = 88) or TBI (n = 79). Patient and donor characteristics are listed in Table1. Details regarding the patients and treatment have been previously reported.9
Conditioning.
Busulfan 1 mg/kg was administered four times per day for 4 consecutive days from day -8 to day -5. As prophylaxis against seizures, the patients received diazepam, clonazepam, or clorazepam from day -8 to day -5. Cyclophosphamide 60 mg/kg was administered on days -4 and -3. Alkalization and forced diuresis with 3 L intravenous (IV) fluid/m2/24 h was used during busulfan and cyclophosphamide treatment. In addition, during cyclophosphamide treatment, uromitexan was administered at 120% to 150% of the cyclophosphamide daily dose, divided into three to four doses. Intrathecal (IT) methotrexate (MTX) 12 mg was also administered on two occasions before BMT. Patients randomized to TBI received cyclophosphamide 60 mg/kg on 2 consecutive days and TBI 10 Gy in one session at Huddinge Hospital or TBI fractionated from 11.3 to 12 Gy at the other centers.
Prophylaxis against graft-versus-host disease and central nervous system leukemia.
The patients received four doses of IV MTX combined with cyclosporin (CsA).13 CsA was discontinued from 2 to 12 months after BMT in the absence of graft-versus-host disease (GVHD). Patients with acute lymphoblastic leukemia (ALL) and patients with AML types M4 and M5 received IT MTX 12 mg on day 32 and then once every other week until day 102, according to the Seattle protocol.1 In Helsinki, IT MTX was started after platelet recovery. Patients with a history of central nervous system (CNS) leukemia also received IT MTX every eighth week for 1.5 years.
Definitions and grading of complications.
Acute GVHD was graded from 0 to IV according to previously published criteria.1 Symptomatic hemorrhagic cystitis was defined as symptoms from the bladder combined with hematuria. Veno-occlusive disease (VOD) of the liver was defined as an increase in bilirubin greater than 2 mg/dL (>34 mmol/L) with at least two of the following: hepatomegaly, ascites, and a body weight gain exceeding 5%.14 Chronic GVHD was defined as present or not.15 Cataracts were diagnosed by an ophthalmologist during yearly visits.16 Obstructive bronchiolitis was defined to include the symptoms of dyspnea, coughing, and wheezing in association with pulmonary function tests showing airflow obstruction.17 Open-lung biopsies were not performed routinely. Alopecia in long-term survivors was recorded as none, partial, or total.
Statistics.
The analysis was performed on March 4, 1998, with an observation time of 5 years to 9 years and 2 months. Mean values were compared with Student’s t test. The distribution was compared with the χ2 test and corrected with Yates’ method. The cumulative time to various complications and survival rate were analyzed by the life-table method.18 Differences in survival, leukemia-free survival (LFS), etc, between subgroups were studied with the log-rank test, taking censored data into account.19 Patients with incomplete follow-up data were considered censored observations.
RESULTS
Complications.
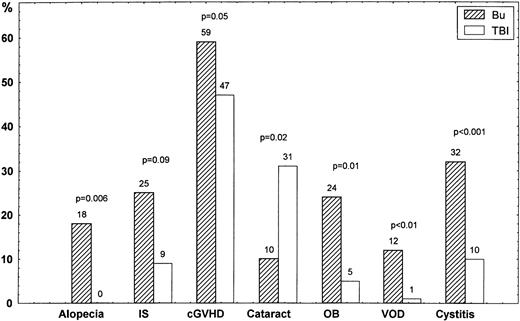
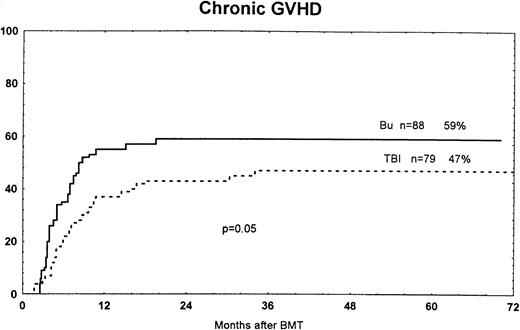
The cumulative incidence of VOD was 12% in patients treated with busulfan and 1% in those who received TBI (P < .01; Fig1). The overall incidence of hemorrhagic cystitis was 32% in busulfan-treated patients and 10% in the TBI group (P < .001). Grades II to IV acute GVHD occurred in 29% of the busulfan group and 25% of the TBI group. Grades III to IV acute GVHD developed in 15% and 6% of the two groups, respectively (P = .09). The cumulative incidence of chronic GVHD at 7 years was 59% in the busulfan group and 47% in the TBI group (P = .05; Fig 2). At long-term follow-up study, 13 of 51 (25%) were on immunosuppressive therapy (CsA and/or steroids) in the busulfan group, versus 5 of 56 (9%) in the TBI group (P = .09; Fig 1).
Complications (%) in patients randomized to busulfan (Bu) or TBI regarding alopecia, long-term immunosuppression (IS) with CsA and/or steroids (>5 years), chronic GVHD (cGVHD), cataract, obstructive bronchiolitis (OB), liver VOD, and hemorrhagic cystitis.
Complications (%) in patients randomized to busulfan (Bu) or TBI regarding alopecia, long-term immunosuppression (IS) with CsA and/or steroids (>5 years), chronic GVHD (cGVHD), cataract, obstructive bronchiolitis (OB), liver VOD, and hemorrhagic cystitis.
Time to and cumulative incidence of chronic GVHD among patients randomized to treatment with busulfan (Bu) or TBI. The difference was significant (P = .05).
Time to and cumulative incidence of chronic GVHD among patients randomized to treatment with busulfan (Bu) or TBI. The difference was significant (P = .05).
Among 45 long-term survivors in the busulfan group, 20 had unchanged or normal hair growth and 17 developed partial and eight total alopecia. In TBI-treated patients, 46 had no alopecia, five partial alopecia, and none total alopecia (P < .001).
Obstructive bronchiolitis was diagnosed in 14 of 54 (26%) busulfan patients, compared with three of 58 (5%) TBI patients (P = .005; Fig 1). In the busulfan group, 11 patients with obstructive bronchiolitis also had chronic GVHD and 2 did not. None of 3 patients with obstructive bronchiolitis in the TBI group experienced chronic GVHD. The correlation between chronic GVHD and obstructive bronchiolitis was not statistically significant in these patients (P = .14).
Cataracts were diagnosed in 5 of 48 (10%) long-term survivors in the busulfan group and 16 of 52 (31%) in the TBI group (P = .02). In the TBI group, cataracts were found in 11 who were treated with TBI in one session versus 5 with fractionated TBI (P = .007). Three patients underwent surgery for cataracts in the busulfan group, versus 11 in the TBI group.
Outcome of various study parameters.
The incidence of interstitial pneumonitis was 18% in the busulfan group and 16% in the TBI group (P = .51). Among long-term survivors in the busulfan group, the median Karnofsky score was 90 (range, 50 to 100), compared with 100 (80 to 100) in the TBI group (P = .062). Karnofsky scores in the busulfan group were 50 (n = 1), 70 (n = 2), 80 (n = 8), 90 (n = 11), and 100 (n = 19). In the TBI group, Karnofsky scores were 80 (n = 4), 90 (n = 16), and 100 (n = 30). Hypothyroidism developed in 3 patients in the busulfan group and 6 in the TBI group (nonsignificant [NS]).
At long-term follow-up evaluation, serum creatinine increased from pre-BMT to a median of 9 (−20 to +75) mmol/L in the busulfan group (n = 51) versus 5 (−36 to +79) mmol/L in the TBI group (n = 57) (P = .3). A median change in the lung vital capacity of −0.2 (−1.8 to 0.7) L occurred in the busulfan group (n = 25), compared with 0 (−1.2 to 1.8) L in TBI patients (n = 23) (P = .098). In the 5 children in each group who were long-term survivors, the median increase in height was 28 (12 to 32) cm in the busulfan group, compared with 17 (0 to 24) cm in the TBI group. Busulfan-treated children gained a median of 14 (8 to 29) kg in weight, versus 7 (−2 to 12) kg in TBI-treated children (NS).
Cause of death.
There were 40 deaths in the busulfan group and 29 in the TBI group. The primary cause of death is listed in Table2. The incidence of death from GVHD was 22% in the busulfan group (11 acute and 5 chronic GVHD), compared with 3% (1 acute and 1 chronic GVHD) in the TBI group (P < .001).
Transplantation-related mortality.
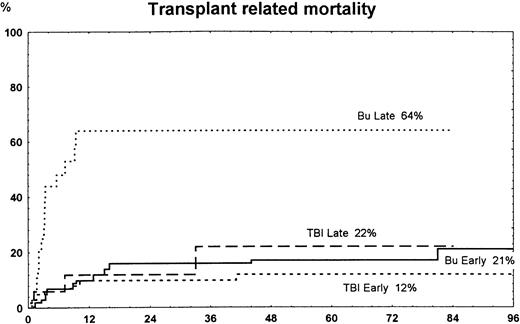
The overall 7-year transplantation-related mortality (TRM) was 34% in the busulfan group and 14% in the TBI group (P = .01). In patients with early disease, TRM was similar in the busulfan group versus the TBI group (NS). In high-risk patients, 7-year TRM was 64% in busulfan patients and 22% in TBI patients (P = .004) (Fig3).
Time to and cumulative incidence of TRM among patients randomized to treatment with busulfan (Bu) or TBI for early disease (first remission or first chronic phase) or late disease (later stages). The difference was significant for Bu late versus TBI late (P = .004) and Bu late versus Bu early or TBI early (P < .001).
Time to and cumulative incidence of TRM among patients randomized to treatment with busulfan (Bu) or TBI for early disease (first remission or first chronic phase) or late disease (later stages). The difference was significant for Bu late versus TBI late (P = .004) and Bu late versus Bu early or TBI early (P < .001).
Survival and relapse.
Overall survival did not differ significantly in the busulfan group compared with the TBI group (Table 3). However, in high-risk patients, survival was lower in the busulfan group versus the TBI group (P = .005).
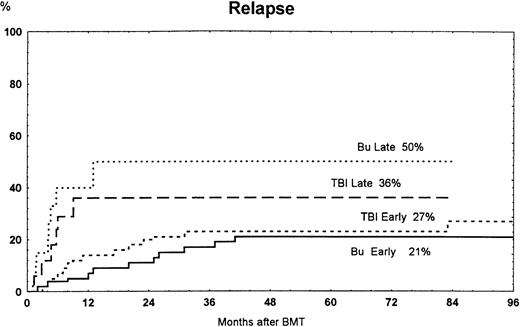
The overall 7-year cumulative incidence of relapse was the same in patients treated with busulfan or TBI (Table 3). The incidence of relapse was not significantly different in patients with early and advanced disease (Fig 4).
Time to and cumulative incidence of relapse among patients randomized to treatment with busulfan (Bu) or TBI for early disease (first remission or first chronic phase) or late disease (later stages). Bu late versus Bu early were significantly different (P = .001).
Time to and cumulative incidence of relapse among patients randomized to treatment with busulfan (Bu) or TBI for early disease (first remission or first chronic phase) or late disease (later stages). Bu late versus Bu early were significantly different (P = .001).
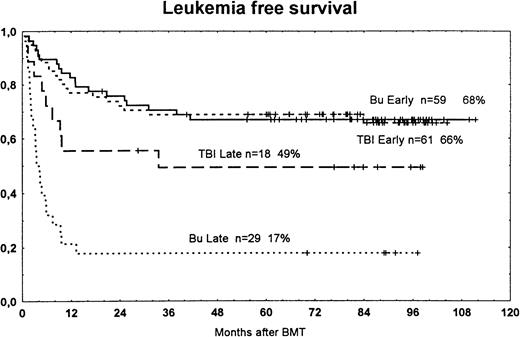
In patients with early disease, LFS was similar in patients treated with busulfan versus TBI (Fig 5). However, in patients with more advanced disease, LFS was worse in the busulfan group. In the subgroup analysis of patients with AML, ALL, or CML, there were no significant differences between those randomized to busulfan or TBI (Table 1).
Actuarial LFS among patients randomized to treatment with busulfan (Bu) or TBI for early disease (first remission or first chronic phase) or late disease (later stages). The survival curves are significantly different between groups with late disease (P = .009). Bu early versus Bu late, P < .001; TBI early versus TBI late, P < .055. Tic marks represent surviving patients.
Actuarial LFS among patients randomized to treatment with busulfan (Bu) or TBI for early disease (first remission or first chronic phase) or late disease (later stages). The survival curves are significantly different between groups with late disease (P = .009). Bu early versus Bu late, P < .001; TBI early versus TBI late, P < .055. Tic marks represent surviving patients.
DISCUSSION
TBI induces several unwarranted late toxic effects such as pneumonitis, cataract, endocrinologic disturbances, secondary malignancies, and decreased growth in children.20-23 However, even if the pattern with regard to late toxicity differs between busulfan and TBI, it seems that busulfan is more toxic. The busulfan-treated patients had more VOD and hemorrhagic cystitis, which may be related to more direct tissue toxicity. This may also explain why we found a higher risk for chronic GVHD in the busulfan group than in the TBI group. The trend for more severe acute GVHD in the busulfan group also may have paved the way for chronic GVHD.24,25 The higher risk of chronic GVHD in the busulfan group cannot be explained by a difference in immunosuppression. Both treatment arms followed the same immunosuppressive protocol, and in particular, MTX IV and IT was given to the same number of patients in both study arms. Because of more GVHD, there was a trend for prolonged immunosuppression in busulfan-treated patients (P = .09; Fig 1). An increased risk of VOD in patients treated with busulfan versus TBI was also found in other studies.23
Alopecia was much more common in busulfan-treated patients, occurring in 56%, compared with only 10% with partial alopecia in the TBI group (P < .001). There seems to be a correlation between the busulfan level and alopecia, and by monitoring busulfan levels, this side effect may be reduced.26 Interstitial pneumonitis did not differ between the two groups. However, obstructive bronchiolitis was more common in busulfan-treated patients. There is a correlation between chronic GVHD and obstructive bronchiolitis.17 This correlation was not statistically significant in this study, possibly due to the small number of patients (P = .14). Furthermore, in the TBI group, none of three patients with obstructive bronchiolitis had chronic GVHD.
Cataracts are a well-known complication of TBI, occurring in about 80% of patients receiving TBI in one session but at a significantly lower rate in patients receiving fractionated TBI.16 In the TBI group in this study, 16 (32%) developed cataract; most received TBI in one session. However, it cannot be excluded that cataract formation may appear later in patients receiving fractionated TBI. Five patients in the busulfan arm developed cataract, and three of these had chronic GVHD. Steroids used for treatment of chronic GVHD may also induce cataract.
The incidence of relapse did not differ significantly between the two groups, in accordance with previous studies.8-10,12,23Busulfan induced increased toxicity in various tissues, including the liver, urinary tract, and lung. These effects resulted in a significantly increased incidence of TRM among the high-risk patients treated with busulfan—as high as 64%. Our study suggests that busulfan may be unsuitable for patients with high-risk disease who have received several courses of chemotherapy before BMT. Santos et al7 also reported a poor outcome in high-risk patients treated with busulfan. In their study, cyclophosphamide 200 mg/kg was administered, compared with 120 mg/kg in our study. However, in a retrospective study by the European Group for Blood and Marrow Transplantation (EBMT), there was no difference in outcome when busulfan was combined with cyclophosphamide 200 or 120 mg/kg.23 In the EBMT study and four other randomized trials, there was no increase in TRM in high-risk patients treated with busulfan versus TBI.8-10,12 A possible explanation for the high toxicity and TRM in our high-risk patients may be the short interval between the last busulfan dose and cyclophosphamide treatment. If cyclophosphamide is started less than 24 hours after the last busulfan dose, cyclophosphamide concentrations in plasma are elevated, which may increase toxicity.27 Furthermore, the outcome in patients with more advanced disease must be viewed with caution because of the small sample size, only 29 treated with busulfan and 18 treated with TBI. There is a correlation between high busulfan levels in plasma and TRM.28 Therefore, monitoring of the busulfan level and subsequent adjustment of the dose may reduce toxicity. Together with a prolonged interval (>24 hours) between the last busulfan dose and cyclophosphamide administration, this may improve the outcome, especially in patients with advanced disease.
In patients with early disease, the survival rate was most encouraging, about 70% at 7 years. When patients with AML, ALL, and CML were evaluated separately, there was no difference in outcome between those treated with busulfan or TBI. This is in accordance with the majority of randomized trials and the retrospective EBMT analysis.8,10,12,23 However, in the French multicenter study in patients with AML, busulfan-treated patients had a lower 3-year probability of survival at 47%, compared with 72% for the TBI group (P < .01).9
To conclude, we found that busulfan treatment is associated with several late toxic side effects, eg, chronic GVHD, alopecia, and obstructive bronchiolitis. Cataract was more common in the TBI group. In patients with early disease, busulfan was as effective as TBI, but in high-risk patients, busulfan increased TRM and decreased survival.
ACKNOWLEDGMENT
We thank Inger Hammarberg for excellent manuscript preparation. We thank the staff at the Bone Marrow Transplant Units in Copenhagen, Helsinki, Huddinge, Lund, and Turku and Östra Hospital in Gothenburg for patient care.
Supported by grants from the Swedish Cancer Foundation (0070-B96-10XAC), Children’s Cancer Foundation (1994-060), Swedish Medical Research Council (K97-06X-05971-17A), FRF Foundation, Cancer och Trafikskedates Riksforbund, Tobias Foundation, Ellen Bachrach Foundation, and Sigrid Juselius Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to O. Ringdén, MD, PhD, Karolinska Institute, Division of Clinical Immunology, F79, Huddinge Hospital, SE-141 86 Huddinge, Sweden; e-mail: olle.ringden@immunlab.hs.sll.se.