Abstract
We have recently shown that C5b-6 binds to the erythrocyte membrane via an ionic interaction with sialic acid before the addition of C7 and subsequent membrane insertion. In this study we assessed the role of anionic lipids in the binding of the terminal complement proteins to the membrane and the efficiency of subsequent hemolysis. Human erythrocytes were modified by insertion of dipalmitoyl phosphatidylcholine (DPPC), dipalmitoyl phosphatidylserine (DPPS), dipalmitoyl phosphatidylethanolamine (DPPE), or dipalmitoyl phosphatidic acid (DPPA). Lipid incorporation and the hemolytic assays were done in the presence of 100 μmol/L sodium orthovanadate to prevent enzymatic redistribution of lipid. We found that the neutral lipids, DPPC and DPPE, did not affect C5b-7 uptake or hemolysis by C5b-9. In contrast, the two acidic phospholipids, DPPS and DPPA, caused a dose-dependent increase in both lysis and C5b-7 uptake. We conclude that the presence of anionic lipids on the exterior face of the membrane increases C5b-7 uptake and subsequent hemolysis. It is known that sickle cell erythrocytes have increased exposure of phosphatidylserine on their external face and are abnormally sensitive to lysis by C5b-9. The data presented here provide a plausible mechanism for this increased sensitivity.
THE MEMBRANE ATTACK complex of the complement system consists of five proteins: C5, C6, C7, C8, and C9.1-4 Upon activation, these proteins expose hydrophobic residues, insert into membranes, and form transmembrane channels. The process of channel formation can be divided into two phases: the initial interaction between the cell membrane, C5b-6, and C7 to form a stably inserted C5b-7 complex; and the subsequent addition of C8 and multiple C9 molecules to form a transmembrane channel.1-4
It is well established that the terminal complement proteins interact with membrane lipids via hydrophobic interactions.2,5,6Ionic interactions between C5b-6 and the cell membrane are also important.7,8 We recently showed that C5b-6 binds to sialic acid moieties on glycophorin, and that this binding increases the membrane uptake of C5b-6 and subsequent hemolysis by C5b-9.8
Silversmith and Nelsestuen7 used light scattering to show that the interaction of C5b-6 and C5b-7 with phospholipid vesicles is dependent on the lipid headgroup composition of the vesicles. C5b-6 bound to vesicles made of phosphatidic acid or phosphatidylglycerol but did not bind to vesicles composed of phosphatidylserine (PS), phosphatidylcholine (PC), or phosphatidylinositol.7
The major lipids of the erythrocyte membrane are sphingomyelin (SM), PC, phosphatidyl ethanolamine (PE), and PS.9 Under normal circumstances, the distribution of erythrocyte membrane lipids is asymmetrical; SM and PC are localized to the external lipid monolayer, while PS and PE are localized to the interior leaflet.9This nonequilibrium condition is maintained in part by the slow rate of “flip-flop” of lipids between the inner and outer monolayer, and in part by a lipid “flippase” that transports the amino-phospholipids PE and PS from the outer monolayer to the inner monolayer.10,11 The actual structure of this lipid flippase is not known, but it is inhibited by vanadate.9 11
One pathological condition in which the normal distribution of phospholipids is disrupted is sickle cell anemia. Membranes of deoxygenated sickle erythrocytes have significant quantities of PS and PE in the outer monolayer and a compensatory increase in PC in the inner monolayer.12 Sickle red cells that have undergone repeated cycles of oxygenation/deoxygenation assume an irreversibly sickled shape, and in contrast to the reversibly sickled cell, these cells have abnormal phospholipid asymmetry even under fully oxygenated conditions.12
It is well known that sickle cell erythrocytes are capable of activating complement via the alternative pathway due to the exposure of PS and PE on the outer monolayer.13,14 Test and Woolworth15 showed that erythrocytes from sickle cell anemia patients also showed a marked increase in susceptibility to lysis by the terminal complement proteins, C5b-9. The mechanism of this increased sensitivity to complement was due to increased binding of C5b-7 to the surface of the sickled erythrocytes.15
Based on our studies showing that sialic acid caused increased binding of C5b-7 to erythrocytes, we would predict that the presence of anionic lipids on the exterior monolayer of the erythrocytes may also increase lysis by increasing C5b-7 uptake.
To test this theory directly we incorporated exogenous synthetic lipids with different headgroups into normal human erythrocytes. We find that presence of anionic lipid in the outer monolayer of the erythrocytes increases both deposition of C5b-7 and subsequent lysis with the addition of C8 and C9, thus strengthening the hypothesis that ionic interactions between C5b-6 and the membrane are important in modulating membrane damage by the terminal complement proteins.
MATERIALS AND METHODS
Buffers and solutions.
All buffers were prepared with glass-distilled water and ultrafiltered before use. Phosphate-buffered saline (PBS): 150 mmol/L NaCl, 5 mmol/L sodium phosphate, pH 7.4; DGVB2+: veronal-buffered saline containing 71 mmol/L NaCl, 139 mmol/L dextrose, 2.5 mmol/L sodium veronal (pH 7.4), 0.1% gelatin, 0.15 mmol/L CaCl2, and 1 mmol/L MgCl2; GVB-EDTA: veronal-buffered saline containing 142 mmol/L NaCl, 2.5 mmol/L sodium veronal (pH 7.4), 0.1% gelatin, and 10 mmol/L EDTA.
Reagents.
Human erythrocytes were collected by venipuncture from volunteers. Blood was initially collected in buffered EDTA solution, washed, and stored in DGVB+2. C5b-6 was purchased from Advanced Research Technologies (San Diego, CA). C7, C8, and C9 were purchased from Quidel (San Diego, CA) or Advanced Research Technologies. Dipalmitoyl phosphatidylcholine (DPPC), dipalmitoyl phosphatidylserine (DPPS), dipalmitoyl phosphatidyl ethanolamine (DPPE), and dipalmitoyl phosphatidic acid (DPPA) were obtained from Avanti (Alabaster, AL). The fluorescent derivatives, nitrobenzoxadiazole (NBD)-DPPC, NBD-DPPS, NBD-DPPE, and NBD-DPPA were obtained from Avanti. The structure of these synthetic derivatives was similar in that the R1 fatty acid was 16:0 and the R2 fatty acid was 6:0 with the NBD group at the end of the acyl chain.14C-DPPC was obtained from New England Nuclear (Boston, MA).
Radiolabeling.
C7 was iodinated using IodoGen (Pierce Chemical Co, Rockford, IL) and125I (New England Nuclear). The specific activity was between 0.3 and 3.2 × 107 cpm/μg.
Hemolytic assays.
Hemolytic assays were performed as previously described.16Briefly, one volume of erythrocytes (1.5 × 108/mL) was incubated with one volume of C5b-6 (3.33 μg/mL) or buffer at 30°C for 30 minutes. One volume of C7 (2 μg/mL) was then added and the mixture incubated at 30°C for an additional 15 minutes. Cells were then washed and incubated with guinea pig serum in the presence of 10 mm EDTA at 37°C for 60 minutes. Lysis was determined spectrophotometrically. Percent lysis was calculated as (EXP− BK)/(CL − BK), where EXP is the OD412 of the sample containing C5b-9, BK is the OD412 of erythrocytes treated with C5b-7 only, and CL is the OD412 of 100% lysis.
Lipid incorporation.
We incorporated specific lipids into the external leaflet of the erythrocyte membrane by incubating human erythrocytes with phospholipid vesicles. Lipid uptake was monitored by incorporation of fluorescent-labeled phospholipid. An individual dipalmitoyl phospholipid (DPPA, DPPS, DPPE, or DPPC) was mixed with its fluorescent derivative (NBD-DPPA, NBD-DPPS, NBD-DPPE, or NBD-DPPC). Experiments were also performed with 14C-DPPC and unlabeled DPPC. Molar ratios of unlabeled lipid to fluorescent probe were 3:1. In the case of14C-DPPC, the molar ratio of unlabeled to labeled lipid was 8:1. Phospholipid/probe mixtures were dissolved in chloroform and dried under nitrogen. Vesicles were then prepared by addition of PBS and sonication for 10 minutes at room temperature with a probe sonicator. Contaminating large multilamellar vesicles were removed from the suspension by centrifugation at 2,000g for 15 minutes. Erythrocytes (final concentration 4.5 × 107/mL) were incubated with 15 mL of phospholipid vesicles for 30 minutes at 37°C. The lipid concentration was varied between 0 and 10 μg/mL. When cells were incubated with higher concentrations of lipid, the cells became excessively fragile, leading to increases in background lysis. In addition, when higher concentrations of lipid vesicles were incubated with cells, the lipid tended to bind to the cells as vesicles rather than incorporating into the membrane. Lipid incorporation and the hemolytic assays were done in the presence of 100 μmol/L sodium orthovanadate. This concentration has been shown to inhibit the human erythrocyte “flippase,” thus preventing enzymatic redistribution of lipid.9-11
A standard curve was generated by removing cells with a known concentration of lipid, lysing the cells with 1% sodium dodecyl sulfate (SDS) and reading the fluorescence in a Perkin-Elmer (Norwalk, CT) Fluorimeter (excitation λ, 470 nm; emission λ, 525 nm). Lipid incorporation was estimated reading the fluorescence of 107 lipid-enriched cells in the presence of 1% SDS and comparison with the standard curve.
To determine whether adherent vesicles were present in the treated erythrocyte preparations, fluorescence of treated erythrocytes was determined in the presence and absence of 2% Triton X-100. Because of he self-quenching properties of the labeled phospholipids, the phospholipid vesicles show about a 10-fold increase in fluorescence after lysis. Lipid that has inserted into the erythrocyte membrane is dequenched by dilution with membrane lipids, and its fluorescence is not increased by detergent solubilization. The erythrocytes used for complement activation studies did not show increased fluorescence after lysis, indicating minimal contamination with intact vesicles.17
Incorporation of 14C DPPC was done in a similar fashion except that the molar ratio of 14C DPPC to cold DPPC was 1:8. 14C was counted in a liquid scintillation counter (Beckman, Fullerton, CA) with quench correction.
Uptake of 125I-C7.
Phospholipid-enriched erythrocytes were incubated with C5b-6 and125I-C7 to assess C5b-7 uptake. A total of 0.1 mL of 1.5 × 108 erythrocytes were incubated with C5b-6 at 30°C for 30 minutes in GVB-EDTA. 125I-C7 was then added and the mixture incubated at 30°C for an additional 15 minutes. Cells were then pelleted at 750g for 3 minutes in a Eppendorf tabletop centrifuge (Brinkman, Old Westbury, NY). Erythrocytes were washed once with GVB-EDTA and pelleted at 750g. This procedure pellets only whole cells and not erythrocyte membranes that have been lysed; thus, we measured the C7 counts that were bound to cells, not ghost membranes. Cells were then lysed with 5 mmol/L sodium phosphate (pH 7.5) and membranes pelleted at 16,000g, washed once with PBS, and membranes pelleted at 16,000g and counted for125I. Background binding was estimated by incubating the cells in the presence of 125I-C7, but in the absence of C5b-6. Background counts were generally about 10% of the counts in the presence of C5b-6 and were subtracted from the total counts per minute to give specific counts per minute.
RESULTS
PS increases C5b-7 uptake and lysis by C5b-9.
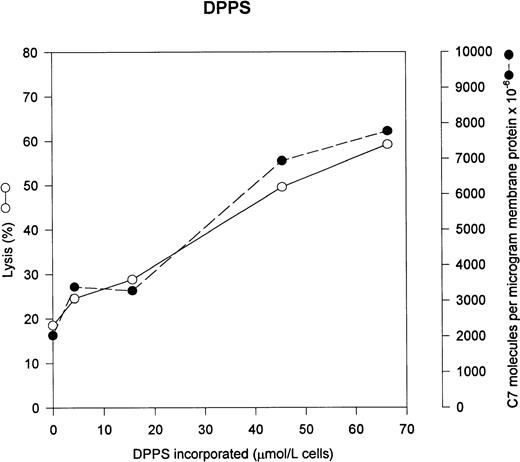
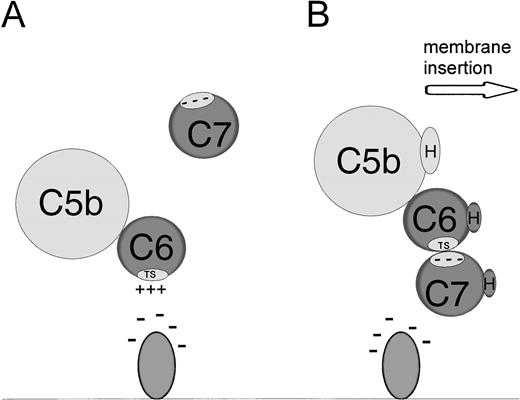
We wished to determine the effect of lipids with different head groups on uptake of C5b-7 and eventual lysis by C5b-9. Based on our earlier data, we would predict that the presence of anionic lipids such as DPPS and DPPA would increase both C5b-7 uptake and lysis. Figure 1 shows that incorporation of DPPS significantly increases both the uptake of C5b-7– and C5b-9–mediated lysis in a dose-dependent fashion. Under normal conditions, the human erythrocyte contains 446 μmol/L of PS per liter of cells, however virtually none of the PS is exposed on the outer monolayer.18
PS increases C5b-7 uptake and lysis by C5b-9. Various concentrations of PS were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 14% for the highest concentration of DPPS.
PS increases C5b-7 uptake and lysis by C5b-9. Various concentrations of PS were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 14% for the highest concentration of DPPS.
Phosphatidic acid increases C5b-7 uptake and lysis by C5b-9.
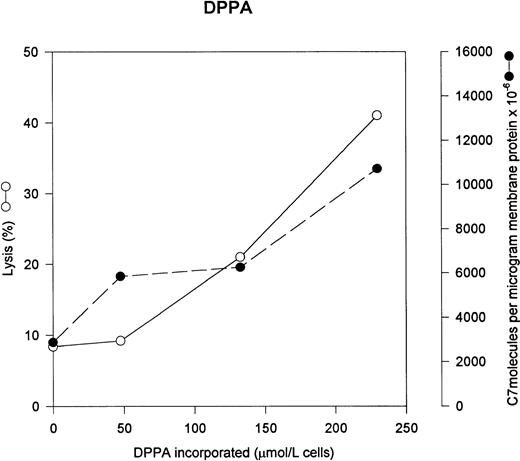
Figure 2 shows that DPPA also increased C5b-7 uptake and subsequent lysis by C5b-9. Phosphatidic acid is a minor component of the erythrocyte membrane (less than 80 μmol/L phosphatidic acid per liter of cells), thus the added PA represents a significant increase in the PA content of the erythrocyte.18
Phosphatidic acid increases C5b-7 uptake and lysis by C5b-9. Various concentrations of phosphatidic acid were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 18% for the highest concentration of DPPA.
Phosphatidic acid increases C5b-7 uptake and lysis by C5b-9. Various concentrations of phosphatidic acid were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 18% for the highest concentration of DPPA.
PC has no effect on C5b-7 uptake and lysis by C5b-9.
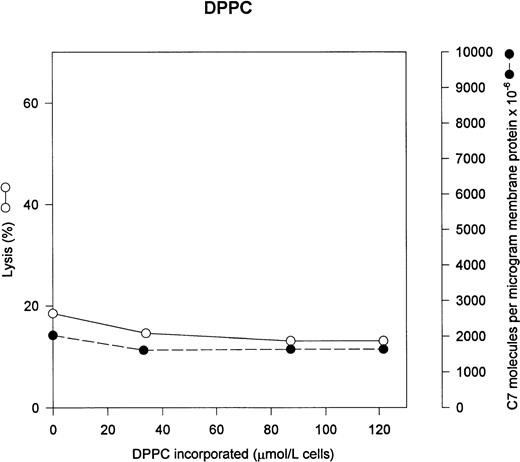
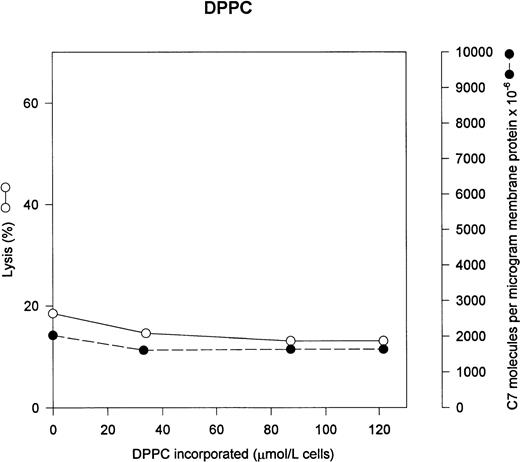
The two major neutral phospholipids of the erythrocyte are PC and PE. PC is normally the predominant phospholipid on the exterior face of the erythrocyte membrane. Figure 3 shows that addition of exogenous PC to the erythrocytes has no effect on either C5b-7 uptake or lysis by C5b-9. In normal erythrocytes, PC content is 944 μmol/L cells.18
PC has no effect on C5b-7 uptake and lysis by C5b-9. Various concentrations of PC were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 12% for the highest concentration of DPPC.
PC has no effect on C5b-7 uptake and lysis by C5b-9. Various concentrations of PC were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 12% for the highest concentration of DPPC.
PE has no effect on C5b-7 uptake and lysis by C5b-9.
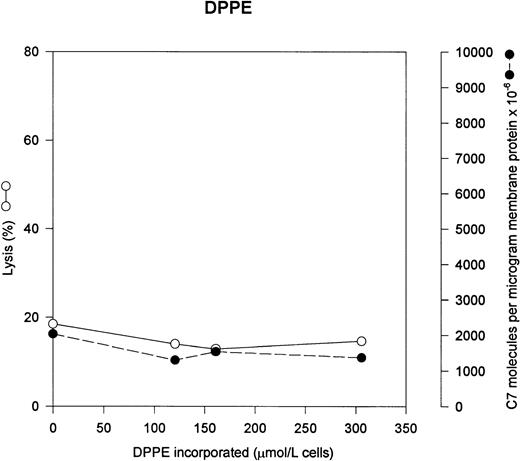
PE is the other major neutral phospholipid of the erythrocyte membrane. Under normal circumstances, the vast majority of the PE is localized to the interior leaflet of the lipid bilayer. In Fig 4, we incorporated increasing quantities of PE into the erythrocyte, and as can be seen, there is no increased lysis or increased uptake of C5b-7. Erythrocyte membranes contain 1040 μmol of PE per liter of cells; however, only 20% of the PE is present on the outer leaflet of unmodified erythrocytes (208 μmol/L). Thus the added PE is a significant percentage of the exposed PE.18
PE has no effect on C5b-7 uptake and lysis by C5b-9. Various concentrations of PE were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 8% for the highest concentration of DPPE.
PE has no effect on C5b-7 uptake and lysis by C5b-9. Various concentrations of PE were incorporated into erythrocytes in the presence of vanadate. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 8% for the highest concentration of DPPE.
Using 14C PC as a marker for total PC gives results identical with the fluorescent probe.
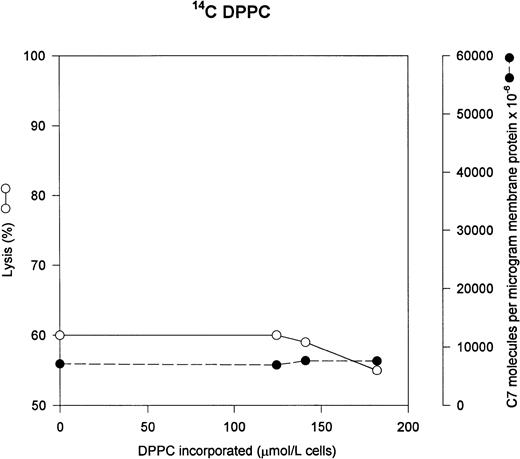
We wished to verify our results using a nonfluorescent probe to be sure our results were not affected by the presence of the fluorescent NBD moiety, so we used 14C PC. The results are shown in Fig 5. The use of the radioactive probe gave results that were identical to the fluorescent probe: addition of DPPC causes no increase in either C5b-7 uptake or lysis by C5b-9.
14C PC has no effect on C5b-7 uptake and lysis by C5b-9. DPPC was incorporated into the erythrocytes as in Fig3; however, we used 14C-DPPC as a marker of DPPC incorporation. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 5% for the highest concentration of DPPC.
14C PC has no effect on C5b-7 uptake and lysis by C5b-9. DPPC was incorporated into the erythrocytes as in Fig3; however, we used 14C-DPPC as a marker of DPPC incorporation. The cells were washed and used in a hemolytic assay to determine percent lysis (○). The same cells were used to measure uptake of C5b-7 using 125I-C7 (•). Lysis before the addition of C8 and C9 varied between 0% for the buffer control and 5% for the highest concentration of DPPC.
DISCUSSION
The results of this study show that the presence of anionic lipids on the external face of the erythrocyte membrane results in increased hemolysis by the terminal complement proteins. The increased lysis is due to increased deposition of C5b-7, as the C5b-7 uptake parallels the amount of increased lysis. Addition of two neutral phospholipids, DPPC and DPPE, showed no increase in either hemolysis or in C5b-7 uptake. In comparing the slopes of both lysis and C5b-7 uptake, DPPS is somewhat more efficient than DPPA in C5b-7 binding and subsequent hemolysis. This indicates that negative charge density alone is not responsible for the increased uptake of C5b-7. This result is similar to what we found in the case of C5b-6 binding to sialic acid. The negative charge of the sialic acid was essential for the interaction; however, other structural modifications also affected the ability of gangliosides to interact with C5b-6.8 In similar fashion, the serine moiety may serve to increase the affinity of C5b-6 for the membrane.
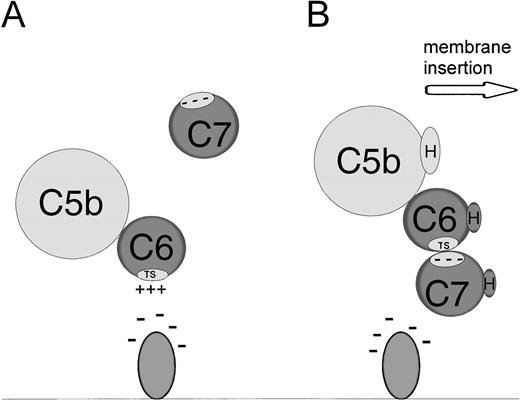
These data provide further evidence for the importance of ionic interactions between C5b-6 and anions on the cell membrane. Our model of this interaction is shown in Fig 6. When C5 is cleaved by the classical or alternative pathway C5 convertase, C5a is released into the fluid phase and C5b remains bound to the convertase.19 C5b then binds to C6 to form C5b-6. This complex then dissociates from the C5 convertase and binds to the anionic lipid headgroups via ionic forces. When C7 binds to C5b-6, the ionic bond between C5b-6 and the membrane is disrupted, C5b-7 exposes hydrophobic residues and the C5b-7 complex inserts into the membrane.
Model for interaction between membrane anions, C5b-6 and C7. (A) Binding of C5b-6 to anionic lipids via the grouped positive charges of the conserved TS1 domain in C6. (B) The effect of C7 addition. Binding of C7 via anionic residues disrupts the membrane-C5b-6 interaction and leads to exposure of hydrophobic residues, resulting in the dissociation of the C5b-7 complex from the anionic molecule and insertion into the lipid bilayer. TS represents the highly conserved TS1 domain in C6. H represents the hydrophobic domains of C5b, C6, or C7.
Model for interaction between membrane anions, C5b-6 and C7. (A) Binding of C5b-6 to anionic lipids via the grouped positive charges of the conserved TS1 domain in C6. (B) The effect of C7 addition. Binding of C7 via anionic residues disrupts the membrane-C5b-6 interaction and leads to exposure of hydrophobic residues, resulting in the dissociation of the C5b-7 complex from the anionic molecule and insertion into the lipid bilayer. TS represents the highly conserved TS1 domain in C6. H represents the hydrophobic domains of C5b, C6, or C7.
The amino acid sequence or sequences of C5b-6 that interacts with membrane anions is not known. The terminal complement proteins share several structural domains that are found in other proteins, including the thrombospondin type 1 motif (TS1).20 C6 has three and C7 has two TS1 domains. This sequence of amino acids binds to negatively charged molecules such as heparin, heparan sulfate proteoglycan, sulfatides, and cholesterol sulfate and contain grouped positive charges making them likely candidates to bind to anions.
Previous studies have investigated the role of lipid structure in complement lysis. Shin and coworkers showed that increasing the fluidity of the membrane increased the efficiency to lysis by C5b-9.21-23 These studies focused on altering the hydrophobic domain of the membrane in distinction to the current studies that examine the hydrophilic domain of the lipid molecule. Obviously both are important in controlling susceptibility of membranes to damage by C5b-9.
One pathological condition in which there is exposure of PE and PS on the outer monolayer of erythrocytes is sickle cell disease. The mechanism of this flip-flop is unclear and may be due to recently identified calcium-dependent phospholipid “scramblase,” as it is known that sickle cell erythrocytes do have elevated levels of calcium.24
Test and Woolworth found that sickle erythrocytes are abnormally sensitive to lysis by C5b-9 and that this abnormal sensitivity was due to increased deposition of C5b-7. Our current data supply a likely mechanism for the increased sensitivity of sickle erythrocytes to hemolysis by C5b-9.
There are some interesting similarities in the ways the terminal complement proteins and the alternative pathway activation proteins interact with membrane lipids. Exposure of PS and PE on the external membrane leaflet leads to increased activation of the alternative complement pathway.13 Thus, the exposure of PS on the external face of the sickle erythrocyte makes these cells more susceptible to complement lysis by at least two mechanisms, increased activation of the alternative pathway and increased channel formation.
Supported by a Veteran’s Administration Merit Review and a fellowship from the Charles Culpeper Foundation.
Address reprint request to Michael Whitlow, MD, PhD, Dermatology Service, Veteran’s Administration Medical Center, 423 E 23rd St, New York, NY 10010-5050.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.