Abstract
Glucocorticoid hormones (GCH) have been implicated as regulators of T-lymphocyte growth and differentiation. In particular, it has been reported that GCH can induce thymocyte apoptosis. However, the molecular mechanisms responsible for this GCH-induced death have not been clarified. In this work, the biochemical events associated with apoptosis induced by Dexamethasone (Dex), a synthetic GCH, in normal mouse thymocytes, have been analyzed. Results indicate that Dex-induced thymocyte apoptosis is attributable to an early ceramide generation caused by the activation of an acidic sphingomyelinase (aSMase). Caspase activity plays a crucial role in Dex-induced apoptosis and is downstream the aSMase activation in that inhibition of the early ceramide generation inhibits caspase activation and thymocyte death. Moreover, Dex treatment rapidly induces diacylglycerol (DAG) generation, through a protein kinase C (PKC) and G-protein–dependent phosphatidylinositol-specific phospholipase C (PI-PLC), an event which precedes and is required for aSMase activation. Indeed, PI-PLC inhibition by U73122 totally prevents Dex-induced aSMase activity, ceramide generation, and consequently, caspase activation and apoptosis. All these effects require Dex interaction with GCH receptor (GR), are countered by the GR antagonist RU486, and precede the GCH/GR-activated transcription and protein synthesis. These observations indicate that GCH activates thymocyte death through a complex signaling pathway that requires the sequential activation of different biochemical events.
REGULATION OF T-cell survival is important as a physiological mechanism involved in determining the development of the immune response, and also as one of the mechanisms that can contribute to control the expansion of T-cell tumors. Moreover, it is well accepted that apoptosis plays a relevant role at the thymus level where massive cell death occurs in a continuous selection process.1-3 Among different signals and stimuli involved in the regulation of T-cell death, glucocorticoid hormones (GCH) have been shown to regulate apoptosis of thymocytes and normal and neoplastic T lymphocytes.4-9 GCH-induced apoptosis involves the binding of GCH to their receptor (GCH receptor [GR] ), as the absence, deficiency, or malfunction of the latter can abrogate GCH-induced cell death.10-14 The presence at the thymus level of the enzymes responsible for steroid synthesis and the defects of T-cell development observed in GR-less mice, further suggest a physiological role of GCH in the control of thymocyte selection.15
GCH, which by themselves are apoptosis activators and induce thymocyte death, can also counteract thymocyte death activated by other stimuli such as antigen–T-cell receptor (TCR) interaction.9,16 Although possible mechanisms responsible for the protection effect of GCH against apoptosis have been recently proposed,16-18 the molecular mechanisms underlying the GCH-activated induction of apoptosis are unknown.
Previous studies have shown that GCH can affect sphingolipid metabolism. In particular, Dexamethasone (Dex), a synthetic GCH, increases sphingomyelinase (SMase) activity in rat epididymal fat cells,19 human neutrophils,20 and HeLa cells.21 GCH also increase the activities of phosphatidylcoline:ceramide cholinophosphotransferase and sphingomyelinase in 3T3-L1 fibroblasts,22,23 and in Epstein-Barr virus (EBV)-infected B lymphocytes.24 25 However, the role of those metabolic changes in cell death decision or the subsequent execution of apoptosis is currently unknown.
The induction of SMase activity as an effect of the GCH treatment may have major consequences in cell death induction. Signaling through the sphingomyelin (SM) pathway is mediated via generation of ceramide, which acts as a second messenger in stimulating a variety of cellular functions.26-28 Studies on the involvement of the SM signaling system showed that several cytokines including tumor necrosis factor (TNF) α,29-31CD95/Fas/APO-1,32-35 and environmental stresses, such as ionizing radiation, ultraviolet-C, and heat, and oxidative stress36-38 induce rapid ceramide generation while effecting an apoptosis response.
In the present study, the early biochemical events triggered by Dex and their role in thymocyte apoptosis have been characterized. We present evidence that Dex/GR interaction activates a complex signaling pathway required for cell death induction. In particular, an early generation of ceramide, through the sequential activation of phosphatidylinositol-specific phospholipase C (PI-PLC) and acidic SMase (aSMase), is required for Dex-induced caspase activation and apoptosis.
MATERIALS AND METHODS
Cell system and treatments.
Thymocytes, from 4- to 6-week-old C3H/HeN mice, were enriched by passage through nylon wool columns. The effect of several agents on Dex-induced apoptosis, ceramide generation, and phospholipase activity was evaluated. These were RU486, a glucocorticoid receptor antagonist39; U73122 (Calbiochem, La Jolla, CA), a PI-PLCβ inhibitor40; D609 (Kamiya, Thousand Oaks, CA), a phosphatydylcholine-specific phospholipase C (PC-PLC) inhibitor41; monensin (Sigma, St Louis, MO),42 NH4Cl (Sigma),43 and bafilomycin A1 (a generous gift of Prof Cesare Montecucco, Department of Biomedical Science, University of Padova, Italy)44 to inhibit aSMase activity; the broad serine/threonine kinase inhibitor H7 dihydrochloride (H7; Calbiochem) and the highly specific calphostin-C (Calbiochem) to inhibit protein kinase C (PKC) activity45,46; and Z-Val-Ala-Asp (Ome)-monofluoroketone (Z-VAD-FMK) (Calbiochem), an inhibitor of caspases, including caspase-1.47 In general, cells were incubated with inhibitors for 30 minutes before the addition of Dex, at the concentrations indicated in the figure legends.
Apoptosis evaluation by propidium iodide solution.
Apoptosis was measured by flow cytometry as described elsewhere.48 After culturing, cells were centrifuged, and the pellets were gently resuspended in 1.5 mL hypotonic propidium iodide solution (PI, 50 μg/mL in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma). The tubes were kept at 4°C in the dark overnight. The PI-fluorescence of individual nuclei was measured by flow cytometry with standard FACScan equipment (Becton Dickinson, Mountain View, CA). The nuclei traversed the light beam of a 488-nm argon laser. A 560-nm dichroid mirror (DM 570) and a 600-nm band pass filter (band width 35 nm) were used to collect the red fluorescence caused by PI DNA staining, and the data were recorded in logarithmic scale in a Hewlett Packard (HP 9000, model 310; Palo Alto, CA) computer. The percentage of apoptotic cell nuclei (subdiploid DNA peak in the DNA fluorescence histogram) was calculated with specific FACScan research software (Lysis II).
Ceramide mass measurement (diacylglycerol kinase assay).
Aliquots of 5 × 106 cells were suspended in 1 mL of RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine, and antibiotics, and treated for the indicated times with Dex in the presence or absence of inhibitors. Treatment was stopped by immersion of samples in methanol/dry ice (−70°C) for 10 seconds, followed by centrifugation at 4°C in a microfuge. To measure ceramide levels, pellets were dissolved in a buffer containing Tris-HCl 20 mmol/L pH 7.4), 1 μmol/L phenylmethylsulfonyl fluoride (PMSF), 10 μmol/L leupeptin, 10 μmol/L pepstatin, and 1 μmol/L aprotinin. After incubation for 5 minutes at 4°C, the cells were sonicated (5W, 80% output, 1 minute and 50 seconds, alternating 10-second sonication and 10-second pause) with a Vibracell sonicator (Sonic and Materials Inc, Danbury, CT), and centrifuged for 30 minutes at 14,000 rpm at 4°C. The supernatants were then collected, and protein concentration was determined through the Pierce Micro BCA assay kit (Pierce, Rockford, IL), with bovine serum albumine standards. Lipids were extracted by the sequential addition of 400 μL methanol, 500 μL chloroform, and 200 μL water. Samples were stirred for 2 minutes on a vortex-mixer and centrifuged at 13,000 rpm for 10 minutes. The extraction and centrifugation steps were repeated twice. The organic phases, obtained from different extraction steps, were collected, washed once with 1 mL of solvent system containing chloroform/methanol/water (3/48/47, by volume), essiccated under nitrogen, and then incubated withEscherichia coli diacylglycerol kinase (DAG kinase assay kit and 32P-adenosine triphosphate (ATP) gamma (specific activity 3 Ci/mmol; Amersham, Arlington Heights, IL). Ceramide phosphate was then isolated by thin layer chromatography using CHCl3/CH3OH/CH3COOH (65/15/5, vol/vol/vol) as solvent. Authentic ceramide from bovine brain (Ceramide Type III, nonhydroxy fatty acid ceramides; Sigma) was identified by autoradiography at retardation factor (Rf) = 0.25. Specific radioactivity of ceramide-1-phosphate was determined by scintillation counting of corresponding spots scraped off the gel. Quantitative results for ceramide production were obtained from comparing the experimental values with a linear curve of the ceramide standards and are expressed as picomoles of ceramide-1-phosphate/106 cells.
In vitro acidic and neutral SMase analysis.
Aliquots of 6 × 106 cells/mL were treated for the indicated times with Dex (10−7 mol/L). Treatment was stopped by immersion of samples in methanol/dry ice (−70°C) for 10 seconds followed by centrifugation at 4°C in a microfuge. To measure neutral SMase (nSMase), pellets were dissolved in a buffer containing 20 mmol/L HEPES (pH 7.4), 10 mmol/L MgCl2, 2 mmol/L EDTA, 5 mmol/L dithiothreitol (DTT), 0.1 mmol/L Na3VO4, 0.1 mmol/L Na2MoO4, 30 mmol/Lp-nitrophenylphosphate, 10 mmol/L β-glycerophosphate, 750 mmol/L ATP, 1 μmol/L PMSF, 10 μmol/L leupeptin, 10 μmol/L pepstatin, and 0.2% Triton X-100. After incubation for 5 minutes at 4°C, the cells were sonicated as described above and centrifuged for 30 minutes at 14,000 rpm at 4°C. The supernatants were then collected, and protein concentration was determined through the Pierce Micro BCA assay kit with bovine serum albumine standards. Proteins (50 to 100 μg) were incubated for 2 hours at 37°C in a buffer containing 20 mmol/L HEPES (pH 7.4), 1 mmol/L MgCl2, and 0.32 μL of N-methyl-14C SM (0.04 μCi/mL, specific activity 56.6 mCi/mmol; Amersham). To measure aSMase, after treatment, the cells were washed, and the pellet was resuspended in 200 μL of 0.2% Triton-X-100 and incubated for 15 minutes at 4°C. The cells were sonicated, and the protein concentration was assayed. Fifty to one hundred micrograms of protein were incubated for 2 hours at 37°C in a buffer (50 μL final volume) containing 250 mmol/L sodium acetate, 1 mmol/L EDTA (pH 5.0), and 0.32 μL of N-methyl-14C SM (0.04 μCi/mL, specific activity 56.6 mCi/mmol; Amersham). The reaction was stopped by the addition of 250 μL chloroform:methanol (2:1, by volume). The lipids were extracted as described above. The organic phase, obtained in the different extraction steps, was collected and washed once with 1 mL chloroform:methanol:water (3:48:47, by volume), to totally remove free radioactive phosphorylcholine. The aqueous phases were collected, transferred to scintillation vials, and routinely counted by liquid scintillation counting. The counts/minute represented the choline phosphate generated from SM hydrolysis. The organic phase was analyzed on thin-layer chromatography (TLC) plates by using chloroform:methanol:ammonia hydroxide (7 N):water (85:15:0.5:0.5, by volume). The hydrolysis of SM was quantitated by autoradiography and liquid scintillation and expressed as picomoles SM hydrolyzed/106cells.
Ceramide synthase assay.
Assay of ceramide synthase activity was performed as previously described.49 After treatment with Dex for different times (5 to 180 minutes), thymocytes were collected and resuspended in 300 μL of homogenization buffer (25 mmol/L HEPES (pH 7.4), 5 mmol/L EGTA, 50 mmol/L NaF, 10 μg/mL leupeptin, and 10 μg/mL soybean trypsin inhibitor), disrupted by sonication, and lysates were centrifuged at 800g for 5 minutes. Protein concentrations in the postnuclear supernatants were determined through the Micro BCA protein assay reagent kit (Pierce), with bovine serum albumine standards. Proteins of 75 μg were incubated in a 1-mL reaction mixture containing 2 mmol/L MgCl2, 20 mmol/L HEPES (pH 7.4), 20 μmol/L defatted bovine serum albumine (Sigma), 20 μmol/L dihydrosphingosine, 70 μmol/L unlabeled palmitoyl-coenzime A, and 3.6 μmol/L (0.2 μCi) [1-14C]palmitoyl-coenzyme A (55 mCi/mmol; Amersham). Dihydrosphingosine was dried under N2 from a stock solution in 100% ethanol and dissolved with sonication in the reaction mixture before addition of cell extracts. The reaction was started by addition of palmitoyl-coenzyme A, incubated at 37°C for 1 hour, and then stopped by extraction of lipids using 2 mL of chloroform/methanol (1/2, by volume). Lower phase was removed, concentrated under N2, and applied to a silica gel 60 TLC plate. Dihydroceramide was resolved from free radiolabeled fatty acid using a solvent system of chloroform/methanol/3.5 N ammonium hydroxide (85/15/1), identified by autoradiography based on comigration with ceramide standards (stained with iodine vapor), and quantified by liquid scintillation counting. The amount of palmitoyl-CoA consumed did not exceed 5% of total.
PI-PLC and PC-PLC activity assay.
PI-PLC and PC-PLC activities were determined in vitro by their ability to hydrolyze 14C-PC or 14C-PI vesicles, respectively, to generate DAG. Cells were treated for the indicated times with Dex (10−7 mol/L) in the presence or absence of the PC-PLC inhibitor, D609 (50 μg/mL),41 or the PI-PLC inhibitor, U73122 (2.5 μmol/L).40 Treatment was stopped by immersion of samples in methanol/dry ice (−70°C) for 10 seconds followed by centrifugation at 4°C in a microfuge. The pellets were then resuspended in 250 mmol/L Tris-HCl buffer (pH 7.4), containing 10 μmol/L PMSF, 100 μmol/L bacitracin, 1 mmol/L benzamidine, 1 μmol/L aprotinin, 10 μmol/L leupeptin, 10 μmol/L pepstatin, and 5 μg/mL soybean trypsin inhibitor. Cells were lysed by sonication with a cell sonifier. Radiolabeled PC or PI vesicles were prepared by sonicating (5 minutes, 5W, and 80% output) L-3-phosphatidyl [N-methyl-14C] choline-1,2,dipalmitoyl (specific activity 56 mCi/mmol; Amersham) or L-3-phosphatidylinositol-1stearoyl-2[14C]arachidonoyl for the detection of released DAG through PC-PLC or PI-PLC, respectively. Vesicles were resuspended at 10 μmol/L in the reaction buffer (50 mmol/L Tris-HCl [pH 7.4], 5 mmol/L CaCl2, 5 mmol/L MgCl2, and 0.01% fatty acid free–bovine serum albumin [BSA]). Whole cell lysate (50 to 100 μg proteins) was added to 250 μL reaction buffer containing the vesicles, incubated at 37°C for 1 hour, and the reaction buffer was stopped by the addition of 250 μL chloroform:methanol:acetic acid (4:2:1, by volume). To separate the organic from the aqueous phase, 250 μL of H2O, 250 μL of CHCl3, and 100 μL of KCl were added, and the mixture was centrifuged at 4,000 rpm in a microfuge for 5 minutes. The organic phase was removed, dried under nitrogen, resuspended in 200 μL chloroform, and applied to a silica gel TLC plate (Merck, Darmstadt, Germany), with an automatic applicator (Linomat IV; CAMAG, Muttenz, Germany). Samples were chromatographed in chloroform:methanol:acetic acid:water (100:60:16:8) to separate the parent phospholipids from the product of PC-PLC and PI-PLC, ie, DAG. Authentic standards were cochromatographed with the lipid extracts to locate the compounds of interest by exposure to iodine vapor. Radioactive spots, visualized by autoradiography and corresponding to standards, were scraped from the plate and counted by liquid scintillation. Radioactive measurements were converted to picomoles of product by using the specific activity of substrate. Blank values obtained from controls lacking cell proteins were subtracted from the experimental values. PC-PLC or PI-PLC activities were expressed as picomoles DAG produced/106 cells.
CPP32 activity assay.
The CPP32/Caspase-3 protease activity was assayed by using the ApoAlert CPP32 Caspase-3 Colorimetric Assay Kit (Clontech Laboratories, Inc, Palo Alto, CA), based on spectrophotometric detection at 405 nm of the chromophore p-nitroanilide ( pNA) after cleavage from the labeled substrate Asp-Glu-Val-Asp (DEVD)-pNA. The units of protease activity can be quantitated using a standard curve established with the chromogenic molecule. Briefly, the cells (2 × 106/mL), after treatment with Dex (10−7 mol/L) for 8 hours, in the presence or absence of inhibitors, were collected and resuspended in a lysis buffer. Cellular lysates were then incubated in a microplate in the presence of conjugated protease substrate for 1 hour at 37°C and then analyzed using a 96-well plate reader. To verify that the signal detected was attributable to protease activity, the effect of the irreversible CPP32 inhibitor DEVD-FMK on the Dex-induced caspase activity was also tested. Thus, the cell lysates from Dex-induced samples were treated with CPP32 inhibitor before incubation with the substrate, according to the manufacturer’s instructions. Caspase activity is expressed as nanomoles pNA/106cells.
Statistical analysis.
For in vitro data analysis, the Student’s t-test was performed by the STATPAC Computerized Program (STATPAC Inc, Minneapolis, MN), and a P value less than .05 was used as the significance criterion.
RESULTS
Dex treatment induces thymocyte apoptosis and ceramide generation.
Dex treatment caused a dose-dependent increase in the concentration of endogenous ceramide, which correlated well with the dose-related Dex-induced apoptosis showed in Fig1A. In Figs 1B and C the results are reported showing ceramide generation from thymocytes incubated for 15 minutes in the presence of various concentrations of Dex (ranging from 10−7 to 10−12 mol/L), obtained by treating lipid extracts with DAG kinase for quantitation of ceramide-1-phosphate amounts. A basal level of ceramide was evident in the untreated control and further significantly increased after Dex treatment (P < .001, 10−7, 10−8, and 10−10 mol/L compared with untreated control). Moreover, the dose able to induce maximal ceramide generation was 10−7 mol/L and corresponded to Dex concentration able to induce maximal levels of apoptosis in these cells (Fig 1A). As for apoptosis, levels of ceramide declined almost to the basal level at the Dex concentrations of 10−12 mol/L, being not significantly different from control value (P = .33).
(A) Dex-induced apoptosis. Mouse thymocytes incubated for 18 hours with or without different doses of Dex (10−7 to 10−12 mol/L), were processed for DNA content analysis by propidium iodide staining. Nuclei were analyzed with a FACScan cytofluorymeter (Becton Dickinson, Mountain View, CA). Percentage numbers of hypodiploid nuclei are reported for each condition. Data shown are representative of one out of four experiments. (B) Ceramide generation after Dex treatment. Thymocytes were treated with Dex at indicated doses for 15 minutes. Lipids were then extracted, subjected to DAG kinase assay, and separated by TLC. Radioactive spots were visualized by autoradiography, scraped from the plate, and counted by scintillation counting. Data shown are representative of one out of three experiments. (C) Quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. Standard deviations <10% of the mean values are omitted for clarity.
(A) Dex-induced apoptosis. Mouse thymocytes incubated for 18 hours with or without different doses of Dex (10−7 to 10−12 mol/L), were processed for DNA content analysis by propidium iodide staining. Nuclei were analyzed with a FACScan cytofluorymeter (Becton Dickinson, Mountain View, CA). Percentage numbers of hypodiploid nuclei are reported for each condition. Data shown are representative of one out of four experiments. (B) Ceramide generation after Dex treatment. Thymocytes were treated with Dex at indicated doses for 15 minutes. Lipids were then extracted, subjected to DAG kinase assay, and separated by TLC. Radioactive spots were visualized by autoradiography, scraped from the plate, and counted by scintillation counting. Data shown are representative of one out of three experiments. (C) Quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. Standard deviations <10% of the mean values are omitted for clarity.
The kinetics of ceramide induction after Dex treatment were also evaluated. Thymus cells were treated with Dex for different times, and lipid extracts from cell suspensions were prepared and subjected to ceramide level determination through DAG kinase assay. Figure2A shows that Dex caused a rapid ceramide level increase, which was evident within 5 minutes, reached a maximum at 15 minutes, and declined after 2 hours of treatment. Values relative to Dex-treated samples were significantly different from corresponding control values at all the analyzed times (P < .01).
(A) Kinetics of ceramide generation induced by Dex treatment. Thymocytes were treated with Dex (10−7 mol/L) for different times. Lipids were then extracted, subjected to DAG kinase assay, and separated by TLC. Radioactive spots were visualized by autoradiography, scraped from the plate, and counted by scintillation counting. On the left, the quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106cells are reported (mean values of three different experiments in duplicate; standard deviations, less than 10% of the mean values, are omitted for clarity). On the right side, a representative autoradiography of a ceramide chromatogram is shown. (B) C2-ceramide–induced apoptosis. Thymocytes were incubated for 18 hours with 10 μmol/L C2-ceramide in the presence or absence of actinomycin-D (Act-D; 2.5 μg/mL) and cycloheximide (CHX; 50 μg/mL) and analyzed for apoptosis. Percentage numbers of hypodiploid nuclei are reported for each condition. Data shown are representative of one out of two experiments. (C) Dex-induced SMase activation. Acidic (on the left) and neutral (on the right) SMase activity induced by Dex treatment at the indicated times. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. The mean values from two determinations are reported. Standard deviation values were lower than 3% of mean values. The results are representative of one out of three separate experiments.
(A) Kinetics of ceramide generation induced by Dex treatment. Thymocytes were treated with Dex (10−7 mol/L) for different times. Lipids were then extracted, subjected to DAG kinase assay, and separated by TLC. Radioactive spots were visualized by autoradiography, scraped from the plate, and counted by scintillation counting. On the left, the quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106cells are reported (mean values of three different experiments in duplicate; standard deviations, less than 10% of the mean values, are omitted for clarity). On the right side, a representative autoradiography of a ceramide chromatogram is shown. (B) C2-ceramide–induced apoptosis. Thymocytes were incubated for 18 hours with 10 μmol/L C2-ceramide in the presence or absence of actinomycin-D (Act-D; 2.5 μg/mL) and cycloheximide (CHX; 50 μg/mL) and analyzed for apoptosis. Percentage numbers of hypodiploid nuclei are reported for each condition. Data shown are representative of one out of two experiments. (C) Dex-induced SMase activation. Acidic (on the left) and neutral (on the right) SMase activity induced by Dex treatment at the indicated times. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. The mean values from two determinations are reported. Standard deviation values were lower than 3% of mean values. The results are representative of one out of three separate experiments.
In Fig 2B, the results are reported of a representative experiment showing that thymocytes undergo apoptotic death on exposure to cell-permeant synthetic C2-ceramide (10 μmol/L) for 18 hours. Similar results were obtained with thymocytes exposed to synthetic ceramide (10 μmol/L) for 1 hour, extensively washed, and cultured for 17 additional hours in fresh medium. In this experiment, the percentage of apoptosis was as follows: untreated controls, 17%; ceramide treated, 65%. By contrast, the structural analogue C2-dihydroceramide (10 μmol/L) was totally ineffective, thus supporting the stereospecificity of ceramide (not shown, Obeid et al30). Moreover, apoptosis induced by exogenous ceramide was inhibited by treatment with mRNA (actinomycin-D) and protein synthesis (cycloheximide) inhibitors (Fig 2B).
Dex induces activation of both acidic and neutral SMases.
The contribution of acidic and neutral SMase to Dex-induced ceramide generation in thymocytes was investigated. The two classes of SMases can be defined based on in vitro pH optima: aSMase requires pH of 4.5 to 5.5 and DAG for activation, whereas nSMase prefers pH of 7.0 to 7.5.50-52 In addition, aSMase, consistent with its subcellular localization in vivo, is particularly resistant to the action of proteases and phosphatases, unlike nSMase, which is in turn associated with the outer cell membrane or within the cytosol.53-55 nSMase does not require DAG for activation and may require Mg2+. Moreover, the residual aSMase activity at neutral pH was totally abolished in the presence of ATP and β-glycerophosphate. Thus, the analysis of SMases in different buffers (see Materials and Methods) allows to discriminate between aSMase and nSMase activity. Cellular extracts from mouse thymocytes treated for different times (5 to 180 minutes) with Dex 10−7 mol/L were incubated with radiolabeled SM vesicles to detect aSMase or nSMase activity. Results of a representative experiment presented in Fig 2C show that both aSMase and nSMase activities could be detected in extracts from untreated cells and significantly increased after Dex treatment. In particular, the Dex-induced augmentation of aSMase activity was indeed evident and statistically significant (P < .001) as early as 5 minutes from Dex addition, was maximal at about 15 to 30 minutes, and returned to the basal level within 180 minutes. Augmentation of nSMase activity was detectable and statistically significant (P < .02) at 30 minutes, reached a peak at 120 minutes after treatment, and then decreased.
Taken together, these data indicate that Dex treatment induces two different temporal waves of SM hydrolysis and consequent ceramide generation through the sequential activation of aSMase and nSMase.
Ceramide synthase was not influenced by Dex treatment.
The possibility that Dex-induced ceramide level increase in thymocytes could also be caused by the activation of ceramide synthase was assessed. This enzyme, also known as sphinganine N-acyl transferase,50 catalyzes the condensation of sphinganine and fatty acyl-coenzyme A to form dihydroceramide, which is rapidly oxidized to ceramide. This pathway seemed to be slightly activated in control thymocytes (data not shown). This constitutive activity (about 5 to 8 pmol ceramide/106 cells) did not change when cells were incubated for 180 minutes and could account, together with constitutive SMase activity (Fig 2C), for the basal ceramide generation observed in these cells. On the other hand, Dex treatment did not significantly change the ceramide synthase activity in our experimental conditions and during the analyzed time interval (5 to 180 minutes; not shown), thus indicating that the above described Dex-induced ceramide increase could not be attributable to a stimulation of this pathway.
Role of acidic compartment in Dex-induced ceramide generation and apoptosis.
Because of the lack of a specific inhibitor, we used some agents able to undirectly inhibit aSMase through different mechanisms.42-44 In fact, to evaluate the possible contribution of aSMase and nSMase to Dex-induced apoptosis, thymocytes were treated for different times with Dex 10−7 mol/L in the presence of three different endolysosomotropic agents such as monensin (10 μmol/L), NH4Cl (8 mmol/L), or bafilomycin A1 (1 μmol/L), able to raise, through different mechanisms, the pH to neutrality in endolysosomal compartments, thus impeding the activation of lysosomal enzymes, including aSMase.35,56 As expected, pretreatment with monensin, a Na+ ionophore able to form stable complexes with monovalent cations,42 prevented the Dex-induced activation of aSMase (Fig 3A, left panel) without affecting nSMase (Fig 3A, right panel), as assayed in vitro on radiolabeled SM vesicles at 15 and 120 minutes, respectively. Moreover, monensin treatment was able to inhibit the early (5 to 15 minutes) ceramide generation caused by the activity of the aSMase, but did not affect the ceramide level increase observed later (120 minutes) and caused by the activity of nSMase (Fig3B). Similar results (not shown) were obtained with NH4Cl, a weak base that increases vacuolar pH,43 and with the highly specific inhibitor of the H+-ATPase pump, able to alkalinize lysosomes and late endosomes, bafilomycin A1 (1 μmol/L).44 Moreover, monensin, as well as bafilomycin A1, showed a strong protective effect on Dex-induced apoptosis, being able to totally abrogate thymocyte death (Fig 3C). Similar results were obtained with NH4Cl (not shown).
Effect of monensin on Dex-induced ceramide generation, aSMase and nSMase, and apoptosis. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of monensin (10 μg/mL). (A) Effect of monensin on acidic (on the left) and neutral (on the right) SMase activity induced by Dex treatment for 15 or 120 minutes, respectively. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. Standard deviations, less than 5% of the mean values, are omitted for clarity. (B) Ceramide levels in thymocytes treated for 15 minutes with Dex (10−7 mol/L) in the presence or absence of monensin. The quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106 cells, are reported (mean values of three different experiments in duplicate; standard deviations, less than 10% of the mean values, are omitted for clarity). (C) Effect of monensin or bafilomycin A1 (1 μmol/L) on Dex-induced thymocyte apoptosis at 18 hours. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments.
Effect of monensin on Dex-induced ceramide generation, aSMase and nSMase, and apoptosis. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of monensin (10 μg/mL). (A) Effect of monensin on acidic (on the left) and neutral (on the right) SMase activity induced by Dex treatment for 15 or 120 minutes, respectively. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. Standard deviations, less than 5% of the mean values, are omitted for clarity. (B) Ceramide levels in thymocytes treated for 15 minutes with Dex (10−7 mol/L) in the presence or absence of monensin. The quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106 cells, are reported (mean values of three different experiments in duplicate; standard deviations, less than 10% of the mean values, are omitted for clarity). (C) Effect of monensin or bafilomycin A1 (1 μmol/L) on Dex-induced thymocyte apoptosis at 18 hours. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments.
These findings suggest that Dex-induced thymocyte death is caused by aSMase activation responsible for the early ceramide generation.
Dex-induced activation of aSMase is dependent on PI-PLC activity.
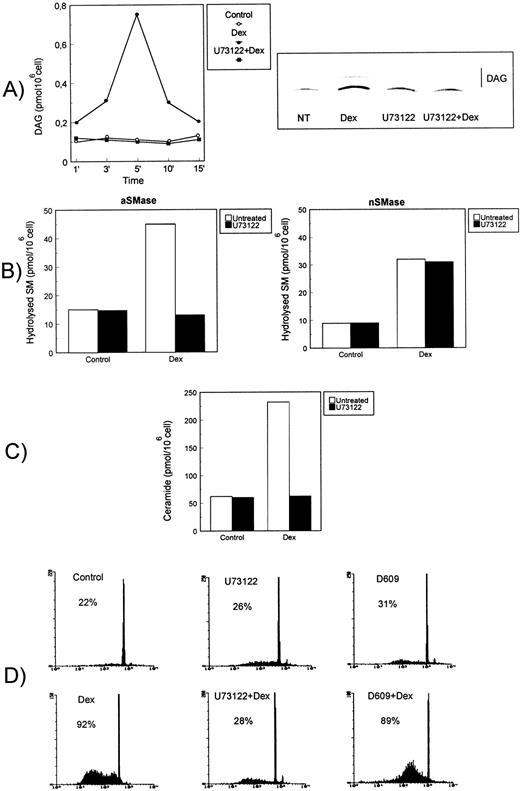
It has been previously reported that aSMase, associated with the lysosomal intracellular compartment or the caveolae, requires DAG for activation.50-52 The possible effect of Dex on the activity of DAG-generating enzymes, such as PI-PLC or PC-PLC, was directly addressed by evaluating the enzymatic activities in vitro using appropriate radiolabeled substrates and TLC analysis of the reaction products. In Fig 4, results are reported showing that Dex induced PI-PLC activity in mouse thymocytes. In particular, augmentation of PI-PLC activity was evident and statistically significant (P < 0.001) at 1 minute, peaked at 5 minutes, and then declined within 15 minutes after Dex treatment (Fig 4A, left panel). Furthermore, Dex-induced PI-PLC activation could be totally blocked by pretreatment of cells with U73122, a selective PLCβ inhibitor able to interfere with the G-protein-PLC coupling.40 Figure 4A (right panel) shows the results of a representative TLC autoradiography relative to the U73122-inhibitory effect of Dex-induced DAG generation at 5 minutes. No induction of PC-PLC activity could be detected in extracts from thymocytes treated with Dex (not shown).
PI-PLC activity involvement in Dex-induced thymocyte apoptosis. Effect of U73122 on (A) Dex-induced PI-PLC activity, (B) aSMase (left) and nSMase (right), (C) ceramide generation, and (D) apoptosis. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of U73122 (2.5 μmol/L) or D609 (50 μg/mL). (A) Dex-induced PI-PLC activity. Cell extracts were reacted with radiolabeled PI vesicles, and then DAG released was separated by TLC, visualized by autoradiography, scraped from the plate, and counted by scintillation counting. PI-PLC activity is expressed as picomoles DAG/106 cells (left side). Mean values of three different experiments in duplicate are reported. Standard deviations (SD), less than 3% of the mean values, are omitted for clarity. On the right side, a representative autoradiography of a DAG chromatogram is shown. (B) Acidic (on the left) and neutral (on the right) SMase activity induced after 15 or 120 minutes of Dex treatment, respectively. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. SD, less than 5% of the mean values, are omitted for clarity. (C) Ceramide levels in thymocytes treated for 15 minutes with Dex (10−7 mol/L) in the presence or absence of U73122. The quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106cells, are reported (mean values from three experiments in duplicate; SD values, less than 10% of mean values, are omitted for clarity). (D) Effect of U73122 and D609 on Dex-induced thymocyte apoptosis, as detected after an 18-hour culture. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments.
PI-PLC activity involvement in Dex-induced thymocyte apoptosis. Effect of U73122 on (A) Dex-induced PI-PLC activity, (B) aSMase (left) and nSMase (right), (C) ceramide generation, and (D) apoptosis. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of U73122 (2.5 μmol/L) or D609 (50 μg/mL). (A) Dex-induced PI-PLC activity. Cell extracts were reacted with radiolabeled PI vesicles, and then DAG released was separated by TLC, visualized by autoradiography, scraped from the plate, and counted by scintillation counting. PI-PLC activity is expressed as picomoles DAG/106 cells (left side). Mean values of three different experiments in duplicate are reported. Standard deviations (SD), less than 3% of the mean values, are omitted for clarity. On the right side, a representative autoradiography of a DAG chromatogram is shown. (B) Acidic (on the left) and neutral (on the right) SMase activity induced after 15 or 120 minutes of Dex treatment, respectively. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. SD, less than 5% of the mean values, are omitted for clarity. (C) Ceramide levels in thymocytes treated for 15 minutes with Dex (10−7 mol/L) in the presence or absence of U73122. The quantitative results for ceramide-1-phosphate levels, expressed as picomoles/106cells, are reported (mean values from three experiments in duplicate; SD values, less than 10% of mean values, are omitted for clarity). (D) Effect of U73122 and D609 on Dex-induced thymocyte apoptosis, as detected after an 18-hour culture. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments.
PI-PLC seemed to be crucial for Dex-induced aSMase activation. Indeed, the pretreatment of cells with U73122 totally blocked Dex-induced aSMase activation assayed at 15 minutes (Fig 4B, left panel), without affecting the nSMase assayed at 120 minutes (Fig 4B, right panel), suggesting that PI-PLC and aSMase activation are two sequentially related steps of the same pathway. In accordance, ceramide generation was strongly compromised in Dex-treated cells cultured in the presence of U73122 (Fig 4C) as a result of inhibition of the early ceramide peak (15 minutes) attributable to aSMase. Of note, the presence of U73122 in Dex-treated cells totally prevented steroid-induced apoptosis, whereas D609, a PC-PLC inhibitor,41 did not (Fig 4D).
These results indicate that Dex treatment activates a G-protein–dependent PI-PLC, but not PC-PLC. PI-PLC activation leads to a sequence of events including DAG release, aSMase activation, ceramide generation, and apoptosis.
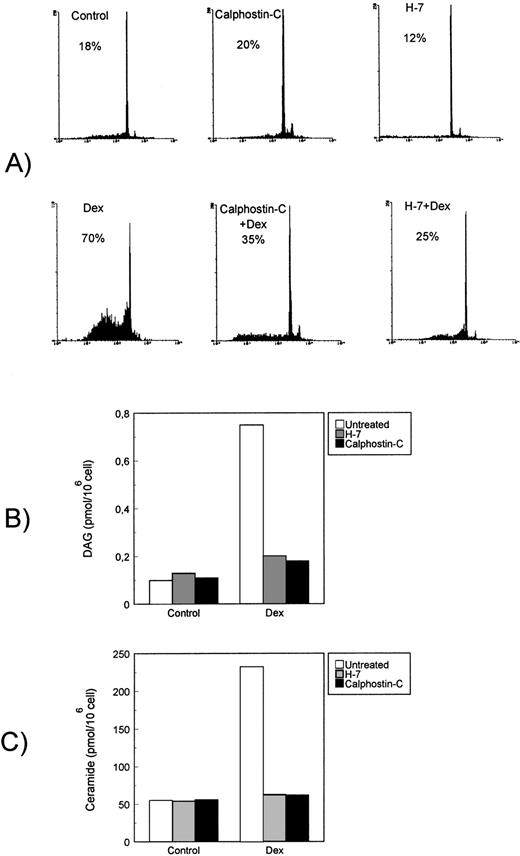
Dex-induced DAG and ceramide generation is countered by PKC inhibitors H7 and calphostin-C.
Dex-induced apoptosis is also dependent on PKC activity, and previous studies indicated that PKC inhibitors, including the broad serine/threonine kinase inhibitor H-745 and the more specific calphostin-C,46 completely counter thymocyte apoptosis.57-59 To evaluate the role of PKC activity in the Dex-induced thymocyte apoptosis, we tested the possible effect of H7 and calphostin-C on Dag and ceramide generation. Results indicate that both H7 and calphostin-C, at concentrations able to inhibit Dex-induced apoptosis (Fig 5A), also prevented DAG (Fig5B) and ceramide generation (Fig 5C). These results suggest that Dex treatment induces PKC activity that is then involved in the apoptotic transduction pathway upstream PI-PLC activation.
Effect of PKC inhibitors on Dex-induced apoptosis and signaling pathway. Effect of H7 and calphostin-C on (A) Dex-induced apoptosis, (B) DAG, and (C) ceramide generation. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of H7 (50 μmol/L) or calphostin-C (1 μmol/L).
Effect of PKC inhibitors on Dex-induced apoptosis and signaling pathway. Effect of H7 and calphostin-C on (A) Dex-induced apoptosis, (B) DAG, and (C) ceramide generation. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of H7 (50 μmol/L) or calphostin-C (1 μmol/L).
Dex-induced proteases activity is dependent on the PI-PLC/aSMase pathway.
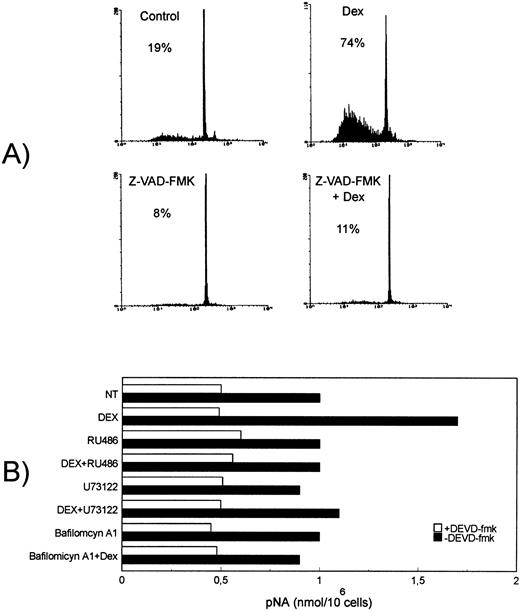
It has been previously suggested that several interleukin-1β–converting enzyme (ICE)-family cysteine proteases (caspases) play a prominent role in GCH-induced thymocyte apoptosis.60 To further evaluate the caspase role in the Dex-induced thymocyte apoptosis, we tested the possible effect of Z-VAD-FMK, a highly specific cell-permeable, irreversible inhibitor of caspases including caspase-1, also able to inhibit the activation of ProCPP32 to its active form.47 Results indicate that Z-VAD-FMK (50 μmol/L) totally inhibited Dex-induced thymocyte apoptosis (Fig 6A), whereas ceramide generation was not influenced (data not shown).
Dex-induced apoptosis is dependent on caspase activation, an event downstream Dex/GC receptor binding and PI-PLC/aSMase pathway induction. (A) Effect of the caspase inhibitor, Z-VAD-FMK (50 μol/L), on Dex-induced apoptosis, analyzed after 18-hour culture. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments. (B) Thymocytes were treated for 8 hours at 37°C with Dex (10−7 mol/L) in the presence or absence of RU486 (10−6 mol/L), U73122 (2.5 μmol/L), or bafilomycin A1 (1 μmol/L). Crude cell lysates were then assayed for CPP32/Caspase 3 activity by using a colorimetric assay based on spectrophotometric detection of the chromophore pNA after cleavage from the labeled substrate DEVD-pNA. Caspase activity is expressed as nanomoles pNA/106 cells. The results are the mean values of two determinations (standard deviation < 2%). The results are representative of one out of three separate experiments.
Dex-induced apoptosis is dependent on caspase activation, an event downstream Dex/GC receptor binding and PI-PLC/aSMase pathway induction. (A) Effect of the caspase inhibitor, Z-VAD-FMK (50 μol/L), on Dex-induced apoptosis, analyzed after 18-hour culture. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments. (B) Thymocytes were treated for 8 hours at 37°C with Dex (10−7 mol/L) in the presence or absence of RU486 (10−6 mol/L), U73122 (2.5 μmol/L), or bafilomycin A1 (1 μmol/L). Crude cell lysates were then assayed for CPP32/Caspase 3 activity by using a colorimetric assay based on spectrophotometric detection of the chromophore pNA after cleavage from the labeled substrate DEVD-pNA. Caspase activity is expressed as nanomoles pNA/106 cells. The results are the mean values of two determinations (standard deviation < 2%). The results are representative of one out of three separate experiments.
We also performed experiments to evaluate the possible role of PI-PLC/aSMase pathway in protease activation. For this purpose, thymocytes were treated for 8 hours at 37°C with Dex (10−7 mmol/L) in the presence or absence of U73122 (2.5 μmol/L) or bafilomycin A1 (1 μmol/L), and then CPP32/caspase 3 activity was evaluated.
In vitro experiments showed that Dex significantly (P < .002) augmented the basal CPP32 activity, expressed as nanomoles pNA/106 cells, and that the PI-PLC inhibitor U73122 and the aSMase inhibitor bafilomycin A1 inhibited this augmented CPP32/caspase 3 activity (Fig 6B). The inhibition of CPP32 activity observed in the presence of the irreversible CPP32 inhibitor DEVD-FMK allowed to verify the assay specificity.
These results indicate the Dex-induced caspase activation, required for apoptosis, is downstream and dependent on the PI-PLC/aSMase pathway.
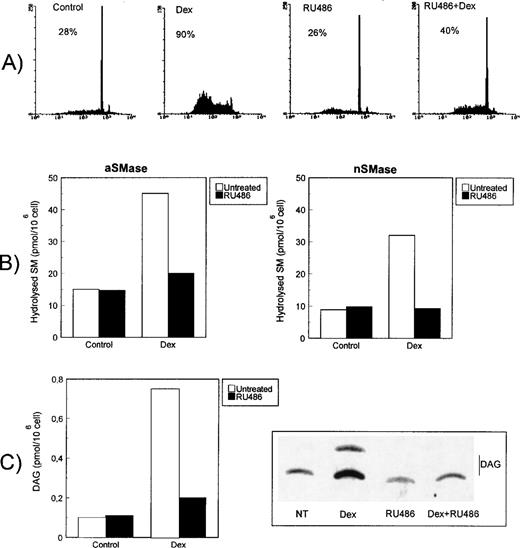
Dex-induced ceramide generation and caspase activation are dependent on binding to GR.
It has previously been shown that Dex-induced apoptosis is dependent on GR binding and is inhibited by the receptor antagonist RU486.39 61 We performed experiments to evaluate the effect of RU486 on ceramide generation and apoptosis. Figure7A shows the results of a representative experiment confirming that RU486 inhibits Dex-induced apoptosis. Furthermore, Dex-induced aSMase (Fig 7B, left panel), nSMase (Fig 7B, right panel), and PI-PLC (Fig 7C) activation as abrogated in the presence of RU486. As expected, RU486 also prevented ceramide generation (not shown) and CPP32 activity (Fig 6B) induced by Dex, indicating that signals responsible for ceramide generation and caspase activation require the Dex/GR interaction.
RU486 inhibition of Dex-induced apoptosis and signaling pathways. (A) Effect of RU486 (10−6 mol/L) on Dex-induced apoptosis as detected after an 18-hour culture. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments. (B) Effect of RU486 on Dex-induced acidic (left) and neutral (right) SMase activities after 15 or 120 minutes of Dex treatment, respectively. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. SD, less than 10% of the mean values, are omitted for clarity. (C) Effect of RU486 on Dex-induced PI-PLC activity at 5 minutes. Cell extracts were reacted with radiolabeled PI vesicles, and then DAG released was separated by TLC, visualized by autoradiography, scraped from the plate, and counted by scintillation counting. PI-PLC activity is expressed as picomoles DAG/106 cells. Mean values of three different experiments in duplicate are reported. SD, less than 3% of the mean values, are omitted for clarity.
RU486 inhibition of Dex-induced apoptosis and signaling pathways. (A) Effect of RU486 (10−6 mol/L) on Dex-induced apoptosis as detected after an 18-hour culture. Percentage numbers of hypodiploid nuclei are reported for each condition. The results are representative of one out of three separate experiments. (B) Effect of RU486 on Dex-induced acidic (left) and neutral (right) SMase activities after 15 or 120 minutes of Dex treatment, respectively. Hydrolyzed SM was quantitated and expressed as picomoles/106 cells. Mean values of three different experiments in duplicate are reported. SD, less than 10% of the mean values, are omitted for clarity. (C) Effect of RU486 on Dex-induced PI-PLC activity at 5 minutes. Cell extracts were reacted with radiolabeled PI vesicles, and then DAG released was separated by TLC, visualized by autoradiography, scraped from the plate, and counted by scintillation counting. PI-PLC activity is expressed as picomoles DAG/106 cells. Mean values of three different experiments in duplicate are reported. SD, less than 3% of the mean values, are omitted for clarity.
Dex-induced apoptosis, but not DAG and ceramide generation and caspase activation, is inhibited by mRNA and protein synthesis inhibitors.
It has been previously reported that Dex-induced apoptosis is countered by mRNA and protein synthesis inhibitors.5 62 We performed experiments to evaluate the possible effect of mRNA and protein synthesis inhibitors on ceramide generation and CPP32 activity. For that purpose, thymocytes were treated with Dex, Dex plus actinomycin-D, or Dex plus cycloheximide. Results of a representative experiment indicate that both actinomycin-D and cycloheximide inhibited the Dex-induced apoptosis (Fig 8A), but not the Dex-induced DAG and ceramide generation (Fig 8B) and induction of CPP32 activity (Fig 8C). These data confirm previously reported results suggesting that transcription and protein synthesis are required for Dex-induced apoptosis and also show these events are downstream in the pathway after DAG and ceramide generation and caspase activation.
Effect of mRNA and protein synthesis inhibitors on Dex-induced apoptosis and signaling pathway. Effect of actinomycin-D (Act-D; 2.5 μg/mL) and cycloheximide (CHX; 50 μg/mL) on (A) Dex-induced apoptosis, (B, left) DAG, (B, right) ceramide generation, and (C) CPP32 activity. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of Act-D or CHX.
Effect of mRNA and protein synthesis inhibitors on Dex-induced apoptosis and signaling pathway. Effect of actinomycin-D (Act-D; 2.5 μg/mL) and cycloheximide (CHX; 50 μg/mL) on (A) Dex-induced apoptosis, (B, left) DAG, (B, right) ceramide generation, and (C) CPP32 activity. Thymocytes were treated with Dex (10−7 mol/L) for the indicated times in the presence or absence of Act-D or CHX.
DISCUSSION
In the present study, we show results indicating that an early generation of ceramide, caused by the sequential activation of PI-PLC and aSMase, is required for Dex-induced thymocyte apoptosis. In particular, both induction of ceramide and cell death are dose-dependent with doses ranging between 10−7 mol/L and 10−12 mol/L, being evident also at low Dex concentrations (10−10 mol/L, Fig 1). Moreover, thymocytes are sensitive to the apoptosis induction by soluble cell permeant ceramide (Fig 2B). As for most of the GCH-mediated effects, treatment with the GR antagonist RU48639,61 results in the complete inhibition of Dex-induced ceramide generation and apoptosis (Fig 7). Dex-induced ceramide generation is caused by SMase activation. Interestingly enough, both aSMase and nSMase, respectively, are activated, although at different times, within 5 minutes and 30 minutes after Dex treatment (Fig 2). However, the inhibition of aSMase activity through agents (monensin, NH4Cl, or bafilomycin A1) able to cause, by different mechanisms, alkalinization of endolysosomal compartment,42-44 counteracts Dex-induced apoptosis (Fig3). These results indicate that the early ceramide generated by aSMase (5 minutes) is necessary and sufficient to signal Dex-induced cell death.
Our findings further indicate that nSMase and aSMase are independently activated by Dex treatment and that the ceramide generated from the action of each SMase may be involved in separate and distinct signaling events, as previously suggested (as a review, see Riboni et al50). The different contribution of aSMase and nSMase in apoptosis induction could thus be attributed to the distinct cellular compartmentalization of these enzymes. Ceramide generated from nSMase seems to remain associated to or near the plasma membrane facilitating the targeting plasma membrane–associated proteins, whereas the ceramide generated through the action of the aSMase is associated with an endosomal/lysosomal compartment.
Experiments performed to analyze the possible mechanisms involved in Dex-induced aSMase activation, indicate that Dex treatment rapidly induces DAG generation (Fig 4A), an event which precedes and is required for aSMase activation.50-52 Dex-induced DAG generation is caused by PI-PLC, but not by PC-PLC activation. This Dex-induced PI-PLC is prevented by U73122 (Fig 4), a G-protein–PLC coupling inhibitor,40 and by PKC inhibitors (Fig 5), thus suggesting that Dex may act by inducing the activation of PLCβ, an enzyme regulated by α subunits of G proteins (Gq family) and phosphorylation.63-65 Interestingly, PI-PLC inhibition through U73122 also totally prevented Dex-induced aSMase activity, ceramide generation, and apoptosis (Figs 4B, C, and D). Our preliminary data suggest that a pertussis-toxin unsensitive G-protein, activated by Dex, is responsible for PLCβ activation. Indeed, pertussis-toxin treatment of thymocytes did not influence either Dex-induced PLCβ activation or apoptosis (unpublished results). Of note, GCH have in the past been shown to regulate G-protein level and activity in different cell systems, thus modulating several enzymes.66,67 In particular, GCH-induced Gαq-11-protein regulation modulates PLC signal transduction.68 Similar mechanisms could be involved in the Dex-induced activation of thymocyte death. Studies are in progress to further analyze the mechanisms underlying the proposed Dex-induced G-protein activation potentially responsible for PI-PLC activity.
Activation of proteases has been postulated as a general feature of apoptosis.69,70 Many proteins have been reported to be cleaved during apoptosis, including fodrin, terminin, the protein component of the U1 small nuclear ribonucleoprotein, and poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP).71 Ceramide has recently been shown to induce cleavage of PARP, a nuclear enzyme that converts nicotinamide adenine dinucleotide (NAD) to ADP-ribose polymers.72 At the onset of apoptosis, PARP is cleaved to an 85-kD apoptosis-specific fragment in different cells, including thymocytes. The protease pro-ICE (YAMA/CPP32b/apopain), a mammalian homolog of theCaenorhabditis elegans death gene ced-3, has been identified as the protease that cleaves PARP during apoptosis.73-75 The finding that ceramide is able to induce PARP cleavage provides the first evidence of the signal transduction pathway leading to activation of the ICE-like proteases.76
The role of ICE-like proteases as mediators of glucocorticoid-induced apoptosis, even if currently receiving great attention, has not been completely clarified (as a review, see Distelhorst77). A specific involvement of CPP32 has been recently proposed in Dex-induced thymocyte apoptosis.60 In our hands, aSMase-dependent ceramide generation is required for caspase activation, an event involved in the progression of the apoptotic signal. Our results show that the Z-VAD-FMK, a highly specific, cell-permeable, irreversible inhibitor of caspases, including caspase 1, able to prevent the activation of ProCPP32 to its active form,47 counteracts DEX-induced thymocyte death (Fig 6A). Moreover, the inhibition of the signaling pathway required for Dex-induced ceramide generation prevents CPP32 activity increase (Fig 6B).
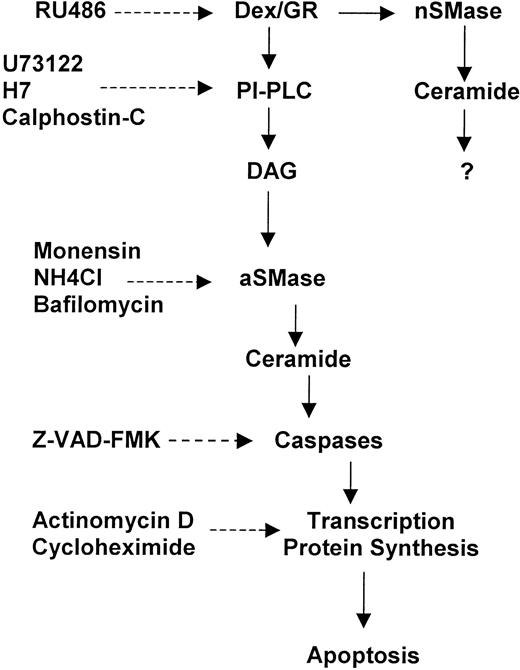
The results described here indicate that GCH lead to a rapid activation of a number of biochemical events involved in apoptosis signaling. In particular, Dex causes a rapid PI hydrolysis through a G-protein–activated PLC, which is upstream the aSMase-dependent ceramide generation and caspase activation (Fig9).
Sequential signaling events in Dex-induced thymocyte apoptosis. (-----) = Inhibition.
Sequential signaling events in Dex-induced thymocyte apoptosis. (-----) = Inhibition.
The influence of the GCH/GR system in the SMase pathway may represent a general signaling mechanism shared by a number of cytoplasm receptors. GR is a member of a large family of transcription factors that share as a common feature a zinc finger structure able to recognize specific DNA sequences.78-80 Interestingly, both 1α,25-dihydroxyvitamin D3 and retinoic acid, which bind specific receptors belonging to the same receptor superfamily of GR,79 are able to induce in different cell systems an increase of ceramide, which has been associated with crucial events such as differentiation and apoptotic death.81-88
Either transcription-dependent or transcription-independent GCH-induced apoptosis has been previously described.5,62,89 However, mRNA and/or protein synthesis inhibitors counteract thymocyte apoptosis, suggesting that transcription events, consequent to Dex/GR interaction, are required for apoptosis induction.5 77 In our experiments, treatment with actinomycin-D or cycloheximide, although it completely inhibited Dex-induced thymocyte apoptosis, did not influence the signaling pathway including DAG release, ceramide generation, and caspase activation (Fig 8), thus suggesting that all these events precede the Dex-induced transcription regulation required for thymocyte apoptosis. Moreover, as shown in Fig 2B, apoptosis induced by exogenous ceramide is inhibited by mRNA and protein synthesis inhibition, further suggesting that transcription is a downstream event required for Dex-induced apoptosis. Interestingly enough, these observations indicate that both Dex and ceramide, which is also generated after Dex treatment, induce thymocyte apoptosis in a transcription/translation-dependent manner. However, whether ceramide pathway affects the Dex/GR-mediated transcription is a matter of debate and requires further studies.
In conclusion, although the role of transcription in Dex-induced apoptosis remains to be clarified,77 future studies will be addressed to analyze the molecular mechanisms linking the signaling pathway described here to transcription regulation.
The knowledge of the molecular events involved in the GCH-mediated apoptosis in thymocytes and T lymphocytes is important for the understanding of mechanisms contributing to the immune response regulation. Moreover, these observations can be informative for the development of new pharmacological approaches aimed to control neoplastic T-cell growth.
Supported by AIRC, Milan, Italy, and P.F. Biotecnologie, CNR, Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Carlo Riccardi, MD, PhD, Department of Clinical and Experimental Medicine, University of Perugia, Via del Giochetto, 06100 Perugia, Italy.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal