Abstract
We have recently shown that stimulation of glycoprotein (gp) 130, the membrane-anchored signal transducing receptor component of IL-6, by a complex of human soluble interleukin-6 receptor (sIL-6R) and IL-6 (sIL-6R/IL-6), potently stimulates the ex vivo expansion as well as erythropoiesis of human stem/progenitor cells in the presence of stem cell factor (SCF). Here we show that sIL-6R dose-dependently enhanced the generation of megakaryocytes (Mks) (IIbIIIa-positive cells) from human CD34+ cells in serum-free suspension culture supplemented with IL-6 and SCF. The sIL-6R/IL-6 complex also synergistically acted with IL-3 and thrombopoietin (TPO) on the generation of Mks from CD34+ cells, whereas the synergy of IL-6 alone with TPO was barely detectable. Accordingly, the addition of sIL-6R to the combination of SCF + IL-6 also supported a substantial number of Mk colonies from CD34+ cells in serum-free methylcellulose culture, whereas SCF + IL-6 in the absence of sIL-6R rarely induced Mk colonies. The addition of monoclonal antibodies against gp130 to the suspension and clonal cultures completely abrogated the megakaryopoiesis induced by sIL-6R/IL-6 in the presence of SCF, whereas an anti-TPO antibody did not, indicating that the observed megakaryopoiesis by sIL-6R/IL-6 is a response to gp130 signaling and independent of TPO. Furthermore, human CD34+ cells were subfractionated into two populations of IL-6R–negative (CD34+ IL-6R−) and IL-6R–positive (CD34+ IL-6R+) cells by fluorescence-activated cell sorting. The CD34+IL-6R− cells produced a number of Mks as well as Mk colonies in cultures supplemented with sIL-6R/IL-6 or TPO in the presence of SCF. In contrast, CD34+ IL-6R+cells generated much less Mks and lacked Mk colony forming activity under the same conditions. Collectively, the present results indicate that most of the human Mk progenitors do not express IL-6R, and that sIL-6R confers the responsiveness of human Mk progenitors to IL-6. Together with the presence of functional sIL-6R in human serum and relative unresponsiveness of human Mk progenitors to IL-6 in vitro, current results suggest that the role of IL-6 may be mainly mediated by sIL-6R, and that the gp130 signaling initiated by the sIL-6R/ IL-6 complex is involved in human megakaryopoiesis in vivo.
HEMATOPOIESIS IS A HIGHLY complex process mediated by cytokines, by which blood cells of various lineages are produced from a small population of stem cells. The development of megakaryocytes (Mks) from their stem/progenitor cells is one of the least understood aspects of hematopoiesis. The recent cloning of thrombopoietin (TPO), the ligand for c-Mpl receptor, and the findings that TPO acts through c-Mpl to promote proliferation and maturation of Mk progenitors, have provided impetus for the study of Mk biology.1-4 Extensive data published recently support that TPO acts as the primary regulator of Mk development and platelet production in vivo.5 However, the facts that although Mpl knock-out mice are thrombocytopenic, they do not suffer a bleeding diathesis,6,7 and that transcription factor NF-E2–deficient mice developed absolute thrombocytopenia independent of action of TPO,8 suggest that additional factors act in vivo to promote Mk development and platelet production.
In recent years, three well-characterized cytokines, interleukin-6 (IL-6), IL-11, and leukemia-inhibitory factor (LIF) have been shown to have various effects on megakaryocyte development in vitro and in vivo.9-12 These three cytokines together with ciliary neurotrophic factor (CNTF), oncostatin M (OSM), and recently cloned cardiotrophin-1 (CT-1), form a subset of cytokines with structural and functional similarities that share glycoprotein (gp)130, a 130 kD transmembrane glycoprotein with a large intracytoplasmic domain, as their signal transducing receptor component.13,14 gp130 has been shown to be ubiquitously expressed in various tissue and organs, whereas the ligand-specific receptor components (α-chain) display a more limited expression.14,15 Our recent studies have shown that gp130 was expressed in almost all human CD34+ cells, and that a complex of sIL-6R/IL-6, but not IL-6 or sIL-6R alone, can activate this glycoprotein and transduce the functional signals that stimulate the hematopoietic progenitor expansion as well as erythropoiesis in the presence of stem cell factor (SCF) in vitro, thus suggesting a novel role of gp130 signaling in human hematopoiesis.16-18
To examine the potential role of sIL-6R with IL-6 through gp130 signaling in human megakaryopoiesis in vitro, we have examined the effect of a complex of sIL-6R/IL-6 on Mk production and colony formation from purified human CD34+ cells in serum-free suspension and methylcellulose cultures. In the present study, we show that sIL-6R confers the IL-6 responsiveness of human Mk progenitors, and that a complex of sIL-6R/IL-6, but not sIL-6R or IL-6 alone, significantly stimulates the proliferation and differentiation of human Mk progenitors through gp130 signaling in the presence of SCF.
MATERIALS AND METHODS
Cell preparation.
Human umbilical cord blood (CB) samples, collected according to institutional guidance, were obtained during normal full-term deliveries. Mononuclear cells (MNCs) were separated by Ficoll-paque (Pharmacia LKB, Uppsala, Sweden) density-gradient centrifugation after depletion of phagocytes with Silica (IBL, Fujioka, Japan).
Receptor, cytokines, and antibodies.
Recombinant human IL-6 and sIL-6R were prepared as described.19 Recombinant human SCF was kindly provided by Amgen Biologicals (Thousand Oaks, CA). Recombinant human TPO, IL-3 and EPO were generously provided by Kirin Brewery (Tokyo, Japan). All the cytokines were pure recombinant molecules and were used at concentrations that induced optimal response in methylcellulose culture of human hematopoietic cells. These concentrations are 100 ng/mL of SCF, 4 U/mL of TPO, and 200 U/mL of IL-3. Preparation of antihuman gp130 monoclonal antibodies (MoAbs) (GPX7, GPX22 and GPZ35) and anti-IL-6R MoAb (MT26) has been described.20,21 The three anti-gp130 MoAbs recognize different epitopes on gp130 and have been shown to inhibit IL-6–mediated biological response through inhibition of the IL-6–induced association of gp130 and IL-6 receptors.22 The rabbit antihuman TPO neutralizing polyclonal antibody was kindly provided by Kirin Brewery. The anti–IL-6R MoAb was labeled with biotin (Pierce Chemical Co, Rockford, IL) according to conventional methods. Mouse fluorescein isothiocyanate (FITC)-labeled monoclonal immunoglobulin (Ig)G1 antibody specific for CD34(anti-HPCA-2), FITC- and biotin-labeled irrelevant IgG1 MoAb, and SA-PE (phycoerythrin-conjugated streptavidin) were provided by Becton Dickinson (San Jose, CA).
Purification of CD34+ cells.
Human CB MNCs were resuspended at 3-5 × 107/mL in phosphate-buffered saline (PBS) and mixed with Dynabeads M-450 CD34 (Dynal AS, Oslo, Norway), with a bead to cell ratio of 1:1. The cell-bead suspension was resuspended and incubated at 4°C for 30 minutes with gentle rotation. After incubation, the cell-bead volume was expanded and placed in a DYNAL Magnetic Particle Concentrator (MPC) to collect the Dynabeads M-450 CD 34/rosetted cells. The rosetted cells were incubated with DETACHaBEAD CD34 (Dynal) at room temperature for 45 minutes to detach the Dynabeads M-450 CD34 from the positively selected cells. The released cells (CD34+), collected by placing the tube in MPC, were further evaluated by flow cytometric analysis and colony assay. Eighty-five percent to 95% of the cells separated were CD34+ by flow cytometric analysis.
Flow cytometry and cell sorting.
Cell sorting based on the CD34 and IL-6R markers was performed with MNCs. Cells were first incubated with biotin-labeled IL-6R MoAb in ice for 30 minutes. After washing, the cells were then incubated simultaneously with FITC–anti-CD34 MoAb and SA-PE for another 30 minutes. After two washings, the cells were suspended in α-medium (Flow Laboratories, Rockville, MD) at concentrations of 5 to 10 × 105/mL and separated by cell sorting. Cells were sorted on a FACS Vantage flow cytometer (Becton Dickinson). A morphologic gate including about 25% of the events and all the CD34+ cells was determined on two-parameter histograms (side scatter and forward scatter). Compensation for two-color labeled samples was set up with single-stained samples. Positivity or negativity for IL-6R antigen among CD34+ cells was determined using control cells labeled with the biotin-PE and FITC-labeled irrelevant IgG1 MoAb. Cells were sorted into CD34+ IL-6R− and CD34+ IL-6R+ fractions. Purity of sorted populations as verified by reanalysis was greater than 95%.
Suspension culture.
Purified CD34+ cells were incubated in serum-free suspension culture as we recently described.16 17 One mL of culture mixture containing 2000 CD34+ cells, α-medium, 2% pure bovine serum albumin (BSA) (Sigma, St Louis, MO), 10 μg/mL of insulin (Sigma), 200 μg/mL of transferrin (Sigma), 0.01mmol/L 2-mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), 40 μg/mL of low-density lipoprotein (Sigma), and different combinations of cytokines was incubated in 24-well tissue plates (Nunc, Kamstrup, Denmark) at 37°C in a humidified atmosphere flushed with 5% CO2, 5% O2, and 90% N2. At weekly intervals, cultures were demidepopulated by removal of half the culture volume, which was then replaced by newly prepared medium with the same combinations of cytokines. Cells in the collected medium were washed, counted, cytocentrifuged, and stained. Total Mks generated at various time points in the suspension culture were calculated based on the proportion of the IIbIIIa+ cells in cytospin preparations and the total cell number induced by each combination. For blocking studies, anti-gp130 MoAbs, anti-TPO Ab, and their control IgG were added at the beginning of the culture.
Clonal culture.
CD34+ cells were incubated in triplicate at concentrations of 500 cells/mL in serum-free methylcellulose culture as previously reported with minor modification.23-25 One mL of culture mixture containing cells, α-medium, 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 2% pure-BSA (Sigma), 300 μg/mL of human transferrin, 160 μg/mL of soybean lecithin (Sigma), 96 μg/mL of cholesterol (Nacalai Tesque, Kyoto, Japan), 10 μg/mL of insulin, 0.05 mmol/L 2-mercaptoethanol and various combinations of cytokines with or without sIL-6R, was plated in each 35-mm Lux standard nontissue culture dish and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air.
The pure Mk colonies were divided into two subtypes according to their size: colony forming unit-megakaryocyte (CFU-Mk)–derived colonies and burst forming unit-megakaryocyte (BFU-Mk)–derived colonies. CFU-Mk–derived colonies were scored as such when they had 4 to 50 Mks, whereas BFU-Mk–derived colonies were scored when they had more than 50 Mks. Megakaryocyte-mixed colonies including granulocyte-macrophage-megakaryocyte (GMM), erythroid-megakaryocyte (EM), and granulocyte-erythroid-macrophage-megakaryocyte (GEMM) colonies, were scored according to the criteria reported previously.23-25 The Mks in colonies were determined by observation on an inverted microscope, and were typically large cells that had nongranular, translucent cytoplasm and highly refractile cell membranes. To assess the accuracy of the in situ identification, individual colonies were lifted with an Eppendorf micropipette under direct microscopic visualization, spread on glass slides using a cytocentrifuge (Cytospin II; Shandon Southern, Sewickley, PA), and stained for morphological examination.
Cytochemical and immunological staining.
Cytocentrifuge preparations from suspension culture and methylcellulose culture were stained for the observation of cellular morphology. Staining with May-Grünwald-Giemsa was performed by conventional method. Immunostaining with the alkaline phosphatase antialkaline phosphatase (APAAP) method using MoAbs of anti-gpIIbIIIa was performed as described previously.26 Briefly, cytocentrifuged samples were fixed with buffered formalin-acetone at 4°C, washed with Tris buffer saline (Wako, Osaka, Japan), and preincubated with normal rabbit serum to saturate the Fc receptors on the cell surface. After washing, the samples were successively incubated with mouse MoAbs and rabbit antimouse IgG Ab (Medical and Biological Laboratories, Nagoya, Japan), and then reacted with calf intestinal alkaline phosphatase-mouse monoclonal antialkaline phosphatase complex (Dako, Osaka, Japan). Alkaline phosphatase activity was detected with naphthol AS-TR phosphate sodium salt (Sigma) and fast red TR salt (Sigma) in pH 7.6, 40 mmol/L barbital buffer (Wako) containing levamisole (Sigma) to inhibit nonspecific alkaline phophatase activity. Positive cells were stained with reddish granules.
Statistical analysis.
For the statistical comparison in scoring the numbers of Mks, and that of various Mk colonies, Student’s t-test was applied. The significant level was set to 0.05.
RESULTS
Soluble IL-6R stimulates Mk production from human CD34+ cells in serum-free suspension culture dose-dependently in the presence of IL-6 and SCF.
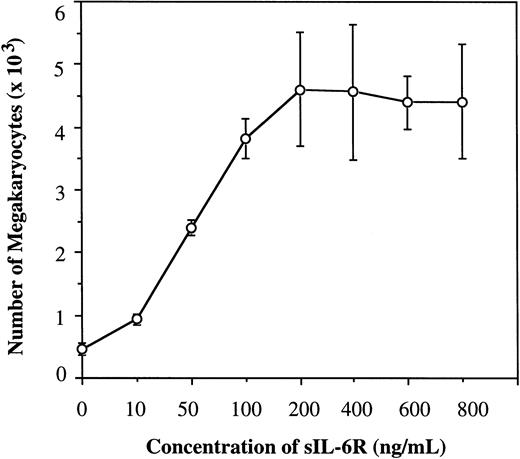
To determine the effect of sIL-6R on human Mk progenitors, we first examined its effect on the Mk generation from human CB CD34+ cells in the presence of 100 ng/mL of IL-6 and SCF in serum-free culture. The culture was kept up to 1 week and the generated Mks were detected by immunostaining the cytospin preparations with MoAb of anti-IIbIIIa. As shown in Fig 1, the addition of sIL-6R in the presence of IL-6 significantly enhanced Mk generation in a dose-dependent manner. The increase in the number of Mks was detectable at concentrations of sIL-6R as low as 10 ng/mL, and reached a plateau at 200 ng/mL. In the absence of sIL-6R, IL-6 also induced the generation of Mks, but to much less extent. The same results were also obtained when CD34+ cells purified from human bone marrow MNC were used. These results clearly indicate that sIL-6R is functional and capable of stimulating Mk generation in the presence of IL-6 and SCF, and that sIL-6R at 200 ng/mL appears to be the optimal concentration for the generation of Mks in serum-free suspension culture in the presence of SCF and IL-6.
Effect of sIL-6R on Mk generation from human CD34+ cells in suspension culture in the presence of 100 ng/mL of IL-6 and SCF. 2,000 CB CD34+ cells were initiated in the culture and results were examined at day 7. Total Mks generated at each concentration of sIL-6R were calculated based on the proportion of IIbIIIa+ cells on cytocentrifuge preparations and total cell number. Results are obtained from three separate experiments. Standard deviations are represented by error bars.
Effect of sIL-6R on Mk generation from human CD34+ cells in suspension culture in the presence of 100 ng/mL of IL-6 and SCF. 2,000 CB CD34+ cells were initiated in the culture and results were examined at day 7. Total Mks generated at each concentration of sIL-6R were calculated based on the proportion of IIbIIIa+ cells on cytocentrifuge preparations and total cell number. Results are obtained from three separate experiments. Standard deviations are represented by error bars.
Effects of sIL-6R and IL-6 in combination with various cytokines on the Mk generation in suspension culture.
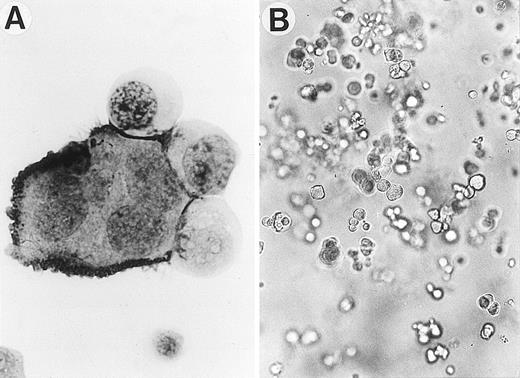
To examine the sIL-6R and IL-6–mediated Mk generation in more detail, we performed CD34+ cell suspension culture supplemented with sIL-6R, and IL-6 in combination with various cytokines, over a period of 2 weeks with weekly analysis of the generated cells. The total number of Mks generated at each time point by various combinations of cytokines is shown in Table1. Soluble IL-6R and IL-6 alone or in combination failed to support cell growth and no Mks were detected in their cultures. A combination of IL-6 and SCF induced a small number of Mks, but a striking increase in Mks was observed when sIL-6R was added to the combination. The addition of sIL-6R to the combination of SCF + IL-6 enhanced the Mk generation about 13- and 45-fold at days 7 and 14, respectively, with the maximal generation of Mks at day 14. A typical Mk induced by the three factors in suspension culture and positively immunostained with anti-IIbIIIa MoAb, is shown in Fig 2A. The addition of sIL-6R to the combination of IL-3 + IL-6, also significantly enhanced the production of Mks, but to a lesser extent than that to the combination with SCF. The addition of sIL-6R to the combination of TPO + IL-6 increased Mk numbers about 2- and 5-fold at days 7 and 14, respectively, although no significant synergistic effect was observed between IL-6 and TPO. Thus, while having no effects alone, the complex of IL-6/sIL-6R potently stimulated the Mk generation from human CD34+ cells in combination with either SCF, IL-3, or TPO in suspension culture with the most pronounced synergy, in terms of the fold increase of Mks after addition of sIL-6R, observed in combination with SCF at day 14. Norol et al27 recently showed that a combination of TPO with either IL-3 or SCF appeared to be the optimal combination in the Mk production from adult peripheral blood and bone marrow CD34+ cells. In the present study, our results also showed that these combinations exert synergistic action on the Mk production from CB CD34+ cells. A combination of TPO + SCF is more potent than that of TPO + IL-3, and addition of both sIL-6R and IL-6 but not IL-6 alone to the combination of TPO + SCF greatly enhanced Mk production (Table 1).
Development of Mk generation from CB CD34+cells by sIL-6R, IL-6, and SCF. (A) A typical Mk generated in serum-free suspension culture was positively immunostained with anti-IIbIIIa MoAb by APAAP technique. Magnification: ×1000. (B) A representative Mk colony derived from CD34+ cells in serum-free methylcellulose culture (original magnification ×100).
Development of Mk generation from CB CD34+cells by sIL-6R, IL-6, and SCF. (A) A typical Mk generated in serum-free suspension culture was positively immunostained with anti-IIbIIIa MoAb by APAAP technique. Magnification: ×1000. (B) A representative Mk colony derived from CD34+ cells in serum-free methylcellulose culture (original magnification ×100).
The effect of sIL-6R/IL-6 in combination with other cytokines on human Mk generation was also tested. No synergies were found between the complex of sIL-6R/IL-6 with granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), IL-1β, transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), macrophage inflammatory protein-1 (MIP-1), or tumor necrosis factor (TNF), in the presence or absence of SCF (data not shown). The effects of other members of the IL-6 family (gp130-stimulatory cytokines) on megakaryopoiesis were also tested. IL-11 and LIF had comparable effects to IL-6 in the presence of SCF on the generation of Mks, whereas no Mks were observed in the cultures supplemented with either OSM or CNTF in the presence or absence of SCF. The addition of IL-11, LIF, CNTF, and OSM alone or in combination to the culture supplemented with sIL-6R, IL-6, and SCF, did not affect the megakaryopoiesis induced by the three factors (data not shown), indicating that the observed effect on Mk generation through gp130 stimulation was provided specifically by a complex of IL-6/sIL-6R.
Mk colony formation by sIL-6R and IL-6.
It is likely that the stimulatory effects of sIL-6R and IL-6 on Mk generation in suspension culture were provided by the proliferation and differentiation of Mk progenitors. To examine this possibility, methylcellulose clonal assay of CD34+ cells was performed (Table 2). Although SCF alone or in combination with IL-6 stimulated no or few Mk colonies from the human CD34+ cells, the addition of sIL-6R to the combination of SCF and IL-6 significantly enhanced Mk colony formation. The Mk colonies induced by the three factors usually consisted of 10 to 300 Mks. A large Mk colony containing about 250 cells induced by sIL-6R, IL-6 and SCF is shown in Fig 2B. The nature of the Mks was confirmed by immunostaining of cytospin preparations with MoAb against IIbIIIa. In addition to a number of pure Mk colonies, the combination of sIL-6R, IL-6, and SCF also induced a large number of megakaryocyte-mixed colonies, in which the cells of other lineages such as granulocyte, macrophage, and/or erythroid cells, in addition to a number of Mks, were also present (GMM, GEMM, or EM), suggesting a complex of IL-6/sIL-6R in the presence of SCF acts on Mk progenitors as well as more primitive progenitors to promote their proliferation and differentiation. In accordance with the finding obtained in suspension culture, the addition of sIL-6R also enhanced the Mk colony formation induced by IL-3 and IL-6. Although no significant synergistic effect was observed between IL-6 and TPO, the addition of sIL-6R to the combination of IL-6 + TPO did increase the size and number of Mk colonies. A larger number of BFU-Mk–derived colonies developed in the culture supplemented with a combination of sIL-6R, IL-6, and TPO than in that with TPO alone. In the presence of SCF, IL-6/sIL-6R in combination with TPO induced the largest number of megakaryocyte-mixed colonies, most of which were GEMM, in addition to a number of pure Mk colonies, suggesting that the IL-6/sIL-6R complex and TPO, apart from their effects on Mk progenitors, also synergistically act on more primitive progenitors (CFU-GEMM) in the presence of SCF.
sIL-6R and IL-6 stimulate megakaryopoiesis independent of the action of TPO.
To verify the involvement of membrane-anchored gp130 in the sIL-6R/IL-6–mediated megakaryopoiesis and to exclude the possibility that the observed megakaryopoiesis by sIL-6R/IL-6 was mediated by TPO, we performed blocking studies using MoAbs against gp130 as well as neutralizing Ab against TPO. The addition of anti-gp130 MoAbs to the culture dose-dependently inhibited the Mk production supplemented with a combination of sIL-6R, IL-6, and SCF although no effects of control IgG were detectable, whereas anti-TPO neutralizing Ab had no effect on the Mk generation in the same condition (Fig 3A). By contrast, although anti-TPO Ab efficiently inhibited the TPO-dependent megakaryopoiesis, anti-gp130 MoAbs failed to affect the Mk generation induced by TPO and SCF (Fig3B). Similar results were also observed in the clonal assay, in which the anti-gp130 MoAbs, but not TPO Ab, abrogated the Mk colonies induced by sIL-6R, IL-6, and SCF (Fig 4A,B). These results clearly indicated that the observed megakaryopoiesis mediated by a sIL-6R/IL-6 complex was provided by the interaction of sIL-6R/IL-6 with membrane-anchored gp130 and was independent of the action of TPO.
Effects of various concentrations of anti-gp130 MoAbs (○) and TPO-neutralizing Ab (•) on the generation of Mks from CD34+ cells stimulated by a complex of IL-6/sIL-6R (A), or TPO (B) in serum-free suspension culture in the presence of SCF. The data from isotype control IgG for anti-TPO Ab (□) and anti-gp130 MoAb (▵) were also presented. 2,000 CD34+ cells were cultured in suspension condition and the antibodies were added at the beginning of the culture. Total Mks was calculated based on the proportion of IIbIIIa+ cells on the cytospin preparation and total cell number at day 10 of culture. The absolute number of Mks produced in the wells without Abs were presented (*) and estimated as control data. Data indicate the ratio of the total Mks in each well treated with antibodies or control IgG to those from control, and are expressed as percent (%) of the control.
Effects of various concentrations of anti-gp130 MoAbs (○) and TPO-neutralizing Ab (•) on the generation of Mks from CD34+ cells stimulated by a complex of IL-6/sIL-6R (A), or TPO (B) in serum-free suspension culture in the presence of SCF. The data from isotype control IgG for anti-TPO Ab (□) and anti-gp130 MoAb (▵) were also presented. 2,000 CD34+ cells were cultured in suspension condition and the antibodies were added at the beginning of the culture. Total Mks was calculated based on the proportion of IIbIIIa+ cells on the cytospin preparation and total cell number at day 10 of culture. The absolute number of Mks produced in the wells without Abs were presented (*) and estimated as control data. Data indicate the ratio of the total Mks in each well treated with antibodies or control IgG to those from control, and are expressed as percent (%) of the control.
Effects of anti-gp130 MoAbs (A) and TPO-neutralizing Ab (B) on the Mk clonal growth from CD34+ cells supported by a complex of IL-6/sIL-6R in serum-free methylcellulose culture in the presence of SCF. 500 CD34+ cells purified from cord blood were initiated and various concentrations of Abs were added at the beginning of the culture. Mk colonies including CFU-Mk, BFU-Mk, MK-Mix were scored at day 11. The number of Mk colonies indicates mean ± SD from triplicate cultures.
Effects of anti-gp130 MoAbs (A) and TPO-neutralizing Ab (B) on the Mk clonal growth from CD34+ cells supported by a complex of IL-6/sIL-6R in serum-free methylcellulose culture in the presence of SCF. 500 CD34+ cells purified from cord blood were initiated and various concentrations of Abs were added at the beginning of the culture. Mk colonies including CFU-Mk, BFU-Mk, MK-Mix were scored at day 11. The number of Mk colonies indicates mean ± SD from triplicate cultures.
Most of the human Mk progenitors express little or no IL-6R, and sIL-6R can confer their responsiveness to IL-6.
The effects of sIL-6R/IL-6 on human megakaryopoiesis together with the results of blocking studies by anti-gp130 MoAbs suggest that most of the human Mk progenitors may not express IL-6R but do express the signal transducer gp130. Indeed, our recent studies, using FACS analysis, have shown that almost all of the CD34+ cells express gp130 whereas 50% to 70% of the CD34+ cells do not express IL-6R.18 To examine the different effects of sIL-6R and IL-6 on the generation of Mks from IL-6R-positive and negative CD34+ cells, we performed suspension culture of CD34+ IL-6R+ and CD34+IL-6R− cells sorted by FACS (Fig 5A). In cultures of CD34+IL-6R− cells, the addition of sIL-6R to the combination of IL-6 and SCF dramatically increased the total number of Mks, whereas CD34+ IL-6R+ cells failed to do so under the same conditions. Moreover, although TPO alone was capable of stimulating CD34+ IL-6R− cells to generate a number of Mks, Mks generated from CD34+IL-6R+ cells by TPO were hardly detectable (Fig 5B). Similar results were obtained in the methylcellulose assay, in which CD34+ IL-6R− but not CD34+IL-6R+ cells gave rise to a number of pure Mk and megakaryocyte-mixed colonies (data not shown). Collectively, these findings indicate that most Mk progenitors do not express IL-6R, and that sIL-6R confers the IL-6 responsiveness of human Mk progenitors on which gp130 but not IL-6R is expressed.
Different effects of IL-6/sIL-6R on the generation of Mks from CD34+ IL-6R− and CD34+IL-6R+ cells. (A) Selection of CD34+IL-6R− and CD34+ IL-6R+ cells from human umbilical cord blood MNCs by flow cytometry. R2: CD34+ IL-6R+, R3: CD34+IL-6R− cells. (B) Megakaryocyte generation from the two populations of the CD34+ cells. 2,000 CD34+IL-6R− cells and CD34+ IL-6R+cells sorted by FACS were initiated in serum-free culture, respectively. The total number of Mks generated by each combination was determined at day 10. Results are from one representative experiment. Similar results were obtained in three additional experiments.
Different effects of IL-6/sIL-6R on the generation of Mks from CD34+ IL-6R− and CD34+IL-6R+ cells. (A) Selection of CD34+IL-6R− and CD34+ IL-6R+ cells from human umbilical cord blood MNCs by flow cytometry. R2: CD34+ IL-6R+, R3: CD34+IL-6R− cells. (B) Megakaryocyte generation from the two populations of the CD34+ cells. 2,000 CD34+IL-6R− cells and CD34+ IL-6R+cells sorted by FACS were initiated in serum-free culture, respectively. The total number of Mks generated by each combination was determined at day 10. Results are from one representative experiment. Similar results were obtained in three additional experiments.
DISCUSSION
Hematopoietic cytokines control numerous aspects of hematopoiesis through binding to specific receptors on the surface of target cells. Most cytokine receptors in the hematopoietic system consist of a multichain complex, a ligand-binding chain and a signal-transducing chain, the latter of which is usually used in common by several receptor complexes. gp130, a membrane-anchored 130 kD glycoprotein with large transmembrane and intracellular domains, originally identified as the signal transducing receptor component of IL-6, is shared in common by the receptor complexes of IL-11, LIF, CNTF, OSM, and CT-1.14,28,29 Subsequent cloning of the cDNA encoding murine gp130 showed the ubiquitous expression of gp130 in every murine organ, including heart, spleen, kidney, lung, liver, and brain, whereas the expression of various ligand-binding chains displayed a more limited distribution. In the human hematopoietic system, information on what cytokine receptors are normally expressed on stem/progenitor cells remains incomplete. The potential role of gp130, which can be initiated by a complex of IL-6/sIL-6R, in normal human cells remains largely unknown. In our recent study a complex of IL-6/sIL-6R through gp130 signaling in the presence of SCF, was found to stimulate expansion of human primitive progenitor cells and erythropoiesis from human stem/progenitor cells in vitro. In the present study, we showed that sIL-6R with IL-6, but not IL-6 or sIL-6R alone, significantly stimulates the proliferation and differentiation of human Mk progenitors through gp130 signaling in the presence of SCF. A recent study showed that the production of murine Mks in response to SCF, IL-6, and IL-11 alone or in combination was eliminated by neutralizing the biological activity of TPO, suggesting that the effects of SCF, IL-6, and IL-11 on megakaryocytes were indirect and mediated by TPO.30 However, this may not be the case with human hematopoietic progenitor cells when SCF, IL-6, and sIL-6R are employed, as shown in the present study. The results of the blocking experiments by TPO-neutralizing Ab and anti-gp130 MoAbs clearly indicate that the observed effect of sIL-6R/IL-6 on human megakaryopoiesis is provided directly by gp130 signaling in the presence of SCF independent of TPO. Thus, the present study may provide new information as to the mechanisms that control the development of human megakaryocytes.
The stimulatory effect of sIL-6R on the megakaryopoiesis in the presence of IL-6 and SCF, and the lack of this effect in the culture without sIL-6R, are reminiscent of our recent findings that sIL-6R confers IL-6 responsiveness to human hematopoietic primitive and erythroid progenitors.16,17 Our flow cytometric analysis of the expression of gp130 and IL-6R on CD34+ cells has provided a plausible explanation for this observation.18The distinct responsiveness of CD34+IL-6R− and CD34+ IL-6R+ cells to IL-6/sIL-6R and TPO as shown in the present study, clearly indicates that most of the Mk progenitors are included in the IL-6R− populations, and that the activation of the signal pathway of gp130 in these Mk progenitors can only be achieved by a complex of IL-6/sIL-6R, but not by IL-6 alone. Because it is conceivable that most of the Mk progenitors express both gp130 and c-Mpl, the activation of either signal pathway can contribute to megakaryopoiesis. The activation of gp130 (homodimerization), induced by a complex IL-6/IL-6R, is believed to result in the juxtaposition of the cytoplasmic regions that appear to initiate a downstream signaling cascade such as RAS/MAPK and JAK2/STAT1,3 leading to cellular response.14 Interestingly, recent studies showed that TPO also induces activation of JAK2, STAT1, STAT3, and STAT5 through c-Mpl signaling,31-33 suggesting that the ability to activate JAK/STAT pathway may underline the effects common to c-Mpl signaling by TPO and the gp130 signaling by IL-6/sIL-6R.
It is interesting to note that gp130 signaling plays a role in both erythropoiesis17 and megakaryopoiesis in the presence of SCF. In recent years, several lines of evidence support a common origin of erythroid and Mk lineages. For example, erythroleukemia cell lines express markers of Mk differentiation, and erythroid and megakaryocytic cells display a number of common surface markers and transcription factors including GATA-1, NF-E2, and Tal/SCL.34-36 EPO and TPO are structurally related growth factors. EPO possesses megakaryopoietic activity although as a physiological regulator of erythropoiesis.23,37 Similarly, recent studies also show that TPO enhances proliferation of erythroid progenitors.38,39 The present study together with our previous one,17 suggests that both erythroid and megakaryocyte progenitors express gp130 and respond to gp130 signaling induced by IL-6/sIL-6R, adding further evidence for the hypothesis that the progenitors within the two lineages respond to overlapping signals.
Recently cloned c-Mpl ligand (TPO) has been shown to be the primary regulator of megakaryocyte development in vivo. However, the failure of the Mpl (TPO receptor) knock-out to absolutely eliminate marrow megakaryocytes or circulating platelets argues that alternative routes to platelet production exist.6,7 Our results suggest that the three factors of sIL-6R, IL-6, and SCF may contribute to human TPO-independent megakaryopoiesis in vivo. This hypothesis is supported by the following: (1) Soluble IL-6R, IL-6, and SCF are detectable in human serum, and the half-maximal effect of sIL-6R observed in the present study was 50 ng/mL, which is within the physiological range.40-43 In fact, a serum concentration of sIL-6R at approximately 50 ng/mL in MRL/lpr mice was reported to mediate IL-6 signal in IL-6R− gp130+cells.44 Given the dissociation constant of IL-6 and sIL-6R at approximately 10−9 mol/L, it is assumed that the IL-6 will be almost completely complexed with sIL-6R in serum.45 (2) gp130 and IL-6–knock-out mice have decreased number of Mk progenitors and megakaryocytes.46,47 (3) SCF-deficient mice have a pronounced abnormality of megakaryocytes.48,49 (4) IL-6-sIL-6R double transgeneic mice have extramedullary expansion of hematopoietic progenitor cells and a strong increase of peripheral platelets and other blood cells.50 Taken together, the current results suggest that although TPO is the principal regulator in the development of Mk, gp130 and c-kit signaling may play a role in megakaryopoiesis in vivo. The TPO-independent megakaryopoiesis and platelet production observed in Mpl knock-out mice might be provided, at least in part, by the combined signals through the gp130 and c-kit.
Supported by grants from Ministry of Education, Sciences, Sports, and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tatsutoshi Nakahata, MD, DM Sci, Department of Clinical Oncology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108, Japan; e-mail:nakahata@ims.u-tokyo.ac.jp.