Abstract
Agnogenic myeloid metaplasia (AMM) is a chronic myeloproliferative disorder in which patients with poor prognostic features, receiving conventional treatments, have a median survival of less than 3 years. In this retrospective multicenter study, we analyze the results and try to define the indications for allogeneic stem cell transplantation in AMM. From January 1979 to November 1997, 55 patients with a median age of 42 years were transplanted from HLA-matched related (n = 49) or alternative (n = 6) donors for AMM. A multivariate analysis was conducted to identify factors associated with posttransplant outcome. The median posttransplant follow-up was 36 months (range, 6 to 223). The 5-year probability of survival was 47% ± 8% for the overall group, and 54% ± 8% for patients receiving an unmanipulated HLA-matched related transplant. The 1-year probability of transplant-related mortality was 27% ± 6%. Hemoglobin level ≤100 g/L and osteomyelosclerosis before transplant adversely affected the outcome. The probability of developing grade III-IV acute graft-versus-host disease (GVHD) was 33% ± 8%. Sixteen of 45 patients developed extensive chronic GVHD. At last follow-up, 22 patients were in complete histohematologic remission. Treatment failure was observed in 13 cases. Age at transplant and karyotype were predictors of treatment failure. Allogeneic stem cell transplantation is an effective treatment leading to cure in a substantial number of patients with AMM. A better characterization of the variables affecting the posttransplant outcome should lead to a decreased transplant-related mortality and an improvement in these results.
AGNOGENIC MYELOID metaplasia (AMM) is a clonal hematopoietic stem cell disorder characterized by bone marrow fibrosis, extramedullary hematopoiesis, splenomegaly, and a leukoerythroblastic blood picture.1,2 With median survival ranging from 3 to 5 years, AMM has the worst outcome among the chronic myeloproliferative disorders.3-11 Dupriez et al11 recently proposed a scoring system, which identifies three distinct prognostic groups. In the low-risk group (hemoglobin level >100 g/L and leukocyte count between 4 and 30 × 109/L), patients had a median survival of 93 months, whereas patients from the intermediate-risk (hemoglobin level ≤100 g/L or leukocyte count <4 or >30 × 109/L) and high-risk (hemoglobin level ≤100 g/L and leukocyte count <4 or >30 × 109/L) groups had median survivals of 26 and 13 months, respectively. To date, whether using hydroxyurea, α-interferon, androgens or corticosteroids, standard therapies apart from transfusions have not been shown to prolong the survival of patients with AMM.12-17 Splenectomy can be performed when a huge spleen is painful or implicated in the worsening of cytopenias. However, its morbidity and mortality rates are not negligible,18-20 and Barosi et al20 have shown that this procedure might be associated with an increased risk of acute transformation. In any case, palliative strategies are not satisfactory for intermediate or high-risk patients less than 55 years of age, whose median expected survival is less than 3 years.21
Allogeneic stem cell transplantation is a potential treatment approach for AMM. We have recently reported encouraging results with the use of this high-risk strategy in small series of selected patients,22 23 with apparent cure in some cases. However, the small size of these studies precluded an analysis of factors influencing engraftment, the occurrence of severe graft-versus-host disease (GVHD), and treatment failure. Therefore, in this collaborative study, we have evaluated the results of 55 allogeneic stem cell transplants, determined the variables affecting the outcome, and tried to define indications for this aggressive approach in patients with AMM.
MATERIALS AND METHODS
Patients.
Between January 1979 and November 1997, 55 consecutive patients (39 men and 16 women) from the European Blood and Marrow Transplantation Registry, the Fred Hutchinson Cancer Research Center, and five other non-European centers, representing 28 centers worldwide, were allografted for AMM. All patients fulfilled the diagnostic criteria for AMM proposed by the Polycythemia Vera Study Group.24Patients with a previous history of polycythemia vera, bcr-abl rearrangement without Philadelphia chromosome, or acute transformation before transplant were not included. Two histopathologists performed a centralized review of bone marrow biopsies performed at diagnosis and before transplant to exclude frank acute transformation and other causes of myelofibrosis, such as acute megakaryoblastic leukemia and myelodysplastic syndromes with myelofibrosis. For the 12 patients in whom it was not possible to review the biopsy specimens, histopathological reports were obtained. Patient and disease characteristics before transplant are listed in Table 1. Eight patients had a previous history of essential thrombocythemia. Median age at transplant was 42 years (range, 4 to 53) with two patients being less than 20 years of age at that time. The median time from diagnosis to transplant was 21 months (range, 2 to 266). Immediately before transplant, 35 patients had a hemoglobin level ≤100 g/L, 22 had leukocyte counts <4 × 109/L, and 21 had platelet counts <100× 109/L. Cytogenetic abnormalities were detected in 13 of the 45 assessable karyotypes (29%). Marrow fibrosis was graded using the scale proposed by Sultan et al.25 Briefly, marrow fibrosis was grade I if increased cellularity and reticulinic fibrosis were observed, grade II if the overall cellularity was decreased with collagen fibrosis, and grade III when there was osteomyelosclerosis. At last bone marrow biopsy performed before transplant, grade I marrow fibrosis was observed in 11 cases, grade II marrow fibrosis in 17 cases, and osteomyelosclerosis in 27 cases.
Transplant procedure.
Characteristics of the transplant procedures are listed in Table 2. Thirty-five patients received a conditioning regimen including fractionated or single-dose total body irradiation. HLA matched related donors were used in 49 cases, and HLA mismatched related or six loci matched unrelated donors in six cases. According to institutional protocols, growth factors were used during the early phase after graft infusion in seven cases.
The day of graft infusion was defined as day 0. The day of neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of at least 0.5 × 109/L. Primary graft failure was diagnosed if the neutrophil engraftment endpoint was not reached by day 50. The day of platelet engraftment was defined as the first day with a platelet count of at least 50 × 109/L without transfusion for a week.
Criteria of response.
Complete hematologic remission was defined as disappearance of all clinical signs (including constitutional symptoms, splenomegaly, and hepatomegaly), peripheral blood and cytogenetic abnormalities attributable to the disease. Complete histohematologic remission was defined as the combination of a complete hematologic remission with disappearance of marrow fibrosis, whereas partial histohematologic remission was considered when a complete hematologic remission was achieved, but with partial regression of marrow fibrosis. Treatment failure was considered when there was disease recurrence or persistence.
Statistical analysis.
Survival was assessed on the date of last patient contact and analyzed on July 1, 1998. Deaths occurring the first year posttransplant, which were not attributable to the disease, were considered as events for the assessment of the 1-year transplant-related mortality. The probabilities of hematopoietic recovery, acute GVHD, overall survival, transplant-related mortality, and treatment failure were calculated from date of transplant, according to the Kaplan-Meier product-limit method.28 To determine factors affecting these endpoints, variables found significant at a P value ≤.05 in the two-tailed log-rank test were subsequently introduced in a Cox proportional hazards model.29 The Fisher’s exact test was used to individualize variables associated with the occurrence of extensive chronic GVHD.
RESULTS
Hematopoietic recovery.
Fifty of the 55 patients had hematopoietic recovery. One of them, because of nonstable engraftment, received a “boost” of peripheral blood stem cells without further immunosuppressive conditioning, followed by splenectomy, before achieving stable engraftment. Four patients died before day 50, between day 21 and day 32, without reaching the neutrophil engraftment endpoint, and one patient had primary graft failure. The median time to reach the neutrophil engraftment endpoint was 20 days (range, 11 to 50), with a Kaplan-Meier estimated probability of 82% ± 5% to obtain neutrophil recovery by day 30. In multivariate analysis, pretransplant splenectomy (P < .001; relative risk, 3.6; 95% confidence interval, 1.8 to 7.2), absence of osteomyelosclerosis (P = .01; relative risk, 0.42; 95% confidence interval, 0.2 to 0.8), and a high number of nucleated cells infused (P = .025; relative risk, 1.1; 95% confidence interval, 1 to 1.2) were the factors associated with a shorter time to neutrophil recovery. The median time to reach the platelet engraftment endpoint was 28 days (range, 10 to 393). Splenectomy (P < .0001; relative risk, 4.6; 95% confidence interval, 2.2 to 9.7) and absence of osteomyelosclerosis (P = .004; relative risk, 0.35; 95% confidence interval, 0.17 to 0.72) were the factors associated with a shorter time to achieve platelet recovery in multivariate analysis.
GVHD.
The probability of developing grade II-IV acute GVHD was 60% ± 7% and grade III-IV acute GVHD, 33% ± 8% for the overall group of patients. In univariate analysis, osteomyelosclerosis observed before transplant was the only variable associated with the occurrence of grade III-IV acute GVHD (P = .04). Twenty-seven of 45 assessable patients experienced chronic GVHD, which was extensive in 16 cases, and limited in 11. Extensive chronic GVHD was more frequently observed in patients who had developed grade III-IV acute GVHD (P < .001). Recipient age at transplant, time from diagnosis to transplant, splenectomy before transplant, and total body irradiation were not significantly associated with grade III-IV acute GVHD or extensive chronic GVHD.
Survival.
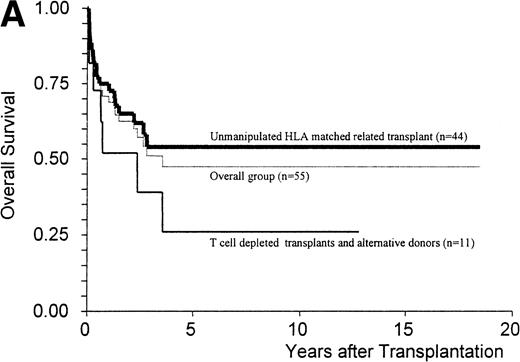
The Kaplan-Meier estimate of survival at 5 years was 47% ± 8% for the overall group and 54% ± 8% for patients receiving an unmanipulated HLA matched related graft (n = 44) (Fig 1A). The median duration of follow-up for the patients alive at that time was 36 months (range, 6 to 223), with nine patients alive more than 5 years after transplant. Twenty-five patients died of infection (n = 5), chronic GVHD (n = 5), disease progression (n = 5), acute GVHD (n = 4), solid organ failure (n = 3), lymphoproliferative disorder (n = 2), and graft failure (n = 1). The Kaplan-Meier estimate of the 1-year transplant-related mortality for the overall group of patients was 27% ± 6% and for the patients receiving an unmanipulated HLA matched-related transplant, 22% ± 6%.
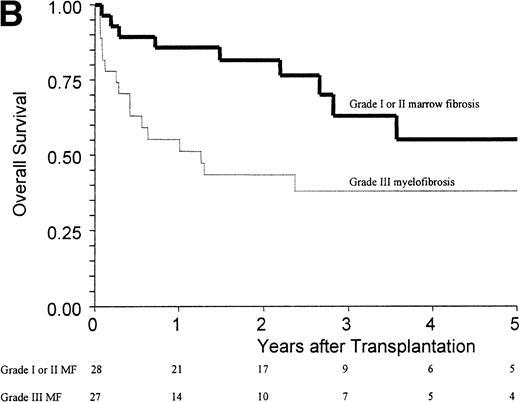
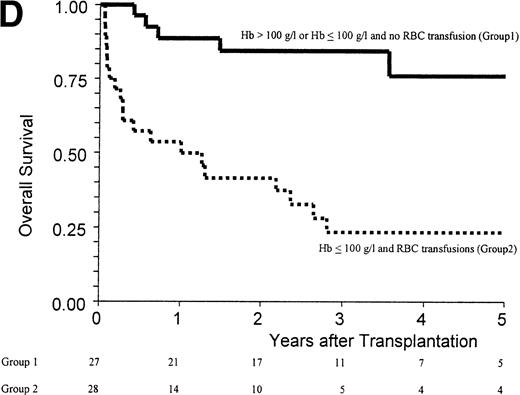
(A) Kaplan-Meier estimates of the probability of survival in 55 patients who received an allogeneic stem cell transplant for AMM. The 5-year probability of overall survival was 47% for the overall group, 54% for patients receiving an unmanipulated HLA matched related transplant, and 26% for those receiving a T-cell–depleted graft or a graft coming from an alternative donor (P = .18). (B) Outcome according to the severity of myelofibrosis (MF) before transplant. The 5-year probability of survival was 55% if there was grade I or II marrow fibrosis, and 38% if there was grade III marrow fibrosis (osteomyelosclerosis) (P = .027). (C) Outcome according to the score proposed by Dupriez et al before transplant. The 5-year probability of survival was 83% for the patients in the low-risk group, 43% for the patients in the intermediate-risk group, and 31% for the patients in the high-risk group (P = .018). (D) Outcome according to the hemoglobin level at transplant and the requirement of RBC transfusions before transplant. The 5-year probability of survival was 76% for patients with hemoglobin level >100 g/L or with hemoglobin level ≤100 g/L and no RBC transfusion before transplant, and 23% for patients with hemoglobin level ≤100 g/L receiving pretransplant RBC transfusions (P < .0001).
(A) Kaplan-Meier estimates of the probability of survival in 55 patients who received an allogeneic stem cell transplant for AMM. The 5-year probability of overall survival was 47% for the overall group, 54% for patients receiving an unmanipulated HLA matched related transplant, and 26% for those receiving a T-cell–depleted graft or a graft coming from an alternative donor (P = .18). (B) Outcome according to the severity of myelofibrosis (MF) before transplant. The 5-year probability of survival was 55% if there was grade I or II marrow fibrosis, and 38% if there was grade III marrow fibrosis (osteomyelosclerosis) (P = .027). (C) Outcome according to the score proposed by Dupriez et al before transplant. The 5-year probability of survival was 83% for the patients in the low-risk group, 43% for the patients in the intermediate-risk group, and 31% for the patients in the high-risk group (P = .018). (D) Outcome according to the hemoglobin level at transplant and the requirement of RBC transfusions before transplant. The 5-year probability of survival was 76% for patients with hemoglobin level >100 g/L or with hemoglobin level ≤100 g/L and no RBC transfusion before transplant, and 23% for patients with hemoglobin level ≤100 g/L receiving pretransplant RBC transfusions (P < .0001).
Factors assessed in univariate analysis for a potential impact on the 1-year transplant-related mortality and the 5-year overall survival are listed in Table 3. Hemoglobin level ≤100 g/L, need for red blood cell (RBC) transfusions, osteomyelosclerosis, and a high-risk score at transplant were the pretransplant variables associated with a worse outcome (Fig 1B and C). The prognostic value of the hemoglobin level at transplant was neither supported by an older recipient age at transplant (P = .76) nor by a higher incidence of grade III-IV acute GVHD (P = .20). Among the patients with a hemoglobin level ≤100 g/L before transplant, groups of different prognosis were distinguished according to the number of RBC transfusions required before transplant (Fig 1D). In the multivariate analysis, the main pretransplant variable affecting the outcome at 5 years was hemoglobin level ≤100 g/L at transplant (Table 4). This variable, and the presence of osteomyelosclerosis before transplant, were significantly associated in the Cox model with the outcome of the 45 patients who received an unmanipulated HLA matched-related transplant.
Considering posttransplant variables, grade III-IV acute GVHD and extensive chronic GVHD had the strongest effect on the outcome. GVHD was the main cause of death for patients who had osteomyelosclerosis before transplant (8 of 16 deaths).
Posttransplant disease status.
Disease status assessment after transplant is summarized in Table 5. Thirty-nine patients were in complete hematologic remission at last follow-up time, 22 among them were in complete histohematologic remission. Complete resolution of marrow fibrosis was observed between 21 days and 23 months after transplant (median, 6 months). Ten relapses were diagnosed between 4 and 67 months posttransplant (median, 12 months); and three patients had persistent disease after transplant. The 5-year probability of treatment failure was 36% ± 10% for the overall group and 28% ± 8% for the patients receiving an unmanipulated HLA matched related graft. The 5-year probability of event-free survival (events being death or treatment failure) was 39% ± 7% for the overall group and 48% ± 8% when unmanipulated HLA matched transplants were used. An older age at transplant, presence of a cytogenetic abnormality before transplant, and absence of grade II-IV acute GVHD were factors associated with treatment failure in multivariate analysis (Table 6). Eight of the patients with treatment failure were alive at last follow-up time, from 1 to 108 months after detection of treatment failure (median, 9 months), one in complete remission after a second transplant. Five patients who relapsed had died at a median of 9 months (range, 4 to 31).
DISCUSSION
This retrospective multicenter study of allogeneic stem cell transplantation for AMM documents an important advance in the management of a disease otherwise uniformly fatal within a median of 5 years. First of all, this study confirms and extends previous reports showing that allogeneic stem cell transplanta-tion is the only approach, which leads in the majority of cases to a complete hematologic remission, and for a substantial number of these, to long-lasting complete histohematologic remissions22,23,30-38 and probably to cure. The 5-year overall survival of 54% for patients receiving an unmanipulated HLA matched related graft is particularly encouraging, given the fact that the 5-year actuarial survival rate reported in the largest series of AMM patients treated by conventional, nonaggressive, but only palliative, approaches was 40%.11
Second, this study is the first one to identify risk factors that are significantly associated with posttransplant outcome. In particular, using multivariate analysis, we found that the presence of osteomyelosclerosis and hemoglobin level ≤100 g/L at the time of transplant were independent predictors for decreased survival. The reason for increased mortality in patients with osteomyelosclerosis is unclear, as this group had similar characteristics and transplant procedures as compared with the group of patients without osteomyelosclerosis. The main reason might be the high incidences of grade III-IV acute and extensive chronic GVHD seen in patients with osteomyelosclerosis. The release of fibronectin and vitronectin from the abnormal extracellular matrix of the bone marrow fibrotic tissue39 after the conditioning regimen could explain this relationship between severe GVHD and osteomyelosclerosis, as these integrins can activate T cells via an antigen-independent pathway.40,41 The hemoglobin level at diagnosis has been widely recognized as the main variable affecting the outcome of patients with AMM receiving conventional treatments.4-11 We also found that a hemoglobin level ≤100 g/L before transplant adversely affected the outcome. Severe anemia might have been a marker of a heavier pretransplant transfusional support, as the survival was worse among anemic patients who received more than 10 RBC units compared with less or nontransfused anemic patients.
Third, this study shows that the risk of treatment failure after an allogeneic transplant for AMM is of concern because the 5-year probability of treatment failure was 28% for patients receiving an unmanipulated HLA matched related transplant. We found in multivariate analysis that recipient age at transplant, presence of a cytogenetic abnormality, and the absence of grade II-IV acute GVHD were associated with a higher risk of treatment failure. The significant association between grade II-IV acute GVHD and a low treatment failure rate leads one to suspect a graft-versus-AMM effect. This hypothesis could be supported in the near future by the results of donor lymphocyte infusions recently performed in four patients with AMM (data not shown). The identification of the pretransplant karyotype as a predictor of treatment failure individualizes a group of patients with cytogenetic abnormalities, for whom T-cell depletion, a procedure usually associated with an increased risk of relapse in other indications, should probably not be recommended.
Finally, the presence of severe marrow fibrosis and the absence of splenectomy performed before transplant have been individualized as the two main variables having a significant impact on the speed of posttransplant hematopoietic recovery. However, it has to be emphasized that osteomyelosclerosis was not associated with an increased incidence of graft failure, even if total body irradiation was not used during the conditioning regimen. Furthermore, marrow fibrosis was a reversible process, with impressive regressions, most often complete, occurring during the first year posttransplant. Splenectomy was also associated with a faster hematopoietic recovery, without an increased rate of severe GVHD. Therefore, splenectomy as an elective before transplant might be an attractive option in case of spleen enlargement. However, because of the significant morbidity and mortality of this procedure in AMM and of the absence of survival benefit in our series, it seems preferable to restrict splenectomy to patients with osteomyelosclerosis or a huge spleen, eg, situations in which engraftment could be significantly delayed.
As for patients with chronic myeloid leukemia in chronic phase, physicians caring for patients with AMM face a difficult decision when considering the optimal timing for a transplant. The prognostic score recently described by Cervantes et al,42 which is derived from the analysis of a large cohort of patients less than 55 years of age at diagnosis, and based on hemoglobin level, presence of blasts in peripheral blood and/or constitutional symptoms, should be of some use. This prognostic score identifies patients with a poor expected outcome according to conventional approaches (median survival <33 months if two of these factors are observed). For these patients, it seems reasonable to consider a transplant shortly after diagnosis, as the results of this curative approach in high-risk patients receiving less than 10 RBC transfusions before transplant were at least similar to the one reported in the study of Dupriez et al,11 where only palliative treatments were given.
For asymptomatic patients with no cytogenetic abnormalities, no significant cytopenias, and an expected median survival greater than 14 years with palliative treatments, “to be or not to be transplanted” early remains a dilemma. The identification in multivariate analysis of the recipient age at transplant as the main predictive factor of treatment failure could lead to consideration of the transplant earlier in the course of the disease. Better results were obtained when transplants were performed before patients developed poor prognostic features, and earlier transplants in younger patients should decrease the incidence of severe GVHD. However, because transplant-related mortality was 27% at 1 year in this series and because the life expectancy of the low-risk patients receiving palliative treatments, another less “straightforward” option could be to propose a transplant when the hemoglobin level has fallen below 100 g/L, or when an isolated cytogenetic abnormality, constitutional symptoms, or blasts in peripheral blood appear. Therefore, when considering low-risk patients, it is not currently possible to give a clear-cut message as to when a transplant should be performed. Until larger series with longer follow-up time show that early transplant is beneficial to low-risk patients, the proposal of Clift et al43 regarding allogeneic transplantation for chronic myeloid leukemia in chronic phase could be applied to low-risk patients with AMM, in that “the ultimate decision should first reflect the patient’s approach to the disease.”43
Drawing a parallel with the dramatic improvement of the allogeneic transplant results for chronic myeloid leukemia, we can expect that, with a more precise definition of the factors affecting the posttransplant outcome, better results will be achieved with this strategy in AMM over the next decade.
ACKNOWLEDGMENT
The authors thank Prof Claude Chastang for his advice concerning the statistical analysis and Anja van Biezen for her help in collecting the data from the European centers.
Additional participating institutions and their principal investigators. Inderjeet Dokal (Hammersmith Hospital, London, UK), Norbert Ifrah (C.H.R.U. Angers, Angers, France), Jean-Pierre Jouet (Hôpital Claude Huriez, Lille, France), Manuel Abecasis (Instituto Portugues Oncologia, Lisboa, Portugal), Jane F. Apperley (Hammersmith Hospital, London, UK), J.J. Cornellissen (Dr Daniel Den Hoed Cancer Center, Rotterdam, The Netherlands), Maher K. Gandhi (Addenbrooke’s Hospital, Cambridge, UK), John M. Goldman (Hammersmith Hospital, London, UK), Nicole Gratecos (Hôpital de l’Archet I, Nice, France), Shunro Kai (Hyogo College of Medicine, Nishinomiya, Japan), Mathieu Kuentz (Hôpital Henri Mondor, Créteil, France), Bruno Lioure (Hôpital Haute-Pierre, Strasbourg, France), Per Ljungman (Huddinge Hospital, Huddinge, Sweden), Aleksandaar Mijovic (King’s College School of Medicine and Dentistry, London, UK), Ricardo Pasquini (Hospitale de Clinicas, Curitiba, Brazil), Raymond L. Powles (Royal Marsden Hospital, Sutton Surrey, UK), Christoph H. Rossbach (St Petersburg, FL), Tapani Ruutu (Helsinki University Central Hospital, Helsinki, Finland), Norbert Schmitz (Christian-Albrechts-University, Kiel, Germany), HervéTilly (Centre Anti-Cancéreux Henri Becquerel, Rouen, France), Eric Deconinck (Hôpital Jean Minjoz, Besançon, France), Paolo Di Bartolomeo (Ospedale Civile, Pescara, Italy), Augustin Ferrant (Cliniques Universitaires St Luc, Brussels, Belgium), François Guilhot (Hôpital la Miletrie, Poitiers, France), Gerald Marit (Hôpital du Haut-Lévêque, Pessac, France), Mauricette Michallet (Hôpital Edouard Herriot, Lyon, France), M. Mistrik (University Hospital, Bratislava, Slovakia), H. Grant Prentice (Royal Free Hospital, Hampstead London, UK), John D. Shepherd (Vancouver Hospital and Health Sciences Centre, Vancouver, Canada), Harry Shouten (University Hospital Maastricht, Maastricht, The Netherlands), Anne M. Smith (London Hospital, London, Ontario, Canada).
Additional participating institutions and their principal investigator are listed in the .
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Philippe Guardiola, MD, Service de Greffe de Moelle “Trèfle 3,” Hôpital Saint-Louis, 1 avenue Claude Vellefaux, F-75475 Paris Cedex 10, France; e-mail:Phguardiol@aol.com.