Abstract
Better understanding of hemostasis will be possible by the identification of new lineage-specific stimuli that regulate platelet formation. We describe a novel functional megakaryocyte receptor that belongs to a family of ionotropic glutamate receptors of theN-methyl-d-aspartate (NMDA) subtype responsible for synaptic neurotransmission in the central nervous system (CNS). Northern blotting and reverse-transcriptase polymerase chain reaction (RT-PCR) studies identified expression of NMDAR1 and NMDAR2D type subunit mRNA in rat marrow, human megakaryocytes, and MEG-01 clonal megakaryoblastic cells. Immunohistochemistry and in vivo autoradiographic binding of the NMDA receptor-specific antagonist MK-801 confirmed that megakaryocytes expressed open channel-forming NMDA receptors in vivo. Western blots indicated that megakaryocyte NMDAR1 was either unglycosylated or only glycosylated to low levels, and of identical size to CNS-type NMDAR1 after deglycosylation with endoglycosidase F/peptide-N-glycosidase F. In functional studies, we demonstrated that NMDA receptor activity was necessary for phorbol myristate acetate (PMA)-induced differentiation of megakaryoblastic cells; NMDA receptor blockade by specific antagonists significantly inhibited PMA-mediated increases in cell size, CD41 expression, and adhesion of MEG-01 cells. These results provide evidence for a novel pathway by which megakaryocytopoiesis and platelet production may be regulated.
THROMBOPOIESIS FOLLOWS a complex sequence of events beginning with megakaryocyte differentiation from pluripotent bone marrow stem cells and culminating with the release of platelets into the circulation from megakaryocyte cytoplasmic fragments.1 The major regulator of this process is thec-Mpl ligand thrombopoietin.2-4 In addition, a variety of pleiotropic hematopoietic growth factors acting on different cells of the megakaryocytic lineage promote the growth and maturation of megakaryocytes.5 Despite these advances, the precise cell-signaling events underlying the regulation of megakaryocytopoiesis are incompletely understood.
In a recent study to investigate responses of bone cells to mechanical load in vivo, we identified a glutamate/aspartate transporter (GLAST), previously only detected in the central nervous system (CNS).6 GLAST performs an essential function during glutamate-mediated synaptic neurotransmission by acting as a high-affinity reuptake system to remove released glutamate from the synaptic cleft, thus preventing overstimulation of postsynaptic glutamate receptors. The presence of GLAST in bone led us to hypothesize that glutamate signaling may also have a function outside the CNS. To explore this hypothesis initially, we have investigated the expression of glutamate receptor types in bone and bone marrow. One cell type in bone marrow expressingN-methyl-d-aspartate (NMDA)-type glutamate receptors was the megakaryocyte, which suggests a role for glutamate signaling in megakaryocyte maturation and platelet formation.
Glutamate is the predominant excitatory amino acid in the vertebrate CNS, mediating synaptic neurotransmission through diverse ionotropic and metabotropic receptors. Three subtypes of the ionotropic receptors have been described: α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), kainate, and NMDA glutamate receptors, which are classified according to their pharmacologic responses to these agonists.7 NMDA receptors have received particular attention as principal participants in synaptic plasticity, which is believed to underlie key neuronal events such as development, learning, and memory8-10; in vivo, they function as a heteropentamer of NMDAR1 subunits with one of the four structurally related NMDAR2 subunits (A, B, C, or D).11 In addition, overstimulation of NMDA receptors, causing excessive Ca2+ influx, is thought to promote neuronal degeneration and its clinical sequelae.12 The NMDA-type glutamate receptors we have identified on megakaryocytes form open channels in vivo and have functional activity in a human megakaryocyte-like cell line. These data suggest that an auxiliary glutamate-mediated signaling pathway may be involved in the regulation of megakaryocytopoiesis.
MATERIALS AND METHODS
Cell culture.
Culture media and supplements were obtained from GIBCO-BRL (Paisley, UK). MEG-01 cells were maintained in RPMI 1640 medium (as supplied contains 140 μmol/L L-glutamate) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, and 10% fetal calf serum at 37°C in 5% CO2/95% air atmosphere. Differentiation was induced by culturing MEG-01 cells in the presence of 10−7 mol/L phorbol myristate acetate (PMA) for up to 3 days.13
Megakaryocytes were generated ex vivo from hematopoietic progenitors. Briefly, CD34+ cells were isolated from umbilical cord blood by a magnetic immunoselection technique (Milteny Biotec, Auburn, CA) and cultured in Iscove’s modified Dulbecco’s medium supplemented with minimum essential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 2 mg/mL L-asparagine, 0.2% bovine serum albumin, 10% human cord blood platelet-poor plasma, and 10 ng/mL thrombopoietin. After 14 days, megakaryocytic differentiation was assessed by flow cytometry as previously described.14
Reverse-transcriptase polymerase chain reaction and Northern blotting.
Total RNA was isolated from MEG-01 cells and bone marrow using Trizol reagent (GIBCO-BRL). First-strand cDNA was synthesized using Superscript RNase H reverse transcriptase (RT) (GIBCO-BRL). The following polymerase chain reaction (PCR) primers were used: 5′-AATGACCCCAGGCTCAGAAAC-3′ and 5’-TGAAGCCTCAAACTCCAGCAC-3′, which amplify a product of 201 bp and correspond to position 2001 to 2222 of rat brain NMDAR1 (accession no. U08261), 5′-AGGTGTTCTATCAGCGTG-3′ and 5′-TGTAGCTGTGCATGTCAG-3′, which amplify a product of 619 bp and correspond to position 1570 to 2189 of rat brain NMDAR2D (accession no. L31612), and 5′-GATGTCTTCCAAGTATGCGGA-3′ and 5′-GGGAATCTCCTTCTTGACCAG-3′, which amplify a product of 667 bp and correspond to position 992 to 1658 of human NMDAR1 (accession no. L05666).
PCR was performed for 35 cycles of 94° for 30 seconds, 50°C for 30 seconds, and 72°C for 30 seconds. The PCR products were subcloned into pCR11 using the TA cloning kit (Invitrogen, Groningen, The Netherlands) or into pGEM-T (Promega, Southampton, UK), and sequenced using the ABI 377 DNA sequencer (Perkin Elmer, Warrington, UK).
The cloned human NMDAR1 cDNA was used as a probe for Northern blotting of RNA extracted from megakaryocytes derived from CD34+progenitor cells using the technique described earlier. Northern blots were performed using 2 μg total RNA extracted from cultures containing greater than 90% megakaryocytes.
Western blotting.
Brain homogenates and MEG-01 cells were lysed in 50 mmol/L Tris, 10% glycerol, 1 mmol/L EDTA, 0.5 mmol/L dithiothreitol (DTT), 1% sodium dodecyl sulfate (SDS), and separated by SDS-polyacrylamide gel electrophoresis (PAGE). Western blotting was performed using a monoclonal antibody against NMDAR1 (clone 54.1; Pharmingen, San Diego, CA). This antibody identifies an extracellular peptide sequence that is conserved among NMDAR1 splice variants. Deglycosylation was achieved by preincubating samples with endoglycosidase F/peptide-N-glycosidase F (Oxford Glycosystems, Abingdon, UK) for 18 hours at 37°C.
Immunolocalization.
Adult rat ulnae and neonatal rat tibiae used for immunolocalization studies were dissected out, dipped in 10% polyvinyl alcohol (PVA) (Sigma, Poole, UK), and immediately frozen in chilled n-hexane (−70°C). The tissues were mounted in 10% PVA on brass chucks and 5- to 7-μm sections were cut using a Brights cryostat (Brights Instrument, Huntington, UK). Sections were collected on Vectorbonded slides (Vector Laboratories, Peterborough, UK) and stored at −35°C until use. Before immunolocalization, the sections were fixed in 4% paraformaldehyde for 5 minutes and endogenous peroxidase activity depleted with 3% hydrogen peroxide (Sigma) for 30 minutes. A further preincubation was performed with 10% normal horse serum (Vector Laboratories) for 30 minutes to block nonspecific antibody binding. The sections were incubated for 30 minutes with anti-NMDAR1 monoclonal antibody (Pharmingen; 1 μg/mL) followed by biotinylated horse anti-mouse secondary antibody (Vector Laboratories; 1:200 dilution) for 10 minutes and avidin-biotinylated-peroxidase reagent (ABC Elite, Vector Laboratories; 1:50 dilution) for 15 minutes. Peroxidase activity was visualized with 0.5 mg/mL 3, 3’-diaminobenzidine (Sigma) and 0.3% hydrogen peroxide as substrate. All dilutions were made up in phosphate-buffered saline (PBS), pH 7.4, and incubations were performed at room temperature with three PBS washes between each incubation. Negative controls received the same concentrations of normal mouse IgGs (Vector Laboratories) in place of primary antibody. Some sections were counterstained with hematoxylin before mounting in glycerol/PBS. Similar immunolocalizations were performed on blood and marrow smears from normal human subjects and on cytospins of MEG-01 cells.
Acetylcholinesterase activity was used to identify rat megakaryocytes. Unfixed sections were incubated in 0.1 mol/L acetate buffer, pH 6.0, containing 3 mmol/L copper sulfate, 5 mmol/L sodium citrate, 0.5 mmol/L potassium ferricyanide, and 0.5 mg/mL acetylthiocholine iodide, for up to 2 hours at room temperature in a humidified chamber. The slides were then rinsed in buffer and fixed in 4% paraformaldehyde. For colocalization with NMDAR1, acetylcholinesterase-stained sections were blocked for 30 minutes with 10% goat serum (Vector Laboratories), then incubated with the primary NMDAR1 antibody (Pharmingen; 1 μg/mL overnight at 4°C). Antibody binding was detected by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody (Sigma; 1:100 dilution) for 45 minutes at room temperature and the mounted slides were viewed under UV light illumination.
For immunofluorescent localization of glutamate transporters on MEG-01 cells, cytospins were fixed in 4% paraformaldehyde for 5 minutes, blocked with 10% goat serum for 30 minutes, and rabbit polyclonal antibodies against GLAST, GLT-1, and EAAC1 (provided by Dr Jeffrey Rothstein, Johns Hopkins University, Baltimore, MD) were applied to sections (0.1 to 0.5 μg/mL, overnight, 4°C). Antibody binding was identified using FITC-conjugated goat anti-rabbit secondary antibody (Sigma; 1:50 dilution) for 45 minutes, mounted, and viewed under UV illumination.
Radioligand binding.
Male rats (50 to 70 g) were injected intracardially with 15 μg/kg (in 0.9% saline) of tritiated MK-801 ([3-3H]-MK-801; 23.9 Ci/mmol; Dupont NEN, Stevenage, UK), a specific noncompetitive NMDA receptor antagonist. After 15 minutes, the animals were killed and flushed with saline by intracardiac perfusion until the tissues were blanched, to remove unbound ligand. To control for nonspecific labeling, three rats were injected intracardially with a 60-fold excess of cold MK-801 (1 mg/kg dizocilipine maleate; Research Biochemicals International, Natick, MA) 15 minutes before administration of radiolabeled MK-801. Tissues were then rapidly removed, frozen, and sections cut as described previously.
Sections were dehydrated through graded alcohols, dipped in EM-1 emulsion (Amersham International, Little Chalfont, UK), and cooled on an ice tray for 30 minutes. They were then air-dried in desiccated light-tight boxes at room temperature for 4 hours, and finally exposed at 4°C for up to 24 weeks. Some 3H-MK-801–labeled tissue sections were subjected to the NMDAR1 immunoperoxidase localization protocol described earlier, before dipping in emulsion.
Flow cytometry.
CD41 (GPIIb/IIIa) expression on MEG-01 cells was determined following PMA treatment in the presence or absence of 100 μmol/L MK-801, using flow cytometry and anti-human CD41 monoclonal antibody (clone 5B12; Dako, High Wycombe, UK), which identifies glycoproteins IIb and IIIa. MEG-01 cells (1 to 5 × 106 cells) were centrifuged, washed in cold phenotyping buffer (PB; calcium and magnesium-containing PBS with 0.1% bovine serum albumin), and blocked with PB containing 1 μg/mL normal rabbit IgGs for 10 minutes. Following centrifugation, the cells were incubated with anti-human CD41 (1:100 dilution) in 100 μL PB for 30 minutes on ice. Following washing with PB, 50 μL goat anti-mouse FITC-conjugated secondary antibody (Sigma; 1:100 dilution) was added and the cells were incubated for a further 30 minutes on ice. After a final wash with PB, the cells were resuspended in 1 mL PB for analysis. FITC control samples were treated identically except that they received the same concentration of normal mouse IgGs in place of primary antibody. Live cells were analyzed with a Coulter XL flow cytometer (Coulter, Miami, FL) using light-scatter parameters. The percentage of CD41+ cells was evaluated according to an FITC gate set at 1% on samples stained with the control antibody.
Similar experiments were repeated using an antibody that recognizes a different epitope on the GPIIb/IIIa complex. FITC-conjugated monoclonal antibody Y2/51 (Dako), which detects the megakaryocytic lineage-specific antigen CD61 (glycoprotein IIIa), was used as previously described.14 Antigen expression was determined using a FACScan instrument and Lysis II software (Becton Dickinson, Mountain View, CA). Light-scatter parameters were used to exclude dead cells. Relative antigen expression was estimated on live cells using the median fluorescence intensity. The fluorescence of cells stained with an FITC-conjugated isotype-matched control antibody was subtracted from all determinations.
Adhesion assays.
Adhesion assays were performed as described by De Luca et al15 with minor modifications. Briefly, MEG-01 and CMK cells (2 × 105 cells/mL) were cultured in 96-well plates with and without PMA (10−7 mol/L) for 72 hours in the presence of varying concentrations (0 to 100 μmol/L) of MK-801 or the competitive glutamate receptor antagonist D-AP5 (D(-)-2-amino-5-phosphonopentanoic acid; Research Biochemicals International). Medium was then removed and the wells were rinsed three times with PBS to remove nonadherent cells. Adherent cells were fixed in 70% ethanol (15 minutes) and stained with 0.5% crystal violet (25 minutes), followed by extensive washing with water to remove unbound dye. Dye was eluted by the addition of 50% ethanol/0.1 mol/L sodium citrate, pH 4.2. Absorbances were measured on a plate reader (Dynatech MR5000; Dynatech, Billingshurst, UK) at 570 nm.
The methylthiotetrazole (MTT) assay was used to determine cell viability.16 Briefly, parallel cultures were grown as described earlier in phenol red-free RPMI 1640 growth medium. After 72 hours, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Thiazol blue; Sigma) solution was added directly to the wells to give a final concentration of 1 mg/mL and the plates were incubated for a further 3 hours. The cells were lysed with 0.05N HCl containing 10% SDS and absorbances read at 570 nm.
Statistical analysis.
Where indicated, significant differences were determined using Student’s t-test, as described in the text.
RESULTS
Expression of NMDA receptor subunits in ex vivo–generated megakaryocytes and megakaryoblastic cells.
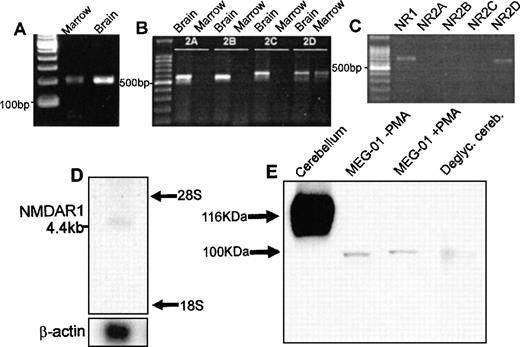
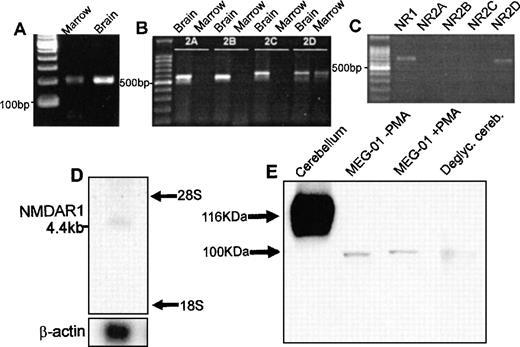
PCR of cDNA from rat marrow amplified single products for the NMDA receptor subunits NMDAR1 and NMDAR2D of predicted size (Fig1A and B). In agreement with these results, amplification of NMDAR1 and NMDAR2D using cDNA from the human megakaryoblastic cell line, MEG-01, identified single products of 667 bp and 619 bp, respectively (Fig 1C). RT-PCR did not identify expression of NMDAR2A, B, or C in rat marrow or MEG-01 cells. All of the PCR products showed 100% sequence homology with corresponding CNS-type NMDA subunits. NMDA receptor mRNA expression was confirmed on Northern blots of total RNA derived from primary human megakaryocytes. As shown in Fig 1D, the NMDAR1 probe hybridized to an mRNA species of approximately 4.4 kb, which matched the size of a previously identified NMDAR1 transcript.17 Western blots of cell lysates from MEG-01 cells showed the presence of major protein bands of approximately 100 kD corresponding to the size of deglycosylated CNS-type NMDAR1 (Fig 1E). Since NMDAR2D is the least characterized of CNS NMDA-type subunits, and no anti-NMDAR2D antibodies yet exist commercially, our immediate studies concentrated on investigating the localization and expression of NMDAR1 in megakaryocytes.
Expression of NMDA receptor subunits by megakaryocytes. RT-PCR amplified products for the NMDAR1 (A) and NMDAR2D (B) receptor subunit from rat bone marrow; NMDAR2A-C were identified in brain only (B). (C) RT-PCR confirmed expression of NMDAR1 (NR1) and NMDAR2D (NR2D) subunits by MEG-01 cells. (D) Northern blot analysis of total RNA (2 μg) from primary human megakaryocytes using an NMDAR1 probe, which identified a 4.4-kb mRNA species. (E) Western blot analysis of NMDAR1 protein in rat cerebellum, MEG-01 cells (cultured in the absence or presence of 10−7 mol/L PMA for 3 days) and N-deglycosylated rat cerebellum. CNS-type NMDAR1 was of expected size,17 116 kD, which was reduced to ∼100 kD following N-deglycosylation. Major MEG-01 NMDAR1 protein bands were detected at ∼100 kD, matching N-deglycosylated CNS-type NMDAR1 size.
Expression of NMDA receptor subunits by megakaryocytes. RT-PCR amplified products for the NMDAR1 (A) and NMDAR2D (B) receptor subunit from rat bone marrow; NMDAR2A-C were identified in brain only (B). (C) RT-PCR confirmed expression of NMDAR1 (NR1) and NMDAR2D (NR2D) subunits by MEG-01 cells. (D) Northern blot analysis of total RNA (2 μg) from primary human megakaryocytes using an NMDAR1 probe, which identified a 4.4-kb mRNA species. (E) Western blot analysis of NMDAR1 protein in rat cerebellum, MEG-01 cells (cultured in the absence or presence of 10−7 mol/L PMA for 3 days) and N-deglycosylated rat cerebellum. CNS-type NMDAR1 was of expected size,17 116 kD, which was reduced to ∼100 kD following N-deglycosylation. Major MEG-01 NMDAR1 protein bands were detected at ∼100 kD, matching N-deglycosylated CNS-type NMDAR1 size.
Megakaryocytes express NMDAR1 protein in vivo.
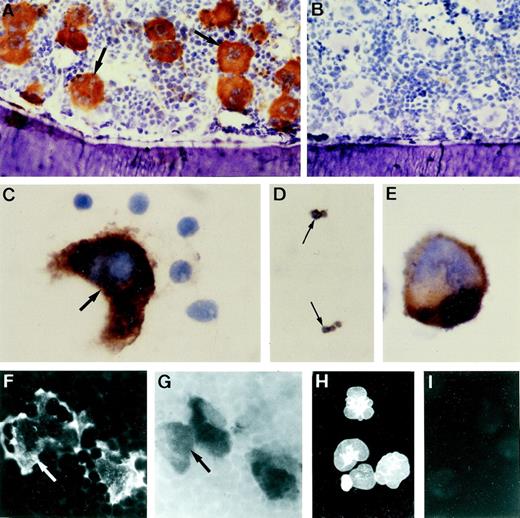
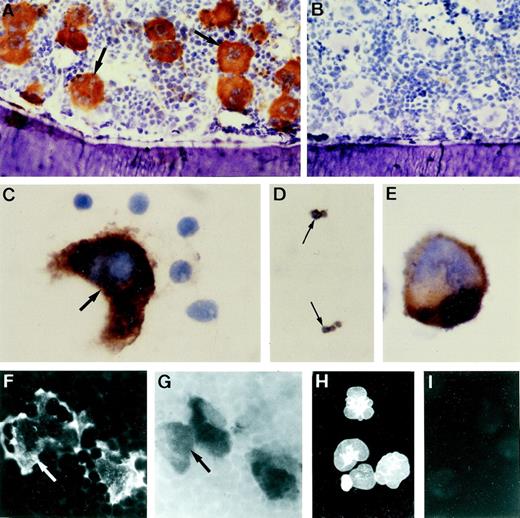
Specific NMDAR1 immunoreactivity was observed on megakaryocytes in sections of neonatal and adult rat bone (Fig2A) and spleen (data not shown). Positive staining was also detected on megakaryocytes in smears of mouse and human marrow on human platelets and on cytospins of MEG-01 cells (Fig2C through E). Colocalization studies showed that NMDAR1 staining was present only on acetylcholinesterase-positive cells, and that all acetylcholinesterase-positive megakaryocytes displayed NMDAR1 staining regardless of stage of maturation (Fig 2F and G). Intense immunostaining for the glutamate transporter GLAST was seen on MEG-01 cells (Fig 2H), although expression of the related transporters, GLT-1 and EAAC1, was not observed on these cells.
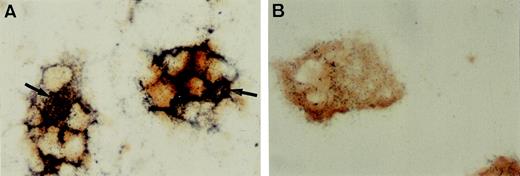
Immunolocalization of NMDAR1 on megakaryocytes. (A) Cryosection of rat bone indicating intense NMDAR1 immunoreactivity (brown reaction product) on bone marrow megakaryocytes (arrows). (B) Antibody control, hematoxylin counterstain. Positive immunostaining was also identified on human megakaryocytes (C, arrow), human platelets (D, arrows), and MEG-01 cells (E). (F and G) Colocalization studies showed that cells displaying NMDAR1 immunofluorescence in rat bone marrow (F, arrow) were acetylcholinesterase-positive (G, arrow). (H) MEG-01 cells demonstrated strong immunostaining for the glutamate transporter, GLAST. (I) MEG-01 antibody control. Original magnification: A and B, ×250; C, D, and E, ×1,000; F and G, ×500; H and I, ×200. Fig 3. Binding of the specific NMDA channel blocker, MK-801 to megakaryocytes in vivo. Autoradiography studies localized 3H-MK-801, identified as numerous black silver particles (A, arrows) to NMDAR1-positive (brown staining) megakaryocytes in rat ulna cryosections. Ligand binding was displaced by excess cold MK-801 (B). Original magnification × 1,000.
Immunolocalization of NMDAR1 on megakaryocytes. (A) Cryosection of rat bone indicating intense NMDAR1 immunoreactivity (brown reaction product) on bone marrow megakaryocytes (arrows). (B) Antibody control, hematoxylin counterstain. Positive immunostaining was also identified on human megakaryocytes (C, arrow), human platelets (D, arrows), and MEG-01 cells (E). (F and G) Colocalization studies showed that cells displaying NMDAR1 immunofluorescence in rat bone marrow (F, arrow) were acetylcholinesterase-positive (G, arrow). (H) MEG-01 cells demonstrated strong immunostaining for the glutamate transporter, GLAST. (I) MEG-01 antibody control. Original magnification: A and B, ×250; C, D, and E, ×1,000; F and G, ×500; H and I, ×200. Fig 3. Binding of the specific NMDA channel blocker, MK-801 to megakaryocytes in vivo. Autoradiography studies localized 3H-MK-801, identified as numerous black silver particles (A, arrows) to NMDAR1-positive (brown staining) megakaryocytes in rat ulna cryosections. Ligand binding was displaced by excess cold MK-801 (B). Original magnification × 1,000.
Radioligand binding demonstrates open NMDA receptor channels.
MK-801 binds specifically to channel-forming NMDA receptors in the CNS. As shown in Fig 3A, when animals were injected with [3H]-MK-801, and binding was examined in bone marrow sections, prominent radioactivity was detected on bone marrow megakaryocytes, which colocalized with NMDAR1 antibody staining. The number of developed silver grains present on megakaryocytes was markedly reduced when the animals were pretreated with an excess of cold MK-801 (Fig 3B).
Functional studies.
MEG-01 cells undergo further differentiation when treated with PMA.13 As shown earlier, these cells were found to express NMDA receptor subunits by RT-PCR (Fig 1C) and Western blotting (Fig 1E) and immunostaining (Fig 2E). Having demonstrated that megakaryocytes bind MK-801 in vivo, a specific noncompetitive antagonist of the CNS-type NMDA receptor, we investigated the effect of this in MEG-01 cells.
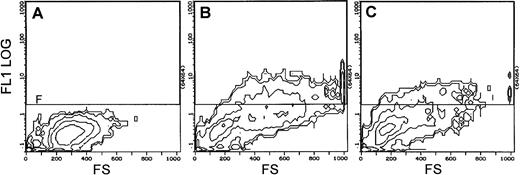
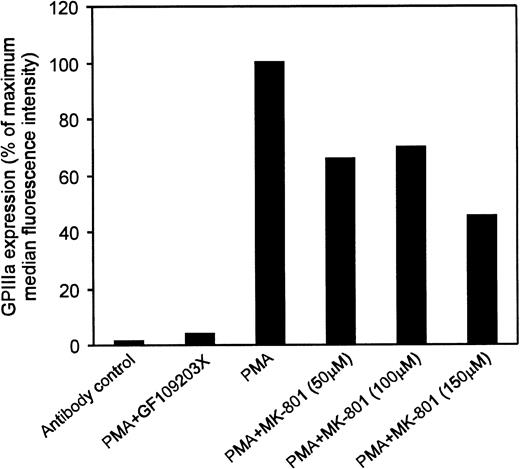
Flow cytometric analyses showed that 100 μmol/L MK-801 inhibited PMA-mediated increases in cell size and CD41 (GPIIb/IIIa) expression. Thus, in the presence of MK-801, mean forward scatter was significantly reduced from 480 to 326 nm (P < .05), and CD41+events from 34% to 20% (P < .005) (Fig4 and Table1). To confirm these observations further, experiments were repeated using an alternative antibody recognizing a different epitope on GPIIIa. These studies again demonstrated that MK-801 caused a 30% to 55% inhibition of GPIIIa expression in PMA-stimulated cultures of MEG-01 cells (Fig5).
Effect of the NMDA channel blocker, MK-801, on CD41 (GPIIb/IIIa) expression by MEG-01 cells. Charts show flow cytometric analyses of CD41 expression by (A) untreated MEG-01 cells, (B) MEG-01 cells treated with PMA (10−7 mol/L), and (C) MEG-01 cells treated with PMA (10−7 mol/L) and MK-801 (10−4 mol/L) for 3 days. Representative contour plots are shown indicating forward-scatter (FS, cell size) against CD41-FITC fluorescence (FL1). Events lying within the F gate indicate CD41 positivity. PMA-stimulated increases in cell size and CD41 expression (compare A and B) are reduced in the presence of MK-801 (C). See Table1.
Effect of the NMDA channel blocker, MK-801, on CD41 (GPIIb/IIIa) expression by MEG-01 cells. Charts show flow cytometric analyses of CD41 expression by (A) untreated MEG-01 cells, (B) MEG-01 cells treated with PMA (10−7 mol/L), and (C) MEG-01 cells treated with PMA (10−7 mol/L) and MK-801 (10−4 mol/L) for 3 days. Representative contour plots are shown indicating forward-scatter (FS, cell size) against CD41-FITC fluorescence (FL1). Events lying within the F gate indicate CD41 positivity. PMA-stimulated increases in cell size and CD41 expression (compare A and B) are reduced in the presence of MK-801 (C). See Table1.
Effect of MK-801 on GPIIIa expression in MEG-01 cells. MEG-01 cells were incubated in the presence of the indicated compounds for 3 days. Results expressed as percentage of the increase in the median fluorescence intensity observed in the presence of PMA alone. Addition of the PKC inhibitor GF109203X (3 μmol/L) was used as a positive control and fluorescence intensity of samples not treated with PMA was subtracted from values obtained in the presence of PMA. Results indicated that 50 to 150 μmol/L MK-801 caused a 30% to 55% inhibition of PMA-induced increase in GPIIIa expression.
Effect of MK-801 on GPIIIa expression in MEG-01 cells. MEG-01 cells were incubated in the presence of the indicated compounds for 3 days. Results expressed as percentage of the increase in the median fluorescence intensity observed in the presence of PMA alone. Addition of the PKC inhibitor GF109203X (3 μmol/L) was used as a positive control and fluorescence intensity of samples not treated with PMA was subtracted from values obtained in the presence of PMA. Results indicated that 50 to 150 μmol/L MK-801 caused a 30% to 55% inhibition of PMA-induced increase in GPIIIa expression.
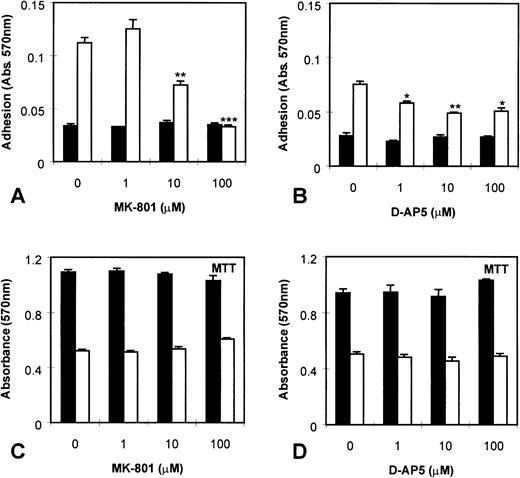
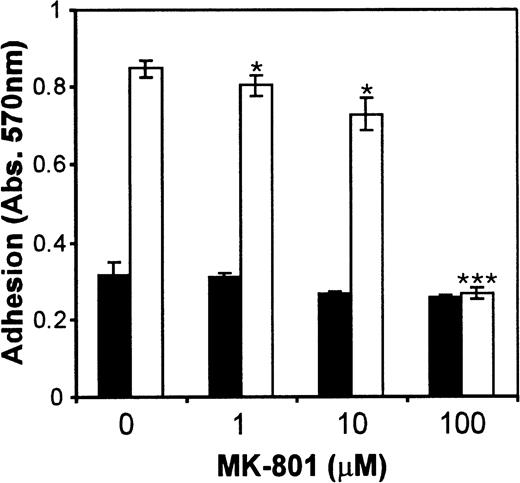
In addition, we investigated the effect of NMDA receptor blockade on MEG-01 cell adhesion. It was found that PMA markedly increased MEG-01 adhesion, and that this effect was significantly inhibited (P< .005) by MK-801 in a dose-dependent manner (Fig6A). In addition, another NMDA receptor antagonist, D-AP5, which competitively blocks glutamate binding to receptors, also caused a significant (P < .01) inhibition of PMA-mediated adhesion (Fig 6B). MK-801 and D-AP5 did not affect cell numbers or viability in MTT assays of parallel cultures (Fig 6C and D).
Effect of NMDA receptor antagonists on MEG-01 adhesion. MEG-01 cells were cultured in the absence (▪) or presence (□) of PMA (10−7 mol/L). (A) The noncompetitive antagonist, MK-801, and (B) the competitive antagonist, D-AP5, caused significant dose-dependent inhibition of PMA-stimulated adhesion. MTT assays of parallel cultures indicated that MK-801 (C), and D-AP5 (D), had no effect on cell numbers or viability. Values are means ± SEM (n = 4). *P < .05, **P < .01, ***P < .005v controls (t-test).
Effect of NMDA receptor antagonists on MEG-01 adhesion. MEG-01 cells were cultured in the absence (▪) or presence (□) of PMA (10−7 mol/L). (A) The noncompetitive antagonist, MK-801, and (B) the competitive antagonist, D-AP5, caused significant dose-dependent inhibition of PMA-stimulated adhesion. MTT assays of parallel cultures indicated that MK-801 (C), and D-AP5 (D), had no effect on cell numbers or viability. Values are means ± SEM (n = 4). *P < .05, **P < .01, ***P < .005v controls (t-test).
To rule out the possibility that the inhibition of PMA-induced adhesion was a characteristic of this particular cell line, the effect of MK-801 was also investigated in another megakaryocytic cell line. Figure7 shows that treatment of CMK cells with 1 to 100 μmol/L MK-801 caused a significant (P < .005) dose-dependent inhibition of PMA-mediated cell attachment.
Effect of the NMDA receptor antagonist, MK-801 on CMK adhesion. Treatment of CMK cells with various concentrations of MK-801 in the absence (▪) or presence (□) of PMA (10−7mol/L) caused a significant dose-dependent inhibition of PMA-induced adhesion. Values are means ± SEM (n = 4). *P < .05, ***P = .001 v controls (t-test).
Effect of the NMDA receptor antagonist, MK-801 on CMK adhesion. Treatment of CMK cells with various concentrations of MK-801 in the absence (▪) or presence (□) of PMA (10−7mol/L) caused a significant dose-dependent inhibition of PMA-induced adhesion. Values are means ± SEM (n = 4). *P < .05, ***P = .001 v controls (t-test).
DISCUSSION
Identification of additional regulatory signals governing megakaryocyte maturation and platelet release is fundamental to our understanding of thrombopoiesis. Megakaryocytic expression of an NMDA-type glutamate receptor provides evidence for an additional level of control in this regulatory hierarchy. Although NMDA receptors are concentrated at CNS synaptic junctions, expression outside the CNS is not without precedent. Similar functional receptors have been described in guinea pig ileum, rat adrenal gland, islet cells of rat pancreas,18-24 and bone cells25, indicating that glutamate receptors may have a more widespread distribution, including some nonneuronal peripheral tissues. Megakaryocytes are not classical excitable cells, yet they interact directly with several peripheral neurotransmitters. They express receptors, and display storage and transporter mechanisms for serotonin (5-HT)26-30 mimicking central serotonergic neurons,31 and megakaryocytes interact with dopamine in a similar manner.32,33 Receptors for vasoactive intestinal peptide have been demonstrated on megakarocytes and platelets34 and the same cells express neuropeptide Y.35 In addition, megakaryocytes synthesize acetylcholinesterase,36,37 which may play a role in megakaryocytopoiesis,38 although classically it is responsible for terminating cholinergic neurotransmission at central and peripheral synapses, by hydrolyzing acetylcholine. It is noteworthy that the codistribution of acetylcholinesterase with glutamate receptors at noncholinergic synaptic junctions enhances glutamate-mediated signaling,39,40 possibly through direct interaction with NMDA receptors,41 and such synergy may also operate in megakaryocytes. Similarly, activation of serotonin 5-HT2 receptors, which can stimulate growth and colony formation in megakaryocytes,30 also enhances ion currents through isolated NMDA receptor channels.42
Our findings may help to reinterpret the observation that platelets express a high-affinity binding site for the NMDA receptor antagonist phencyclidine.43 44 Results from those studies suggested that this binding site was not related to CNS NMDA receptors. In contrast, in this work we have provided compelling evidence for functional megakaryocyte NMDA receptors. Furthermore, we have observed immunoreactivity for NMDAR1 on platelets.
The NMDA receptors expressed by megakaryocytes are open channel-forming structures, as evidenced by their binding of MK-801, which cannot bind to closed channels, confirming in vivo functionality. It is likely that these receptor complexes are composed predominantly of NMDAR1-type subunits, although our finding that NMDAR2D-type subunit transcripts are also expressed suggests that native megakaryocyte NMDA receptors form as heteromeric assemblies of NMDAR1/NMDAR2D subunits, consistent with current theories regarding NMDA receptor assembly in central neurones.11 An interesting observation is that the level of glycosylation in megakaryocyte NMDA receptors is reduced or absent compared with CNS-type, although the functional significance of this is not entirely clear. It may be that the enhanced stability that carbohydrate moieties would provide to NMDA receptors in the synaptic membrane, where precise orientation and localization is essential, is deemed unnecessary in megakaryocytes, which display evenly distributed receptors across their cell surfaces, susceptible to multidirectional agonist stimulation.
The finding that NMDA receptors are present on bone marrow megakaryocytes is highly suggestive that glutamate-like signaling mechanisms are important for these cells. This view is reinforced by our recent finding that in vivo, megakaryocytes exist in intimate contact with cells bearing the glutamate transporter proteins GLT-1 and GLAST, which are found on mononuclear bone marrow cells and osteoblasts, respectively.6 These transporters are essential requirements for functional glutamate-mediated communication; at CNS synaptic junctions, they initiate the reuptake of released glutamate into nearby cells, terminating neurotransmission.45 Furthermore, we have demonstrated expression of NMDA-type glutamate receptors by osteoblasts and osteoclasts,25 pointing to the existence of a multicellular glutamate signaling pathway in bone.
Megakaryocyte-like MEG-01 cells also express NMDA receptors and GLAST, suggesting that autocrine glutamate signaling pathways operate in these cell cultures. In vitro studies with MEG-01 cells indicated that functional NMDA receptors are necessary to allow these cells to progress through PMA-induced differentiation. The differentiation-promoting effects of PMA are believed to be achieved through activation of protein kinase C (PKC).13,46,47 There is substantial evidence that NMDA receptor activity is regulated by PKC,48-52 and that NMDA ion currents are positively modulated by phorbol esters.40,51,53,54 It is possible therefore, that PMA-induced PKC activation may stimulate NMDA receptor activity in MEG-01 cells to promote their differentiation. This is consistent with our finding that PMA-stimulated increases in cell size and CD41 expression by MEG-01 cells were significantly reduced by blockade of NMDA receptors with the specific antagonist, MK-801 (Figs 4and 5). Increased adhesiveness is an additional feature of PMA-induced differentiation of megakaryoblastic cell lines and primary megakaryocytes.55-60 The striking effect that MK-801 and D-AP5 had on inhibiting PMA-stimulated MEG-01 adhesion suggests that one of the primary actions of megakaryocyte NMDA receptors may lie in regulating cell-cell and cell-matrix adhesion, both critical elements of megakaryocyte and platelet function. As β1-integrins are likely mediators of PMA-induced adhesion of erythroleukemia cell lines,56 the relationship between megakaryocyte NMDA receptor activation and the expression of these and other adhesion molecules is currently being investigated.
This work demonstrates that megakaryocytes express ionotropic receptors that have distinct similarities to NMDA-type glutamate receptors of the CNS, although detailed pharmacokinetic studies have not yet been performed. The strong implication that these receptors are involved in megakaryocyte adhesion and differentiation stimulates further research to determine the precise physiologic role of megakaryocyte NMDA receptors.
ACKNOWLEDGMENT
The authors thank Dr Jeffrey Rothstein (Johns Hopkins University, Baltimore, MD) for kindly providing the antibodies to GLAST, GLT-1, and EAAC1. We also thank Nigel Raven and Pamela Wiseman (York District Hospital) for provision of human marrow smears.
Supported by the Arthritis Research Campaign, Wellcome Trust, Medical Research Council, and Smith and Nephew.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paul G. Genever, PhD, Department of Biology, PO Box 373, University of York, York YO10 5YW, UK; e-mail:pg5@york.ac.uk.