Proliferation, differentiation, and survival of erythroid progenitor cells are mainly regulated by stem cell factor (SCF) and erythropoietin (Epo). Using normal human progenitors, we analyzed the role of Ca2+-sensitive protein kinase C (PKC) subtypes and of G-protein–coupled receptor ligands on growth factor–dependent DNA synthesis. We show that stimulation of DNA synthesis by the two growth factors requires activation of PKC. Inhibitors of Ca2+-activated PKC subtypes blocked the growth factor–induced 3H-thymidine incorporation. SCF and Epo caused no significant translocation of PKC into the membrane, but treatment of intact cells with either of the two cytokines resulted in enhanced activity of immunoprecipitated cytosolic PKC. Stimulation of PKC with the phorbol ester PMA mimicked the cytokine effect on DNA synthesis. Epo-, SCF-, and PMA-induced thymidine incorporation was potently inhibited by thrombin (half-maximal inhibition with 0.1 U/mL). This effect was mediated via the G-protein-coupled thrombin receptor and the Rho guanosine triphosphatase. Adenosine diphosphate caused a modest Ca2+-dependent stimulation of DNA synthesis in the absence of cytokines and specifically enhanced the effect of SCF. Cyclic 3′,5′-adenosine monophosphate exerted a selective inhibitory effect on Epo-stimulated thymidine incorporation. Our results define PKC as major intermediate effector of cytokine signaling and suggest a role for thrombin in controlling erythroid progenitor proliferation.
THE DEVELOPMENT OF hematopoietic progenitor cells from pluripotent stem cells into specific terminally differentiating lineages is associated with a strictly regulated program of sequentially changing sensitivities toward a number of cytokine growth factors.1 Proliferation and survival in early cells committed to the erythroid lineage is mainly governed by stem cell factor (SCF) and erythropoietin (Epo), while other factors (eg, interleukin-3 [IL-3], IL-6, granulocyte-macrophage colony-stimulating factor [GM-CSF], or IL-11) may play supportive roles.2-4 Late erythroid progenitors (CFU-E) in the final stage of proliferation (before terminal differentiation) depend exclusively on Epo.
Promotion of cell growth by most hematopoietic cytokines, including Epo, is associated with the activation of the JAK/STAT pathway involving a specific set of cytosolic tyrosine kinases (JAKs) and transcription factors (STATs). In addition, cytokines may stimulate nonreceptor tyrosine kinases from various other families (Src, Fes, Tec, Syk) and the downstream effectors Ras, Raf-1, and mitogen-activated protein kinases (MAPK). By contrast, the receptors for SCF, c-Kit, and M-CSF belong to the receptor tyrosine kinase family where tyrosine autophosphorylation creates binding sites for downstream effector proteins.5 Moreover, it is increasingly appreciated that the activation of various protein kinase C (PKC) isoforms, in addition to the protein tyrosine kinases mentioned above, may represent an essential element in the signaling pathways of several cytokines (eg, IL-3, M-CSF, G-CSF, thrombopoietin, Epo) during hematopoietic cell development.6-10 In IL-3– and GM-CSF–dependent human myeloid cells, some evidence has accumulated suggesting that the PKC-linked signaling cascade is required to inhibit apoptosis.11,12 In GM progenitors, PKCα appears to promote macrophage lineage commitment.10
Much less is known about mechanisms that limit cell proliferation in response to cytokines. Such negative feedback is required to ensure the tight control of terminally differentiated cell numbers in any one lineage. Several reports suggested that at least in the megakaryoid/erythroid lineage, such inhibitory signals could be mediated by G-protein–coupled receptor agonists. In human megakaryoblastic cell lines, cAMP and the multifunctional serine protease thrombin were shown to reduce cell growth.13,14Similarly, in primary human megakaryocyte progenitor cultures, thrombin, acting via its G-protein–coupled receptor, exerted a selective growth inhibition.15 Convergence on the same PKC isoforms may provide a possible basis for crosstalk between cytokine– and G-protein–linked pathways. Studies in primary human erythroid progenitors from our own laboratory had shown a strong, PKC-mediated, potentiating effect of thrombin on Gs-stimulated adenylyl cyclase activity.16 However, these experiments did not address possible consequences of this latter effect for cell growth and differentiation.
Most earlier studies on G-protein– and/or PKC–linked modulation of cell development have used either permanent cell lines derived from transformed hematopoietic cells or progenitor cells from mice rather than normal human progenitors. Therefore, it is still unclear to what extent G-protein–mediated signals and/or PKC-dependent mechanisms contribute to the overall regulation of nontransformed hematopoietic cell development. In the present work, experiments were performed in suspension cultures of primary human erythroid progenitors maintained in serum-free medium. We assessed the interactions of SCF and of Epo, the most prominent growth factors in these cells, with endogenous PKC isoforms as well as with thrombin, adenosine diphosphate (ADP) and 8-Bromo-cyclic 3′,5′-adenosine monophosphate (8-Br-cAMP) on the level of cellular DNA synthesis. In addition, we have analyzed possible feedback interactions between known actions of thrombin on cAMP formation or on intracellular Ca2+ release and cytokine-dependent effects. Our results suggest that thrombin potently antagonizes the actions of Epo and of SCF on growth and survival of erythroid progenitors, mainly due to an interaction downstream of PKCα. ADP enhances DNA synthesis induced by SCF but not the one induced by Epo, whereas cAMP inhibits Epo- but not SCF-induced DNA synthesis.
MATERIALS AND METHODS
Erythroid progenitor cell culture.
CD34+ cells were obtained from three sources: (1) blood donated by healthy volunteers, (2) blood collected after informed consent from a patient treated for primary hemochromatosis with phlebotomy at regular intervals, (3) surplus CD34+ cells collected, after a G-CSF challenge, by centrifugal elutriation from leukemia patients in remission. Our study has been reviewed and approved by the ethical committee of the Bern University Medical Faculty. No significant difference with respect to any parameter measured in the present study was noted between CD34+ cells from these different sources. Therefore, results from all types of preparations were pooled. Isolation of CD34+ cells from peripheral blood by density gradient centrifugation followed by a negative panning technique with anti-CD2, -CD11b, and -CD45 monoclonal antibodies followed our previously published method.16Purified CD34+ cells were cultivated for 6 to 8 days at a density of 0.5 to 1 × 106 cells/mL either in medium supplemented with 10% fetal calf serum together with SCF, Epo, IL-3, GM-CSF, and Hemin16 or in serum-free Iscove Medium (Iscove’s modification of Dulbecco’s minimal essential medium; IDMEM). IDMEM was supplemented with 20% BIT-9500 serum substitute, containing bovine serum albumin, insulin, and transferrin (StemCell Technologies, Vancouver, British Columbia, Canada), amphotericin B (1 μg/mL), penicillin/streptomycin (50 U + 50 μg/mL), pyruvate (1 mmol/L), mercaptoethanol (100 μmol/L), human LDL (35 μg/mL), MEM essential amino acids, MEM nonessential amino acids, and MEM vitamins (IDMEM-BIT medium). SCF (50 ng/mL), Epo (0.5 U/mL), and dexamethasone (1 μmol/L) were used as growth factors. As in avian progenitor cells,17 dexamethasone prolongs the proliferation phase of human progenitors in the presence of SCF and Epo and retards terminal erythroid differentiation.
3H-thymidine incorporation.
After a growth period of 5 to 6 days, the cells were washed and resuspended in IDMEM-BIT medium for 15 to 16 hours in the absence of any growth factors. At the end of the starvation period, the cells were distributed into 96-well plates (0.8 to 4 × 104 cells per well) and supplemented with growth factors and/or other experimental compounds and 3H-thymidine (1 μCi/mL, triplicate cultures for each condition). After a further incubation period of 24 hours, thymidine incorporation was measured using a modified version of a published method.18 Briefly, the cells from each well were sedimented, resuspended in 0.5 mL 10% trichloroacetic acid (TCA), and kept on ice for 30 minutes. The precipitate was collected by centrifugation at 14,000g, washed once with 0.5 mL TCA, and dissolved in 0.25 mL tissue solubilizer (Solutron; Kontron, Zurich, Switzerland). The incorporated3H was measured by liquid scintillation counting. The total amount of 3H-uptake was corrected for cell numbers per culture and normalized with respect to the value in cells maintained in the absence of growth factors.
Cellular Ca2+ determinations.
Cellular calcium transients were measured with the Fura-2 (1-[2-(5-caboxyoxazol-2-yl)-6-aminobenzofuran-5oxyl]-2-(2′-amino-5′-methylphenoxy)-ethane-N,N,N′N′-tetraacetic acid)-method,19 using a LS-50B (Perkin-Elmer, Norwalk, CT) dual-wavelength spectrofluorometer as previously described.20 For calibration, maximum and minimum fluorescence ratios were determined after cell lysis in digitonin (12.5 μmol/L) in the presence of 1 mmol/L Ca2+ alone or together with 20 mmol/L Tris-buffered EGTA. The WinLab 2.0 software package (Perkin-Elmer) was used to calculate free cellular calcium concentrations at any given time assuming an apparent Kd(dissociation constant) value for the Fura 2-Ca2+ complex of 224 nmol/L.
PKC assays.
Cells maintained in IDMEM-BIT supplemented with SCF, Epo, and dexamethasone were washed once in IDMEM and then starved in IDMEM-Bit in the absence of growth factors. After 16 hours the cells were stimulated for 60 minutes with different combinations of growth factors.
Determination of total PKC activity.
Cells were washed once in IDMEM, resuspended (1 to 2 × 107/mL) in IDMEM containing 0.5 mg/mL bovine serum albumin (BSA), and stimulated for 3 minutes with thrombin (5 U/mL) or with phorbol 12-myristate 13-acetate (PMA) (10 nmol/L). The cells were then sedimented and resuspended in 400 μL of ice-cold buffer A (20 mmol/L Tris-Cl, 2 mmol/L EDTA, 1 mmol/L dithiothreitol [DTT], 0.25 mmol/L phenylmethylsulfonyl fluoride [PMSF], pH 7.5). To separate cytosolic and particulate fractions, the cells were disrupted by two freezing/thawing cycles using liquid nitrogen and then centrifuged at 10,000g for 1 minute at room temperature (RT). The supernatant represented the cytosolic fraction. The pellet, representing the particulate fraction, was washed once in buffer A and solubilized in 500 μL of the same buffer containing 0.5% Triton X-100. Solubilization was further improved by 2 × 15-second sonification at a scale setting of 18 μm peak to peak (MSE Ultrasonic Disintegrator Mk2; MSE Scientific Instruments, Crawley, UK). Insoluble material was removed by sedimentation (20 minutes, 100,000g, 4°C). Following a published protocol,21 cytosolic and solubilized particulate fractions were further purified on diethylaminoethyl (DEAE)-Sephacel (Sigma) columns (2 mL). No PKC activity could be measured without this purification step. Before the enzyme assay, the proteins from the particulate fraction were fivefold concentrated by ultrafiltration (Centricon 30 concentrators; Amicon/Millipore, Volketswil, Switzerland). The reaction mixture contained (in 100 μL) 40 μL sample (80 to 150 μg protein), 20 μg histone III-SS, 20 μg phosphatidylserine, 100 μmol/L diacylglycerol (DC8), 1 mmol/L EGTA, 5 mmol/L MgCl2, 100 μmol/L sodium orthovanadate, 2 mmol/L PMSF, 100 μmol/L adenosine triphosphate (ATP), 2 μCi [γ32P]ATP (3,000 Ci/mmol), and 20 mmol/L Tris-Cl (pH 7.5 at 4°C). To measure the activity of Ca2+-dependent isoforms of PKC, 1.5 mmol/L CaCl2 was added to the above mixture.
Determination of subtype-specific PKC activity.
Samples with 150 to 200 μg protein from the cytosolic fraction were resuspended in buffer A supplemented with 150 mmol/L NaCl and 200 mmol/L sodium orthovanadate (buffer B). PKC subtypes were immunoprecipitated with specific monoclonal antibodies at 4°C according to standard protocols as provided by the manufacturer (Transduction Laboratories, Lexington, KY). After 16 hours the immunoprecipitate was collected on 20 μL of Protein G Plus-agarose beads. The beads were washed three times with 500 μL of buffer B and then used for the PKC assay as described above.
Immunoblotting.
Cellular proteins were solubilized as previously described,20 separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% acrylamide), and electrophoretically transferred to nitrocellulose membranes. PKC isoforms were detected using subtype-specific antibodies. Binding was visualized by the enhanced chemiluminescence assay (ECL kit; Amersham/Pharmacia, Rainham, UK) with horseradish peroxidase–conjugated anti-mouse IgG as secondary antibody.
Data analysis.
Differences between mean values of groups were tested for statistical significance either with Student’s t-test or, where applicable, with one-way analysis-of-variance (ANOVA). Using the statistical functions of the Prism 2.04 program (GraphPad Software, San Diego, CA) within the ANOVA analysis, Dunnet’s test was applied to compare multiple groups to one control while Bonferroni’s test was applied to compare selected pairs of groups. P < .05 was considered significant. The same software was used for nonlinear least square fitting of data points.
Materials.
Analytical grade biochemical reagents were purchased from Merck ABS (Dietikon, Switzerland) or Fluka (Buchs, Switzerland). Tissue culture reagents, media and fetal calf serum were obtained from GIBCO/Life Technologies (Basel, Switzerland) or from Sigma (Buchs, Switzerland). BIT-9500 serum substitute was obtained from CellSystems (St Katharinen, Germany). Human recombinant SCF and Epo were gifts from Immunex (Seattle, WA) and Cilag (Schaffhausen, Switzerland), respectively. PKC subtype antibodies were products of Transduction Laboratories (Lexington, KY). Protein G Plus-agarose was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies, thrombin, PGE1, phorbol esters, histone III-SS, and LDL were from Sigma. The PKC inhibitors bisindolylmaleimide (GF-109203X) and Gö6976 [12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole] were from LC Laboratories/Alexis (Läufelfingen, Switzerland). SFLLRN thrombin receptor peptide (TRP) was from Bachem (Basel, Switzerland). Recombinant hirudin was the product of Fluka. Botulinus C3 toxin was purchased from Calbiochem (JURO Supply, Lucerne, Switzerland). Density gradient media (Percoll, Ficoll-Paque) for the isolation of CD34+ cells from peripheral blood was obtained from Pharmacia (Dübendorf, Switzerland). Monoclonal antibodies for cell panning procedures (anti-CD2, -CD11b, -CD45) were prepared from the respective mouse hybridoma cell lines available from ATCC (Rockville, MD).
RESULTS
Several hematopoietic cytokines (eg, IL-3, GM-CSF, Epo) have been reported to enhance PKC activity in addition (or subsequent) to their effects on tyrosine kinases. Therefore, an interaction on the level of PKC could form the basis of crosstalk between G-protein– and cytokine–linked signaling pathways. In the experiments described below, we asked first whether Epo- or SCF-induced DNA synthesis, as measured by 3H-thymidine incorporation, was sensitive to PKC activation or inhibition and whether and how it could be modulated by thrombin. A second part covers the results with ADP and with 8-Br-cAMP, a membrane-permeable derivative of cAMP. Both of these compounds are known to mimic partial reactions in the complex signaling network activated by thrombin.
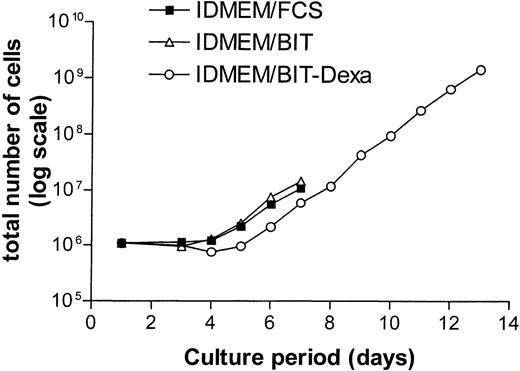
In growth factor–supplemented IDMEM-medium containing serum (10% FCS), most CD34+ cells advanced to the CFU-E stage by day 6 and entered terminal differentiation by day 8.16 In accordance with earlier results,22 we observed that a serum-free medium containing the serum substitute BIT (20%) together with Epo and SCF delayed terminal differentiation (50% benzidine-positive, Hb-producing cells at day 9) and supported proliferation beyond day 9. The phase of exponential growth could be further extended to 13 days while simultaneously slowing terminal differentiation (60% benzidine-positive cells at day 13) by additional supplementation of the serum-free IDMEM-BIT medium with dexamethasone (Dexa, 1 μmol/L, Fig 1). For controls, these conditions ensured a relatively constant rate of DNA synthesis for the duration of the experiment (day 6-8 of suspension culture). Epo- or SCF-dependent DNA synthesis was studied using two different protocols. In most experiments, all growth factors were removed from the medium on day 6 for a starvation period of 16 hours. At the end of this period, Epo and/or SCF were re-added. Any prolongation of the starvation period resulted in massive cell death. In other experiments, only Epo and Dexa were withdrawn on day 6 or 7 while the cells were maintained on SCF alone for 24 hours (SCF cells). DNA synthesis was then measured after re-addition of Epo or of other compounds of interest. With SCF as the sole growth factor, the rate of proliferation decreased but most cells survived for 24 hours.
Effect of dexamethasone on the growth rate of normal human erythroid progenitor cells in serum-free medium. CD34+ cells were incubated in IDMEM supplemented with either 15% fetal calf serum (FCS, ▪) or 20% BIT-9500 (BIT, serum substitute, ▵). All cells received SCF (50 ng/mL). The medium concentration of Epo was maintained at 5 U/mL in FCS and BIT while it was reduced to 1 U/mL in BIT/Dexamethasone (Dexa)-medium (○). In addition, the latter medium contained 1 μmol/L of dexamethasone. For detailed composition of media see Materials and Methods. Starting at day 5, the cells were split 1:2 on each successive day, always adding ½ volume of fresh medium. Cells grown in the absence of dexamethasone entered terminal differentiation after day 8.
Effect of dexamethasone on the growth rate of normal human erythroid progenitor cells in serum-free medium. CD34+ cells were incubated in IDMEM supplemented with either 15% fetal calf serum (FCS, ▪) or 20% BIT-9500 (BIT, serum substitute, ▵). All cells received SCF (50 ng/mL). The medium concentration of Epo was maintained at 5 U/mL in FCS and BIT while it was reduced to 1 U/mL in BIT/Dexamethasone (Dexa)-medium (○). In addition, the latter medium contained 1 μmol/L of dexamethasone. For detailed composition of media see Materials and Methods. Starting at day 5, the cells were split 1:2 on each successive day, always adding ½ volume of fresh medium. Cells grown in the absence of dexamethasone entered terminal differentiation after day 8.
Effects of growth factors and of thrombin on DNA synthesis in starved progenitors.
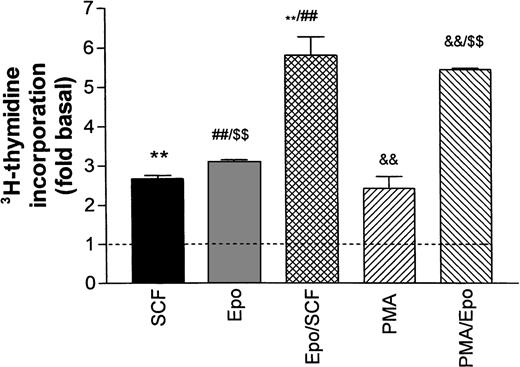
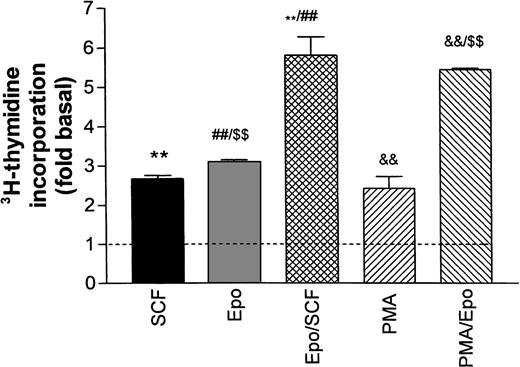
Figure 2 illustrates the stimulation of DNA synthesis in growth factor–starved cells after re-addition of Epo and SCF either individually or in combination. All values are normalized with respect to basal thymidine incorporation in the absence of growth factors (dotted line in Fig 2). Epo (0.5 U/mL) and SCF (50 ng/mL) both caused a twofold to threefold increase in DNA synthesis that was not significantly changed in the additional presence of Dexa. The combined effects of Epo and SCF were additive. No further stimulation was obtained by increasing the Epo concentration to 5 U/mL (not shown). The phorbol ester PMA (2 to 5 nmol/L) stimulated DNA synthesis to a level comparable to the one reached with SCF or Epo, whereas the combination of PMA and Epo was also additive. The poor resistance of progenitors to growth factor deprivation prohibited any further reduction in baseline DNA synthesis by a more extensive starving procedure.
Stimulation of DNA synthesis in human erythroid progenitors by various growth factors. Acid-precipitable3H-thymidine incorporation was measured 24 hours after addition of growth factors to cells maintained for 16 hours in growth factor–depleted medium (‘starved’). Basal thymidine incorporation in the absence of added growth factors is indicated by the dashed line. The columns give mean values ± SEM from six separate cultures, except for PMA/Epo, which is the mean of three cultures. Statistical differences between group means was tested by one-way ANOVA followed by Bonferroni’s test to compare individual pairs of columns labeled **, ##, $$, &&. All tested differences were significant on the P< .001 level.
Stimulation of DNA synthesis in human erythroid progenitors by various growth factors. Acid-precipitable3H-thymidine incorporation was measured 24 hours after addition of growth factors to cells maintained for 16 hours in growth factor–depleted medium (‘starved’). Basal thymidine incorporation in the absence of added growth factors is indicated by the dashed line. The columns give mean values ± SEM from six separate cultures, except for PMA/Epo, which is the mean of three cultures. Statistical differences between group means was tested by one-way ANOVA followed by Bonferroni’s test to compare individual pairs of columns labeled **, ##, $$, &&. All tested differences were significant on the P< .001 level.
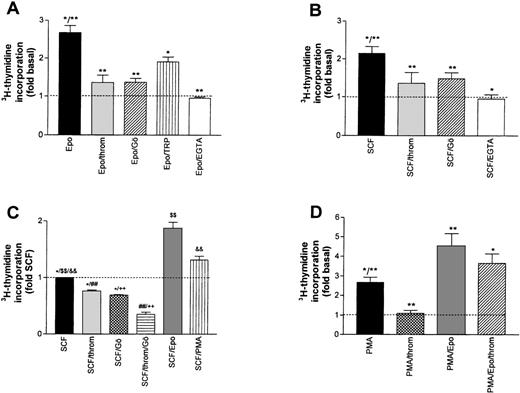
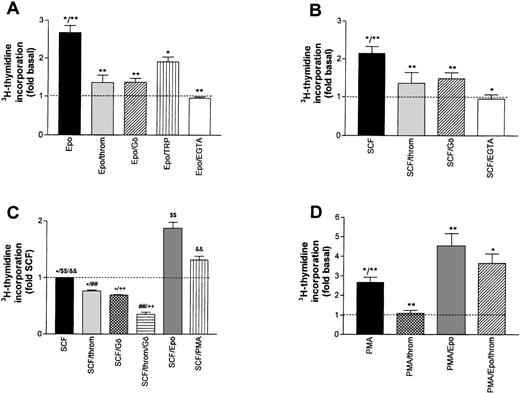
A possible contribution of PKC activation to the overall stimulating effect of Epo and SCF was assessed by either blocking Ca2+-dependent isoforms of the enzyme with Gö6976,23 or by inhibiting both Ca2+-dependent and -independent isoforms with bisindolylmaleimide (BIM, GF-109203X24). The effect of Gö 6976 (1 μmol/L) is shown in Fig 3A and B. Mean Epo- or SCF-induced DNA synthesis was reduced by 78.0% and 58.0%, respectively. A similar level of inhibition was reached with BIM (not shown). These results point to a major contribution of PKC activation to the overall growth stimulation by either Epo or SCF. Consistent with a dominant role for Ca2+-dependent PKC isoforms in this effect, complexing extracellular Ca2+ with EGTA during the stimulation period with Epo or SCF reduced DNA synthesis to the level of untreated cells.
Inhibition of growth factor-stimulated DNA synthesis in human erythroid progenitors in the presence of thrombin (throm) and Gö 6976 (Gö, an inhibitor of Ca2+-dependent PKC subtypes). (A) Inhibition of Epo (0.5 U/mL)-induced DNA synthesis by thrombin (2 U/mL), Gö 6976 (1 μmol/L), thrombin receptor peptide SFLLRN (TRP, 100 μmol/L) and by lowering the extracellular Ca2+ concentration from 1.2 mmol/L to about 10−7 mol/L by adding 4 mmol/L EGTA. Reduction of Epo-stimulated 3H-thymidine incorporation was significant for all agents (**P < .01; *P < .05 by ANOVA followed by Dunnett’s test). Data show mean values ±SEM of six to nine different experiments. The inhibition by EGTA was measured in six cultures from two batches of cells. The dotted line gives basal thymidine incorporation in the absence of added growth factors. (B) Experiment analogous to (A) but measuring the inhibition of SCF (50 ng/mL)-stimulated DNA synthesis. Columns show mean values ±SEM of three to nine different experiments, except for the effect of thrombin alone, which was measured in three separate cultures from one batch of cells. All agents significantly reduced SCF-stimulated DNA synthesis (*P < .05; **P < .01 by paired t-test). (C) Effects of thrombin (2 U/mL), Gö 6976 (1 μmol/L), Epo (0.5 U/mL), and PMA (10 nmol/L) on cells maintained for 24 hours in serum-free medium with SCF (50 ng/mL) as only growth factor (no starving). Groups normalized with respect to DNA-synthesis in the sole presence of SCF. Differences between groups were significant onP < .05 (*) and P < .01 ($$, &&, ##, ++) levels (ANOVA/Bonferroni). (D) Effects of thrombin and of Epo on phorbol ester (PMA)-stimulated DNA synthesis. PMA (10 to 100 nmol/L) was present during both the 16-hour starvation and the 24-hour experimental periods, while thrombin (2 U/mL) and Epo (0.5 U/mL) were first added at the end of the starvation period. The dotted line corresponds to basal thymidine incorporation in the absence of PMA. The values give means ±SEM of six to nine cultures from two to three different batches of cells. Thrombin and Epo caused significant changes of PMA-induced DNA synthesis (*P < .05; **P < .01 by ANOVA and Dunnett’s test). Addition of thrombin (2 U/mL) to the combination of PMA and Epo did not result in a significant change of thymidine incorporation.
Inhibition of growth factor-stimulated DNA synthesis in human erythroid progenitors in the presence of thrombin (throm) and Gö 6976 (Gö, an inhibitor of Ca2+-dependent PKC subtypes). (A) Inhibition of Epo (0.5 U/mL)-induced DNA synthesis by thrombin (2 U/mL), Gö 6976 (1 μmol/L), thrombin receptor peptide SFLLRN (TRP, 100 μmol/L) and by lowering the extracellular Ca2+ concentration from 1.2 mmol/L to about 10−7 mol/L by adding 4 mmol/L EGTA. Reduction of Epo-stimulated 3H-thymidine incorporation was significant for all agents (**P < .01; *P < .05 by ANOVA followed by Dunnett’s test). Data show mean values ±SEM of six to nine different experiments. The inhibition by EGTA was measured in six cultures from two batches of cells. The dotted line gives basal thymidine incorporation in the absence of added growth factors. (B) Experiment analogous to (A) but measuring the inhibition of SCF (50 ng/mL)-stimulated DNA synthesis. Columns show mean values ±SEM of three to nine different experiments, except for the effect of thrombin alone, which was measured in three separate cultures from one batch of cells. All agents significantly reduced SCF-stimulated DNA synthesis (*P < .05; **P < .01 by paired t-test). (C) Effects of thrombin (2 U/mL), Gö 6976 (1 μmol/L), Epo (0.5 U/mL), and PMA (10 nmol/L) on cells maintained for 24 hours in serum-free medium with SCF (50 ng/mL) as only growth factor (no starving). Groups normalized with respect to DNA-synthesis in the sole presence of SCF. Differences between groups were significant onP < .05 (*) and P < .01 ($$, &&, ##, ++) levels (ANOVA/Bonferroni). (D) Effects of thrombin and of Epo on phorbol ester (PMA)-stimulated DNA synthesis. PMA (10 to 100 nmol/L) was present during both the 16-hour starvation and the 24-hour experimental periods, while thrombin (2 U/mL) and Epo (0.5 U/mL) were first added at the end of the starvation period. The dotted line corresponds to basal thymidine incorporation in the absence of PMA. The values give means ±SEM of six to nine cultures from two to three different batches of cells. Thrombin and Epo caused significant changes of PMA-induced DNA synthesis (*P < .05; **P < .01 by ANOVA and Dunnett’s test). Addition of thrombin (2 U/mL) to the combination of PMA and Epo did not result in a significant change of thymidine incorporation.
Surprisingly, in the same assay, thrombin also acted as a strong inhibitor reducing the Epo- and the SCF-dependent stimulation by 88.5% and 68.5%, respectively (Fig 3A and B, second bar).* In the absence of growth factors, thrombin reduced basal DNA synthesis by 55% (not shown). These effects were most likely mediated by the G-protein–coupled thrombin receptor because the thrombin receptor peptide SFLLRN (TRP), mimicking the endogenous tethered receptor agonist, also significantly inhibited the effect of Epo (albeit with lower efficacy, Fig 3A). Moreover, the inhibitory effect of thrombin was almost completely blocked by hirudin, a specific inhibitor of thrombin’s proteolytic activity (compare inset to Fig 4). The combined effect of Epo and SCF on DNA synthesis was less sensitive to either thrombin- or Gö6976-mediated inhibition (8% ± 3% and 34% ± 4%, respectively). However, if applied together, thrombin and Gö 6976 reduced the Epo/SCF effect by 65% ± 7% (not shown). The effect of thrombin was not obliterated by either maintaining the cells in the continuous presence of SCF (SCF cells, see above) or by growing the cells in the absence of Dexa, thus allowing more rapid differentiation. Addition of thrombin (2 U/mL) or Gö 6976 (1 μmol/L) to SCF cells reduced DNA synthesis by 27% ± 4% and 31% ± 2%, respectively. These effects were additive. Mean inhibition in the joint presence of thrombin and Gö 6976 reached 64% ± 7% (Fig 3C). After starvation of rapidly differentiating cells grown in the absence of Dexa, thrombin-, TRP-, and Gö 6976-induced an inhibition of Epo- or SCF-supported DNA synthesis that did not differ significantly from the results in Dexa-treated cells documented in Fig 3A and B (not shown).
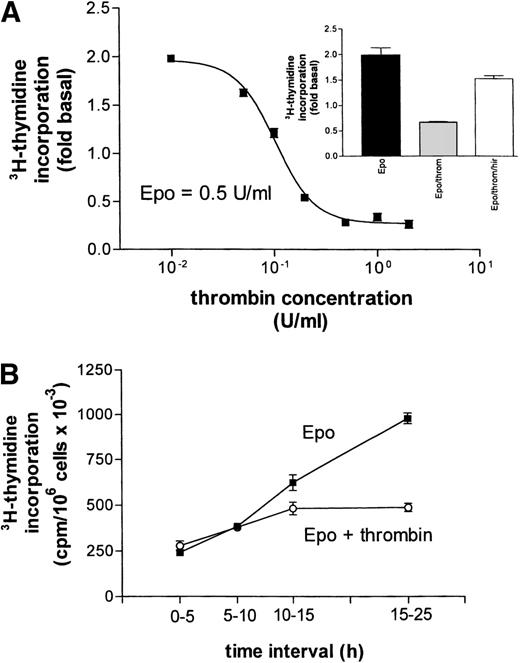
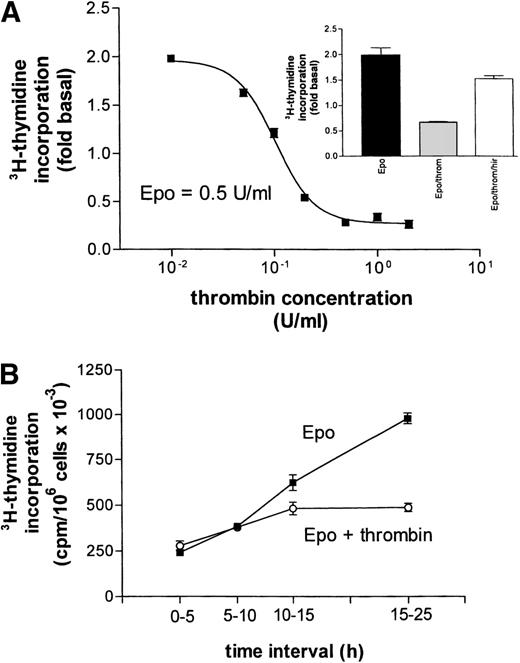
(A) Concentration-response curve for the inhibitory effect of thrombin on Epo (0.5 U/mL)-dependent DNA synthesis. Data points give mean values ±SEM of three separate cultures from one batch of cells. The curve represents a nonlinear least square fit to the data points. (Inset) Reversal of thrombin’s (throm, 2 U/mL) inhibition of Epo-stimulated DNA synthesis by hirudin (hir, 2 U/mL). (B) Time dependence of the inhibitory effect of thrombin on Epo-simulated DNA synthesis. At time zero (end of starvation period) Epo (0.5 U/mL) or Epo together with thrombin (2 U/mL) were each added to 16 cell cultures. A first group of four cultures from each condition received 3H-thymidine at time zero, a second group after 5 hours, a third after 10 hours, and the last group after 15 hours. Thymidine incorporation was always measured 5 hours later, except for the last group, which was exposed to 3H-thymidine for 10 hours. Data points give the mean ±SEM of four cultures.
(A) Concentration-response curve for the inhibitory effect of thrombin on Epo (0.5 U/mL)-dependent DNA synthesis. Data points give mean values ±SEM of three separate cultures from one batch of cells. The curve represents a nonlinear least square fit to the data points. (Inset) Reversal of thrombin’s (throm, 2 U/mL) inhibition of Epo-stimulated DNA synthesis by hirudin (hir, 2 U/mL). (B) Time dependence of the inhibitory effect of thrombin on Epo-simulated DNA synthesis. At time zero (end of starvation period) Epo (0.5 U/mL) or Epo together with thrombin (2 U/mL) were each added to 16 cell cultures. A first group of four cultures from each condition received 3H-thymidine at time zero, a second group after 5 hours, a third after 10 hours, and the last group after 15 hours. Thymidine incorporation was always measured 5 hours later, except for the last group, which was exposed to 3H-thymidine for 10 hours. Data points give the mean ±SEM of four cultures.
On the basis of these results, it could not be decided whether the effect of thrombin resulted from a direct inhibition of one of the Ca2+-dependent PKC isoforms, or from an interaction with some other component of the Epo/SCF signaling cascade. Moreover, it remained unclear whether the effect of thrombin was itself PKC-dependent. To address these questions, we tested the effect of thrombin on PMA- rather than cytokine-activated DNA synthesis under two different conditions. In a first group of experiments, growth factor–starved cells were treated either with PMA or with PMA and thrombin. PMA (2 nmol/L) alone caused a 2.3-fold increase in thymidine incorporation (compare Fig 2). This increase was completely blocked by thrombin or Gö 6976 (not shown). In a second group of experiments, we exposed the cells to high PMA concentrations (10 to 100 nmol/L) during the entire 16-hour starvation period to induce partial PKC inactivation. The results of these latter experiments are shown in Fig 3D. While cell numbers remained constant or decreased by up to 20% during 16-hour starvation in the absence of Epo or SCF, cell numbers increased during this period by 20% to 80% if the medium was supplemented with PMA. The rate of DNA synthesis exceeded the basal level by a factor of 2.7 ± 0.27. The addition of Epo (or of SCF, not shown) increased this value to 5.7 ± 0.39. Thrombin was still able to abolish the stimulation by PMA but, as with the joint application of Epo and SCF, appeared much less efficacious in reducing the combined effect of PMA and Epo. Together these results suggest that the inhibitory effect of thrombin targets one of the ‘conventional’ PKC subtypes or one of their downstream effectors, but is resistant to PMA-induced downregulation of PKC activity.
Thrombin-induced apoptotic cell death has been described recently in primary astrocyte cultures.25 This effect required rather high thrombin concentrations (40 to 200 U/mL) while lower concentrations would protect the cells against a variety of insults, including growth supplement deprivation.26 In erythroid progenitors, even at very low thrombin concentrations, no cell-protective effect was observed (Fig 4A). Half-maximal inhibition of Epo-stimulated DNA synthesis was reached with about 0.1 U thrombin/mL (≈0.5 nmol/L) while a maximal effect could be obtained with 0.5 U. In principle, the observed inhibition of DNA synthesis could result not only from cell death but also from a prolonged delay in the G0-G1 transition after the end of the starvation period. Therefore, we measured thymidine incorporation at different time intervals after the addition of either Epo alone or of Epo together with thrombin (Fig 4B). After a latency period of about 10 hours, the rate of DNA synthesis was markedly increased in the presence of Epo but remained essentially unchanged in the joint presence of thrombin even 15 to 25 hours after addition of the growth factor. These results are compatible with the assumption that thrombin did not act by inducing a time shift in the growth curve but possibly by promoting cell death.
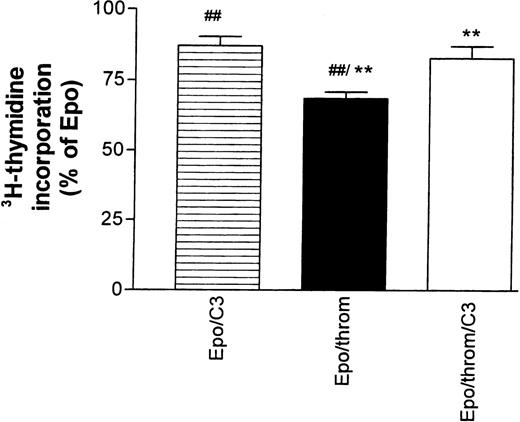
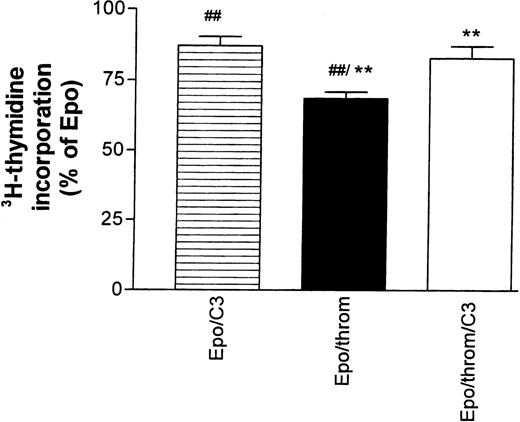
The effects of thrombin in the astrocyte system mentioned above, but also those on endothelial cytoskeletal targets, are mediated, at least partially, by signals that require activation of the small guanosine triphosphate (GTP)-binding protein Rho.25,27,28 They could be inhibited by Clostridium botulinum C3 exoenzyme, a specific inactivator of RhoA.29 30 Therefore, we tested the effect of C3 toxin on the thrombin-mediated inhibition of Epo-simulated DNA synthesis (Fig 5). Progenitor cells were first starved for 16 hours in the presence of 30 to 40 μg/mL toxin.3H-thymidine incorporation was then measured for 24 hours after re-addition of growth factors. The toxin concentration during this second incubation period was reduced to 15 to 20 μg/mL. C3 toxin reduced the stimulatory effect of Epo by 11% ± 4% but antagonized significantly the inhibitory effect of thrombin.
The inhibitory effect of thrombin on Epo-dependent DNA synthesis is reversed by Botulinus C3 exotoxin (C3). Progenitor cell cultures were growth factor–starved for 16 hours in the presence or absence of C3 toxin (30 to 40 μg/mL). At the end of this period, all cultures received Epo (0.5 U/mL). Thrombin was added as indicated in the column legends. The data are normalized with respect to the effect of Epo alone and give the mean ± SEM of nine different cultures from three batches of cells (##P < .01; **P < .01, paired test). Note that addition of C3 toxin by itself caused an 11% ± 3.7% decrease of Epo-stimulated DNA synthesis. See footnote in text for a comment on the low inhibitory efficacy of thrombin in this group of experiments.
The inhibitory effect of thrombin on Epo-dependent DNA synthesis is reversed by Botulinus C3 exotoxin (C3). Progenitor cell cultures were growth factor–starved for 16 hours in the presence or absence of C3 toxin (30 to 40 μg/mL). At the end of this period, all cultures received Epo (0.5 U/mL). Thrombin was added as indicated in the column legends. The data are normalized with respect to the effect of Epo alone and give the mean ± SEM of nine different cultures from three batches of cells (##P < .01; **P < .01, paired test). Note that addition of C3 toxin by itself caused an 11% ± 3.7% decrease of Epo-stimulated DNA synthesis. See footnote in text for a comment on the low inhibitory efficacy of thrombin in this group of experiments.
PKC subtype expression and activity in progenitor cells.
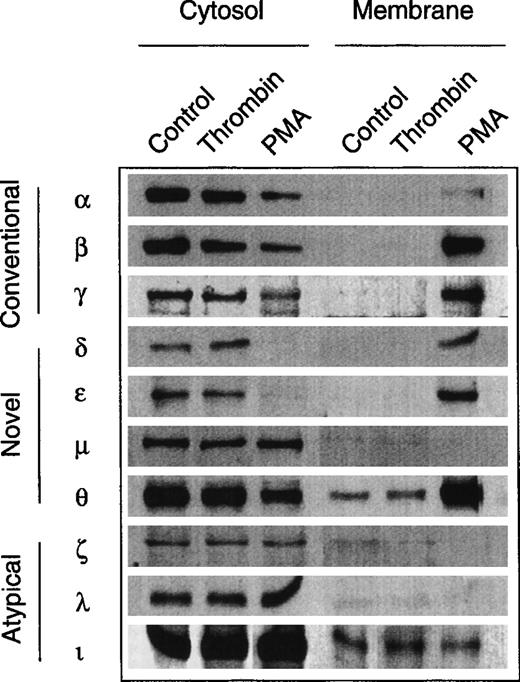
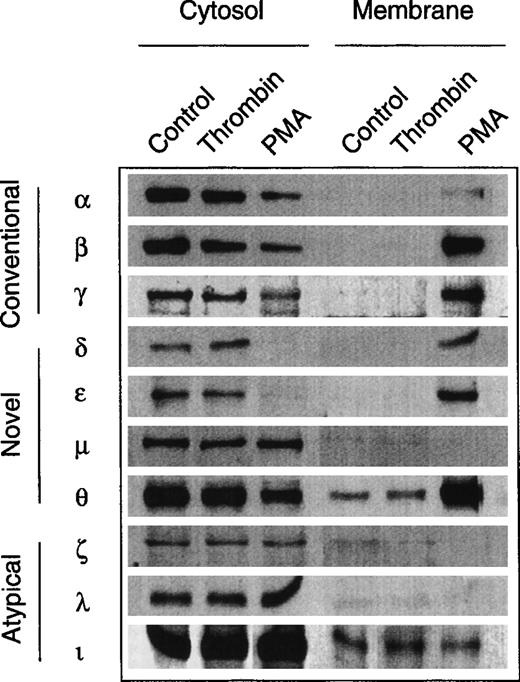
The evidence for an involvement of PKC in the growth response of erythroid progenitors prompted us to study the expression pattern of PKC subtypes and to assess possible subtype-specific interactions with growth factors and thrombin. No such data are available for nontransformed human erythroid progenitors, although several studies have observed multiple PKC subtype expression in human megakaryocytic and erythroleukemic cell lines.31 32Figure 6 shows the result of a screening experiment with a panel of subtype-specific antibodies. Similar to other hematopoietic cells, normal erythroid progenitors express at least 9 of the 12 known PKC subtypes from all three families (‘conventional,’ ‘novel,’ and ‘atypical’). Except for PKCμ (belonging to the nPKC family), all cPKC- and nPKC-subtypes were translocated into the particulate fraction upon activation with PMA. By contrast, stimulation with thrombin was not associated with a detectable translocation of any of the identified PKC subtypes. Similarly, the subcellular distribution of PKCα, β, δ, ε, and θ remained unchanged in the presence of Epo (not shown).
Expression of PKC isoforms in human erythroid progenitor cells. Members of all three subfamilies (conventional, novel, atypical) were detected by immunolabeling of cytosolic and membrane proteins with specific monoclonal antibodies. PMA (10 nmol/L, 3 to 10 minutes) induced a translocation of the PKC isoforms , β, γ, δ, ɛ, and θ to the membrane fraction. By contrast, thrombin (2 to 10 U/mL, 3 to 10 minutes) did not affect the subcellular distribution of the enzymes. Apparent membrane association of θ- and ι-kinases may be due to a minor contamination of the particulate fraction with cytosol.
Expression of PKC isoforms in human erythroid progenitor cells. Members of all three subfamilies (conventional, novel, atypical) were detected by immunolabeling of cytosolic and membrane proteins with specific monoclonal antibodies. PMA (10 nmol/L, 3 to 10 minutes) induced a translocation of the PKC isoforms , β, γ, δ, ɛ, and θ to the membrane fraction. By contrast, thrombin (2 to 10 U/mL, 3 to 10 minutes) did not affect the subcellular distribution of the enzymes. Apparent membrane association of θ- and ι-kinases may be due to a minor contamination of the particulate fraction with cytosol.
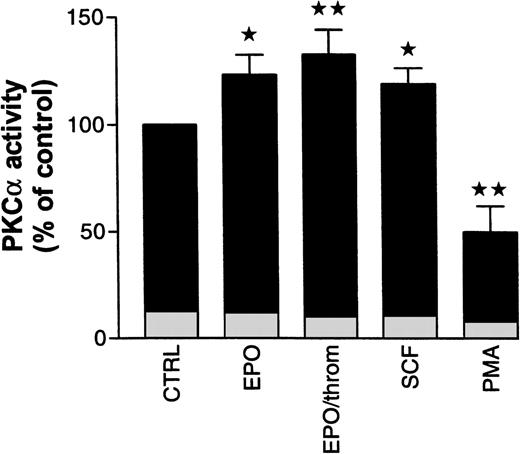
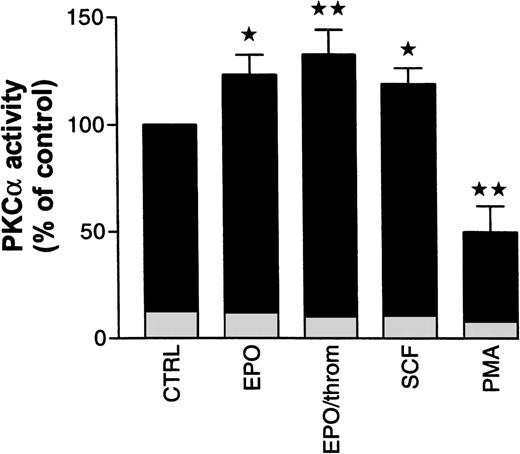
To assess possible PKC subtype-specific effects of Epo, SCF, and thrombin in more detail, we analyzed PKC enzymatic activity after subtype immunoprecipitation with specific antibodies from the cytosolic fraction of progenitor cells. In the following experiments, specific activation or inhibition of PKC subtypes was judged from activity changes in the cytosolic immunoprecipitates alone. Not enough cellular material was available to allow reliable measurements of the subtype enzymatic activity in immunoprecipitates from the solubilized particulate (membrane) fraction. Using cytosolic PKC had the additional advantage of avoiding any detergent treatment of the enzyme. We decided to study mainly PKCα and β because the preceding experiments had shown that the effects of Epo and SCF were associated with these Ca2+-sensitive and Gö 6976-inhibited subtypes. The results of these studies are summarized in Fig 7. Treatment of starved progenitor cells for 1 hour with Epo (0.5 U/mL), with Epo and thrombin (2 U/mL), or with SCF (50 ng/mL) caused a significant increase in the cytosolic activity of PKCα to 123% ± 9.2%, 132.8% ± 11.4%, and 119% ± 7.3%, respectively (n = 5 to 9). The differences between these values are not statistically significant. In one of seven experiments with Epo, no PKCα stimulation was observed. However, significance of the differences was maintained whether or not this experiment was included in the analysis. In two additional experiments, we tested the effect of SCF on immunoprecipitated PKCβ activity but failed to observe any SCF-dependent stimulation (note that the antibody used for immunoprecipitation did not discriminate between βI and βII subtypes). Because PKCα was not detectably associated with the membrane in nonstimulated cells (compare Fig 6), the increase in cytosolic activity was probably not caused by a redistribution of the enzyme but seemed to result from a genuine growth factor–mediated activation (phosphorylation?). As expected, under identical conditions, PMA significantly reduced the cytosolic activity of PKCα by 50% ± 12%, reflecting the partial translocation to the particulate fraction as shown in Fig 6. In a separate group of experiments, where the enzyme was partially purified from the cytosolic and particulate fractions rather than immunoprecipitated (see Materials and Methods), we measured the effect of PMA on total PKC activity in the progenitor cell population. Under these conditions, PMA (10 nmol/L) reduced total Ca2+- and phospholipid-stimulated cytosolic activity within 3 minutes by 35% ± 3.8% (n = 3), while the particulate activity was increased by 37% (n = 1). Because of the limited availability of normal progenitor cells, we complemented these studies with analogous experiments in human erythroleukemia (HEL) cells. Within 3 minutes of PMA (10 nmol/L) treatment, cytosolic PKC activity in these cells decreased by 24% ± 5.2% (n = 6) while particulate activity increased by 38% ± 18% (n = 4). These values are in close agreement with the results from progenitor cells. Together, these observations suggest that activation of PKCα, albeit by different mechanisms, is a shared obligatory step in cytokine- and in PMA-induced stimulation of DNA synthesis.
Effect of Epo in the absence and presence of thrombin, SCF, and PMA on cytosolic PKC activity in human erythroid progenitors. Cells were pretreated for 1 hour with Epo (0.5 to 1 U/mL), with Epo (0.5 U/mL) and thrombin (2 U/mL), with SCF (50 ng/mL), or with PMA (10 nmol/L). Cytosolic PKC was immunoprecipitated with a monoclonal antibody. Enzymatic activity of the precipitate was estimated by measuring phosphate transfer to the substrate histone III-SS. Immunoprecipitates of untreated cells served as controls. Epo with or without thrombin as well as SCF increased, while PMA decreased immunoprecipitated PKC activity. Significance of differences to controls was checked by ANOVA followed by Dunnett’s test (*P< .05; **P < .01). Values give the mean ± SEM from five to nine separate assays from four to seven different batches of cells, except for PMA where the mean from three different cell preparations is given. Basal kinase activity in the absence of cofactors, but in the presence of EGTA, is documented for each condition by the height of the lightly shaded segment at the base of each column.
Effect of Epo in the absence and presence of thrombin, SCF, and PMA on cytosolic PKC activity in human erythroid progenitors. Cells were pretreated for 1 hour with Epo (0.5 to 1 U/mL), with Epo (0.5 U/mL) and thrombin (2 U/mL), with SCF (50 ng/mL), or with PMA (10 nmol/L). Cytosolic PKC was immunoprecipitated with a monoclonal antibody. Enzymatic activity of the precipitate was estimated by measuring phosphate transfer to the substrate histone III-SS. Immunoprecipitates of untreated cells served as controls. Epo with or without thrombin as well as SCF increased, while PMA decreased immunoprecipitated PKC activity. Significance of differences to controls was checked by ANOVA followed by Dunnett’s test (*P< .05; **P < .01). Values give the mean ± SEM from five to nine separate assays from four to seven different batches of cells, except for PMA where the mean from three different cell preparations is given. Basal kinase activity in the absence of cofactors, but in the presence of EGTA, is documented for each condition by the height of the lightly shaded segment at the base of each column.
cAMP and the growth-inhibitory effects of thrombin.
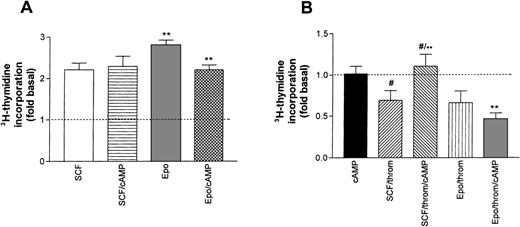
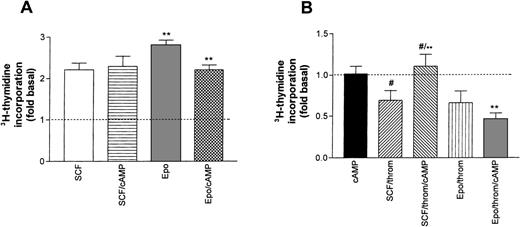
Earlier studies in erythroid progenitors or erythroleukemic cells had shown that thrombin potentiates cAMP formation induced by Gs-coupled receptor agonists.16 33 Therefore, we studied the effect of 8-Br-cAMP, a membrane-permeable cAMP derivative, on SCF- or Epo-promoted thymidine incorporation (Fig 8A). By itself, the addition of 8-Br-cAMP (1 mmol/L) to starved progenitors did not enhance basal DNA synthesis. Similarly, 8-Br-cAMP did not affect SCF-stimulated DNA synthesis. By contrast, 8-Br-cAMP caused a small, though significant, inhibition of Epo-dependent thymidine incorporation. The differential effect of cAMP on SCF and Epo-stimulated cells became more pronounced in the simultaneous presence of thrombin (Fig 8B). 8-Br-cAMP antagonized the thrombin-induced inhibition of SCF-supported DNA synthesis, whereas the effect of thrombin in the presence of Epo was not significantly affected. These results suggest that in Epo-dependent cells, cAMP may amplify inhibitory signals of low thrombin concentrations.
Effect of cAMP on growth factor–induced DNA synthesis in human erythroid progenitors. (A) 8-Br-cAMP (cAMP, 1 mmol/L) in the extracellular medium caused a significant inhibition (**P < .01 by paired t-test) of Epo (0.5 U/mL)-induced thymidine incorporation while SCF (50 ng/mL)-dependent incorporation remained unaffected. Values represent means ± SEM of 6 to 15 separate cultures from 2 to 5 different batches of cells. (B) Effect of 8-Br-cAMP on the thrombin-induced inhibition of Epo and SCF-dependent DNA synthesis. cAMP alone left basal DNA synthesis (dotted line) unchanged (leftmost column). Note that cAMP antagonized the effect of thrombin in the presence of SCF but not in the presence of Epo. Columns show means ± SEM from 6 to 9 separate cultures from 2 to 3 different batches of cells (**P < .001; #P < .05 by t-test).
Effect of cAMP on growth factor–induced DNA synthesis in human erythroid progenitors. (A) 8-Br-cAMP (cAMP, 1 mmol/L) in the extracellular medium caused a significant inhibition (**P < .01 by paired t-test) of Epo (0.5 U/mL)-induced thymidine incorporation while SCF (50 ng/mL)-dependent incorporation remained unaffected. Values represent means ± SEM of 6 to 15 separate cultures from 2 to 5 different batches of cells. (B) Effect of 8-Br-cAMP on the thrombin-induced inhibition of Epo and SCF-dependent DNA synthesis. cAMP alone left basal DNA synthesis (dotted line) unchanged (leftmost column). Note that cAMP antagonized the effect of thrombin in the presence of SCF but not in the presence of Epo. Columns show means ± SEM from 6 to 9 separate cultures from 2 to 3 different batches of cells (**P < .001; #P < .05 by t-test).
The role of cellular Ca2+ transients in the effects of thrombin and of ADP on PKC-dependent DNA synthesis.
Thrombin as well as other G-protein–linked receptor agonists are known to cause transient increases in cellular Ca2+ by releasing Ca2+ from cellular stores of hematopoietic cells.34 The prominent role of Ca2+-sensitive PKC subtypes in mediating cytokine-dependent DNA synthesis might predict an additional growth promoting effect of such Ca2+transients. On the other hand, an inhibitory effect of PKC activation on cellular Ca2+ release and/or store-operated Ca2+ influx has been noted in many cell types.35,36 Therefore, additional experiments were designed to assess the consequences of a transient increase in cellular Ca2+ concentrations on Epo- or SCF-supported DNA synthesis and on thrombin-mediated growth factor antagonism. ADP was selected in addition to thrombin as a second G-protein–coupled receptor agonist because it is known to elicit in native progenitors a strong transient Ca2+ release via a Gq-coupled P2T-type receptor but does not share most of the other properties of thrombin.16 37
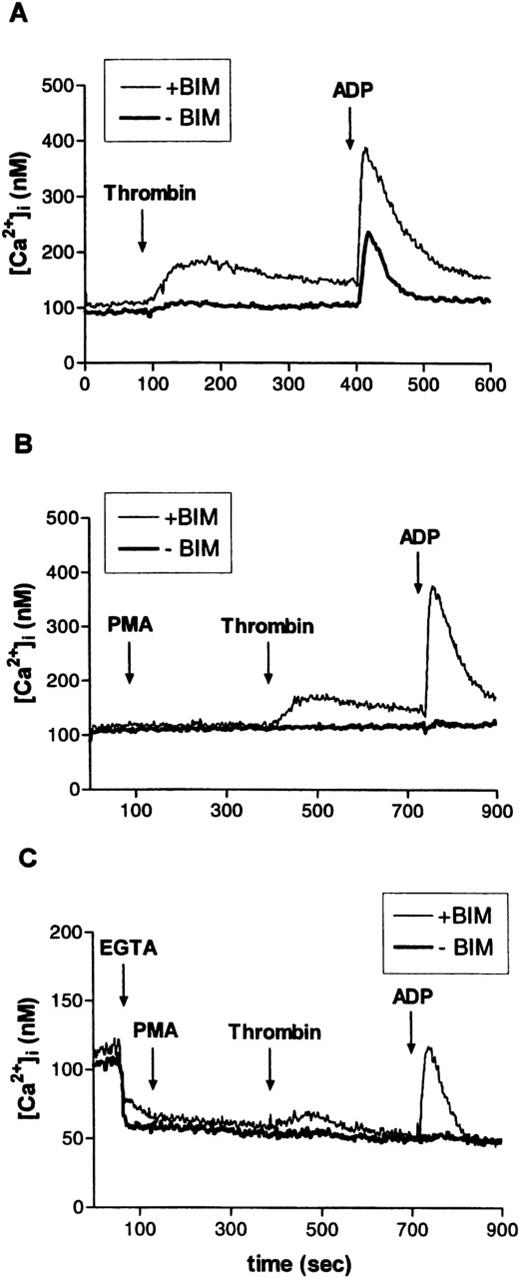
In the experiments documented in Fig 9A through C, erythroid progenitors from serum-free cultures loaded with fura-2 AM (9 μmol/L) were challenged, in succession, with thrombin (2 U/mL) and ADP 20 μmol/L. Control experiments had shown that neither the size nor the time course of the ADP-induced Ca2+ signal was affected by a preceding challenge with thrombin. Before the addition of agonists, part of the cells were pretreated for 5 minutes with the PKC inhibitor bisindolylmaleimide (BIM, 30 μmol/L). Although thrombin had very little effect on cellular Ca2+ levels under control conditions (in the absence of BIM), ADP caused a marked Ca2+ signal. Confirming the results of earlier studies,35 36 BIM significantly enhanced thrombin- and ADP-induced Ca2+ transients (Fig 9A). A similar stimulating effect was seen when BIM was replaced by Gö 6976, the selective inhibitor of Ca2+-sensitive PKC isoforms (not shown). Pretreatment with the PKC activator PMA (3 minutes, 10 nmol/L) completely abolished the responses to thrombin or ADP (Fig 9B). Figure9C documents the effects of thrombin and of ADP in controls and in BIM-treated cells under conditions where the addition of the Ca2+ complexing agent EGTA (2 mmol/L) was used to reduce the extracellular Ca2+ concentration to less than 10−7 mol/L. One minute after EGTA, PMA (10 nmol/L) was added to activate PKC. In this case, when Ca2+ influx was minimized, any increase in cellular Ca2+ must have been caused by a release from cellular stores. Although to a lesser degree, such release was duly observed in the presence of PMA together with the PKC inhibitor but was absent in the presence of PMA alone. The results illustrated in Fig 9A through C clearly suggest that endogenous and/or agonist-stimulated PKC activity will initiate a mechanism that severely dampens any signal linked to cellular Ca2+ transients (eg, the effect of ADP documented in Fig 10).
Effect of PKC activation or inhibition on thrombin- or ADP-induced intracellular Ca2+-transients in human erythroid progenitors. (A) The PKC inhibitor BIM (30 μmol/L) markedly enhanced thrombin (2 U/mL)- or ADP (10 μmol/L)-evoked cellular Ca2+-release. (B) Stimulation of PKC with PMA (10 nmol/L) abolished the effect of thrombin and of ADP. (C) Addition of EGTA (4 mmol/L) to the experimental medium. Ca2+-release from internal stores by thrombin or ADP was similarly blocked by PKC activation. Results are representative for two to eight independent experiments.
Effect of PKC activation or inhibition on thrombin- or ADP-induced intracellular Ca2+-transients in human erythroid progenitors. (A) The PKC inhibitor BIM (30 μmol/L) markedly enhanced thrombin (2 U/mL)- or ADP (10 μmol/L)-evoked cellular Ca2+-release. (B) Stimulation of PKC with PMA (10 nmol/L) abolished the effect of thrombin and of ADP. (C) Addition of EGTA (4 mmol/L) to the experimental medium. Ca2+-release from internal stores by thrombin or ADP was similarly blocked by PKC activation. Results are representative for two to eight independent experiments.
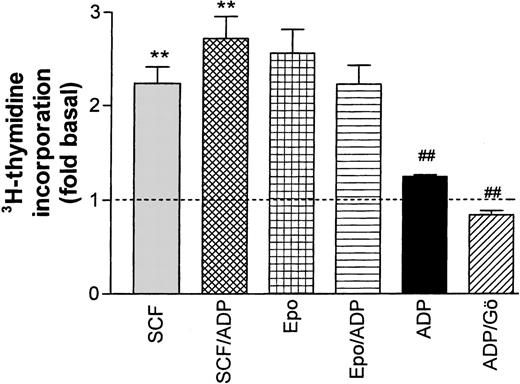
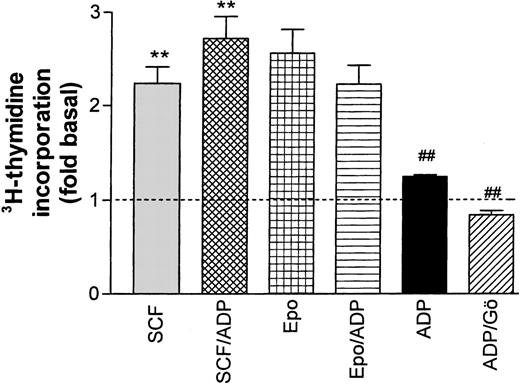
Effect of ADP on Epo- and SCF-induced DNA synthesis in progenitor cells. ADP (20 μmol/L) significantly (**P < .001, paired t-test) enhanced SCF-stimulated thymidine incorporation while no significant effect on Epo-stimulated activity was observed. In the absence of growth factors, ADP had a significant (P < .01) stimulating effect on the basal rate of DNA synthesis (dotted line) that was completely blocked by the PKC inhibitor Gö 6976 (##P < .001). Columns show means ± SEM from five to eight independent experiments.
Effect of ADP on Epo- and SCF-induced DNA synthesis in progenitor cells. ADP (20 μmol/L) significantly (**P < .001, paired t-test) enhanced SCF-stimulated thymidine incorporation while no significant effect on Epo-stimulated activity was observed. In the absence of growth factors, ADP had a significant (P < .01) stimulating effect on the basal rate of DNA synthesis (dotted line) that was completely blocked by the PKC inhibitor Gö 6976 (##P < .001). Columns show means ± SEM from five to eight independent experiments.
In a final set of experiments, the Ca2+ response to ADP was used to test a possible interference between Ca2+transients and cytokine-induced DNA synthesis. Starved progenitor cells were exposed for 24 hours to either ADP (20 μmol/L) alone or to ADP together with SCF or Epo. In the absence of additional growth factors, ADP caused a modest (29.5% ± 0.46%), albeit significant, stimulation of DNA synthesis, which could be completely blocked by the PKC inhibitor Gö 6976. The stimulating effect of ADP was additive to the one of SCF (increase significant at the P < .05 level). By contrast, ADP tended to reduce Epo-dependent DNA synthesis, but this change did not reach significance. In control experiments, we tested the effect of two other G-protein–coupled receptor ligands known to induce cellular Ca2+ transients (platelet-activating factor and uridine triphosphate [UTP]). These agonists, which both caused much less prominent changes in cellular Ca2+ than ADP, had no effect on Epo or SCF-dependent thymidine incorporation (not shown).
We conclude from these results that G-protein–coupled receptor agonists that induce strong Ca2+ transients may indeed modulate cytokine-supported cell proliferation. However, the type of growth factor seems to determine whether stimulating or inhibitory effects will be observed. In the context of erythroid progenitor development, Epo-dependent growth of CFU-E cells might be reduced while growth of earlier SCF-dependent stages could be enhanced.
DISCUSSION
In the context of the present results, three aspects in the development of normal human erythroid progenitor cells appear particularly interesting: (1) the important role of PKCα in the signaling pathways of SCF and Epo, (2) the potent inhibitory effect of thrombin on Epo- and SCF-dependent DNA synthesis, and (3) the modulating actions of changes in cellular cAMP or Ca2+ concentrations on cytokine-dependent growth.
PKC and hematopoiesis.
The contribution of PKC-dependent mechanisms in the cytokine-mediated regulation of hematopoietic cell development and differentiation is increasingly appreciated.38 In particular, it has been observed that activation of PKC will drive several leukemic cell lines into a differentiation program.39 In transformed hematopoietic cell lines or rodent progenitors, subtype nonspecific PKC activators or inhibitors were shown to induce a rather broad and variable spectrum of mitogenic, anti-apoptotic and differentiating (lineage commitment) actions.7,9,40 However, the precise signaling chains leading to these effects often remained undefined. Consequently, because erythropoietic, like myelopoietic, cells express multiple PKC subtypes, a search for subtype-specific functions in cell development has been initiated.31,41,42 In murine myeloid progenitors and in the K562 HEL cell line, activation of PKCα is clearly associated with a differentiation signal.10,43,44Yet, in many cases the results were difficult to interpret because the functional associations of different PKC subtypes appeared to vary depending on the cellular model system used. Thus, convincing evidence has been presented that in Rauscher murine erythroleukemia cells, Epo-dependent cell proliferation requires activation of PKCε8 while in another murine cell line (B6SUt.EP) it seemed to be associated with the activation of PKCβII.45Some discrepancies may also result from the fact that these studies did not use a uniform definition of enzyme ‘activation.’ It is variably quantified by measuring PKC membrane translocation,8,31nuclear translocation,42,45 expression levels,44 or, rarely, by estimating compartmentalized enzymatic activity.46 On the basis of these previous data it would not have been possible to predict the contribution of any PKC subtype to cytokine signaling in nontransformed human progenitors.
Our studies now identify PKCα as a central target for signals from Epo and SCF receptors as well as from G-protein–linked receptors in the regulation of normal human erythroid cell growth and survival. A strong synergism between the two cytokine receptors in promoting erythroid cell colony growth has been described earlier.4,47 However, this effect was ascribed entirely to an SCF receptor-mediated tyrosine phosphorylation of the Epo receptor. SCF-Epo synergism not explained by SCF acting via the Epo receptor pathway has also been observed.48,49 The present results suggest that the two cytokines also cooperate in their activation of PKCα. The levels of DNA synthesis attained by joint application of Epo and SCF were additive and were not reached with maximum effective concentrations of Epo alone (see Fig 2). This observation is consistent with a synergistic PKC activation by SCF and Epo. Similarly, the additive stimulation of DNA synthesis by PMA and Epo (see Fig 2) was probably due to their joint interaction with PKCα because the effect of PMA could be completely inhibited by either Gö 6976 or thrombin. The two compounds seem to antagonize PKCα-mediated stimulation of DNA synthesis via two distinctly different mechanisms. Consequently, they produced additive or even synergistic inhibitory effects on DNA synthesis. The combined effect of SCF and Epo that was partially resistant to inhibition by either Gö 6976 or thrombin was synergistically blocked by the joint action of the two inhibitors (see also Fig 3C for an additive effect). The effect of Gö 6976 results from a competitive interaction at the ATP binding site of Ca2+-sensitive PKC.23 Thrombin, which did not show a direct inhibitory action on PKCα (Fig 7), seems to interfere with a downstream target of this enzyme. This latter effect may be mediated via its stimulating effect on the Rho GTPase (see below). The pathway leading from SCF- or Epo-receptor stimulation to PKCα activation is incompletely understood. Two different mechanisms have been described by which hematopoietic cytokine-dependent tyrosine kinases could stimulate PKC isoforms: (1) increasing the phospholipase C–mediated generation of diacylglycerol,10,50 and (2) tyrosine phosphorylation of the enzyme in a phosphoinositide 3-kinase (PI3-kinase)-dependent manner.51,52 Alternatively, many PKC subtypes can also be activated by direct tyrosine phosphorylation without requiring prior hydrolysis of inositol phospholipids.53 Further studies are needed to establish which of these mechanisms is used in normal erythroid progenitors.
Inhibition of cytokine-mediated DNA synthesis by thrombin.
Thrombin potently inhibited Epo- or SCF-induced DNA synthesis. On one hand this effect requires the proteolytic activity of thrombin because it could be largely blocked by hirudin. On the other hand, it is probably mediated via the G-protein–coupled thrombin receptor rather than by protease activity targeted to another substrate, because significant inhibition was also obtained with the receptor peptide (SFLLRN). This peptide mimics the terminal sequence of the tethered receptor ligand generated by thrombin-induced proteolytic cleavage of the receptor N-terminus and has no enzymatic activity.54Although thrombin is mitogenic for some cell types like vascular smooth muscle cells18 or astrocytes,55 it was shown to reduce proliferation and to promote differentiation in a megakaryoblastic cell line. Also, it inhibited IL-3–supported growth in human megakaryocyte progenitors.15,56 The potency of thrombin in these experiments was similar to the one reported in Fig 4. In the study by Plantier et al,15 thrombin caused no growth inhibition in erythroid progenitors (BFU-E) that were cultivated in the presence of IL-3 and Epo. Like SCF, IL-3 is an activator of PKC.6 Hence, this finding is in accordance with our observation (Fig 3C) that thrombin alone will not block PKC activation mediated by the joint action of two synergistic cytokines. In neuronal cells and astrocytes, thrombin was shown to induce cell protective effects at low concentrations (nanomolar) and apoptosis at high concentrations (micromolar). Both responses appeared to be mediated via the G-protein–coupled thrombin receptor.25 26
The mechanisms underlying the antiproliferative effects of thrombin are incompletely understood. The thrombin receptor is known to interact with G proteins from at least three different families: Gi, Gq, and G12/13.54,57 Recent evidence suggests that thrombin can activate the Rho GTPase RhoA, probably by interacting with a G12 family G protein.28,58 Through its direct target Rho kinase and possibly other effector systems, RhoA is assumed to mediate several downstream effects including activation of PKC and stimulation of DNA synthesis.27 59 Our results with C3 toxin tend to confirm a central role of Rho proteins for the antagonistic effect of thrombin on cytokine-mediated DNA synthesis, although PKCα may not represent a direct target system for thrombin in erythroid progenitors (see Fig 7).
Earlier studies on human progenitor cells in our laboratory had indicated that thrombin potentiated Gs-mediated adenylyl cyclase activity by stimulating a Ca2+-independent protein kinase of the nPKC family.16 An elevation of cellular cAMP levels might well amplify the growth-inhibiting signal of thrombin. However, our results (Fig 8) indicated that only Epo-promoted growth was reduced by 8-Br-cAMP while the effect of SCF was not changed. cAMP-induced growth depression in hematopoietic cells has been repeatedly described.14,60 The cAMP-mediated inhibition of mitogenic signaling has been attributed to a reduction of the Ras-dependent activation of the Raf protein kinase.61Because Epo has been shown to activate Raf in normal progenitors,62 this mechanism may well explain a preferential inhibition of Epo-dependent growth.
The role of cellular Ca2+ transients in modulating DNA synthesis.
Several previous reports have suggested that a cellular Ca2+ signal may be generated by cytokines and is causally related to SCF- and Epo-dependent effects on hematopoietic and, in particular, erythropoietic progenitor cell survival and development.63-65 These findings are consistent with our observation of a prominent role of Ca2+-dependent PKC subtypes in mediating progenitor cell proliferation and might be compatible with a general growth promoting effect of agonist-induced cellular Ca2+ transients. On the other hand, it has been suggested that cellular Ca2+ accumulation may contribute to thrombin-induced cell death.25 Our observations in erythroid progenitors did not show a consistent correlation between cellular Ca2+ levels and thrombin-dependent inhibition of DNA synthesis. PMA completely blocked any thrombin-induced Ca2+ transient, but failed to reduce the inhibitory effect of thrombin on DNA synthesis. By contrast, ADP was the only G-protein–linked receptor agonist that caused a significant, though modest, stimulation of DNA synthesis in the absence of growth factors. Of all G-protein–coupled receptor ligands tested, ADP is known to induce the most prominent increase in cellular Ca2+levels.37 The growth-promoting effect of ADP was completely suppressed by Gö 6976. Therefore, it resembled the effect of SCF and Epo in being linked to Ca2+-sensitive PKC subtypes. Conversely, the ADP-associated Ca2+ signal, although enhancing SCF-dependent DNA synthesis, failed to amplify the Epo signal. This differential interaction confirmed that mechanisms in addition to changes in cellular Ca2+ contribute to the regulation of PKCα activity. On the basis of our findings with cAMP and ADP, it seems that G-protein–linked signals may either promote or inhibit growth, depending on which type of cytokine dominates a particular developmental state.
The relatively weak correlation between agonist-induced changes in cellular Ca2+ and their effects on DNA synthesis supports the view that promotion of progenitor cell growth requires sustained rather than transient PKC stimulation. Prolonged activation of PKC isoforms by cytokines in hematopoietic cells has been well established.7 Similarly, prolonged activation and/or enhanced expression of PKCα has been observed even in the presence of high PMA concentrations for extended time periods.44,66 For the stimulation of DNA synthesis it seemed irrelevant whether PKCα activation occurred via translocation into the particulate (membrane) compartment (associated with a decrease of the cytosolic activity) or via direct cytosolic activation. We observed a shift with the phorbol ester PMA, but Epo and SCF exclusively enhanced cytosolic PKCα activity without inducing significant translocation. Nevertheless, cytokines and PMA both caused a comparable stimulation of thymidine incorporation. The cytokine-mediated percent increase in PKCα activity seems modest. However, it is only slightly lower than the changes in total PKC activity that were observed, both in normal progenitors and in HEL cells, under the same conditions with PMA (a stronger-than-physiologic stimulus). Moreover, the growth factor–induced change in PKC activity in vivo may be much higher if the presumed phosphorylation reaction would interfere with pseudo-substrate inhibition of the cytosolic enzyme.67
Overall, our experiments provide evidence for a functionally relevant convergence of growth-regulatory signals in erythroid progenitors at the level of PKCα. Because of the difficulties of measuring compartmentalized PKC subtype activities directly, we cannot definitely exclude minor contributions of other subtypes to this regulatory network. In any case, PKCα offers a suitable target to link proliferation stimuli with changes in cellular Ca2+concentration as can be induced by ADP or other G-protein–linked receptor ligands. However, as exemplified for thrombin, G-protein–dependent signaling can also provide inhibitory inputs that seem to involve downstream targets of PKC rather than interfering with the cytokine-stimulated PKC activity. This pathway defines a new role for thrombin as a potent inhibitor of late erythroid cell proliferation.
ACKNOWLEDGMENT
We are particularly grateful to Dr A. Tobler (Department of Hematology, University of Bern) for samples of CD34+ cells. Without this support the immunoprecipitation studies requiring large numbers of highly purified progenitor cells would not have been possible.
Note that the efficacy of different thrombin batches was somewhat variable. We used three different batches of bovine and one batch of human thrombin, each of the highest purity that was commercially available. Human thrombin and two batches of the bovine material caused more than 80% reduction of Epo-induced DNA synthesis. However, with one batch of bovine thrombin, maximal inhibition reached only 30%. This value could not be further increased with higher thrombin concentrations. The reason for this variability between different thrombin preparations (also reflected in some of our figures, eg, see Fig 3 versus Fig 5 and Fig 8B) is unknown.
Supported by grants from the Swiss National Science Foundation (No. 31-39678.93) and the Swiss Foundation for Cancer Research (No. KFS 183-9-1995).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
Author notes
Address reprint requests to Hartmut Porzig, MD, Pharmakologisches Institut, Universität Bern, Friedbühlstrasse 49, 3010 Bern/Switzerland; e-mail:hartmut.porzig@pki.unibe.ch.