The CC chemokine RANTES is synthesized, stored, and upregulated in response to interferon-γ (IFN-γ) in human peripheral blood eosinophils. In this report, we propose that RANTES is rapidly mobilized from eosinophil crystalloid granules during agonist-induced degranulation. We stimulated purified eosinophils (>99%) from atopic asthmatics with 500 U/mL IFN-γ to analyze the kinetics of mobilization and release of RANTES (0 to 240 minutes). We used subcellular fractionation, immunogold analysis, two-color confocal laser scanning microscopy (CLSM), and enzyme-linked immunosorbent assay (ELISA) to trace the movement of eosinophil-derived RANTES from intracellular stores to release. RANTES was rapidly mobilized (10 minutes) and released after 120 minutes of stimulation (80 ± 15 pg/mL per 2 × 106 cells). RANTES appeared to be stored in at least two intracellular compartments: the matrix of crystalloid granules, detected by major basic protein and eosinophil peroxidase activities, and a specialized small secretory vesicle present in light membrane fractions. The extragranular RANTES was mobilized more rapidly than that of crystalloid granules during IFN-γ stimulation. This effect was not observed in eosinophils treated with IFN-, interleukin-3 (IL-3), IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), or genistein followed by IFN-γ. Our findings suggest that RANTES may be mobilized and released by piecemeal degranulation upon stimulation, involving transport through a putative pool of small secretory vesicles.
EOSINOPHIL ACCUMULATION is a hallmark of allergic inflammation, particularly within the airway mucosa of asthmatic subjects. Eosinophils are thought to be activated in response to local inflammatory stimuli by releasing an array of mediators. These consist of cytotoxic granule proteins such as major basic protein (MBP), eosinophil cationic protein (ECP), and eosinophil peroxidase (EPO), products of respiratory burst, and lipid mediators.1,2 In addition, eosinophils potentially synthesize or produce up to 18 different cytokines and growth factors, including interleukin-2 (IL-2),3,4 IL-4,5IL-6,6,7 granulocyte-macrophage colony-stimulating factor (GM-CSF),8-10 and RANTES.11-13 A number of these proteins have been shown to exert autocrine effects on eosinophils, including RANTES.8,11 14
RANTES is a CC chemokine, which has been shown to be a potent chemoattractant for CD4+/CD45RO+ T cells, eosinophils, basophils, monocyte/macrophages, and mast cells.15-18 In addition to eosinophils, RANTES is synthesized and/or released by a number of other cell types, such as T cells, platelets, macrophages, endothelial cells, fibroblasts, epithelial cells, and a mast cell line.16,19-23 Cutaneous injection of RANTES was found to induce marked eosinophil recruitment in human subjects, which was more rapid in allergic compared with normal subjects.24 In earlier studies, RANTES has been implicated in delayed-type hypersensitivity reactions19 and in ongoing inflammatory processes in rheumatoid arthritis.20 RANTES may have a role in contributing to the infiltration of inflammatory cells in allergen-challenged airway mucosal tissue in asthma. Although the expression and release of RANTES in tissue and bronchoalveolar lavage (BAL) fluids from resting asthmatic and normal subjects do not differ significantly,25,26 the levels of RANTES were found to be elevated in BAL fluids obtained from asthmatics 4 hours after allergen challenge27 and in nasal fluids obtained from subjects with allergic rhinitis after challenge with a grass pollen extract.28 Increased RANTES secretion correlated strongly with elevated tissue eosinophil numbers in both asthma and rhinitis. Furthermore, RANTES has been shown to upregulate expression of CD11/CD18 on monocytes29 and induce histamine release from human basophils,30 suggesting that it may also have a role in immediate-type allergic responses.
It has previously been shown that peripheral blood eosinophils express mRNA for and release bioactive RANTES in response to serum-coated beads, using immunocytochemistry (ICC), in situ hybridization (ISH), and enzyme-linked immunosorbent assay (ELISA).11 A granular pattern of immunocytochemical staining of eosinophils was observed, suggesting that eosinophils store preformed RANTES in intracellular compartments. Interferon-γ (IFN-γ) was found to upregulate RANTES mRNA and protein expression in eosinophils after 16 hours of stimulation.11 IFN-γ has been shown to be a viable stimulus for eosinophils in a number of studies.6,7,9 31-33
We originally hypothesized that intracellular RANTES was secreted after stimulation by IFN-γ in a time-dependent manner. However, preliminary data indicated that IFN-γ had a more rapid effect on mobilization of RANTES than previously anticipated. Thus, we propose that RANTES is rapidly mobilized from intracellular stores in a piecemeal pattern of degranulation. Eosinophils were purified from peripheral blood obtained from atopic asthmatics and stimulated with recombinant human IFN-γ. The release of RANTES was analyzed in cells using a combination of in vitro assay, RANTES-specific ELISA, immunogold analysis, confocal laser scanning microscopy (CLSM), and subcellular fractionation, and its localization was compared with that of known crystalloid granule proteins MBP and EPO. Our findings suggest that RANTES, a stored product of eosinophils, is readily and selectively released in a pattern akin to piecemeal degranulation after stimulation.
MATERIALS AND METHODS
Materials.
Mouse monoclonal alkaline phosphatase antialkaline phosphatase (APAAP) detection kits were obtained from Dako (Glostrup, Denmark). Adenosine triphosphate (ATP), aprotinin, Nα-p-tosyl-l-arginine methyl ester (TAME), phenylmethylsulfonyl fluoride (PMSF), Fast Red TR, leupeptin, 4-methylumbelliferyl N-acetyl-β-d-glucosaminide, β-nicotinamide adenine dinucleotide (reduced form) (NADH), sodium pyruvate, and tetramethylbenzidine (TMB) substrate solution were purchased from Sigma (Oakville, Ontario, Canada). Genistein was obtained from Calbiochem Corp (San Diego, CA). Recombinant human IFN-α was a kind gift from Dr Aziz Ghahary (Department of Surgery, University of Alberta). Nycodenz was purchased from GIBCO-BRL Life Technologies, Ltd (Grand Island, NY). All reagents used in this study, including media, were negative for lipopolysaccharides (LPS) activity, as determined using the E-Toxate assay (Sigma).
Preparation of eosinophils.
Peripheral blood (100 mL) was obtained from mild atopic asthmatic and atopic nonasthmatic subjects displaying eosinophilia greater than 5% and who were not receiving oral corticosteroids. After red blood cell sedimentation in 5% dextran, remaining cells were subjected to density centrifugation on Ficoll (Pharmacia Biotech, Uppsala, Sweden). Eosinophils were then purified from the granulocyte pellet by immunomagnetic selection using the MACS system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Highly purified CD16−eosinophils (>99%) were obtained by negative selection, depleted of neutrophils using anti-CD16–conjugated immunomagnetic beads as described previously.7,10 34 Contamination by mononuclear cells and lymphocytes was avoided by coincubation with anti-CD14 and anti-CD3–coated micromagnetic beads (Miltenyi Biotec).
In vitro assay of granule protein release from eosinophils.
Purified human eosinophils were aliquoted at 2 × 106cells/tube and treated with 500 U/mL IFN-γ at 37°C for various times in RPMI-1640 (BioWhittaker, Walkersville, MD). The reaction was terminated by placing tubes on ice and centrifuging cells at 400g for 5 minutes at 4°C. Assays were then performed for RANTES immunoreactivity in supernatants using a Quantikine ELISA kit (R & D Systems, Minneapolis, MN) with a detection sensitivity of 31.2 pg/mL. For detection of two other crystalloid granule proteins, EPO and β-hexosaminidase, assays for these were a modification of those performed previously.4,7,10 35 Briefly, EPO activity was assayed using TMB substrate solution by combining 50 μL sample with 150 μL substrate solution in a 96-well microplate and incubating at room temperature for 15 minutes. The reaction was terminated by 50 μL 4 mol/L sulfuric acid and absorbance was read at 450 nm in a spectrophotometric microplate reader (Vmax Kinetic Microplate Reader, Molecular Devices, Sunnyvale, CA). For β-hexosaminidase activity, present in both secretory and lysosomal granules, 50 μL sample was added to each well of a 96-well microplate and mixed with 50 μL substrate solution (1 mmol/L 4-methylumbelliferyl N-acetyl-β-d-glucosaminide in 0.2 mol/L citrate buffer, pH 4.5, and 0.1% Triton X-100) before incubating (37°C, 1 to 2 hours). The reaction was terminated by the addition of 150 μL ice-cold 0.2 mol/L Tris, and the fluorescence (excitation 360 nm, emission 460 nm) measured in a Millipore CytoFluor 2350 plate reader (Millipore, Nepean, Ontario, Canada).
Immunogold labeling and electron microscopy.
This procedure was a modification of a previously described technique.5 Briefly, pelleted isolated eosinophils were fixed in freshly prepared formaldehyde (2% in phosphate buffer [PB], 0.1 mol/L, pH 7.4) for 2 hours, embedded in Lowicryl K4M resin, and subjected to an infiltration procedure involving a progressive lowering of the temperature. Silver sections were cut and picked up onto Formvar-coated copper grids. Before labeling, sections were blocked for 10 minutes with 0.14% glycine in PB. Additional blocking was performed for 10 minutes in 3% horse serum. Grids were then floated on a solution containing 20 μg/mL mouse monoclonal anti-human RANTES antibody (R & D Systems) for 2 hours. A further blocking step was performed using 0.14% glycine in PB. The immunoreactive label was visualized by goat anti-mouse antibody conjugated to gold particles (20 nm diameter; E-Y Laboratories, Inc, San Mateo, CA) at 0.5 μg/mL in PB for 2 hours. Sections were washed three times with 0.14% glycine in PB before being rinsed for 3 minutes with distilled water. For negative controls, we substituted anti-RANTES with mouse IgG1 as the isotype control (20 μg/mL; R & D Systems). Staining with osmium tetroxide was omitted to ensure visualization of gold particles in electron-dense crystalloid granules in eosinophil sections.
Double-labeling and CLSM.
Cytospins of eosinophils (100 μL of 0.5 × 106cells/mL in RPMI supplemented with 20% fetal calf serum [FCS]) were made by spinning slides in a Cytospin 2 (Shandon Ltd, Astmoor, Runcorn, UK) centrifuge (800 rpm for 2 minutes) followed by fixing in 2% paraformaldehyde in phosphate-buffered saline (PBS) for 6 minutes. These fixing and staining procedures were optimized as previously reported36 and are satisfactory for visualization of granule proteins in the absence of a permeabilization step. In our hands, permeabilization agents were detrimental in obtaining optimal cell morphology. Slides were then subjected to a wash step (five washes in Tris-buffered saline [TBS], pH 7.4), followed by incubation in blocking solution (2% bovine serum albumin [BSA]) in a humidified container for 1 hour. Specific antibody binding was performed for 1 hour with TBS containing 5 μg/mL mouse monoclonal anti-human RANTES antibody (R & D Systems). Immunoreactivity to RANTES was detected using 20 μg/mL BODIPY FL-conjugated goat anti-mouse antibody (Molecular Probes, Eugene, OR) as previously optimized by our laboratory.36 Slides were blocked again for 2 hours using 50 μg/mL goat anti-mouse IgG (Molecular Probes) and double-labeled with 1% mouse monoclonal anti-human MBP antibody (BMK-13) in TBS for 1 hour. Bound BMK-13 was detected by incubating 5 μg/mL Texas Red-labeled goat anti-mouse antibody for 1 hour (Caltag Laboratories, San Francisco, CA). Mouse IgG1 (5 μg/mL) was used as an isotype control (R & D Systems). After a final wash step, 10 μL of antibleaching agent (0.4% n-propyl gallate [Sigma] in 3:1 glycerol:TBS) was applied to each slide before coverslip attachment. Slides were examined using a 100× objective under a Leica CLSM (Heidelberg, Germany). Images were collected and processed as previously described.7
Subcellular fractionation.
Purified peripheral blood eosinophils were homogenized by repeated passages through a ball-bearing cell homogenizer, with resulting organelles separated by linear density gradient as described in earlier reports.4,5,7 10 Briefly, at least 5 × 107 purified eosinophils from asthmatics were suspended in ice-cold 0.25 mol/L HEPES-buffered sucrose (containing 10 mmol/L HEPES, 1 mmol/L EGTA, pH 7.4) and centrifuged at 4°C. Cells were resuspended in homogenization buffer (HEPES-buffered sucrose described above, supplemented with 100 μg/mL PMSF and 5 μg/mL each of leupeptin, aprotinin, and TAME, 2 mmol/L MgCl2, and 1 mmol/L ATP) to 10 to 15 × 106/mL, and subjected to 10 to 20 passes through a 12-μm clearance in a ball bearing homogenizer (EMBL, Heidelberg, Germany). The homogenate was centrifuged at 400g for 10 minutes, and the resulting postnuclear supernatant was layered onto an 8-mL linear Nycodenz gradient (0% to 45% Nycodenz dissolved in HEPES-buffered sucrose with protease inhibitor cocktail) in a Beckman 14 × 89 mm Ultra-Clear centrifuge tube (Beckman, Palo Alto, CA). The gradient was subjected to equilibrium density centrifugation at 100,000g for 1 hour at 4°C, and 24 × 0.4 mL fractions were collected from each preparation and stored at −80°C until used.
Marker enzyme assays.
Three marker enzyme assays were used to obtain profiles of specific subcellular organelles in fractions collected from density gradient centrifugation. Activities of EPO and β-hexosaminidase were determined in supernatants and pellets using the same technique described earlier in this section. Cytosolic activity was determined using a modification of a microtiter plate endpoint assay37for lactate dehydrogenase (LDH), where 10 μL of sample was mixed with 80 μL 1 mg/mL NADH and 0.75 mmol/L pyruvate in pH 7.5 phosphate buffer in a microtiter plate and incubated at 37°C for 30 minutes, followed by the addition of 80 μL 0.2 mg/mL 2,4-dinitrophenylhydrazine in 1 mol/L HCl and incubation at room temperature for 20 minutes. The reaction was terminated by the addition of 40 μL 3.5 mol/L NaOH and absorbance read at 450 nm. Plasma membrane activity was determined by dot blot analysis (see below) with monoclonal antibody (MoAb) to CD9 as previously described.4,5,7 10
Dot-blot analysis.
Dot-blot analysis was performed to determine the distribution of CD9 in subcellular fractions of resting and stimulated eosinophils. Anti-CD9 MoAb (purified IgG2a) was kindly provided by Dr A.R.E. Shaw (Cross Cancer Institute, University of Alberta). A mouse monoclonal IgG2a isotype control was used as the negative control for anti-CD9 (Pharmingen Canada, Mississauga, Ontario, Canada). Samples of each fraction (2 μL) were pipetted onto nitrocellulose strips, allowed to dry, and blocked in 5% milk powder. Blocked membrane strips were incubated with 1:1,000 anti-CD9 antibody, and after extensive washings in TBS, pH 7.6 + 0.2% Tween-20, were developed using APAAP staining technique as previously described.10 The fractional activities of anti-CD9 immunoreactivity were assessed by staining density using a gel scanner, given arbitrary units and converted to percentage of total activity in all fractions.
Data presentation.
The bioactivity of eosinophil granule, membrane, and cytosol constituents after fractionation are expressed as frequency distributions as previously described.10 RANTES was quantitatively displayed as pg/fraction and pg/mL in fractions and supernatants, respectively. Statistical comparisons were performed using the Mann-Whitney test (one-tailed) and the Kruskal-Wallis one-way analysis of variance. Results were considered significant whenP < .05.
RESULTS
Immunocytochemistry using APAAP.
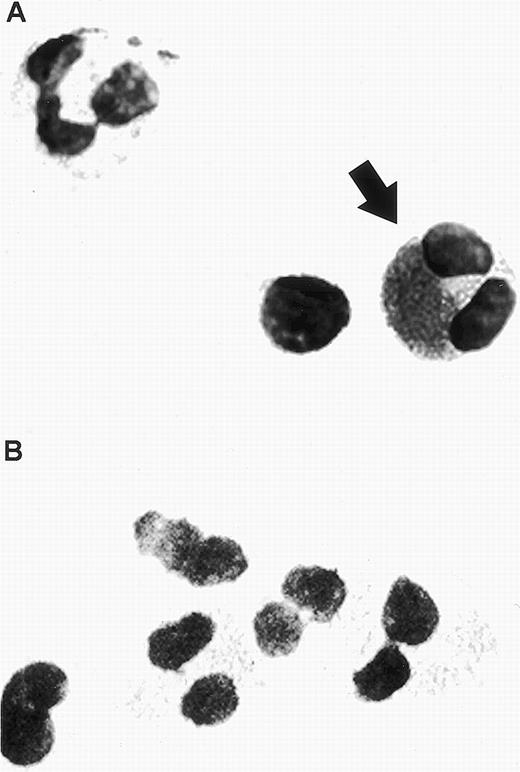
In cytospin preparations of antibody-specific staining of buffy coat from asthmatic subjects, RANTES immunoreactivity was detected in eosinophils using APAAP staining. Morphologically identifiable eosinophils, but not neutrophils or lymphocytes, displayed a mixture of granular and extragranular staining corresponding to RANTES immunoreactivity (Fig 1A). This indicates that while RANTES appears to be stored in association with the unique crystalloid granules of eosinophils, it may also be found in a number of other intracellular compartments. The isotype control antibody (mouse IgG1) was negative (Fig 1B).
Human eosinophil detected in a buffy coat cytospin. (A) The eosinophil was stained specifically with mouse monoclonal anti-human RANTES (20 μg/mL) using APAAP staining, as indicated by the arrow. Two unlabeled cells are also visible within this field, a neutrophil possessing a multilobed nucleus (upper left) and a lymphocyte (left of the stained eosinophil). (B) Isotype control using mouse IgG1 antibody (20 μg/mL). Original magnification × 100.
Human eosinophil detected in a buffy coat cytospin. (A) The eosinophil was stained specifically with mouse monoclonal anti-human RANTES (20 μg/mL) using APAAP staining, as indicated by the arrow. Two unlabeled cells are also visible within this field, a neutrophil possessing a multilobed nucleus (upper left) and a lymphocyte (left of the stained eosinophil). (B) Isotype control using mouse IgG1 antibody (20 μg/mL). Original magnification × 100.
Release of RANTES after IFN-γ stimulation in vitro.
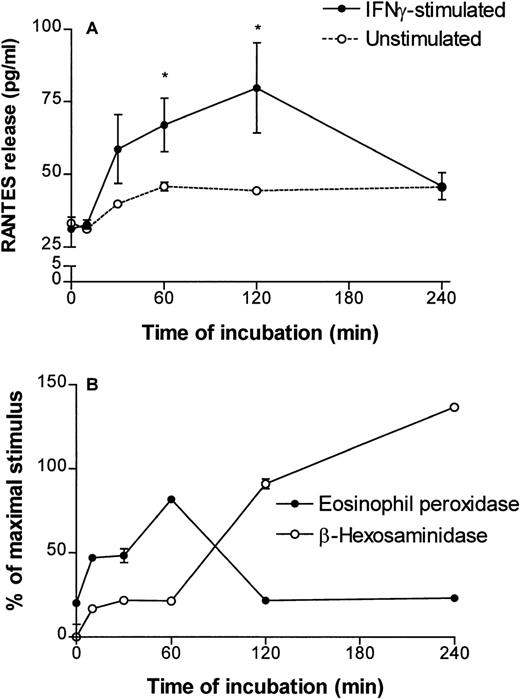
Incubation of eosinophils in culture media containing human recombinant IFN-γ for prolonged periods (up to 24 hours) has been previously demonstrated to upregulate the expression of a number of eosinophil-derived cytokines, including RANTES.11 To evaluate the time course of RANTES release during short periods of stimulation by IFN-γ (500 U/mL), the amount of RANTES released by stimulated cells was measured at 0, 10, 30, 60, 120, and 240 minutes (2 × 106 cells/time point). IFN-γ induced a rapid release of RANTES from human eosinophils, reaching maximal levels after 60 to 120 minutes (four experiments). In a representative experiment, eosinophils released an average of 80 ± 15 pg/mL RANTES after 120 minutes of IFN-γ stimulation (P < .05; Fig 2A). The amount of maximal release varied between the four donors (range, 74.5 to 302 pg/mL). After a 2-hour stimulation, the amount of RANTES detected in supernatants was diminished to baseline values. Unstimulated eosinophils showed no significant spontaneous release of RANTES (Fig 2A). Thus, IFN-γ was found to induce rapid in vitro release of RANTES from human peripheral blood eosinophils. In comparison, the release of EPO was assayed in the same supernatants and plotted as a percentage of release induced by 60 minutes of incubation at 37°C with a maximally stimulating agonist, serum-coated Sephadex (Pharmacia) beads, in a separate sample. EPO release was potently induced by IFN-γ within 60 minutes of incubation and diminished after 120 and 240 minutes of incubation. However, because EPO activity is not always stable after its release,38 39 we also measured β-hexosaminidase activity in these supernatants. β-hexosaminidase activity continued to increase in supernatants during continuous incubation, reaching values exceeding the levels induced by serum-coated Sephadex beads, after 240 minutes of stimulation by IFN-γ (Fig 2B).
Time course of RANTES, EPO, and β-hexosaminidase release from human peripheral blood eosinophils induced by 500 U/mL recombinant human IFN-γ. (A) RANTES immunoreactivity in supernatants of stimulated eosinophils (2 × 106 cells/time point) was detected using a specific ELISA. Values represent averages of triplicate measurements from cells at 0, 10, 30, 60, 120, and 240 minutes of incubation obtained from a representative donor. A similar trend of release was observed in four separate donors. The dotted line represents a single measurement of spontaneous release of RANTES from eosinophils (duplicate values given for times 0 and 60 minutes). *P < .05 compared with RANTES measured in supernatants at the start of the time course by Kruskal-Wallis one-way analysis of variance. The detection sensitivity of the RANTES ELISA was 31.2 pg/mL. (B) IFN-γ also induced the release of eosinophil peroxidase and another granule-stored product, β-hexosaminidase, detected in the same IFN-γ–stimulated supernatants shown in (A). The amount of granule protein release is expressed as a percentage of the release induced by a maximally stimulating agonist (serum-coated Sephadex beads) in another sample. Points and error bars represent the mean and standard error of mean (SEM) of at least three measurements.
Time course of RANTES, EPO, and β-hexosaminidase release from human peripheral blood eosinophils induced by 500 U/mL recombinant human IFN-γ. (A) RANTES immunoreactivity in supernatants of stimulated eosinophils (2 × 106 cells/time point) was detected using a specific ELISA. Values represent averages of triplicate measurements from cells at 0, 10, 30, 60, 120, and 240 minutes of incubation obtained from a representative donor. A similar trend of release was observed in four separate donors. The dotted line represents a single measurement of spontaneous release of RANTES from eosinophils (duplicate values given for times 0 and 60 minutes). *P < .05 compared with RANTES measured in supernatants at the start of the time course by Kruskal-Wallis one-way analysis of variance. The detection sensitivity of the RANTES ELISA was 31.2 pg/mL. (B) IFN-γ also induced the release of eosinophil peroxidase and another granule-stored product, β-hexosaminidase, detected in the same IFN-γ–stimulated supernatants shown in (A). The amount of granule protein release is expressed as a percentage of the release induced by a maximally stimulating agonist (serum-coated Sephadex beads) in another sample. Points and error bars represent the mean and standard error of mean (SEM) of at least three measurements.
Immunogold labeling of RANTES.
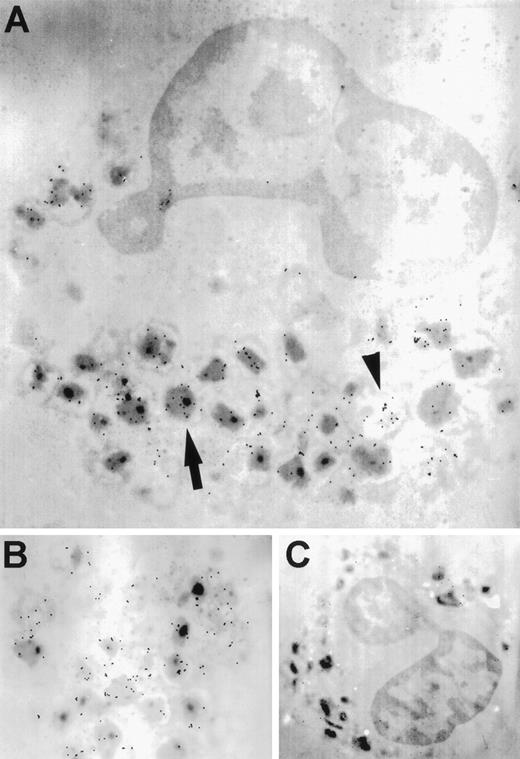
Unstimulated eosinophils exhibited a granular staining pattern for RANTES immunoreactivity, as indicated by the proximity of gold particles to electron-dense granule cores (Fig 3A and B). This pattern of immunolabeling suggests that RANTES may be stored in association with crystalloid granules. In addition, RANTES-specific staining was distributed throughout the cell in the extragranular milieu (shown by the arrowhead in Fig 3A). The isotype control showed negligible background (Fig 3C).
Transmission electron microscopy of immunogold-labeling of RANTES in unstimulated eosinophils. (A) The arrow indicates gold particles associated with electron-dense crystalloid granules, while the arrowhead shows immunogold labeling of extragranular areas. (B) Higher magnification of another cell. (C) Isotype control using mouse IgG1 antibody. Original magnifications: (A), ×9,100; (B), ×34,000; and (C), ×6,900.
Transmission electron microscopy of immunogold-labeling of RANTES in unstimulated eosinophils. (A) The arrow indicates gold particles associated with electron-dense crystalloid granules, while the arrowhead shows immunogold labeling of extragranular areas. (B) Higher magnification of another cell. (C) Isotype control using mouse IgG1 antibody. Original magnifications: (A), ×9,100; (B), ×34,000; and (C), ×6,900.
CLSM.
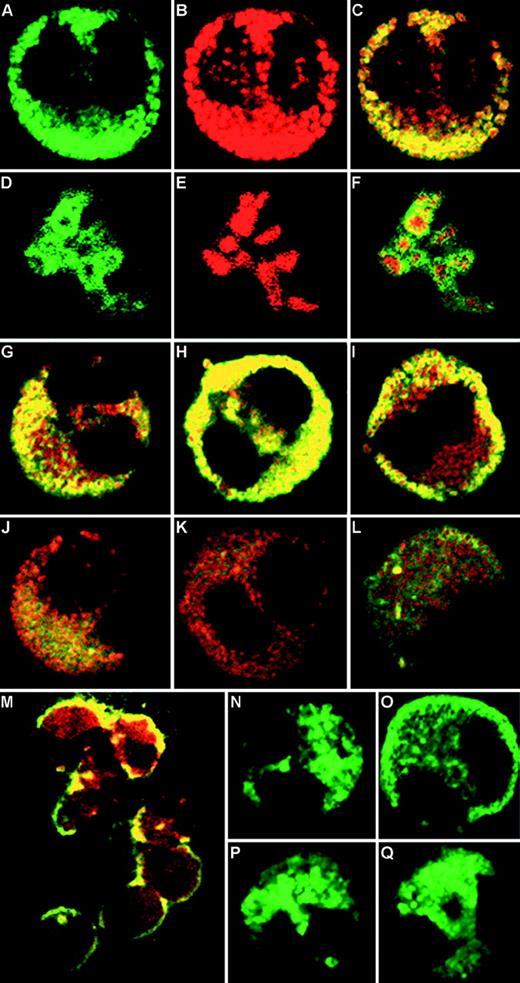
To examine mobilization of stored RANTES using MBP as a marker for eosinophil crystalloid granule, resting and IFN-γ–stimulated eosinophils were subjected to immunofluorescent labeling with appropriate antibodies. Immunostained cells displayed discrete green and red labels, which correspond to BODIPY FL-conjugated RANTES (Fig 4A) and Texas Red–conjugated MBP (Fig4B) immunoreactivity, respectively. Where colors overlapped in the combined images, the immunofluorescence appeared yellow (Fig 4C), suggesting that the two labeled proteins colocalize to the same intracellular compartment. The isotype control exhibited negligible immunoreactivity after subtraction of autofluorescence as previously reported (data not shown36). At higher magnifications, crystalloid granules appeared doughnut-shaped, with red centers (crystalline core MBP) surrounded by green immunofluorescence to RANTES, corresponding to the core and matrix of the crystalloid granules, respectively (Fig 4D through F). There was only partial overlap between MBP and RANTES immunoreactivities in the granules.
CLSM of immunofluorescence staining of eosinophils. (A through C) Unstimulated eosinophils labeled with BODIPY FL indicating RANTES immunoreactivity (A), Texas Red corresponding to MBP (B), and combined images (C). (D through F) Higher magnification of eosinophil crystalloid granules showing matrix-associated doughnut-shaped RANTES immunoreactivity (D), surrounding red-labeled cores of MBP immunoreactivity (E), and combined images of the same structure (F). (G through L) Combined images of RANTES and MBP, depicting time course of IFN-γ (500 U/mL) stimulation, comparing (G) unstimulated cells with those stimulated for 5 minutes (H), 10 minutes (I), 30 minutes (J), 60 minutes (K), and 16 hours (L). (M) Lower magnification of combined images of eosinophils stimulated for 10 minutes with IFN-γ. (N) Single-labeled unstimulated eosinophil, compared with (O) cell after 10 minutes stimulation (500 U/mL IFN-γ). (P) Inhibitory effect of 10−6 mol/L genistein added for 10 minutes before IFN-γ stimulation. (Q) Cell incubated with 1,000 U/mL IFN- for 10 minutes. Original magnification × 100 for all images, except for (M), ×63.
CLSM of immunofluorescence staining of eosinophils. (A through C) Unstimulated eosinophils labeled with BODIPY FL indicating RANTES immunoreactivity (A), Texas Red corresponding to MBP (B), and combined images (C). (D through F) Higher magnification of eosinophil crystalloid granules showing matrix-associated doughnut-shaped RANTES immunoreactivity (D), surrounding red-labeled cores of MBP immunoreactivity (E), and combined images of the same structure (F). (G through L) Combined images of RANTES and MBP, depicting time course of IFN-γ (500 U/mL) stimulation, comparing (G) unstimulated cells with those stimulated for 5 minutes (H), 10 minutes (I), 30 minutes (J), 60 minutes (K), and 16 hours (L). (M) Lower magnification of combined images of eosinophils stimulated for 10 minutes with IFN-γ. (N) Single-labeled unstimulated eosinophil, compared with (O) cell after 10 minutes stimulation (500 U/mL IFN-γ). (P) Inhibitory effect of 10−6 mol/L genistein added for 10 minutes before IFN-γ stimulation. (Q) Cell incubated with 1,000 U/mL IFN- for 10 minutes. Original magnification × 100 for all images, except for (M), ×63.
In time-course experiments, eosinophils stimulated with 500 U/mL IFN-γ for 0, 5, 10, 30, 60 minutes, and 16 hours were fixed and stained for RANTES and MBP immunofluorescence. Interestingly, after 10 minutes of incubation with IFN-γ, RANTES immunofluorescence was redistributed around the periphery of the cells (Fig 4I, M, and O), while that of MBP remained within the core of the secretory granules. The RANTES-specific immunofluorescence became visibly depleted in cells after 1 hour of incubation with IFN-γ, although MBP activity was still detectable (Fig 4K). After 16 hours of incubation, RANTES immunoreactivity returned, indicating that some degree of replenishment may have occurred (Fig 4L). These findings were reproduced in eosinophils from three asthmatic donors, and images represent the labeling pattern of the majority of cells in cytospin preparations.
To test the specificity of the IFN-γ response, eosinophils were incubated with genistein (10−6 mol/L) for 10 minutes before adding 500 U/mL IFN-γ for 10 minutes. Genistein is a broad specificity tyrosine kinase inhibitor used to inhibit early steps in the IFN-γ receptor signaling pathway after ligand binding.40 It fully inhibited the effects of IFN-γ on RANTES immunoreactivity in single-labeled cells (Fig 4P; a stimulated single-labeled cell is shown for comparison in Fig 4O). Inhibition by genistein was detected in greater than 90% of cells examined by CLSM. In confirmation of this, we have also observed that genistein (10−6 mol/L) inhibited RANTES release from eosinophils in vitro (2 × 106) by 32% after 120 minutes of IFN-γ incubation (data not shown). In addition, we incubated eosinophils with IFN-α (1,000 U/mL) for 10 minutes and found that it had no observable effect on the distribution of RANTES immunoreactivity in eosinophils (Fig 4Q). We took advantage of the small numbers of cells required for study by CLSM to examine the effects of IFN-α and genistein on eosinophils, as other in vitro techniques require substantially larger numbers of cells. In a separate assay, recombinant human IL-3 (25 ng/mL), IL-5 (10 ng/mL), and GM-CSF (10 ng/mL) did not induce significant RANTES release from eosinophils after 1 hour of stimulation (data not shown).
Subcellular fractionation.
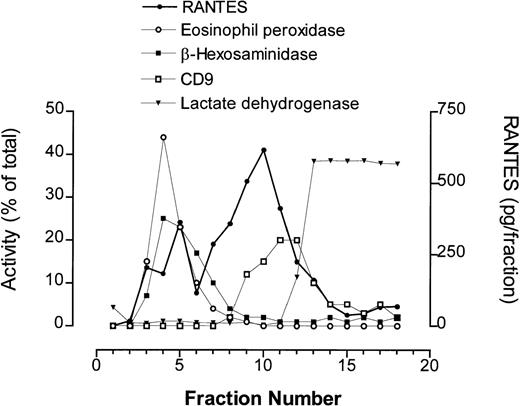
Eosinophils (5 × 107) were homogenized, loaded onto gradients of Nycodenz (0% to 45%) for ultracentrifugation, and fractions containing intact organelles collected for later analysis by ELISA and assays for enzyme activity. Intracellular compartments were identified in gradients by measuring marker enzyme activities within individual fractions (Fig 5). Crystalloid secretory granules were measured using assays for EPO and β-hexosaminidase, while plasma membrane activity was determined by dot-blot analysis of the fractions using anti-CD9. Cytosolic fractions were detected using an endpoint assay for LDH. Fractions with plasma membrane activity are known to contain other light membranes including Golgi compartments, as determined by galactosyl transferase activity measured in subfractionated guinea pig eosinophils.41 We have previously shown that the pellet produced from pooled fractions corresponding to peak granule protein activity, which sediment at high buoyant densities typically observed for crystalloid granules, is enriched in secretory granules.10
Subcellular fractionation of unstimulated peripheral blood eosinophils (5 × 107) obtained from an asthmatic donor. Fractions were collected from a 0% to 45% linear Nycodenz gradient and analyzed for marker enzyme activities to obtain profiles of subcellular compartments. Marker assays used were eosinophil peroxidase (secretory granules), β-hexosaminidase (secretory granules and lysosomal granules), CD9 (plasma membrane), and lactate dehydrogenase (cytosol). Quantification of RANTES was performed by ELISA for each fraction and is expressed as pg/fraction.
Subcellular fractionation of unstimulated peripheral blood eosinophils (5 × 107) obtained from an asthmatic donor. Fractions were collected from a 0% to 45% linear Nycodenz gradient and analyzed for marker enzyme activities to obtain profiles of subcellular compartments. Marker assays used were eosinophil peroxidase (secretory granules), β-hexosaminidase (secretory granules and lysosomal granules), CD9 (plasma membrane), and lactate dehydrogenase (cytosol). Quantification of RANTES was performed by ELISA for each fraction and is expressed as pg/fraction.
In unstimulated eosinophils, RANTES immunoreactivity was detected in at least two separate intracellular compartments (Figs 5 and6A). The first peak of RANTES-specific activity was detected in secretory granule-rich fractions, determined by EPO and β-hexosaminidase activity, while a larger peak was found to be associated with the light membrane fractions, which overlapped with CD9 immunoreactivity. Some CD9 immunoreactivity was visible in the granule fractions, as described earlier,4,10 42 although its optical density was too low to be detected, suggesting that a small amount of CD9 is also intracellularly distributed in the eosinophil. This observation confirmed our results from immunogold labeling and CLSM, which suggested that RANTES immunoreactivity only partially colocalized with secretory granules in unstimulated cells. Unstimulated eosinophils in this example were found to store approximately 72 pg RANTES/106 cells.
Subcellular fractionation of resting and IFN-γ–stimulated eosinophils (5 × 107 per fractionation). These experiments were conducted at different times using purified blood eosinophils from the same donor. Immunoreactivity to RANTES was determined in individual fractions by ELISA and expressed as pg/fraction. Profiles of EPO activity are shown here for comparison. (A) Unstimulated eosinophils, followed by eosinophils stimulated for (B) 10 minutes and (C) 60 minutes with 500 U/mL IFN-γ.
Subcellular fractionation of resting and IFN-γ–stimulated eosinophils (5 × 107 per fractionation). These experiments were conducted at different times using purified blood eosinophils from the same donor. Immunoreactivity to RANTES was determined in individual fractions by ELISA and expressed as pg/fraction. Profiles of EPO activity are shown here for comparison. (A) Unstimulated eosinophils, followed by eosinophils stimulated for (B) 10 minutes and (C) 60 minutes with 500 U/mL IFN-γ.
Stimulation with IFN-γ (500 U/mL) induced a striking change in the distribution of RANTES immunoreactivity in subfractionated eosinophils. RANTES was rapidly depleted from light membrane-associated fractions after 10 minutes of IFN-γ stimulation (Fig 6B), while some immunoreactivity remained within the granule-associated fractions. Eosinophils stored approximately 23 pg/106 cells of RANTES-specific activity after IFN-γ stimulation for 10 minutes, a reduction of 68% compared with unstimulated cells. Moreover, eosinophils stimulated for 60 minutes with IFN-γ showed a marked loss of RANTES immunoreactivity from fractions containing peak secretory granule activity (Fig 6C), which was translocated to plasma membrane-associated fractions at 49% of the quantity measured in unstimulated cells (35 pg RANTES/106 cells). These results are in agreement with those of CLSM, in which eosinophils displayed reduced RANTES activity after stimulation with IFN-γ (Fig 4J and K). Each subfractionation profile was prepared on different occasions from the same donor to allow comparison of control and stimulated cells. IFN-γ–stimulated eosinophils showed similar profiles of EPO activity to that of unstimulated cells (Fig 6), although the peak of EPO activity appeared to be partially diminished after 60 minutes of IFN-γ stimulation.
DISCUSSION
We have shown for the first time that RANTES immunoreactivity in human eosinophils is associated with the matrix of the crystalloid granules. This is based on its close apposition to the crystalline core granule protein marker, MBP. Interestingly, RANTES was also detected in an extragranular compartment distinct from MBP- and EPO-containing granules, which was readily released in response to IFN-γ. We propose that the rapidly mobilizable RANTES is contained within a putative pool of small secretory vesicles that is physically distinct from crystalloid granule.
The profile of RANTES immunoreactivity in fractions from unstimulated eosinophils suggests that the larger peak is likely to be associated with small, light-density vesicles, which possess a greater buoyant density than that of the plasma membrane, as indicated by CD9 immunoreactivity. It is important to note that these fractions do not fully discriminate between endosomal membranes, Golgi, and plasma membrane.41,43 The light membrane fractions are also likely to contain the vesiculotubular structures previously described in eosinophils.44
IFN-γ was observed to activate the release of RANTES from eosinophils in parallel with two other granule-associated proteins, EPO and β-hexosaminidase. Invariably, the levels of RANTES in these supernatants diminished to baseline values after 240 minutes of incubation. Those of EPO were similarly found to become reduced after 120 minutes of incubation (Fig 2). These observations suggest that both RANTES and EPO may be sequestered by surfaces within the assay after their release. Released EPO is likely to be lost to surfaces due to its inherent highly cationic nature (isoelectric point [pI] of 10.8).38,39 In addition, RANTES is rapidly sequestered by cell surface glysoaminoglycans after its secretion, which then foster the adhesion and activation of RANTES-responsive cells.45 In our subfractionation studies, immunoreactivity to RANTES was observed to shift to a very low-density peak after 60 minutes of stimulation with IFN-γ, which was shifted to the right in comparison with the light-density peak of RANTES in unstimulated cells (Fig 6A and C). This shift in the density of RANTES immunoreactivity suggests that released RANTES may be adhering to the glycosaminoglycans coating the surfaces of eosinophils. In support of the possibility that specific eosinophil products may be lost to surfaces during in vitro assay, the noncationic granule-derived enzyme β-hexosaminidase (predicted pI of 5.4 to 5.9) was found to increase in supernatants during incubation with IFN-γ (Fig 2B).
Evidence for the existence of a putative small secretory vesicle in eosinophils was provided in our studies on the effects of IFN-γ on RANTES mobilization by CLSM. RANTES immunoreactivity appeared to be transferred to the periphery of cells during IFN-γ stimulation, apparently to a different vesicular compartment from the crystalloid granules. Moreover, the subcellular fractionation profile of RANTES in IFN-γ–stimulated cells (after 10 minutes) showed that much of the RANTES associated with light density fractions was depleted, while granule-associated RANTES was maintained at a level equivalent to that of unstimulated cells. The observation that IFN-γ exerted such a rapid effect on mobilization and release of eosinophil-derived RANTES was novel and compelling. This is complementary to an earlier report, in which IFN-γ (1,000 U/mL) upregulated RANTES mRNA and protein expression within eosinophils after 16 hours of stimulation11 and provides further support for our recent finding that IFN-γ rapidly elevated IL-6 immunoreactivity in human peripheral blood eosinophils.7 After 60 minutes of stimulation by IFN-γ, nearly all of the detectable RANTES immunoreactivity was colocalized with very light-density membranes as determined by subcellular fractionation, indicating that the crystalloid granule-associated RANTES may have been selectively removed and transported via small secretory vesicles.
These observations suggest that eosinophils possess a unique mechanism for selective, piecemeal release of mediators from the crystalloid granules, probably through exocytosis of a population of small, light-density vesicles. Such small secretory vesicles may be responsible for shuttling crystalloid granule proteins from the granules to the plasma membrane. Selective release of eosinophil granule proteins has been described in earlier reports.46 A similar pattern of piecemeal degranulation has been proposed based on electron microscopy sections of eosinophilic degranulation, in vivo.44 Previous studies have shown that eosinophils undergo degranulation in response to intracellularly applied agonists, for example, guanosine 5′-0-(3-thiotriphosphate) (GTPγS),35 47 although the mechanisms regulating exocytotic release have not yet been fully elucidated. We are currently investigating the identity of the putative small secretory vesicles with a view to determining their precise colocalization with known eosinophil-derived intracellular proteins.
IFN-γ has been shown to stimulate eosinophils in vitro, as shown in its ability to augment eosinophil-induced antibody-dependent cellular cytotoxicity (ADCC)31 and induce the expression of FcγRIII (CD16)32 and CD69.33 Further, IFN-γ has been demonstrated to stimulate the release and/or upregulation of a number of cytokines from eosinophils, including IL-3,48IL-6,6,7 GM-CSF,8,9 and RANTES.11Expression of functional IFN-γ receptors on human peripheral eosinophils has been described recently.49 These and future experiments continue to contribute to the intriguing observation that IFN-γ can induce rapid changes in cytokine expression, receptor upregulation, and mediator release in eosinophils.
Activation of RANTES mobilization and release from eosinophils by IFN-γ was specific, as shown by genistein inhibition, a broadly specific tyrosine kinase inhibitor, suggesting that IFN-γ acts on these cells via the IFN-γ receptor, which activates the Jak-STAT pathway.40 Furthermore, other cytokines such as IFN-α, IL-3, IL-5, and GM-CSF were found to have little or no effect on RANTES immunoreactivity or release into supernatants (Fig 4Q; data not shown). The effects of IFN-γ were unlikely to be LPS-mediated because the culture media containing IFN-γ used in this study, as well as all other media, were negative for LPS as determined by routine E-Toxate testing (data not shown).
All eosinophils tested in these experiments were derived from subjects exhibiting atopy. It is possible that the effects of IFN-γ on RANTES mobilization were due to enhanced susceptibility of the cells to IFN-γ because of priming, for example.50 However, we have also detected IFN-γ–induced release of intracellularly stored RANTES from eosinophils obtained from atopic nonasthmatic subjects. Thus, we conclude that it is unlikely that the capacity of eosinophils to generate a differential response to IFN-γ is dependent on the asthmatic status of the donor.
In both human and murine studies, cytokines released during immune and inflammatory reactions have been proposed to follow a dichotomy of Th1 and Th2-type responses depending on the nature of the stimulus delivered to the immune system. Release of IFN-γ has been associated with Th1-type cytokine responses in bacterial and viral infections along with the suppression of atopy.51,52 On the other hand, eosinophils are regarded as Th2-type effector cells with the potential to respond to Th2-type cytokines thought to be associated with the allergic phenotype.2,53,54 However, the distinction between Th1 and Th2 cytokine profiles in most cases of infection and inflammation in humans is less clearcut. Thus, while IFN-γ potently inhibits granulocytic maturation and proliferation in the bone marrow,55 the levels of IFN-γ have been found to be increased in the sera of patients with acute severe asthma, who also exhibit lung and tissue eosinophilia.56,57 The significance of these findings may be that IFN-γ has a role in regulation of eosinophil homeostasis by stimulating fully mature eosinophils locally, while preventing excessive eosinophilia, as suggested by Valerius et al.31 It is tempting to speculate that IFN-γ released from virus-infected inflammatory and immune cells within the airway mucosa in asthmatics may contribute to activation of resident airway eosinophils during viral exacerbation of asthmatic attacks.58
Eosinophil-derived RANTES is likely to play a role in paracrine, autocrine, or juxtacrine signaling after its release, and its bioactivity on other eosinophils, at least in vitro, has previously been demonstrated.11 Besides RANTES, eosinophils contain a number of other cytokines in their secretory granules, such as IL-2, IL-4, IL-5, IL-6, tumor necrosis factor-α (TNF-α), and GM-CSF.4,5,7,10,59 60 Storage of cytokines as preformed mediators within secretory granules and their rapid release after stimulation may lend eosinophils the potential to regulate local inflammatory responses. Many of eosinophil-derived cytokines so far described are produced in smaller proportions than those of other immune cells, such as T cells. However, unlike eosinophils, T cells are not known to have the capacity to store cytokines. Cytokines produced from an overwhelming influx of actively degranulating eosinophils into the airways in asthma, for example, are likely to prolong their own survival and perpetuate the inflammatory response. These exciting observations will be important in expanding our knowledge of the cytokine and chemokine network that regulates the processes of eosinophil activation and subsequent secretion of cytokines, chemokines, and especially granule proteins with their recognized damaging sequelae in allergic inflammation.
ACKNOWLEDGMENT
The authors thank Dr Vera Chlumecky (Department of Cell Biology and Anatomy, University of Alberta), for her assistance in confocal laser scanning microscopy; Richard Sherburne (Department of Medical Microbiology and Immunology), for his help with electron microscopy; and Drs Klaus Erb and Harissis Vliagoftis for their valuable comments on the manuscript. We are grateful to Drs Andrew Shaw and Aziz Ghahary for their gifts of anti-CD9 and IFN-α, respectively.
Supported by the Medical Research Council, Canada, the University of Alberta Hospital Foundation, the Alberta Heritage Foundation for Medical Research, and the Glaxo Heritage Research Laboratory Award. P.L. is a Parker B. Francis Fellow in Pulmonary Medicine and R.M. is an Alberta Heritage Senior Medical Scholar.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Redwan Moqbel, PhD, FRCPath, Pulmonary Research Group, 574 Heritage Medical Research Center, University of Alberta, Edmonton, Alberta, Canada T6G 2S2; e-mail:redwan.moqbel@ualberta.ca.