Neutrophils undergo constitutive apoptosis when aged ex vivo. Recent studies have indicated roles for Fas/CD95 and the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase system in this process. We have investigated the role of protein kinase C (PKC) in neutrophil death. We show that there is proteolysis and activation of the novel isoform PKCδ in aged neutrophils and that this process is accelerated by the addition of an agonistic Fas antibody. PKCδ proteolysis occurs before the onset of any detectable features of apoptosis and pharmacologic inhibition of this enzyme inhibits neutrophil apoptosis. PKCδ cleavage and activation is dependent on caspase-8/FADD-like interleukin-1β converting enzyme (FLICE)–mediated processing of caspase-3/CPP32. Neutrophil survival is prolonged by the addition of broad spectrum (BD.fmk) or caspase-8 targeted (zIETD.fmk) peptide caspase inhibitors. Inhibition of PKCδ does not prevent apoptosis triggered by factor withdrawal in immature hematopoietic cells, including normal human CD34+ progenitors indicating that within a given lineage, the mechanisms of apoptosis may be differentiation-stage–specific. Ex vivo aging of neutrophils leads to the increasing production of reactive oxygen species and this is attenuated in cells treated with either caspase or PKCδ inhibitors. Proteolytically activated PKCδ acts as a molecular link between the Fas/CD95 receptor and the NADPH-oxidase system and plays a central role in regulating the process of neutrophil apoptosis.
NEUTROPHILS PLAY a central role in host defense against infectious microorganisms. The normal human neutrophil has a circulatory half-life of 6 to 10 hours and the production rate is about 1.5 × 109 cells/kg body weight per day.1 After migration into the tissues, cells either undergo spontaneous or activation-induced death by apoptosis and are recognized and ingested by resident macrophages.2 This serves to limit any damage to host tissues after their death. Neutrophils incubated ex vivo undergo constitutive apoptosis with about half the cells dying in the first 24 hours.3 Survival of neutrophils in culture can be prolonged by incubation with cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF),4 granulocyte CSF (G-CSF),5 and interleukin-8 (IL-8)6 and by inflammatory mediators such as bacterial lipopolysaccharide.7
Recent studies have given some insight into the mechanisms involved in neutrophil apoptosis. Liles et al8 showed that neutrophils express significant basal levels of cell surface Fas/CD95, and when cultured, produce Fas ligand, which leads to cell death in an autocrine or paracrine manner. The molecular mechanism of Fas/CD95-induced cell death has been characterized in the last few years.9 After receptor ligation by ligand or agonistic antibody, there is recruitment and stabilization of an adaptor protein known as FADD (Fas-associated death domain protein).10 This interacts with Fas/CD95 via a region known as the death domain, which binds to a homologous section of the intracytoplasmic tail of the receptor. FADD binds via its death effector domain to a member of the cysteinyl aspartate specific protease (caspase) family, FADD-like interleukin-1β converting enzyme (FLICE) or caspase-8.11 This process leads to auto-processing of procaspase-812 to generate an active molecule that is capable of cleaving and activating downstream caspases.13 These enzymes are believed to mediate many of the processes that culminate in programmed cell death by cleaving specific target proteins.14 An increasing number of these substrates have been identified. Some are involved in cell-cycle regulation and DNA repair, such as Rb and DNA-PK, others are structural proteins such as nuclear lamins, and some are proteins involved in signal transduction.15,16 The cleavage of some proteins by caspases can lead to their inactivation,16 but in other instances may lead to increased enzymatic activity. For example, proteolysis leads to activation of hPAK65 (a p21-activated kinase),17 MEKK-1 (an upstream regulator of the JNK pathway),15 and of protein kinase Cδ.18Ectopic transfection of constructs encoding these truncated forms can lead to some of the morphologic appearance of apoptosis.15,17 19 However, their role in apoptosis in primary cells is largely uncertain. In particular, it is unclear whether specific enzyme activation plays a causal role in certain forms of apoptosis, acts to facilitate the process of cell death once apoptosis is triggered, or is merely a consequence of the execution program.
There is evidence that the production of reactive oxygen species (ROS) via the neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase system also plays an important role in apoptosis. Neutrophil apoptosis is inhibited by hypoxia and by the addition of agents such as catalase, which tend to detoxify the products of the neutrophil oxidative burst.20-23 Neutrophils isolated from patients with chronic granulomatous disease (CGD) show a decreased rate of spontaneous cell death, as well as a reduction in apoptosis induced by the addition of agonistic Fas/CD95 antibodies.24Phagocytosis-induced apoptosis in neutrophils, a process regulated by the CD11b/CD18 integrin, is also dependent on the production of ROS.25
Less is known about the intracellular signaling molecules involved in regulating neutrophil survival and apoptosis. Several groups have shown that elevation of cyclic adenosine monophosphate (cAMP) inhibits neutrophil apoptosis.26-28 Agents that elevate cAMP also inhibit neutrophil respiratory burst activity, but it is not clear whether the effects of cAMP on preventing apoptosis are mediated by this mechanism.29,30 Frasch et al31 have shown that neutrophil apoptosis in reponse to stress stimuli (such as ultraviolet [UV] irradiation and hyperosmolarity) can be inhibited by blocking activation of the p38 stress-activated kinase. However, spontaneous or Fas/CD95-induced apoptosis was not affected by inhibiting p38 activity. The protective effects of cytokines such as GM-CSF and G-CSF involve tyrosine kinase activity,32 33 but the relevant downstream pathways have not been identified.
To try and understand further the intracellular signalling pathways that regulate neutrophil survival, we have investigated the role of protein kinase C (PKC) in neutrophil apoptosis. The PKC family consists of at least three groups of enzymes: the classical PKCs (α, β, and γ) that are dependent on diacylglycerol (DAG) and Ca2+for activation; the novel group (δ, ε, η, and θ) dependent on DAG alone and the atypical PKCs (ζ and ι/λ) that do not require either DAG or Ca2+ for activation, but may be regulated by 3-phosphorylated inositides generated by the activity of phosphoinositide 3-kinase(s).34-36 We show that in neutrophils incubated ex vivo, there is caspase-dependent cleavage and activation of the nPKCδ that precedes the onset of the earliest features of apoptosis. Prevention of PKCδ proteolysis by the use of peptide caspase inhibitors or pharmacologic inhibition of PKCδ strongly inhibits spontaneous and Fas/CD95-induced apoptosis by decreasing the production of reactive oxygen species. These effects are not seen in myeloid precursor cells before terminal differentiation. The results suggest an important role for cleavage and activation of PKCδ in neutrophil apoptosis and provide a molecular link between the Fas/CD95 and NADPH-oxidase pathways that are recognized as key participants in neutrophil programmed cell death.
MATERIALS AND METHODS
Materials.
The PKC inhibitors bisindolylmaleimide (GF109203X) and Gö6976 were obtained from Calbiochem (San Diego, CA). Caspase inhibitors BD-fmk, zIETD-fmk, and zVDVAD-fmk were from Enzyme Systems Products (Dublin, CA) The phosphoinositide (PI) 3-kinase inhibitor LY294002 was from Biomol (Plymouth Meeting, PA). Recombinant human (rh) G-CSF was donated by Chugai Pharmaceuticals (London, UK), rhGM-CSF by Behringwerke/Hoechst (Marburg, Germany) and rhIL-3 by Sandoz (Frimley Park, UK). rhIL-6 and stem cell factor were from Peprotech (Rocky Hill, NJ). PKC antibodies were as follows: polyclonal anti-PKCα (C-terminal) from Sigma (St Louis, MO); monoclonal PKCβ (recognizes both βI and βII), γ, ε, θ, and ζ monoclonal antibodies (MoAbs) were from Transduction Labs (Santa Cruz, CA) and polyclonal antibodies (C-terminal) to PKCβΙΙ, PKCδ, and PKCι were from Santa Cruz (Santa Cruz, CA). A polyclonal ERK2 antibody was from Santa Cruz. Agonistic anti-Fas/CD95 (clone CH11) was from UBI (Lake Placid, NY).
Neutrophil isolation, culture, and annexin V binding assay for apoptosis.
Neutrophils from normal volunteers were isolated by dextran sedimentation, Ficoll hypaque density gradient (Amersham Pharmacia, Uppsala, Sweden) and hypotonic lysis of contaminating red blood cells as described previously.37 Cells were finally resuspended in RPMI 1640 supplemented with 1% heat-inactivated fetal calf serum (FCS) and incubated at a density of 106 cells per mL in 24-well tissue-culture plates. At stated time points, cells were washed once with phosphate-buffered saline (PBS) and used in apoptosis quantitation assays. Fluorescein isothiocyanate (FITC)-conjugated annexin V (Boehringer Mannheim, Mannheim, Germany) was used according to the manufacturer’s instructions. Analysis of annexin V binding was performed by flow cytometry (Epics; Coulter, Hialeah, FL)38 and peak fluorescence of positive cells was at least 2 logs greater than the negative fraction.
Cell lysis, gel electrophoresis, and immunoblotting.
Before cell lysis, neutrophils or HL-60 cells were washed once in PBS and incubated in 1 mmol/L diisopropylfluorophosphate (DIFP) for 5 minutes on ice. Cells were pelleted by centrifugation and resuspended in lysis buffer (50 mmol/L HEPES pH 7.5, 100 mmol/L NaCl, 1% Triton X-100, 1 mmol/L EDTA, 1 mmol/L EGTA, 20 mmol/L NaF, 1 mmol/L Na orthovanadate, 1 mmol/L Pefabloc, 10 μg/mL each of aprotinin, pepstatin, and leupeptin). After 5 minutes on ice, cells were centrifuged at 13,000g at 4°C for 10 minutes, assayed for protein concentration (Bio-Rad, Richmond, CA) and the supernatant added to boiling sample buffer and boiled for 10 minutes. Equivalent protein amounts were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide gels followed by transfer to nitrocellulose membranes (Hybond C; Amersham, Arlington Heights, IL). Primary antibodies were incubated with the nitrocellulose membrane for 60 minutes at room temperature at the following dilutions: anti-PKCα (C-terminal) 1:5,000; monoclonal PKCβ (recognizes both βI and βII) 1:1,000; polyclonal (C-terminal) PKCβΙΙ 1:1,000; anti-PKCζ 1:500; anti-PKCδ (C-terminal) 1:2,000; anti-PKCι 1:1,000; anti-ERK2 1:500.
Neutrophil fractionation into apoptotic/nonapoptotic.
Neutrophils were incubated ex vivo for 8 hours as described above. Cells were washed once in annexin V binding buffer (150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2, 10 mmol/L HEPES, pH 7.4) and resuspended in 90 μL of binding buffer plus 10 μL of annexin V microbeads (kind gift of Miltenyi Biotech, Bergisch Gladbach, Germany) per 107 cells. Cells were incubated for 15 minutes at 12°C and then washed once in a large volume of binding buffer. Cells were loaded onto a mini-MACS column, washed with binding buffer, and both positively selected (by magnet) and flow-through cells collected. Cell lysates were made as described above, protein concentrations quantitated, and equal amounts of protein loaded for Western blotting. The purity of cells was verified by using annexin V-FITC and in the magnetically selected fraction 94% ± 3% of cells were annexin V positive and in the flow-through fraction 4% ± 2% were positive.
PKC kinase assay.
At appropriate time points, 5 × 106 neutrophils were processed and Triton X-100 lysates prepared as described above. Immunoprecipitation of PKC isoforms was performed by the addition of 1 μg of a polyclonal anti–C-terminal antibody for 45 minutes and protein A-agarose for 45 minutes. Immunoprecipitates were washed twice with lysis buffer and once with kinase buffer (20 mmol/L Tris.Hcl, pH 7.5, 10 mmol/L MgCl2). Samples were resuspended in a kinase mixture of 50 μL containing kinase buffer plus 20 μmol/L adenosine triphosphate (ATP), 5 μg H1 histone, and 2 μCi [γ-32P]ATP (Amersham). Incubation was for 5 minutes at 30°C and was terminated by the addition of sample buffer and boiling for 10 minutes. Phosphorylated proteins were separated by SDS-PAGE, analyzed by autoradiography, and phosphorylation of substrate histone H1 quantitated using NIH Image software (NIH, Bethesda, MD). In experiments evaluating the effects of caspase inhibitors or agonistic Fas antibody on PKCδ activity, inhibitors were present throughout the cell culture period, but were not added further to the immunoprecipitated PKCδ. In experiments examining the effects of PKC inhibitors, GF109203X and Gö6976, these were present throughout the culture period at a concentration of 1 μmol/L; due to the competitive, reversible nature of these inhibitors, further compound was added to a final concentration of 500 nmol/L to the kinase mixture before the addition of ATP.
In vitro cleavage of PKCδ by caspases.
Purified recombinant caspases 3 and 8 and PKCδ were from Pharmingen (San Diego, CA). A total of 100 ng of PKCδ was incubated with 10 ng of caspase-3 or 150 ng of caspase-8 in a final volume of 50 μL of assay buffer (20 mmol/L 1,4 piperazinediethanesulfonic acid [PIPES], pH7.2, 100 mmol/L NaCl, 10 mmol/L dithiothreitol [DTT], 1 mmol/L EDTA, 0.1% 3-[(chlolamidopropyl) dimethylammonio]-1-propane sulfonate (CHAPS), 10% sucrose) for 2 hours at 37°C. Parallel tubes were incubated with 1 μmol/L AcDEVD-CHO (Bachem, Saffron Walden, UK) as a caspase inhibitor. The reaction was terminated by the addition of sample buffer and proteins were analyzed by SDS-PAGE and immunoblotting using the anti-PKCδ antibody.
CD34 isolation/culture and culture of HL-60 and Jurkat cell lines.
Peripheral blood CD34+ cells were collected and purified from individuals with relapsed/resistant lymphomas undergoing stem cell mobilization for subsequent use after high-dose cytotoxic therapy. Cells were isolated and purified by immunoaffinity techniques as described. CD34+ cells surplus to defined clinical requirements were used for experimental purposes using a protocol with local ethics committee approval. A second round of selection using a mini-MACS column as per the manufacturer’s instructions was performed and yielded cells with 93% purity. Factor withdrawal-induced apoptosis was measured in freshly purified cells by incubation in a defined serum free medium (BIT; Stem Cell Technologies, Vancouver, Canada). To differentiate CD34+ cells, they were cultured in Iscove’s modified Dulbecco’s medium (IMDM)/20% FCS plus recombinant IL-3, IL-6, and stem cell factor (all at 20 ng/mL) for 7 days and then the same mixture supplemented with G-CSF (50 ng/mL) for a further 7 days. Factor withdrawal-induced apoptosis was measured after extensive washing and resuspension in serum-free medium. HL-60 and Jurkat cells were maintained in RPMI plus 10% fetal bovine serum.
Measurement of intracellular hydrogen peroxide.
Neutrophils were incubated in culture conditions as described above. At stated time points, cells were incubated in 5 μmol/L dichlorofluorescein diacetate (DCF-DA) for 60 minutes and then analyzed by flow cytometry. To control for variations in background fluorescence or dye uptake with time, parallel samples were incubated with the flavoprotein inhibitor diphenylene iodonium (DPI; Sigma) at 20 μmol/L for 15 minutes before DCF-DA addition. DPI inhibits oxidase activity, and in pilot experiments, inhibited the f-met-leu-phe–induced activation of the respiratory burst by over 94%. For each experimental condition, the DPI/DCF-DA sample was used as the control to which the DCF-DA alone sample was corrected. Hydrogen peroxide activity was expressed as the ratio of the mean channel fluorescence DCF-DA alone:DPI/DCF-DA. To verify that the addition of caspase inhibitors (BD.fmk, VDVAD.fmk, and IETD.fmk) did not directly affect the DCF-DA assay, they were incubated with neutrophils subsequently stimulated with phorbol ester to activate the respiratory burst. None of the caspase inhibitors reduced the response to phorbol ester in the DCF-DA assay.
RESULTS
The PKC inhibitor bisindolylmaleimide inhibits neutrophil apoptosis.
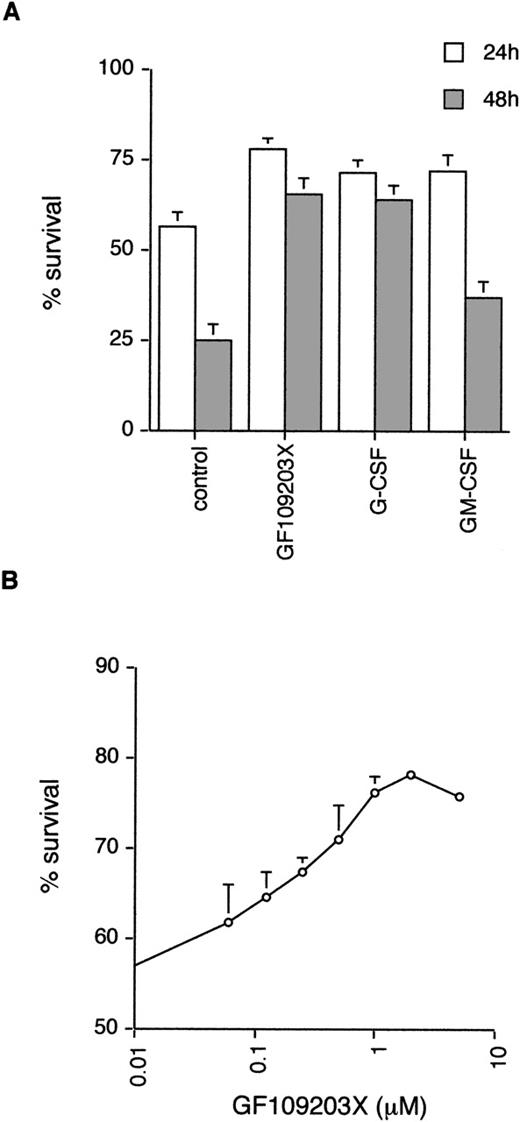
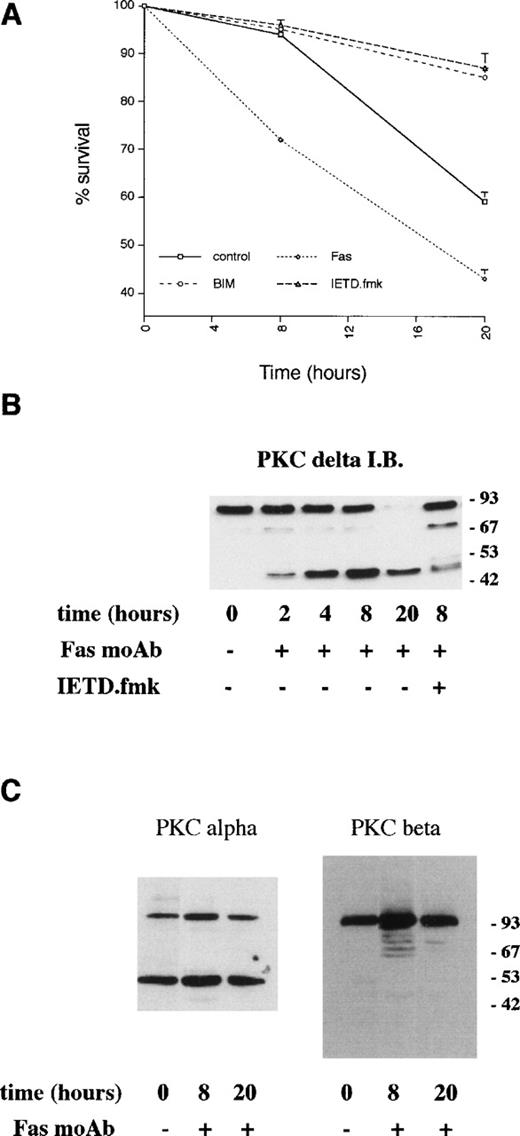
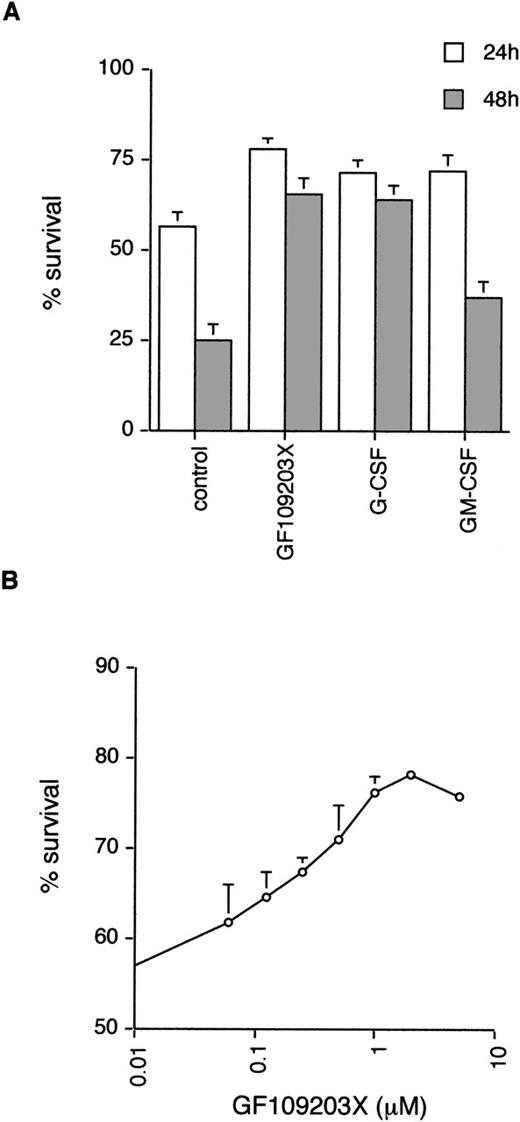
Neutrophils undergo spontaneous apoptosis when incubated ex vivo (Fig 1) with survival of 57% ± 4% at 24 and 25% ± 5% at 48 hours. As has been reported previously, neutrophil survival is promoted by incubation with G-CSF.5Addition of the bisindolylmaleimide PKC inhibitor, GF109203X,39,40 also maintains viability with survival of 78% ± 3% and 65% ± 5% at 24 and 48 hours, respectively (Fig 1), suggesting that activation of PKC is causally involved in neutrophil apoptosis. To investigate which member(s) of the PKC family could be involved in this process, we examined the expression of PKC isoforms by immunoblotting with specific antibodies. Comparison of PKC expression in neutrophils was made with the myeloid cell line HL60, which can be induced to differentiate along the granulocytic pathway (Fig 2).41Of the classical PKCs, neutrophils expressed significant levels of PKCβ (using an antibody that recognizes both βI and βII isoforms), a low level of PKCα and PKCγ was not expressed in either HL60s or neutrophils. PKCδ was the only novel PKC isoform detected. Although HL60 cells express both ζ and ι/λ atypical PKCs, neutrophils express a low level of PKCζ only. These results are consistent with those published by other groups.42-44
Effect of the PKC inhibitor, GF109203X, on neutrophil apoptosis. (A) Neutrophils were incubated in the absence (control) or presence of GF109203X (1 μmol/L), G-CSF (50 ng/mL), or GM-CSF (10 ng/mL) and apoptosis quantified by annexin V binding/flow cytometry at 24 and 48 hours. Mean ± standard error (SE) of 11, 10, and 8 separate experiments for GF109203X, G-CSF, and GM-CSF, respectively. (B) Concentration response curve for effects of GF109203X on neutrophil survival. Cell death was measured at 24 hours by annexin V binding and flow cytometry. Mean ± SE of three separate experiments.
Effect of the PKC inhibitor, GF109203X, on neutrophil apoptosis. (A) Neutrophils were incubated in the absence (control) or presence of GF109203X (1 μmol/L), G-CSF (50 ng/mL), or GM-CSF (10 ng/mL) and apoptosis quantified by annexin V binding/flow cytometry at 24 and 48 hours. Mean ± standard error (SE) of 11, 10, and 8 separate experiments for GF109203X, G-CSF, and GM-CSF, respectively. (B) Concentration response curve for effects of GF109203X on neutrophil survival. Cell death was measured at 24 hours by annexin V binding and flow cytometry. Mean ± SE of three separate experiments.
PKC isoforms expressed in neutrophils and HL-60 cells. Cell lysates from unstimulated freshly isolated neutrophils (PMN) or HL60 cells (20 μg of total protein were loaded per lane) were immunoblotted with various antibodies specific for PKC isoforms. Isoforms that were probed for, but not detected, in either cell type were PKCγ, ɛ, θ. Neutrophil/HL60 expression of the ERK2 MAPkinase is shown for comparison. Lysates from one of two separate experiments analyzed are shown.
PKC isoforms expressed in neutrophils and HL-60 cells. Cell lysates from unstimulated freshly isolated neutrophils (PMN) or HL60 cells (20 μg of total protein were loaded per lane) were immunoblotted with various antibodies specific for PKC isoforms. Isoforms that were probed for, but not detected, in either cell type were PKCγ, ɛ, θ. Neutrophil/HL60 expression of the ERK2 MAPkinase is shown for comparison. Lysates from one of two separate experiments analyzed are shown.
Inhibition of classical PKCs does not affect neutrophil survival.
GF109203X has been shown to inhibit classical and novel PKCs, but to have no effect on the kinase activity of the atypical PKCζ.40,45 PKCζ activity is known to be regulated by 3-phosphorylated inositides generated by the action of phosphoinositide 3-kinase.35 Incubation with the PI 3-kinase inhibitor, LY294002,46 at 20 μmol/L did not attenuate neutrophil apoptosis (control 57% ± 6%, LY294002 60% ± 6% survival at 24 hours, n = 6), further suggesting that PKCζ is unlikely to play a significant role in neutrophil apoptosis. In control experiments, this concentration of LY294002 abolished the activation of the PI 3-kinase target Akt/protein kinase B in response to GM-CSF, and this inhibitory effect was maintained for at least 24 hours (data not shown).
Therefore, the antiapoptotic effects of GF109203X in neutrophils could be attributed to inhibition of the cPKCs α and/or β or of the nPKCδ. The PKC inhibitor, Gö6976, has been shown to inhibit classical PKCs, but to have a limited effect on PKCδ activity.45 47 Incubation with Gö6976 at concentrations up to 10 μmol/L did not inhibit neutrophil apoptosis (control 78% ± 2%, Gö6976 78% ± 3% survival at 24 hours, n = 3). Gö6976 (5 μmol/L) was functional under these conditions as shown by a 68% inhibition of phorbol ester-stimulated neutrophil hydrogen peroxide production as detected by the flow cytometric DCF assay. These results suggest that the effects of GF109203X on neutrophil survival are likely due to inhibition of PKCδ activity.
PKCδ undergoes proteolytic cleavage and activation in neutrophils before the development of features of apoptosis.
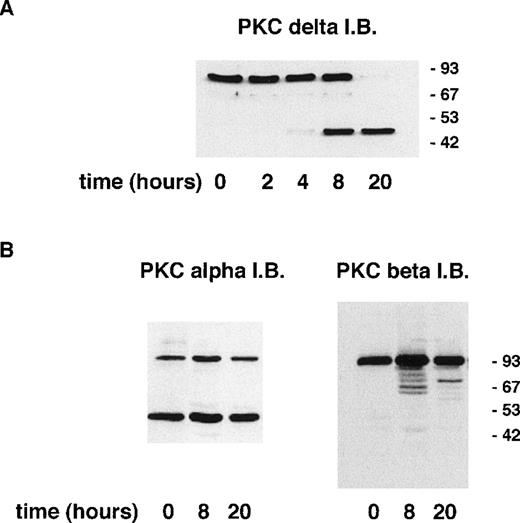
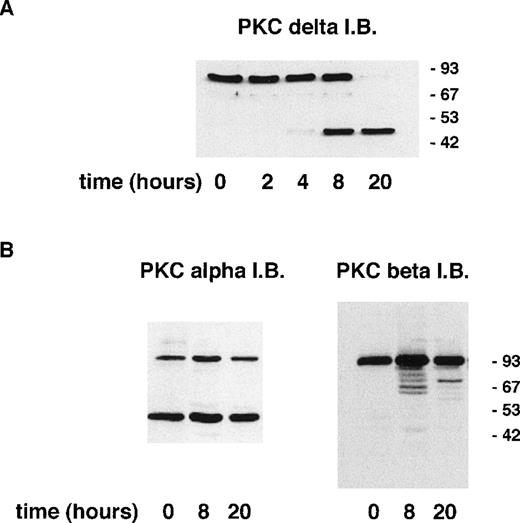
Induction of apoptosis in certain cell lines has been shown to result in the proteolytic cleavage of PKCδ leading to the generation of an active catalytic (C-terminal) fragment.18 Immunoblotting with an antibody raised to the C-terminal portion of PKCδ showed that a significant proportion of neutrophil PKCδ is proteolytically cleaved by 8 hours of incubation and that only the C-terminal fragment (and no full-length protein) can be detected at 20 hours (Fig 3). In contrast, using an anti–C-terminal antibody to PKCβII, which is the major neutrophil isoform,43 48 there was no detectable proteolysis of PKCβΙΙ between 0 and 20 hours of neutrophil incubation. Immunoblotting with an MoAb that recognizes both PKCβ isoforms showed no loss of full-length protein for up to 20 hours (data not shown). Immunoblotting with an anti–C-terminal PKCα antibody showed the presence of a lower molecular weight fragment, but this was present in freshly isolated neutrophils and did not alter with aging in culture. The degree of PKCδ proteolysis was not significantly affected by incubation with GF109203X.
Incubation of neutrophils ex vivo leads to the generation of a C-terminal proteolytic fragment of PKCδ. Lysates prepared from neutrophils aged for the indicated times. Cells (20 μg of total protein loaded per lane) were analyzed by immunoblotting using an antibody that recognizes the C-terminal end of PKCδ (A) or antibodies to the C-terminal portion of PKCs and βII (B).
Incubation of neutrophils ex vivo leads to the generation of a C-terminal proteolytic fragment of PKCδ. Lysates prepared from neutrophils aged for the indicated times. Cells (20 μg of total protein loaded per lane) were analyzed by immunoblotting using an antibody that recognizes the C-terminal end of PKCδ (A) or antibodies to the C-terminal portion of PKCs and βII (B).
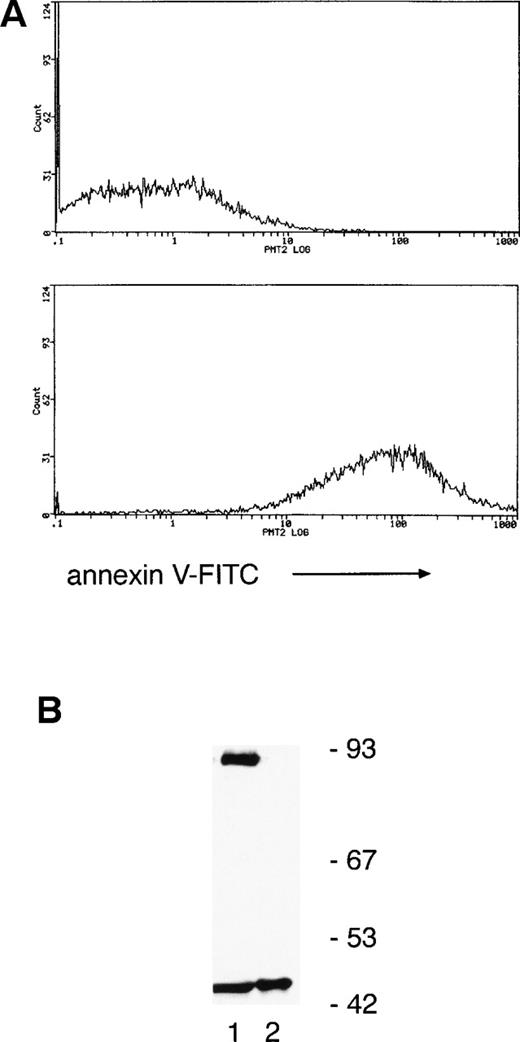
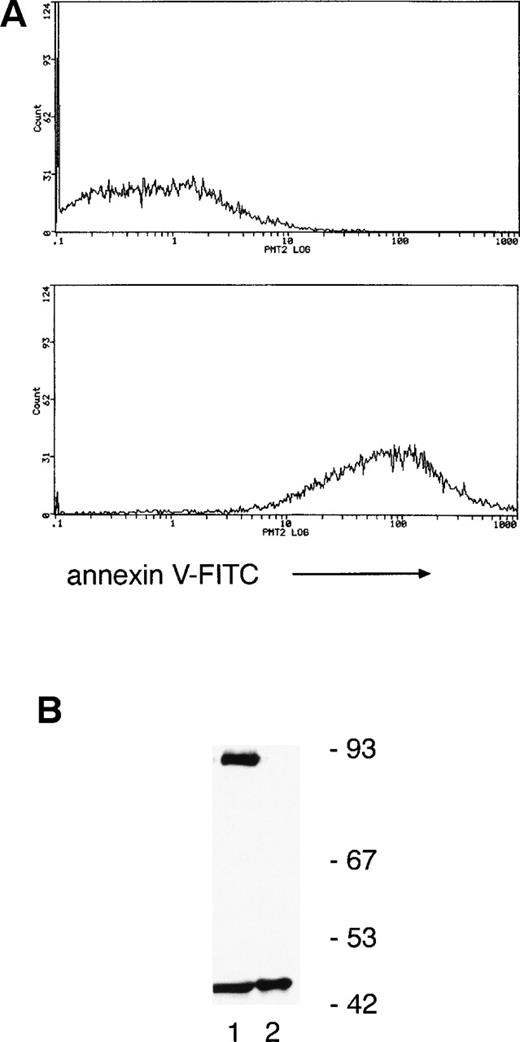
Annexin V binding (a marker of apoptosis) indicated that only 6% ± 3% and 45% ± 5% of neutrophils were apoptotic at 8 and 20 hours, respectively, suggesting that PKCδ proteolysis precedes the onset of cell death. To verify this, annexin V–coated magnetic microspheres were used to separate neutrophils into annexin V negative and positive fractions and whole-cell lysates prepared for immunoblotting. Figure 4 shows that there is a significant proportion of cleaved PKCδ in the annexin V–negative cells and only the C-terminal fragment can be found in annexin V–positive cells.
Separation of neutrophils using annexin V–coated magnetic microspheres shows that PKCδ proteolysis precedes any detectable features of apoptosis. (A) Neutrophils were incubated for 12 hours ex vivo and then separated by using annexin V–coated microspheres, which bind to cells with external exposure of membrane phosphatidylserine, an early marker of apoptosis. Subsequent staining with FITC-conjugated annexin V and flow cytometry shows negative cells in the upper panel and positively selected cells in the lower panel. (B) Lysates prepared from the two fractions were analyzed by immunoblotting with anti-PKCδ antibody. Equal amounts of protein were loaded: lane 1, annexin V negative fraction; lane 2, annexin V positive. This experiment was performed twice with similar results.
Separation of neutrophils using annexin V–coated magnetic microspheres shows that PKCδ proteolysis precedes any detectable features of apoptosis. (A) Neutrophils were incubated for 12 hours ex vivo and then separated by using annexin V–coated microspheres, which bind to cells with external exposure of membrane phosphatidylserine, an early marker of apoptosis. Subsequent staining with FITC-conjugated annexin V and flow cytometry shows negative cells in the upper panel and positively selected cells in the lower panel. (B) Lysates prepared from the two fractions were analyzed by immunoblotting with anti-PKCδ antibody. Equal amounts of protein were loaded: lane 1, annexin V negative fraction; lane 2, annexin V positive. This experiment was performed twice with similar results.
There is increased PKCδ catalytic activity in neutrophils incubated ex vivo.
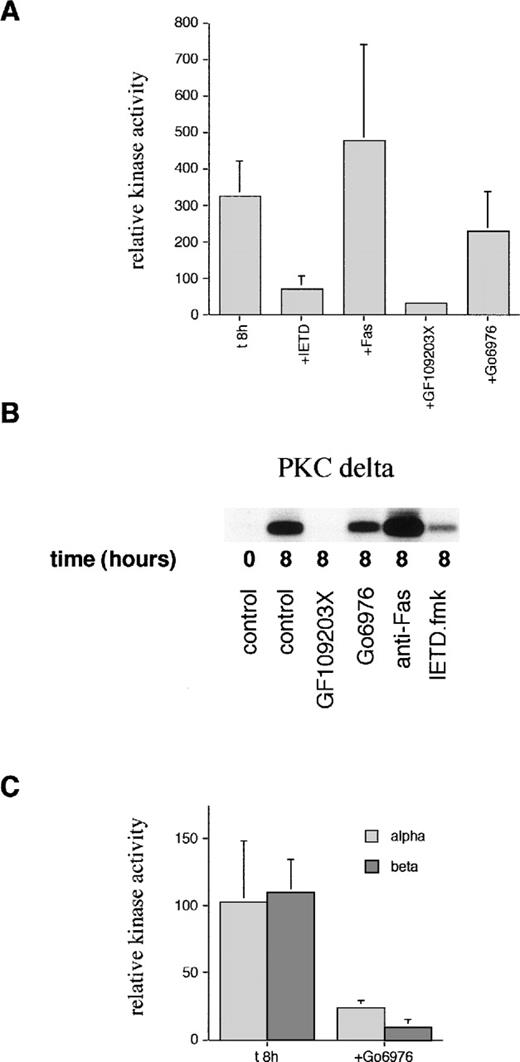
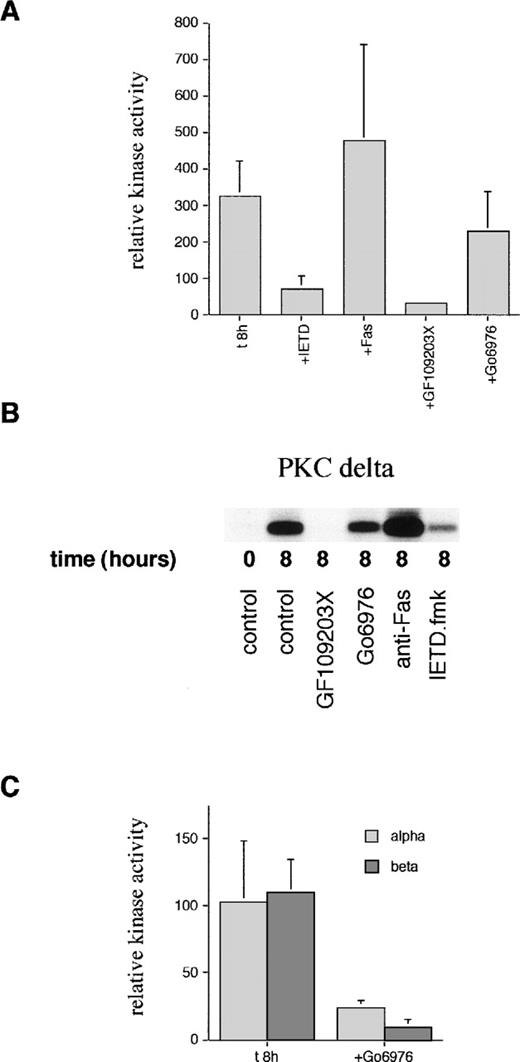
In view of the detection of a C-terminal PKCδ fragment, which could have altered activity, kinase assays were performed on immunoprecipitated PKCδ. Figure 5 shows that there is spontaneous activation of PKCδ detectable at 8 hours of incubation. This activation can be blocked by incubation with the cPKC/nPKC inhibitor, GF109203X, but not with the cPKC inhibitor, Gö6976. In contrast, there was no increase in the activity of PKCα or PKCβ at 8 hours. The basal level of activity of PKCα and PKCβ was inhibited by Gö6976 confirming the activity of this inhibitor.
Measurement of PKC kinase activity in aged neutrophils. (A) Neutrophils were incubated in the absence (t8h) or presence of the caspase-8 inhibitor, IETD.fmk (25 μmol/L), agonistic anti-Fas antibody (500 ng/mL), PKC inhibitor, GF109203X (1 μmol/L), or PKC inhibitor, Gö6976(1 μmol/L) for 8 hours. PKCδ was then immunoprecipitated using an anti–C-terminal antibody from neutrophil lysates and subjected to a kinase assay using histone H1 as a substrate. Due to the competitive, reversible nature of the PKC inhibitors (which would wash out during the immunoprecipitation protocol), further compound was added to a final concentration of 500 nmol/L to the kinase mixture before the addition of ATP. Phosphorylation of substrate was quantified after autoradiography and normalized to the time 0 value (100%). Results are mean ± SE of three separate experiments. (B) Representative autoradiograph from a PKCδ kinase assay. (C) PKCs and βΙΙ were immunoprecipitated using anti–C-terminal antibodies after neutrophil aging for 8 hours in the absence or presence of the PKC inhibitor, Gö6976, and subjected to a kinase assay using histone H1 as a substrate as detailed above. Mean ± SE of three separate experiments.
Measurement of PKC kinase activity in aged neutrophils. (A) Neutrophils were incubated in the absence (t8h) or presence of the caspase-8 inhibitor, IETD.fmk (25 μmol/L), agonistic anti-Fas antibody (500 ng/mL), PKC inhibitor, GF109203X (1 μmol/L), or PKC inhibitor, Gö6976(1 μmol/L) for 8 hours. PKCδ was then immunoprecipitated using an anti–C-terminal antibody from neutrophil lysates and subjected to a kinase assay using histone H1 as a substrate. Due to the competitive, reversible nature of the PKC inhibitors (which would wash out during the immunoprecipitation protocol), further compound was added to a final concentration of 500 nmol/L to the kinase mixture before the addition of ATP. Phosphorylation of substrate was quantified after autoradiography and normalized to the time 0 value (100%). Results are mean ± SE of three separate experiments. (B) Representative autoradiograph from a PKCδ kinase assay. (C) PKCs and βΙΙ were immunoprecipitated using anti–C-terminal antibodies after neutrophil aging for 8 hours in the absence or presence of the PKC inhibitor, Gö6976, and subjected to a kinase assay using histone H1 as a substrate as detailed above. Mean ± SE of three separate experiments.
Spontaneous and agonistic Fas antibody-induced proteolysis and activation of PKCδ is inhibited by peptide caspase inhibitors.
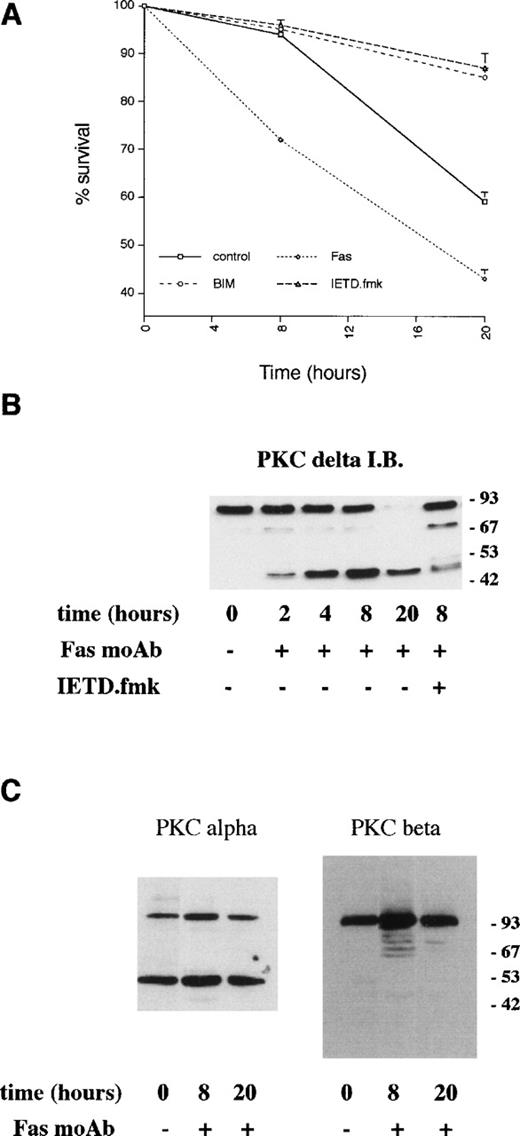
Previous studies have indicated a role for Fas/CD95 in neutrophil apoptosis. Incubation of neutrophils with an agonistic Fas antibody led to more rapid death compared with control cells (Fig 6) with over 30% of neutrophils undergoing apoptosis within 8 hours. Proteolysis of PKCδ was detectable by 2 to 4 hours of incubation with the anti-Fas antibody (Fig 6B), and there was a slight increase in PKCδ kinase activity at 8 hours compared with control cells (Fig 5).
Effects of an agonistic anti-Fas/CD95 antibody on PKCδ proteolysis and neutrophil apoptosis. (A) Neutrophils were incubated in the absence (control) or presence of agonistic Fas/CD95 antibody (500 ng/mL), PKC inhibitor, GF109203X (1 μmol/L), and caspase-8 inhibitor, IETD.fmk (25 μmol/L) and cell survival measured by annexin V binding using a flow cytometric assay. Mean ± SE of three separate experiments. (B) Neutrophils were incubated with agonistic Fas/CD95 antibody (500 ng/mL) with or without preincubation with the caspase-8 inhibitor, IETD.fmk (25 μmol/L) for the indicated times. Lysates were prepared, equal amounts of protein (20 μg) loaded per lane, and immunoblotted with an anti–C-terminal PKCδ antibody. (C) Neutrophils were incubated with agonistic Fas/CD95 antibody (500 ng/mL) for the indicated times. Lysates were prepared, equal amounts of protein (20 μg) loaded per lane, and immunoblotted with anti–C-terminal antibodies to PKC or PKCβ.
Effects of an agonistic anti-Fas/CD95 antibody on PKCδ proteolysis and neutrophil apoptosis. (A) Neutrophils were incubated in the absence (control) or presence of agonistic Fas/CD95 antibody (500 ng/mL), PKC inhibitor, GF109203X (1 μmol/L), and caspase-8 inhibitor, IETD.fmk (25 μmol/L) and cell survival measured by annexin V binding using a flow cytometric assay. Mean ± SE of three separate experiments. (B) Neutrophils were incubated with agonistic Fas/CD95 antibody (500 ng/mL) with or without preincubation with the caspase-8 inhibitor, IETD.fmk (25 μmol/L) for the indicated times. Lysates were prepared, equal amounts of protein (20 μg) loaded per lane, and immunoblotted with an anti–C-terminal PKCδ antibody. (C) Neutrophils were incubated with agonistic Fas/CD95 antibody (500 ng/mL) for the indicated times. Lysates were prepared, equal amounts of protein (20 μg) loaded per lane, and immunoblotted with anti–C-terminal antibodies to PKC or PKCβ.
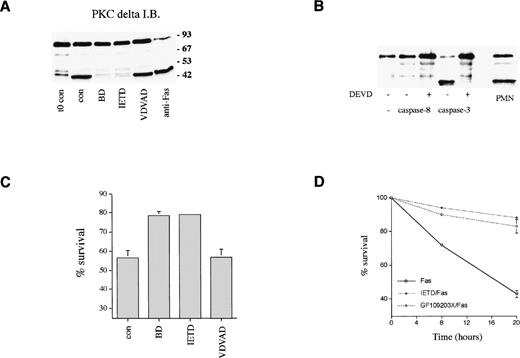
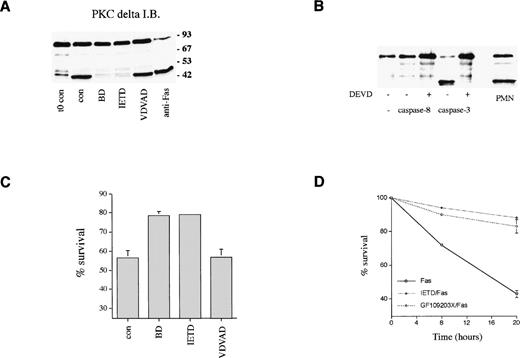
The effect of cell permeable peptide inhibitors of caspase family members14 on PKCδ proteolysis and neutrophil survival was measured. Figure 7A shows that PKCδ proteolysis is markedly inhibited by the broad spectrum caspase inhibitor, boc.aspartyl.fmk (BD.fmk),14,49 and by the caspase-8 inhibitor, IETD.fmk,50 but not by another inhibitor designed to target caspase-2, zVDVAD.fmk.51Caspase-8 is recruited to the intracytoplasmic domain of oligomerized Fas/CD95 via its association with the adaptor protein FADD.11 Previous work has shown that PKCδ can be cleaved by the action of caspase-319; the caspase-8 inhibitor IETD.fmk could potentially block PKCδ proteolysis in intact cells by preventing the processing of caspase-3 by caspase-8. Alternatively, caspase-8 could also cleave PKCδ directly. We incubated purified active caspases 3 and 8 with purified recombinant PKCδ and found that only caspase-3 was capable of cleaving PKCδ, indicating that PKCδ is not a direct target of caspase-8 (Fig 7B). Neutrophil survival was enhanced in the presence of both BD.fmk and IETD.fmk (Figs 6 and 7C). VDVAD.fmk had no effect. IETD.fmk also inhibited anti-Fas–induced PKCδ proteolysis and apoptosis (Figs 6B and 7D).
Role of caspases in PKCδ proteolysis and neutrophil survival. (A) Effect of caspase inhibitors on PKCδ proteolysis in neutrophils. Neutrophils were either lysed immediately after preparation (t0 con) or after 8 hours incubation in the absence (cont) or presence of broad spectrum caspase inhibitor, BD.fmk, the caspase-8 inhibitor, IETD.fmk (both at 25 μmol/L), the caspase-2 inhibitor, VDVAD.fmk (50 μmol/L), or with agonistic Fas antibody (500 ng/mL) as indicated and immunoblotted with anti-PKCδ antibody. (B) Effect of recombinant caspases on PKCδ proteolysis. Purified recombinant caspase-3 or caspase-8 was incubated with recombinant PKCδ ± caspase inhibitor DEVD-CHO and proteolysis estimated by immunoblotting with an anti-PKCδ antibody. For comparison, a lysate from 8-hour aged neutrophils is shown (PMN). (C) Effect of caspase inhibitors on neutrophil survival. Neutrophils were incubated in the absence (cont) or presence of the broad spectrum caspase inhibitor, BD.fmk, the caspase-8 inhibitor, IETD.fmk (both at 25 μmol/L), or the caspase-2 inhibitor, VDVAD.fmk (50 μmol/L) for 20 hours and survival measured by annexin V binding and flow cytometry. Mean ± SE of four separate experiments. (D) Effect of the caspase-8 inhibitor, IETD.fmk, and the PKC inhibitor, GF109203X, on apoptosis induced by an agonistic Fas antibody. Neutrophils were preincubated with IETD.fmk (25 μmol/L) or GF109203X (1 μmol/L) for 15 minutes and then anti-Fas antibody added (500 ng/mL). Cell survival was measured by annexin V binding in a flow cytometric assay. Mean ± SE of three separate experiments.
Role of caspases in PKCδ proteolysis and neutrophil survival. (A) Effect of caspase inhibitors on PKCδ proteolysis in neutrophils. Neutrophils were either lysed immediately after preparation (t0 con) or after 8 hours incubation in the absence (cont) or presence of broad spectrum caspase inhibitor, BD.fmk, the caspase-8 inhibitor, IETD.fmk (both at 25 μmol/L), the caspase-2 inhibitor, VDVAD.fmk (50 μmol/L), or with agonistic Fas antibody (500 ng/mL) as indicated and immunoblotted with anti-PKCδ antibody. (B) Effect of recombinant caspases on PKCδ proteolysis. Purified recombinant caspase-3 or caspase-8 was incubated with recombinant PKCδ ± caspase inhibitor DEVD-CHO and proteolysis estimated by immunoblotting with an anti-PKCδ antibody. For comparison, a lysate from 8-hour aged neutrophils is shown (PMN). (C) Effect of caspase inhibitors on neutrophil survival. Neutrophils were incubated in the absence (cont) or presence of the broad spectrum caspase inhibitor, BD.fmk, the caspase-8 inhibitor, IETD.fmk (both at 25 μmol/L), or the caspase-2 inhibitor, VDVAD.fmk (50 μmol/L) for 20 hours and survival measured by annexin V binding and flow cytometry. Mean ± SE of four separate experiments. (D) Effect of the caspase-8 inhibitor, IETD.fmk, and the PKC inhibitor, GF109203X, on apoptosis induced by an agonistic Fas antibody. Neutrophils were preincubated with IETD.fmk (25 μmol/L) or GF109203X (1 μmol/L) for 15 minutes and then anti-Fas antibody added (500 ng/mL). Cell survival was measured by annexin V binding in a flow cytometric assay. Mean ± SE of three separate experiments.
GF109203X does not inhibit apoptosis in immature hematopoietic cells.
Preincubation with GF109203X decreased the proapoptotic effects of anti-Fas antibody on neutrophils (Fig 7D). In contrast, incubation of Jurkat T-cells with GF109203X before the addition of anti-Fas antibody did not prevent apoptosis with survival of 24% in anti-Fas–treated cells and 11% in GF109203X+anti-Fas cells (measured at 24 hours). In Jurkat cells, PKCδ proteolysis was readily detectable by immunoblotting within 4 hours of Fas stimulation (data not shown). We further investigated the effect of GF109203X on apoptosis triggered by growth factor withdrawal in more primitive hematopoietic cells. GF109203X had no protective effect on factor-withdrawal–induced apoptosis in primary human CD34+ cells (survival at 24 hours in control cells 60%; +GF109203X, 58%). In addition, CD34+ cells were differentiated ex vivo in a growth factor mixture including G-CSF.52 After 14 days, when over 80% of cells were myelocytes and metamyelocytes, growth factors were removed. Under these conditions, PKC inhibition did not ameliorate apoptosis (control, 47%; GF109203X, 50%). These results indicate that the effect of GF109203X on cell death is restricted to terminally differentiated neutrophils.
GF109203X and the caspase inhibitor IETD.fmk inhibit spontaneous production of reactive oxygen species in neutrophils incubated ex vivo.
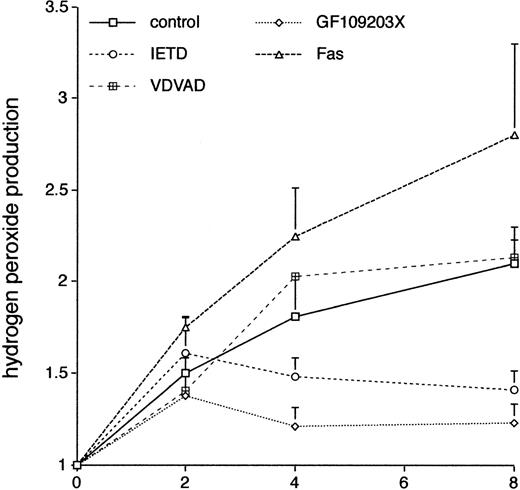
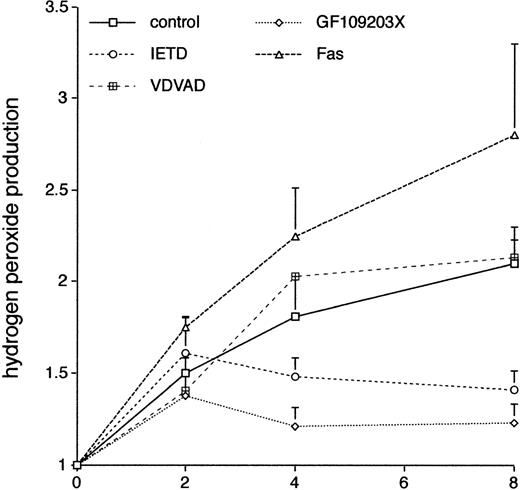
There is increasing evidence that the production of ROS plays a critical role in neutrophil apoptosis; in particular,24neutrophils from patients with chronic granulomatous disease show prolonged neutrophil survival ex vivo. We used the DCF-DA fluorescent probe coupled with flow cytometry37 to look at the effects of GF109203X on neutrophil production of ROS. Figure 8 shows that there was a sustained increase in the spontaneous production of ROS in neutrophils incubated ex vivo and that this was inhibited by incubation with the PKC inhibitor, GF109203X. Incubation with the inhibitor, Gö6976, did not significantly affect ROS production (control, 2.1 ± 0.2-fold increase; Gö6976, 2.43 ± 0.1-fold; duplicate measurements in two separate experiments; mean ± standard deviation [SD]).
The effect of various agents on neutrophil production of ROS. Freshly isolated neutrophils were incubated in the absence (control) or presence of the caspase-8 inhibitor, IETD.fmk (25 μmol/L), the caspase-2 inhibitor, VDVAD.fmk (50 μmol/L), the PKC inhibitor, GF109203X, or an agonistic Fas antibody (500 ng/mL). Relative production of ROS was measured by a flow cytometric assay (DCF-DA) at the indicated time points. Mean ± SE of three separate experiments for VDVAD.fmk and four separate experiments for the rest.
The effect of various agents on neutrophil production of ROS. Freshly isolated neutrophils were incubated in the absence (control) or presence of the caspase-8 inhibitor, IETD.fmk (25 μmol/L), the caspase-2 inhibitor, VDVAD.fmk (50 μmol/L), the PKC inhibitor, GF109203X, or an agonistic Fas antibody (500 ng/mL). Relative production of ROS was measured by a flow cytometric assay (DCF-DA) at the indicated time points. Mean ± SE of three separate experiments for VDVAD.fmk and four separate experiments for the rest.
Incubation with the caspase-8 inhibitor, IETD.fmk, was also associated with a reduction in production of ROS, and this effect was not seen in cells incubated with the caspase-2 inhibitor, VDVAD.fmk, mirroring the effects of these inhibitors on neutrophil survival (Fig 7). In two separate experiments, incubation of neutrophils with the broad spectrum caspase inhibitor, BD.fmk, led to a decrease in ROS production compared with control cells (mean increase at 8 hours in control cells 2.1 ± 0.2 and in BD.fmk-treated cells 1.51 ± 0.1). Production of ROS was more pronounced in neutrophils incubated with an agonistic anti-Fas antibody (Fig 8). These results suggest that in this context ROS production is dependent on caspase activity, which is likely to be triggered via activation of the Fas/CD95 pathway.
DISCUSSION
In this study we have evaluated the role of PKC in neutrophil apoptosis and showed that the novel PKC isoform PKCδ plays an important part in mediating cell death. Neutrophils isolated from normal individuals and incubated ex vivo undergo constitutive apoptosis with about half of the cells dying in the first 24 hours. Other investigators have shown that this phenomenon can be inhibited by incubation with a variety of growth factors and cytokines.4-7 We show that incubation with the PKC inhibitor, GF109203X, also inhibits this form of cell death to levels comparable to cytokines such as G-CSF with only about 20% of cells undergoing apoptosis in the first 24 hours. Of the three major PKC isoenzyme groups, we show that neutrophils contain the classical PKCs α and β, the novel PKCδ, and the atypical PKCζ. This distribution of PKCs is similar to that reported by other groups.42-44
The early development of PKC inhibitors was based on compounds such as staurosporine and H7, which act as catalytic domain inhibitors and are competitive with respect to ATP binding.40 Although these were effective, they lacked specificity. Staurosporine can, for example, inhibit many other types of kinases such as ribosomal S6 kinase, c-AMP–dependent kinase, and the src tyrosine kinase.40 The bisindolylmaleimide inhibitors are synthetic compounds based on staurosporine and have yielded potent and selective PKC inhibitors.39 However, these compounds vary in their potency against different PKC isoforms. The GF109203X compound used in this study has been shown to effectively inhibit the cPKCs α and β and the nPKCs δ and ε at concentrations in the nmol/L range; the 50% inhibitory concentration (IC50) for PKCζ is much higher at about 6 μmol/L.45 This specificity coupled with the distribution of PKC isoforms in neutrophils suggested that the effects of GF109203X on neutrophil survival could be a result of its effects on either the novel isoform PKCδ or the classical isoforms PKCα/β. We used the properties of another inhibitor, Gö6976,45 to discriminate between these possibilities. This inhibitor has been shown to inhibit the cPKC isoforms in the low nmol/L range, but does not affect the activity of PKCδ at concentrations up to 3 μmol/L.45 This failed to have any effect on neutrophil survival, suggesting that PKCδ was the most likely isoform responsible for the proapoptotic effects seen.
In 1995, Emoto et al showed that in U937 cells, induction of apoptosis in response to ionizing radiation was associated with proteolytic cleavage of PKCδ leading to the generation of an approximately 40 kD C-terminal fragment.18 This was catalytically activated due to the loss of the N-terminal inhibitory domain. Subsequent work from the same group showed that this cleavage (at aspartate 330) could be reproduced in vitro using purified caspase-3/CPP32.19 Transfection and transient overexpression of the PKCδ catalytic fragment could induce an apoptotic morphology in HeLa and NIH3T3 cells.19 These results suggested that PKCδ activation by proteolysis could be a participant in the apoptotic program and not just a consequence of the execution phase. Proteolytic activation of PKCδ has been implicated in several models of apoptosis. Caspase-3–dependent activation of PKCδ and PKCα in response to a variety of apoptotic stimuli was reported by Shao et al.53 Mizuno et al54 have shown that in human leukemic cell lines triggered to undergo Fas-mediated apoptosis, the nPKCs, including PKCδ, undergo limited proteolytic cleavage, and that this parallels the activation of caspase-3 and apoptosis. No data showing the effects of PKC inhibition were reported in this report, but in certain cell types, phorbol ester addition alone could induce apoptosis.
More recently, Denning et al55 have reported that in human keratinocytes UV radiation-induced apoptosis is associated with the proteolytic activation of PKCδ and that cell death can be inhibited by GF109203X preincubation. PKCδ proteolytic activation has also been implicated in cerebellar granule cell death.56 We have shown in this report that inhibition of PKC activity with GF109203X does not inhibit all forms of apoptosis, or indeed even all forms of apoptosis triggered by Fas ligation in different cell types. It has recently been shown that induction of apoptosis by Fas can lead to the cleavage of a number of signalling proteins.57 It is likely that caspase-mediated cleavage of different proteins may be critical for the apoptotic phenotype in various cell types and that this may be influenced by their extracellular environment. In neutrophils, cleavage and activation of PKCδ appears to play a crucial role in mediating apoptosis, but this may not be a rate-limiting step in other cell types.
Neutrophils undergoing spontaneous apoptosis show a rapid cleavage of PKCδ such that a significant proportion is cleaved at 8 hours and almost all by 20 hours. In contrast, there is no evidence of proteolysis of PKCα or PKCβ over this time course. The proteolytic changes in PKCδ are accompanied by increased enzyme activity and precede detectable features of apoptosis. At the 8-hour time point, only about 5% of cells display any features of apoptosis. One of the earliest markers of apoptosis is the externalization of plasma membrane phosphatidylserine (PS) detectable by the binding of annexin V.58-60 This PS exposure precedes the morphologic appearances of apoptosis, changes in membrane permeability to agents such as trypan blue and propidium iodide, or the characteristic DNA fragmentation. Functionally PS exposure is one of the mechanisms by which apoptotic cells are recognized by macrophages and targeted for ingestion.61 Separation of annexin V negative and positive neutrophil fractions using magnetic microspheres showed that annexin V negative cells contain a significant proportion of the catalytic PKCδ fragment. These results indicate that proteolytic activation of PKCδ takes place before the onset of apoptosis and that inhibition of PKCδ by GF109203X attenuates the apoptotic process.
Previous work has suggested a role for the Fas/CD95 pathway in neutrophil apoptosis.8,24 This pathway, which was initially thought to have a major role in T-cell regulation alone,9has been shown to be involved in apoptosis in several cell types in response to various inducers of cell death.62-64 Liles et al8 showed that spontaneous neutrophil death in vitro could be inhibited by blocking the Fas/CD95 ligand-receptor interaction and that neutrophils produced FasL suggesting an autocrine/paracrine mechanism of death. Neutrophil apoptosis can be accelerated by incubation with agonistic Fas/CD95 antibodies and this is associated with more rapid PKCδ proteolysis. After oligomerization of Fas/CD95 by ligand or MoAb, there is activation of caspase-8/FLICE, which is recruited to the receptor complex by FADD/MORT-1.11,12,50,65 Caspase-8 is capable of activating other effector caspases, such as caspase-3, by proteolytic removal of a prodomain.13 Thus caspase-8 sits at the apex of the Fas/CD95 pathway and its inhibition has been shown to prevent apoptosis in response to Fas/CD95 ligation.11,50 66
We show that PKCδ proteolysis and activation is inhibited by preincubation with certain peptide caspase inhibitors. These are designed to mimic the preferred substrates of individual family members or to act as general inhibitors.14 The broad spectrum inhibitor, BD-fmk, decreases PKCδ proteolysis and apoptosis as might be expected. The inhibitor, IETD-fmk, targeted to caspase-8,50 also prevents both spontaneous and Fas/CD95-induced PKCδ proteolysis and apoptosis. Using purified recombinant enzymes, we show that caspase-3, but not caspase-8, can cleave PKCδ indicating that the effects of IETD-fmk are due to indirect inhibition of caspase-3 by preventing its activation by caspase-8 in the intact cell. These results confirm and extend those of Ghayur et al19 showing a major role of Fas/CD95 in spontaneous neutrophil apoptosis and indicate that PKCδ proteolysis plays a significant part in this process. The cleavage of PKCδ by caspase-3 in neutrophils where there are no other detectable features of apoptosis suggests that mechanisms to compartmentalize caspase-3 activation are likely to exist in the early stages of programmed cell death.
In addition to Fas/CD95, the neutrophil NADPH-oxidase system has been shown to play an important role in cell death. The production of ROS in other cell types can also predispose to apoptotic death.67Coxon et al25 showed that phagocytosis of opsonized particles by human neutrophils induced apoptosis in a CD11b/CD18 integrin and ROS-dependent manner. Kasahara et al24 showed that neutrophils from patients with CGD had much slower rates of constitutive or Fas/CD95-induced apoptosis compared with normals. Apoptosis of normal neutrophils can be inhibited by incubation with catalase, which leads to the rapid intracellular breakdown of hydrogen peroxide.22,24 We show that neutrophils incubated ex vivo produce increasing amounts of ROS with time and that this increase is inhibited by coincubation with either the PKC inhibitor, GF109203X, with the caspase-8 inhibitor, IETD-fmk, or the broad spectrum caspase inhibitor, BD.fmk. ROS production is not affected by incubation with Gö6976 or with the caspase-2 inhibitor, VDVAD.fmk. This suggests that activation of Fas/CD95 by endogenously produced Fas ligand promotes the formation of ROS in a caspase-8/PKC–dependent manner. Acceleration of the apoptotic process with activating anti-Fas antibodies leads to an increase in ROS production. Triggering the caspase cascade may also lead to the activation and/or dysregulation of other proteins involved in formation of ROS such as the Rac guanine nucleotide dissociation inhibitor D4-GDI68 and the p21-activated kinase PAK65.17 The latter may regulate the NADPH-oxidase complex by phosphorylating p47phox.69 The results shown here suggest that activation of PKCδ is crucial to the production of ROS in this system.
The precise role of PKC in the activation of the neutrophil oxidase system is not clear. The respiratory burst can be efficiently activated by PKC-activating phorbol esters or by synthetic analogues of diacylglycerol.70 These effects can be inhibited by competitive PKC inhibitors such as GF109203X.71 PKC may be involved in the phosphorylation and translocation of the p47 and p67 phox proteins, but the isoforms involved have not been defined.72-76 The data on the effects of PKC inhibitors on the respiratory burst induced by the bacterial peptide, fMLP, are contradictory, but this may relate to the use of nonselective inhibitors or to alternative pathways of oxidase activation.71,74,77-79 One group who used more selective inhibitors has suggested that chemotactic peptide-induced activation of the respiratory burst may depend on the activity of nPKCs,47 of which neutrophils only appear to express the δ isoform.
We have also shown that the effect of GF109203X on cell survival is dependent on the stage of differentiation. Primary peripheral blood-derived CD34+ cells and progeny induced to differentiate down the myeloid pathway in vitro undergo apoptosis on withdrawal of survival factors. This apoptotic pathway is not affected by the PKC inhibitor GF109203X, although CD34+ cells express several PKC isoforms including PKCδ (unpublished data); the only cells to be affected by PKC inhibition are terminally differentiated neutrophils. This may relate to the timing of development of a fully fledged NADPH-oxidase system, which occurs late in myeloid differentiation. In neutrophils, PKCδ acts as a link between the apoptosis-initiating Fas/CD95 receptor and the production of ROS, which amplifies the process of cell death. These results highlight the likelihood that although there are many conserved molecular themes in apoptosis, the precise interplay between component molecules may show significant variations even in cells of the same lineage.
Supported by the Medical Research Council (UK).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Asim Khwaja, MD, Department of Haematology, UCL Medical School, 98 Chenies Mews, London WC1E 6HX, UK; e-mail: a.khwaja@ucl.ac.uk.