We report major responses in 4 of 4 patients with hairy cell leukemia (HCL) who have recently been treated on a phase I trial with the recombinant immunotoxin LMB-2. The immunotoxin, designed to target CD25+ malignancies, is composed of the Fv portion of the anti-Tac (anti-CD25) antibody, fused to a 38-kD truncated form of Pseudomonas exotoxin A, and has previously been called anti-Tac(Fv)-PE38. All 4 HCL patients were resistant to standard and salvage therapies for HCL, including 2-chlorodeoxyadenosine (CdA) and interferon , and all patients responded to LMB-2 after a single cycle. One patient treated with 2 cycles had a complete remission (CR), with regression of HCL cells from the blood and marrow and resolution of splenomegaly and pancytopenia. As is typical for patients in CR after treatment with CdA, minimal residual disease was detectable by flow cytometry of the bone marrow aspirate. This patient has not relapsed after 11 months. Three other patients had 98% to 99.8% reductions in malignant circulating cells. These results represent a proof of principal that targeted therapy with recombinant Fv-containing proteins can be clinically useful. LMB-2 may be an effective new therapy for patients with chemotherapy-resistant CD25+HCL.
VARIABLE DOMAINS make up a small percentage of the molecular weight of an antibody but contain all the amino acids that bind to antigen. In 1988, genetic engineering was used to produce recombinant Fv fragments in which the variable domains of the heavy chain and light chain of the antibody were connected by a peptide linker.1,2 Since then, more than 1,000 reports have described single-chain Fv molecules engineered to target a variety of antigens, many of which are antigens selectively expressed on cancer cells. It has been hoped that these agents, connected to effector molecules such as toxins or radionuclides, could cause regression of malignant disease in patients. Although radiolabeled single-chain Fvs were shown to successfully image tumors in patients,3 no major responses have yet been reported in clinical therapy trials.
Antibodies have been used to target protein toxins to cancer cells as immunotoxins; the toxins are potent enough so that only one or a few molecules need to reach the cytoplasm of target cells to produce cell death.4 Single-chain Fvs fused to toxins are termed recombinant immunotoxins, and are produced by recombinant DNA techniques in Escherichia coli. The molecules are extremely active and have been shown to kill cells with only a few hundred or thousand binding sites.5 The first recombinant immunotoxin produced contained the variable domains of anti-Tac, the monoclonal antibody to the interleukin-2 (IL-2) receptor,6 fused to a truncated form of the bacterial toxin Pseudomonas exotoxin that is devoid of its binding domain.7 For clinical development, a closely related molecule, anti-Tac(Fv)-PE38 (LMB-2), was produced that contains the variable heavy domain (VH) of anti-Tac fused via a 15 amino acid linker to the variable light domain (VL), which in turn is fused to the amino terminus of a 38-kD truncated form (amino acids 253-364 and 381-613) of the toxin.8 LMB-2 has been shown to be cytotoxic toward malignant cells expressing CD25 that were either established as cell lines or directly obtained from patients with hematologic malignancies.9-12 The mode of cytotoxicity appears to include binding to CD25, internalization and processing of the toxin within its translocation domain,13-15 binding of the 35-kD carboxyl terminus of the toxin intracellularly to the KDEL receptor that carries it to the endoplasmic reticulum,16,17translocation of the toxin into the cytoplasm,18,19 and catalytic ADP-ribosylation of elongation factor 2, leading to apoptosis and cell death.20,21 In vivo, LMB-2 produced complete regressions of CD25+ tumors in mice.8Toxicology studies showed that blood levels causing tumor regression in mouse xenografts are well tolerated by monkeys.22 We began phase I testing with LMB-2 in patients with hematologic malignancies in 1996. In this ongoing trial, 4 patients with hairy cell leukemia (HCL) have been treated. The present report focuses on the responses in these 4 HCL patients.
HCL is a malignancy of well-differentiated B lymphocytes that is diagnosed in 500 to 600 patients per year in the United States.23,24 Recent advances in the development of nucleoside analogs to treat HCL have led to long-term clinical complete remissions (CRs) in a high percentage of patients, particularly those treated with deoxycoformycin (DCF) or 2-chlorodeoxyadenosine (CdA).25-28 CRs do not result in a cure, because minimal residual disease is detectable by flow cytometry or by polymerase chain reaction.29 30 Moreover, based on large trials of these agents, 10% to 20% of patients are or will become refractory to chemotherapy and many will die from complications of pancytopenia due to progressive bone marrow involvement. The 4 HCL patients treated with LMB-2 had disease resistant to CdA as well as other established forms of treatment for HCL and had pancytopenia to a degree that necessitated at least palliative therapy. The response in 1 patient (no. 30) was most valuable clinically due to resolution of pancytopenia, lack of infection after treatment, lack of immunogenicity that allowed repeated dosing, and lack of dose-limiting toxicity.
MATERIALS AND METHODS
Patients with HCL were treated as part of a phase I trial of LMB-2 in patients with hematologic malignancies. To be eligible, patients had to have disease refractory to conventional chemotherapy, evidence of CD25 on the surface of the malignant cells, and adequate organ function and had to be resistant to standard chemotherapy. LMB-2 was produced by the Monoclonal Antibody and Recombinant Protein (MARP) facility of the National Cancer Institute (NCI; Frederick, MD) and the investigational new drug (IND) application is held by the Cancer Therapy and Evaluation Program (CTEP) of the NCI. LMB-2 was diluted into 50 mL of 0.2% albumin in 0.9% NaCl and administered as a 30-minute intravenous (IV) infusion administered every other day for 3 doses (QOD × 3). Patients without neutralizing antibodies or progressive disease could be retreated after restaging at monthly intervals. Assessment of disease was performed using fluorescent-activated cell sorting (FACS) analysis of blood and radiological studies. CR was defined as disappearance of evaluable disease lasting at least 4 weeks. A negative FACS analysis was not required. A partial response (PR) required reduction in tumor burden by at least 50% lasting at least 4 weeks, including a ≥50% decrease in malignant cell count and a ≥50% decrease in the sum of the products of perpendicular measurements of malignant solid masses. Responding patients could be retreated providing that they did not develop neutralizing antibodies to LMB-2 as assessed by cytotoxicity assay.31 Patients with greater than 75% neutralization of 1 μg/mL of LMB-2 in the serum were ineligible for further therapy. The maximum number of cycles allowed was arbitrarily set at 10 for patients in PR, and patients attaining CR could receive 2 additional cycles after demonstration of a CR. The 4 HCL patients received LMB-2 at 3 different dose levels (30, 40, and 63 μg/kg IV QOD × 3. The beginning dose in the overall phase I clinical trial was 2 μg/kg QOD × 3, which was chosen because it was 10% of the dose in monkeys, which resulted in no significant toxicity (transaminase elevations). Dose escalation in the phase I trial involved doses of 2, 6, 10, 20, 30, 40, 50, and 63 μg/kg IV QOD × 3 to determine the maximum tolerated dose (MTD). The MTD (40 μg/kg IV QOD × 3) was defined as the dose that is greater than 20% below that at which 2 of 2 to 6 patients incur dose-limiting toxicity (DLT). Patients were not premedicated to prevent fever. The Common Toxicity Criteria of the NCI were used to grade toxicity. DLT was defined as grade III-IV with some exceptions. As in other trials of chimeric toxins,32-36transaminase elevations of 5.1 to 20 times normal were not considered as DLT in the absence of evidence of impaired hepatic function. Hematologic abnormalities were non–dose-limiting in leukemic patients and only grade IV hematologic abnormalities constituted DLT in nonleukemic patients. Fever, which was well tolerated and did not result in suspending therapy, was also not considered dose-limiting.
Plasma levels of LMB-2 were determined by incubating dilutions of plasma with CD25+ SP2/Tac cells37 and comparing cytotoxicity as assessed by {3H}-leucine incorporation with that obtained by an LMB-2 standard.10 Assessment of neutralizing antibodies to LMB-2 was performed by incubating LMB-2 with patient sera and then testing its cytotoxicity against SP2/Tac cells.31 For cytotoxicity analysis of the HCL cells from patients, peripheral blood mononuclear cells were obtained from the blood by Ficoll centrifugation and incubated with different concentrations of LMB-2 at 37°C, and {3H}-leucine incorporation was determined. For binding assays to determine numbers of CD25 sites/cell from Scatchard plots, the Ficoll-purified mononuclear cells were incubated with 0.125, 0.25, 0.5, 1, 2, or 4 nmol/L {125I}-humanized anti-Tac for 1 to 2 hours in the presence or absence of a 100-fold excess of unlabeled LMB-2, as reported previously.5
RESULTS
To test the clinical activity of LMB-2, patients with refractory CD25+ hematologic malignancies were treated in a phase I trial, 4 of whom had HCL. Table 1summarizes the age, years since diagnosis, previous therapy, disease status before LMB-2, CD25 expression, dose of LMB-2, drug-related toxicity encountered, immunogenicity, and response in these patients. All 4 patients had typical HCL based on morphologic and immunocytochemical criteria. Patients had HCL for 12 to 17 years and had each been treated with CdA and interferon α (IFN). Patients no. 15, 32, and 35 had 5, 5, and 2 cycles each of CdA, with decreasing extent and durability of response to later cycles. Patient no. 30 had no response to 1 cycle of CdA. All patients received IFN either before or after CdA. For patient no. 15, IFN was effective before but not after he became resistant to CdA. Of the 3 patients previously treated with splenectomy (patients no. 15, 32, and 35), patients no. 15 and 35 had high circulating HCL counts.
Clinical response of patient no. 30.
Patient no. 30 is a 47-year-old man diagnosed with HCL after presenting with severe anemia (hemoglobin level [Hgb], <5 g/dL). IFN maintained the Hgb at 8 to 10 g/dL for 4 years, but treatment was stopped due to progressive granulocytopenia. He was then treated with CdA without response. He was subsequently treated with frequent blood transfusions for progressive disease-related anemia. Before study entry, his lowest Hgb before transfusion was 3.8 g/dL. He also was treated with imipenem and clarithromycin for pulmonary and cutaneousMycobacterium chelonae. Physical examination was significant for old hyperpigmented skin lesions and an enlarged spleen that was palpable 5 cm below the left costal margin. The white blood cell count (WBC) was 2,070/μL, the granulocyte count was 360/μL, the platelet count was 47,000/μL, and the Hgb was 10.0 g/dL with transfusion. Hairy cells, visible in the peripheral blood film, were quantitated at 480/μL by FACS analysis. Computed tomography (CT) showed an enlarged spleen and precarinal lymphadenopathy. Biapical scarring was observed consistent with chronic mycobacterial infection. He received LMB-2 at 63 μg/kg on days 1, 3, and 5. By day 3, HCL cells were no longer visible in the peripheral blood. By day 8, the spleen was no longer palpable and the marrow biopsy was no longer positive for HCL. By day 31, the spleen size by CT had normalized and thrombocytopenia resolved. Cycle 2 of LMB-2 was begun on day 32 of the first cycle. Granulocytopenia and anemia resolved after cycle 2. The patient achieved a CR with minimal residual disease detectable by FACS and no evidence of progression 13 months after beginning LMB-2.
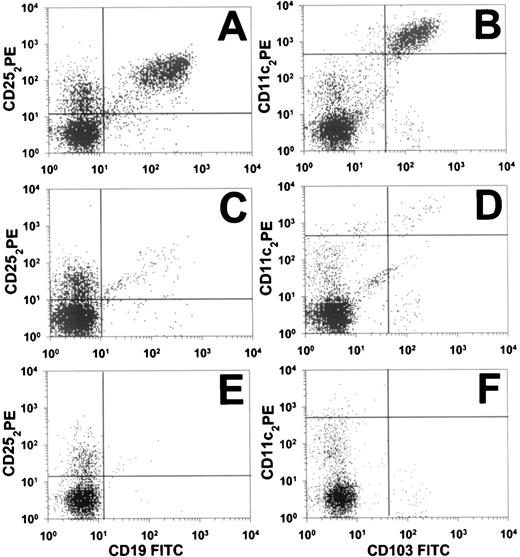
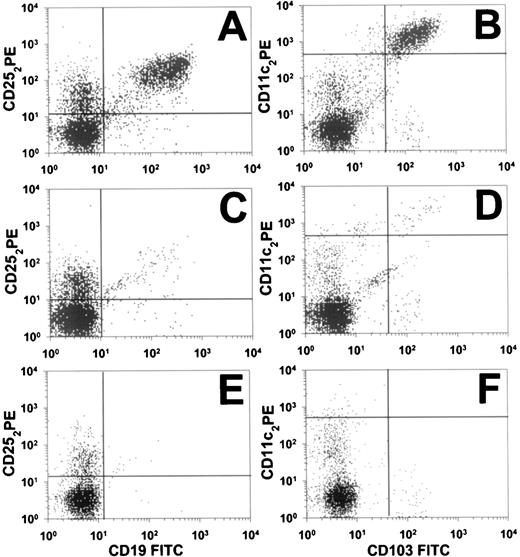
Before study entry, HCL cells from patient no. 30 by FACS were positive for λ light chains and CD103 and stained brightly for CD11c, CD19, CD20, CD22, and CD25, but were negative for CD5, CD10, and CD23. To quantitate the patient’s circulating malignant cells, peripheral blood was examined by 2-color flow cytometry. Figure 1 shows CD19+/CD25+ and CD11c+/CD103+ populations before LMB-2, on day 8, and on day 177 (cycle 2, day 146). The malignant population, originally comprising 35% of the lymphocytes, decreased greater than 90% in 2 days, as shown in Fig 2A, from 480/μL pretreatment to 43/μL on day 3. This effect was from 1 dose of LMB-2. On days 8, 22, and 31, the HCL counts were 3.0, 1.0, and 3.0/μL, respectively, indicating a maximal reduction of 99.8% in the peripheral blood. Flow cytometry on day 8 (shown in Fig 1C and D) indicated a selective depletion of the malignant cells of nearly 3 logs. The CD11c+/CD103+ malignant clone identified in Fig 1D still was brightly positive for CD25, as shown in Fig 1C. After the first 2 doses of cycle 2, the patient’s HCL count decreased further on day 5 to 1.8/μL. After the final dose of cycle 2, on day 8 the HCL count was less than 0.37/μL, which is too low to confirm the presence of malignant cells. The count was 1.9 and 1.1/μL on days 21 and 35 of the second cycle, respectively. Because of the lack of symptoms and limited drug supply, the patient was not retreated. The HCL cell count remained low, being 1/μL on day 117 of cycle 2. By cycle 2, day 146, the HCL cells by flow cytometry were too few to be diagnostic of HCL (Fig 1E and F). Results from λ light chain staining were unable to confirm the presence of circulating malignant cells. Malignant circulating cells could still not be confirmed 29 days later, although by flow cytometry a small population of cells comprising less than 0.01% of lymphocytes was suggestive of persistent HCL (Fig 1E and F). The quantity of these cells, if malignant, would be approximately 0.05 HCL cells per microliter, or nearly 5 logs less than the pretreatment level.
Flow cytometry (FACS) analysis of circulating cells in HCL patient no. 30. At 3 different time points, pretreatment (A and B), cycle 1 day 8 (C and D), and cycle 2 day 145 (E and F), data from 2-color analyses are shown to identify CD25+/CD19+ cells (A, C, and E) or CD11c+/CD103+ cells (B, D, and F). Perpendicular lines were drawn to differentiate malignant (upper-right quadrants) from nonmalignant cells. Horizontal lines in (B), (D), and (F) are drawn above nonmalignant cells that are dimly positive for CD11c.
Flow cytometry (FACS) analysis of circulating cells in HCL patient no. 30. At 3 different time points, pretreatment (A and B), cycle 1 day 8 (C and D), and cycle 2 day 145 (E and F), data from 2-color analyses are shown to identify CD25+/CD19+ cells (A, C, and E) or CD11c+/CD103+ cells (B, D, and F). Perpendicular lines were drawn to differentiate malignant (upper-right quadrants) from nonmalignant cells. Horizontal lines in (B), (D), and (F) are drawn above nonmalignant cells that are dimly positive for CD11c.
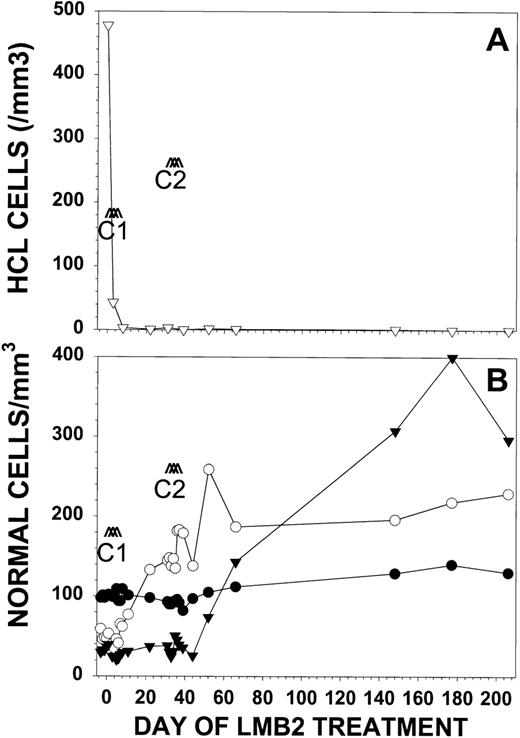
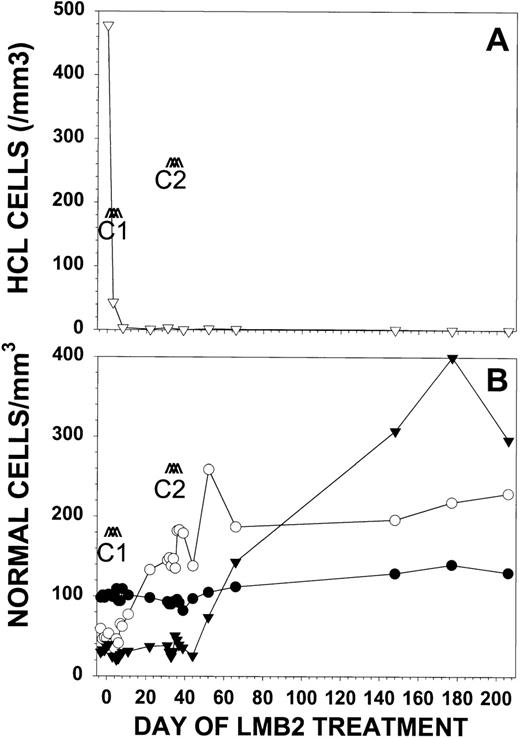
Response of patient no. 30 to LMB-2. Patient no. 30 received LMB-2 at 63 μg/kg IV QOD × 3 for 2 cycles. Cycle 1 began on day 1 and cycle 2 began on day 32. In (A), the number of HCL cells per microliter (▿) as determined by FACS analysis is shown. Normal blood cells are represented in (B) as absolute granulocyte count in cells per microliter × 10−1 (▾), platelet count per microliter × 10−3 (○), and hemoglobin in grams per deciliter × 10 (•).
Response of patient no. 30 to LMB-2. Patient no. 30 received LMB-2 at 63 μg/kg IV QOD × 3 for 2 cycles. Cycle 1 began on day 1 and cycle 2 began on day 32. In (A), the number of HCL cells per microliter (▿) as determined by FACS analysis is shown. Normal blood cells are represented in (B) as absolute granulocyte count in cells per microliter × 10−1 (▾), platelet count per microliter × 10−3 (○), and hemoglobin in grams per deciliter × 10 (•).
With respect to hematologic function before treatment, patient no. 30 was dependent on red blood cell transfusions and had preexisting thrombocytopenia (47,000/μL) and granulocytopenia (360/μL). With LMB-2, the patient became independent of transfusions. The platelet count increased during cycle 1 to 145,000/μL, but the patient remained granulocytopenic, with a granulocyte count of 400/μL (Fig2B). During cycle 2, the pancytopenia improved, with a platelet count of 187,000/μL, a granulocyte count of 1,530/μL, and Hgb of 11.2 g/dL. The patient’s pancytopenia fully resolved during the 6 months off treatment. As shown in Fig 2B, the patient’s hematologic recovery was not associated with even transient hematologic toxicity to LMB-2.
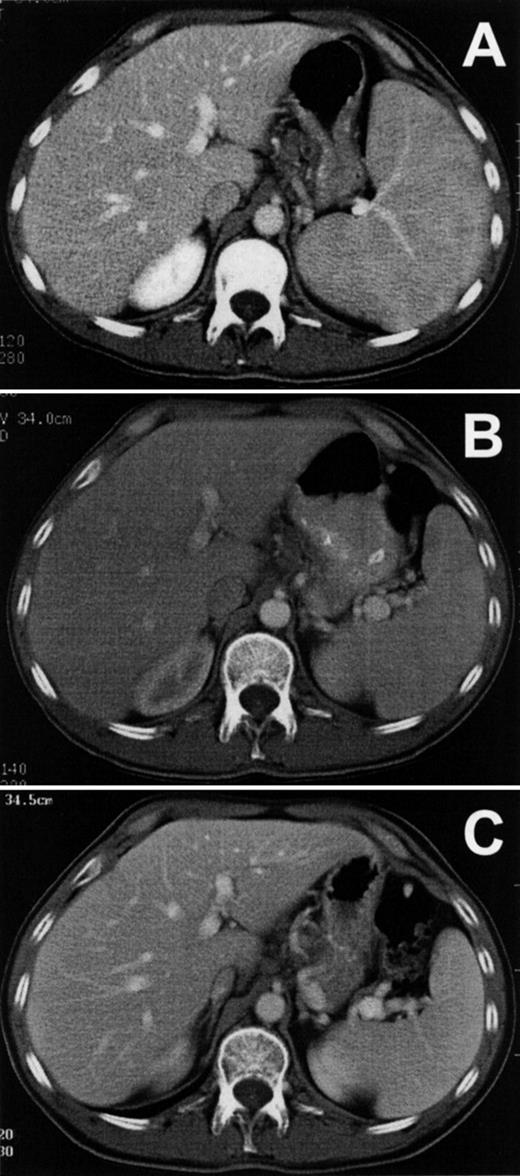
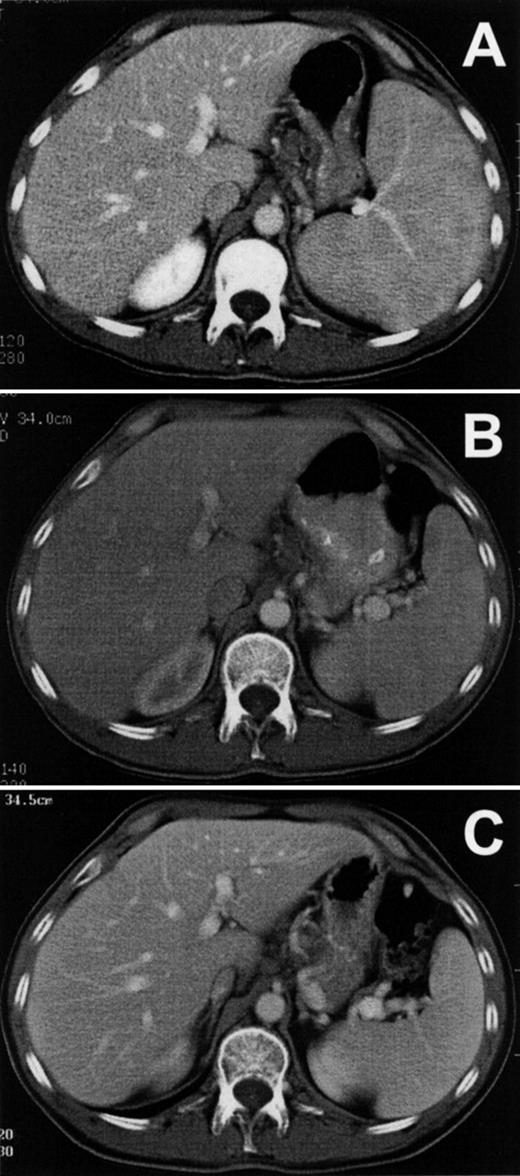
Figure 3A shows the patient’s splenomegaly in axial section before treatment with LMB-2. The cranial-caudal diameter was 16 cm, and after 1 cycle of LMB-2 decreased to a normal size of 11 cm by day 31 (Fig 3B). The spleen size was 9 cm after cycle 2 and showed no evidence of progression during 5 months off treatment, as shown in Fig 3C. The patient had abnormally enlarged precarinal adenopathy (up to 1.6 cm) before LMB-2, which also resolved after the first cycle. The pretreatment bone marrow biopsy was infiltrated with HCL cells, and liquid marrow could not be aspirated. The marrow biopsy by day 8 was no longer positive for HCL and was negative on day 206, showing hypocellularity with adequate normal hematopoietic precursors. However, the marrow aspirate on day 206, which could be obtained for the first time since the patient’s diagnosis, contained 0.15% malignant cells by FACS. Thus, malignant cells outside blood vessels responded to LMB-2, but minimal residual HCL could be detected by FACS in the bone marrow aspirate.
Reduction of spleen size in HCL. Axial sections are shown from computed tomography performed on patient no. 1 before (A) and after (B) the first cycle of LMB-2 and then on day 175 of the second cycle (C).
Reduction of spleen size in HCL. Axial sections are shown from computed tomography performed on patient no. 1 before (A) and after (B) the first cycle of LMB-2 and then on day 175 of the second cycle (C).
Pharmacokinetics of LMB-2.
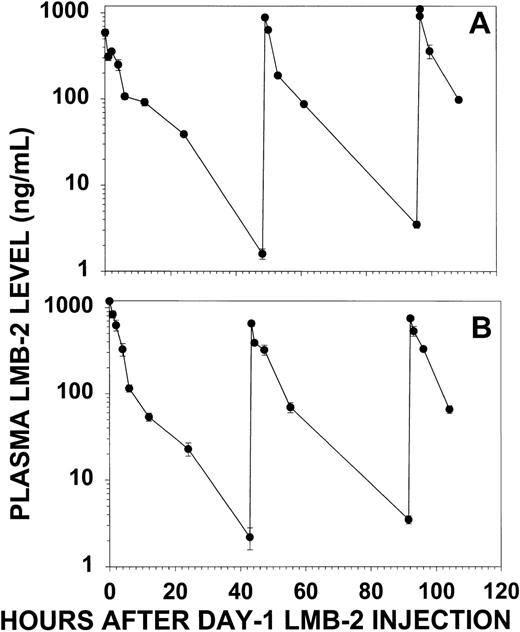
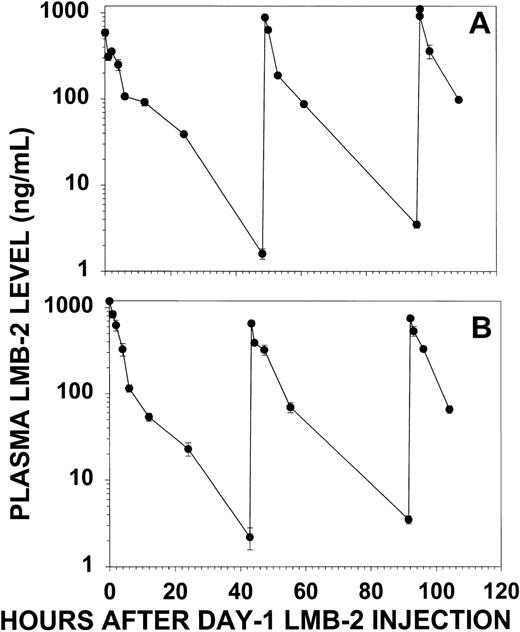
Plasma levels of LMB-2 were quantitated by cytotoxicity assay to determine the amount of cytotoxic activity that was delivered to patient no. 30 by treatment with LMB-2. Figure 4 displays the plasma levels of LMB-2 during the first and second cycles of treatment. The peak plasma levels were 600 to 1,100 ng/mL during cycle 1 and 750 to 1,000 ng/mL during cycle 2. By simple monoexponential decay, the t1/2for day 1 was 380 minutes for cycle 1. The decrease in plasma level for other doses followed a biexponential pattern, and after the first dose of cycle 2, the t1/2α was 77 minutes and the t1/2β was 420 minutes. Levels of LMB-2 remained detectable throughout each cycle, with minimum drug concentrations, obtained 2 days after the first dose, of 1.6 and 2.2 ng/mL for cycles 1 and 2, respectively. These minimum plasma levels are several-fold greater than the concentration of LMB-2 required for 50% inhibition of HCL cells from this patient ex vivo (IC50 = 0.5 ng/mL, see below). Thus, significantly cytotoxic plasma levels of LMB-2 were maintained during the week of treatment by the administration of the immunotoxin as a 30-minute IV infusion QOD × 3.
LMB-2 concentrations in the plasma of patient no. 30 before and after each IV infusion of LMB-2 (63 μg/kg). Plasma levels for cycle 1 (A) and cycle 2 (B) were quantitated by cytotoxicity assay of diluted plasma on SP2/Tac cells. Error bars indicate standard deviations of means of triplicate experiments.
LMB-2 concentrations in the plasma of patient no. 30 before and after each IV infusion of LMB-2 (63 μg/kg). Plasma levels for cycle 1 (A) and cycle 2 (B) were quantitated by cytotoxicity assay of diluted plasma on SP2/Tac cells. Error bars indicate standard deviations of means of triplicate experiments.
Response to LMB-2 in 3 additional HCL patients.
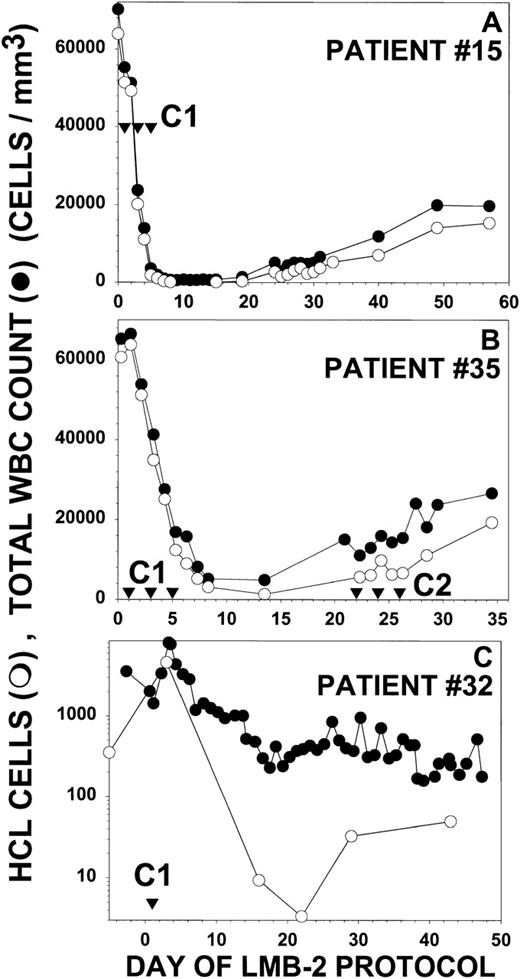
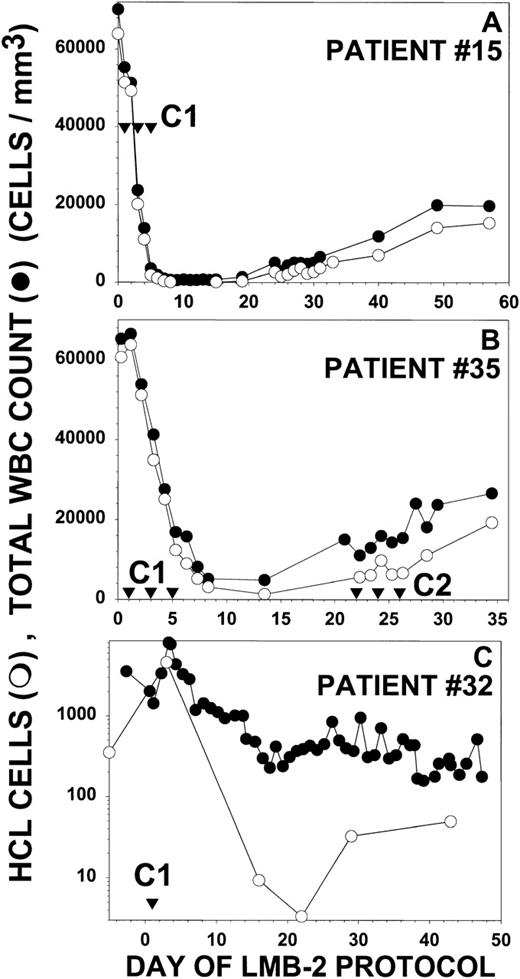
Figure 5 depicts partial responses in 3 additional patients with HCL treated with LMB-2. Each of these 3 patients (no. 15, 32, and 35) did not respond or relapsed after splenectomy, IFN, and CdA, and patient no. 32 also was unresponsive to DCF. Peripheral blood malignant cells, as quantitated by examination of the peripheral blood film and by flow cytometry, constituted evaluable and measurable disease in these patients. As shown in Fig 5A, patient no. 15 had a WBC of 70,200/μL, a granulocyte count of 280/μL, and an HCL count of 63,900/μL before treatment. The platelet count and Hgb were 42,000/μL and 7.2 g/dL, respectively, both transfusion-dependent. He received 30 μg/kg IV QOD × 3 beginning on day 1. The effect of the first dose was observed on day 3, when the WBC decreased to 23,000/μL and the HCL count decreased to 20,100/μL. By day 8, the WBC was 640/μL, with an HCL count of only 115/μL, a reduction of 99.8% from the pretreatment level. The patient was restaged on day 30, with a 95% reduction of HCL from pretreatment. This patient was not retreated because of infections, including an infected intravenous catheter. Patient no. 35 had a WBC count of 65,300/μL, a neutrophil count as low as 838/μL, a platelet count of 47,000/μL, a Hgb of 8.7 g/dL, and an HCL count of 60,700/μL before treatment (Fig 5B). After receiving 40 μg/kg IV QOD × 3, the effect of the first dose, assessed on day 3, was a reduction in the HCL count to 34,900/μL. The maximal response was observed on day 13 with a 98% reduction in HCL cells to 1,380/μL. After 1 cycle of LMB-2 the Hgb increased from 8.7 to 11.4 g/dL and the platelet count increased from 47,000 to 129,000/μL. This patient’s serum before treatment had evidence of a low level of pre-existing antibodies that neutralized 50% of the activity of 200 ng/mL of LMB-2. After the first cycle of LMB-2, the antibody titer increased, and by day 21, it completely neutralized 1,000 ng/mL of LMB-2. A second cycle was begun on day 22, but no biologically active LMB-2 was detected in the blood and no further response was observed with the neutralizing antibodies. Patient no. 32 had a pretreatment WBC of 1,430/μL, a neutrophil count of 470/μL, and an HCL count of 350/μL. The pretransfusion Hgb and platelet count were 7.9 g/dL and 15,000/μL. She received only 1 dose of LMB-2 at 63 μg/kg IV due to DLT, including diarrhea on day 2 and reversible cardiomyopathy from day 5 to 7. As shown in Fig 5C, the malignant count transiently increased from 350 to 4,600/μL on day 3, but by day 16, FACS identified a large number of dead malignant cells in addition to 9.4/μL HCL cells. The maximum response was 3.4/μL HCL cells on day 22, which was less than 1% of the pretreatment count. During the transient increase in WBC and HCL after the first dose, the neutrophil and platelet counts transiently increased from the pretreatment level of 470 and 15,000/μL to a maximum of 1,780 and 155,000/μL, respectively, on day 3.
Response of other HCL patients to LMB-2. For patients no. 15 (A), 35 (B), and 32 (C), the concentrations of circulating WBCs (•) and HCL cells determined by FACS and morphology (○) are shown. Treatment days are shown (▿) for the indicated cycles. Patients no. 15 and 35 in (A) and (B) received LMB-2 at 30 and 40 μg/kg IV QOD × 3, respectively. Patient no. 32 received 1 dose of LMB-2 at 63 μg/kg on day 1. In (C), the Y-axis is depicted showing the log of the WBC and HCL counts to show the wide fluctuation in the counts after the first dose.
Response of other HCL patients to LMB-2. For patients no. 15 (A), 35 (B), and 32 (C), the concentrations of circulating WBCs (•) and HCL cells determined by FACS and morphology (○) are shown. Treatment days are shown (▿) for the indicated cycles. Patients no. 15 and 35 in (A) and (B) received LMB-2 at 30 and 40 μg/kg IV QOD × 3, respectively. Patient no. 32 received 1 dose of LMB-2 at 63 μg/kg on day 1. In (C), the Y-axis is depicted showing the log of the WBC and HCL counts to show the wide fluctuation in the counts after the first dose.
Toxicity of LMB-2 in the HCL patients.
Toxicity in all patients was transient, lasting up to 2 to 3 days after onset. Patient no. 15, who received 30 μg/kg × 3, had grade I transaminase elevations and grade II fever, with peak aspartate transaminase (AST) and alanine transaminase (ALT) values of 44 and 62, respectively, and a maximum temperature (Tmax) of 38.6°C. In patient no. 30, the transaminases were elevated before LMB-2 administration up to an AST of 45 U/L and an ALT of 54 U/L. With 1 cycle of LMB-2 at 63 μg/kg QOD × 3, mild increases in these abnormalities were noted, with a peak AST of 69 U/L and ALT of 66 U/L. The Tmax was only 37.7°C. With the higher plasma levels of LMB-2 for cycle 2 (Fig 4B), toxicity to cycle 2 included non–dose-limiting grade III AST and ALT elevations, up to 241 and 200, respectively, a Tmax of 37.8°C, and transient grade II nausea and vomiting and rash. In patient no. 35, treated at 40 μg/kg QOD × 3, toxicity was limited to transient grade I nausea, rash, and weight gain. The dose in patient no. 32 was 63 μg/kg, but treatment was stopped after 1 dose due to dose-limiting diarrhea (grade III) and grade II nausea and vomiting. Grade I fever was observed with a Tmax of 38.1°C. This patient also had a transient cardiomyopathy without evidence of cardiac necrosis, which became evident on day 5 and resolved in 1 to 2 days. It was not determined whether toxicity in this patient was mediated directly by LMB-2 or by cytokines38 39 produced by normal or malignant cells. However, cardiomyopathy was not observed in preclinical toxicology studies. Hematologic toxicity could not be easily evaluated in patients no. 15 and 32, because these pancytopenic patients were transfusion-dependent before treatment and had no significant change in transfusion requirements during their response. Patients no. 30 and 35 had no significant hematologic toxicity.
CD25 expression by HCL cells and sensitivity to LMB-2 ex vivo.
All 4 HCL patients had malignant cells that were positive for CD25 by flow cytometry. To quantitate the level of CD25 expression on the malignant cells, binding assays were performed using {125I}-anti-Tac(IgG) in the presence or absence of an excess of unlabeled LMB-2. Patients no. 15, 30, and 35 had sufficient numbers of malignant cells in the peripheral blood to perform such studies on fresh malignant cells isolated by Ficoll centrifugation. The number of CD25 sites per cell, as determined from Scatchard plots, is depicted in Table 1. Patient no. 30, who responded best to LMB-2, had 6,200 ± 32 sites/cell. HCL cells from patient no. 15 had 7,200 ± 180 sites/cell. This patient had the same maximal percentage response to 1 cycle as patient no. 30 (99.8% reduction) but far greater numbers of cells were killed. Patient no. 35, whose 98% response was the lowest of the HCL patients treated, had significantly lower CD25 expression at 1,900 ± 180 and 1,400 ± 35 sites/cell measured on day −5 and day 0, respectively. To determine whether the malignant cells in the responding patients were sensitive to LMB-2, they were incubated with LMB-2, as reported previously for chronic lymphocytic leukemia (CLL) cells.5 In patients no. 15, 30, and 35, the concentrations of LMB-2 required for 50% inhibition of protein synthesis (IC50s) were 1.1 ± 0.09, 0.05 ± 0.15, and 3 ± 0.08 ng/mL, respectively. Cytotoxicity required both binding to CD25 and ADP-ribosylation of elongation factor 2 after internalization, because LMB-2 mutants deficient in either binding or ADP-ribosylation activity were inactive on these cells (data not shown). Thus, CD25 expression in these few patients correlated with sensitivity of the malignant cells to LMB-2, which also correlated with clinical response. More patients will need to be treated to determine the significance of this correlation.
DISCUSSION
Four patients with HCL have been treated in a phase I trial of the anti-CD25 recombinant single-chain immunotoxin LMB-2 in patients with CD25+ hematologic malignancies. We report here that major responses occurred in all 4 patients; all patients were resistant to standard and salvage chemotherapy, including CdA. The reductions of malignant cells in the peripheral blood varied from 98% to at least 5 logs. The most durable response met the criteria for CR used in clinical trials of purine analogs in HCL,27 28 although minimal residual disease is known to be present. These are, to our knowledge, the first major responses in cancer patients to a cytotoxic agent that is targeted by an Fv fragment of an antibody.
Although a full report of the phase I trial of LMB-2 in patients with other hematologic malignancies will be made separately, we found that the toxicity, pharmacokinetics, and immunogenicity of LMB-2 in the 4 HCL patients are representative of the other 31 patients so far treated. LMB-2 has been well tolerated without DLT in all 9 patients treated at the 40 μg/kg × 3 dose level with toxicities, including transaminase elevations (8), fever (7), nausea (5), alkaline phosphatase elevations (3), vomiting (2), proteinurea and increased creatinine (2), thrombocytopenia (2), and weight gain (1). Only 6 of 35 patients developed levels of neutralizing antibodies after the first cycle that were high enough to disqualify them from additional cycles of treatment. It is interesting that, whereas immunogenicity is low in HCL, it may be lower in CLL, because no evidence of immunogenicity was observed in 8 patients after a total of 16 cycles. Eliminating immunogenicity in all HCL patients might require using immunosuppressive agents. A clinical trial is currently under way to determine if the B-cell–depleting agent Rituximab40 can prevent patients with solid tumors from becoming immunized to the anti-Ley immunotoxin LMB-1.31 In addition to HCL, partial responses have been observed in adult T-cell leukemia, cutaneous T-cell lymphoma (CTCL), Hodgkin’s disease, and CLL.
HCL is a very treatable albeit incurable malignancy, with long-term complete or partial responses observed with DCF or CdA in most patients. However, up to 10% to 20% of patients become refractory and are in need of more effective salvage therapy.27,28,30 The management of such patients is often problematic, with both disease and chemotherapy-related bone marrow damage limiting therapeutic options. LMB-2 may be particularly useful for chemotherapy-refractory patients with impaired bone marrow function, due to lack of hematopoietic toxicity observed (Fig 2B). LMB-2 could also be combined with conventional chemotherapeutic agents to improve responses in refractory patients. It has recently been reported that a recombinant toxin can be combined with either doxorubicin or ARA-C to synergistically target multiply drug-resistant malignant cells in culture.41 The activity of LMB-2 combined with chemotherapeutic drugs is currently being evaluated ex vivo to explore whether LMB-2 might improve the clinical efficacy of chemotherapeutics against HCL.
Compared with other B-cell leukemias such as CLL, HCL is a particularly sensitive disease to LMB-2 at least in part due to the high CD25 expression on the malignant cells in most patients. However, even cells from patient no. 35, which had relatively low CD25 expression (Table1), were still quite sensitive to LMB-2. HCL cells could be particularly efficient at trafficking the active fragment of the toxin intracellularly to the cytosol or other steps that are part of the intoxication process. Other mechanisms of cell killing cannot be excluded, particularly in the case of patient no. 30, who had a prolonged and improving response. It is possible that a several-log response produced by the cytotoxic effect of LMB-2 corrected the patient’s immune deficiencies, which in turn led to a further multilog reduction of HCL cells several months later. It is notable that the clinical response in HCL patients treated with chemotherapy can occur after many months and improve with time. Because cytoreduction from 1 to 2 cycles of LMB-2 usually did not select for CD25−tumor cells, it should be possible to use additional cycles of LMB-2 to determine whether minimal residual disease can be further reduced or eliminated.
Conventional immunotoxins consisting of monoclonal antibodies chemically connected to toxins have been used to target a variety of tumor types, with some producing at least several clinical responses in patients with hematologic malignancies35,42-49 or with solid tumors.31,50 Recombinant growth factor fusion toxins have also induced responses,51-53 and the IL2-toxin DAB389IL2 is a recently approved salvage therapy for CTCL. Recombinant immunotoxins containing approximately 25-kD Fv fused to a 38- to 40-kD truncated bacterial toxin are close in size to growth factor fusion toxins but can bind to target molecules other than growth factor receptors.10,54 Several of these new agents are now in clinical trials to determine their toxicity and clinical activity. One of these, RFB4(dsFv)-PE38 (BL22),55has just begun to be tested in patients with CD22+malignancies and might be particularly appropriate for the poor-prognosis variant of HCL,56 57 which is usually CD25− but strongly CD22+. In conclusion, the responses seen with LMB-2 serve as proof of principal of the potential clinical use of recombinant immunotoxins and validate efforts to target new antigens with these potent agents.
ACKNOWLEDGMENT
The authors thank Steven Giardina, Toby Hecht, and Daniel Coffman at the MARP (Frederick, MD); Catherine Laurencot, Jay Greenblatt, and Thomas Davis at CTEP; and David Waters and Vickie Marshall at the SAIC (Frederick, MD). We also thank clinical personnel at the NIH clinical center, including research nurses Deborah Pearson, Valerie Dyer, and Miranda Raggio and pharmacist Barry Goldspiel.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ira Pastan, MD, National Cancer Institute, National Institutes of Health, 37/4E16, 37 Convent Dr, MSC 4255, Bethesda, MD 20892.