Gene therapy for inherited disorders is more likely to succeed if gene-corrected cells have a proliferative or survival advantage compared with mutant cells. We used a competitive reconstitution model to evaluate the strength of the selective advantage that Btk normal cells have in Btk-deficient xid mice. Whereas 2,500 normal bone marrow cells when mixed with 497,500 xid cells restored serum IgM and IgG3 levels to near normal concentrations in 3 of 5 lethally irradiated mice, 25,000 normal cells mixed with 475,000 xidcells reliably restored serum IgM and IgG3 concentrations and the thymus-independent antibody response in all transplanted mice. Reconstitution was not dependent on lethal irradiation, because sublethally irradiated mice all had elevated serum IgM and IgG3 by 30 weeks postreconstitution when receiving 25,000 normal cells. Furthermore, the xid defect was corrected with as few as 10% of the splenic B cells expressing a normal Btk. When normal donor cells were sorted into B220+/CD19+ committed B cells and B220−/CD19− cell populations, only the B220−/CD19− cells provided long-term B-cell reconstitution in sublethally irradiated mice. These findings suggest that even inefficient gene therapy may provide clinical benefit for patients with XLA.
MUTATIONS IN THE cytoplasmic tyrosine kinase Btk are responsible for the xid phenotype in mice and X-linked agammaglobulinemia (XLA) in humans.1-7 Although Btk is expressed in all blood cells except T cells and plasma cells,1,2,8-10 adverse effects of mutations in this enzyme are restricted to the B-cell lineage.11-16 Cross-linking of a variety of B-cell surface receptors, including (and perhaps most importantly) the antigen receptor complex,17-25 can activate and phosphorylate Btk, but the exact mechanisms by which mutations in Btk result in the failure of normal B-cell development are not well understood.
Defects in Btk have more severe consequences in the human compared with the mouse. Patients with XLA have profound hypogammaglobulinemia affecting all isotypes, an absence of antigen-specific antibodies, and less than 1% of the normal number of B cells.12,26-29 Bone marrow studies in these patients show normal numbers of pro-B cells but markedly reduced numbers of pre-B cells.15 By contrast, mice with defects in Btk, both knock out mice that do not express Btk and CBA/N (xid) mice that have a spontaneously occurring single amino acid substitution in the pleckstrin homology domain of Btk, have decreased concentrations of serum IgM and IgG3 but normal concentrations of IgG1, IgG2a, and IgG2b.5-7,16 These mice fail to make antibodies to some T-cell–independent antigens but they have normal or near normal titers of antibody to T-cell–dependent antigens. The number of splenic B cells in Btk deficient mice is decreased to 30% to 50% of normal, and there is an absence of a mature B-cell population. However, the numbers of B-cell precursors and immature B cells are normal. It is not clear why mutations in Btk cause a less severe block in B-cell differentiation in the mouse compared with the human, but it is likely that genetic factors play a role. Although mice that are null for CD40 or the nude gene have normal numbers of B cells, mice that are doubly mutant for Btk and CD40 or nu/nu have less than 5% of the normal number of peripheral B cells.30-32
Mice with mutations in Btk can act as useful models to evaluate new strategies for treatment of patients with XLA. As a prelude to studies directed toward gene therapy, several Btk transgenes have been bred into Btk deficient mice.33-35 In one model, murine Btk cDNA transcription was driven by an Ig enhancer and promoter.33,36Xid or Btk−/− mice with 1 copy of this transgene had approximately 25% of the normal amount of Btk as analyzed by Western blot, whereas those with 2 copies had 50% of the normal amount of Btk.36 Mice with either 1 or 2 copies of the transgene had normal numbers of mature splenic B cells, but they had antibody responses to TNP-Ficoll and serum concentrations of IgM and IgG3 that were significantly decreased compared with wild-type controls but improved compared with xid controls.
Drabek et al34 have shown correction of Btk-deficient mice by a human Btk cDNA transgene with regulatory elements from the murine major histocompatibility complex (MHC) class II region. Like the transgene driven by the Ig enhancer and promoter described above, this transgene was not expressed before the pre-B–cell stage of differentiation. In addition, it was expressed in thymic epithelium, activated T cells, monocytes, and at low levels in other tissues. Western blot analysis of splenic lysates from Btk-deficient mice carrying the human Btk transgene demonstrated approximately the same amount of Btk protein as those from wild-type mice. These studies indicate that tight regulation of Btk expression is not required for correction of serum concentrations of IgM and IgG3, the capacity to make antibody to T-cell–independent antigens, or the development of normal numbers of mature B cells. Further, ectopic expression of Btk does not appear to be deleterious. These studies provide support for the possibility that gene therapy for XLA may be a realistic goal.
Current techniques for introducing therapeutic genes into hematopoietic stem cells tend to be inefficient.37,38 However, studies in lethally irradiated xid mice reconstituted with equal mixtures of xid and wild-type marrow indicate that B-cell precursors with normal Btk have a selective advantage in proliferation or survival over Btk-deficient precursors.39 Four months after transplantation, B cells but not other cell lineages are derived exclusively from the normal donor. Studies in women or mice that are heterozygous for the defect in Btk yield similar results. All of the B cells in carrier females are derived from precursors that have the normal X chromosome, the one not bearing the Btk defect, as the active X.13,14 40-42 To examine the strength of the selective advantage that B-cell precursors with normal expression of Btk might have over Btk-deficient cells, we treated lethally or sublethally irradiated xid mice with xid bone marrow supplemented with limiting numbers of bone marrow cells from a wild-type donor and monitored production of IgM, IgG3, and antigen-specific antibody.
MATERIALS AND METHODS
Mice.
CBA/N (xid) and CBA/J (wild-type) mice, which were initially obtained from The Jackson Laboratory (Bar Harbor, ME), were bred and maintained at the St. Jude Children’s Research Hospital Animal Research Center (Memphis, TN). The CBA/N and CBA/J strains diverged from the CBA/Ca background approximately 50 years ago (∼120 generations). The only known difference between the strains is thexid mutation in the CBA/N strain. Only male mice were used in the reconstitution experiments, and recipients and donors were age-matched and used between the ages of 5 to 6 weeks. A137Cs source was used to irradiate mice at a dose rate of 125 rad/min 24 hours before bone marrow transplant.
Preparation of cell suspensions for adoptive transfers and semiquantitative polymerase chain reaction (PCR).
Mice were killed by cervical dislocation and bone marrow cells were flushed from tibias and femurs with phosphate-buffered saline (PBS) supplemented with 5% heat-inactivated fetal calf serum (FCS; JRH Biosciences, Lenexa, KS). After passing the suspension through a 70-μm filter, cells were counted, washed, and resuspended in PBS for injection into xid recipient mice via the lateral tail vein.
For the cell sorting experiments, spleens were harvested into RPMI media supplemented with 5% heat-inactivated FCS and crushed to a single-cell suspension between the frosted ends of 2 microscope slides. Red blood cells were lysed in an equal volume of Gey’s solution (0.83% ammonium chloride, 0.1% potassium bicarbonate) for 10 minutes at room temperature, and the remaining lymphocytes were washed and resuspended in staining buffer (PBS, 1% bovine serum albumin [BSA], 0.1% sodium azide). Bone marrow cells were harvested as described above and resuspended in PBS only. Both samples were blocked for 10 minutes in 10% normal mouse serum, at which time spleen cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti-Thy 1.2 (Pharmingen, La Jolla, CA). Stained cells were washed twice in staining buffer and sorted into B-cell and T-cell populations using a Becton Dickinson FACStar-plus cell sorter (Palo Alto, CA). For all sorted spleen samples, the purity of the sorted B- and T-cell populations was greater than 95%, with less than 2% contaminating cells. Bone marrow cells were stained with phycoerythrin-conjugated anti-B220 and fluorescein isothiocyanate-conjugated anti-CD19 (Pharmingen), washed twice in PBS, and sorted as above. The purity of the B220+/CD19+ population was 95.5% pure, with 3.4% contaminating B220−/CD19−cells. Similarly, the purity of the B220−/CD19− population was 95.8%, with 0.5% contaminating B220+/CD19+ cells.
Serum Ig detection and immunizations.
To determine serum Ig concentrations, mice were bled via the retro-orbital plexus and samples were spun at 4°C for 15 minutes in a micro-centrifuge. Plasma was collected and stored in aliquots at −80°C, and total serum IgM and IgG3 antibody concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA). Ninety-six–well maxisorp plates (NUNC, Roskilde, Denmark) were coated overnight at 4°C with 50 μL of 10 μg/mL polyclonal anti-IgM or anti-IgG3 (Southern Biotechnology Associates, Birmingham, AL). Plates were washed with PBS supplemented with 0.05% Tween 20 and blocked with a 3% BSA solution in PBS for 2 hours at room temperature. After 3 washes, diluted plasma samples were added to the coated wells for overnight incubation at 4°C. Plasma samples were serially diluted in wash buffer supplemented with 0.5% BSA. After overnight incubation, plates were washed and polyclonal alkaline phosphatase-conjugated anti-IgM or anti-IgG3 (Southern Biotechnology Associates) was added to the wells at 1 μg/mL and left at room temperature for 2 hours. The phosphatase substrate, p-Nitrophenyl phosphate (Sigma, St Louis, MO), was dissolved in 100 mmol/L Tris, 100 mmol/L sodium chloride, 5 mmol/L magnesium chloride, and 10% diethanolamine, pH 9.5, and added to the plates for 30 minutes, at which time the OD405 was measured on a Bio-Rad microplate reader (Richmond, CA). Antibody concentrations were calculated by using purified mouse IgM and IgG3 antibodies (Southern Biotechnologies) as standards. The levels of detection for serum IgM and IgG3 were 1 μg/mL and 3 μg/mL, respectively.
To measure the T-cell–independent immune response, mice were immunized with 50 μg NP-Ficoll (Biosearch Technologies, Novato, CA) in PBS injected intraperitoneally. Plasma samples were obtained 10 days postimmunization and NP-specific antibody titers were determined by ELISA. Plates were coated with 10 μg/mL NP-BSA (Biosearch Technologies, Novato, CA), and diluted serum samples were assayed as described above. Antibody titer was calculated as the fold increase compared with the average preimmunization titers of the normal CBA/J control mice.
Semiquantitative PCR.
DNA was extracted from freshly isolated bone marrow cells and sorted splenic B cells and T cells using the QIAamp tissue kit (Qiagen, Valencia, CA). Genomic PCR primers GACTGTGGAAGAAGGAGC and GGCATAGAGTGAGTTCTTAC were used to amplify Btk exon 2 from 200 ng of genomic DNA in the presence of 32P using the following cycling conditions: 95°C for 45 seconds, 60°C for 1 minute, and 72°C for 1 minute, repeated 15 times. The xid mutation, a C to T transition in exon 2 of Btk, results in the loss of a HhaI restriction site, making it possible to distinguish between the wild-type and xid alleles by digesting the 558-bp PCR product with Hha I (New England Biolabs, Beverly, MA). For the wild-type allele, 3 fragments of 222, 45, and 291 bp are seen, whereas the xid allele gives 2 fragments of 222 and 336 bp. Fragments were separated on a 6% polyacrylamide gel; the gel was dried and exposed to x-ray film or a storage phosphor screen (Molecular Dynamics, Eugene, OR) for quantitation of signal strength using the Molecular Dynamics phosphorimager and imagequant software. By comparing the intensity of the wild-type–specific 291-bp fragment with that of the common 222-bp fragment, the degree of chimerism was calculated in the reconstituted mice. The sensitivity of the assay was determined by preparing known mixtures of xid and wild-type genomic DNA, with the percentage of wild-type DNA in the mixtures being 0.1%, 0.5%, 1%, 5%, 10%, and 50%. The lowest dilution of normal DNA that still gave a signal in the reaction was 0.5%.
RESULTS
Restoration of B-cell function in xid mice can be achieved with 25,00 or fewer normal cells.
The strength of the selective advantage of B-cell precursors with normal Btk over those with mutant Btk was examined in a murine model in which lethally irradiated (900 rad) CBA/N xid mice with mutant Btk were reconstituted with xid bone marrow supplemented with limiting numbers of cells from an MHC-matched, closely related strain of mice with normal Btk, CBA/J. The importance of the ratio of normal to mutant precursors versus the absolute number of normal precursors was evaluated by infusing xid mice with 5.0 × 106 or 0.5 × 106 cells of which either 5% or 0.5% were from the CBA/J wild-type donor. Five mice were included in each group, and mice were analyzed individually. Serum concentrations of IgM and IgG3, the isotypes most severely affected by the Btk mutation, were measured at 6-week intervals.
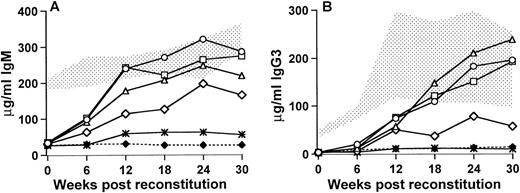
Xid mice that received 250,000 (5% of 5.0 × 106) bone marrow cells from the wild-type control CBA/J mice demonstrated an increase in serum IgM by 6 weeks posttransplant and by 12 weeks after the transplant all of the mice had serum IgM concentrations that were within the normal range seen in the untreated CBA/J control mice (Fig 1A). These mice maintained their elevated IgM levels to 30 weeks posttransplant, at which stage the average concentration was 286.8 ± 35.5 μg/mL compared with 327.1 ± 40.1 μg/mL for the wild-type controls and 32.5 ± 7.8 μg/mL for the xid control mice. Mice that received 25,000 wild-type cells, either as 0.5% of 5.0 × 106 cells or as 5% of 0.5 × 106 cells, were very similar to the mice receiving 250,000 wild-type cells, although there was a slight delay in IgM production in mice that received 0.5% of the total bone marrow cells as wild-type cells. By 30 weeks posttransplant, the 0.5% group had an average serum IgM concentration of 219.1 ± 58.2 μg/mL and the 5% group averaged 274.3 ± 32.1 μg/mL. In contrast, mice that received 2,500 cells (0.5% of 0.5 × 106) showed a variable response. Three of the mice demonstrated an increase in serum IgM such that by 30 weeks after transplant the concentration of IgM was 30% to 90% of normal (164.7 ± 86.0 μg/mL). The remaining 2 mice did not differ from the untreated xid mice 54.6 ± 29.7. The concentrations of serum IgG3 followed a similar trend. At 6 weeks posttransplant, the concentrations of IgG3 were similar to the untreated xidcontrol; however, in the mice that received at least 25,000 wild-type cells, an increase in IgG3 was seen by 12 weeks after the transplant and by 18 weeks after transplant, and the concentrations of IgG3 were within the normal range seen in control mice with normal Btk. By 30 weeks posttransplant, mice that received 250,000 wild-type cells had 201.8 ± 72.9 μg/mL serum IgG3 compared with 187 ± 92.6 μg/mL for the wild-type and 9.8 ± 5.1 μg/mL for the xidcontrols. Similarly mice that received 25,000 normal cells as 0.5% of 5 × 106 or 5% of 0.5 × 106 had 239.0 ± 110.6 μg/mL and 192.8 ± 66.5 μg/mL, respectively. In the group of mice that received 2,500 wild-type bone marrow cells, the 3 mice that demonstrated an increase in IgM also showed an increase in IgG3 (67.0 ± 37.2 μg/mL). The remaining 2 mice in this group were similar to the Btk mutant xid mice (10.5 ± 5.1 μg/mL). These findings indicate that relatively small numbers of wild-type cells can have a significant effect on serum Ig concentrations.
Serum IgM (A) and IgG3 (B) concentrations in reconstituted and control mice measured at 6-week intervals postreconstitution. Time (in weeks) postreconstitution is on the x-axis and Ig concentration in micrograms per milliliter is on the y-axis. The stippled area represents the range seen in 9 CBA/J normal control mice and the (⧫) denotes the CBA/N xid control levels (5 mice). For the reconstituted mice that were all lethally irradiated (900 rad, 5 mice per group), (○) and (▵) denote mice that received 5.0 × 106 total cells, of which 5% or 0.5% were normal, respectively; (□) corresponds to mice that received 0.5 × 106 total cells, of which 5% were normal; (◊) indicates the 3 responding mice that received 0.5 × 106 total cells, of which 0.5% were normal, and (*) shows the 2 mice in this group that did not have significantly increased serum IgG3.
Serum IgM (A) and IgG3 (B) concentrations in reconstituted and control mice measured at 6-week intervals postreconstitution. Time (in weeks) postreconstitution is on the x-axis and Ig concentration in micrograms per milliliter is on the y-axis. The stippled area represents the range seen in 9 CBA/J normal control mice and the (⧫) denotes the CBA/N xid control levels (5 mice). For the reconstituted mice that were all lethally irradiated (900 rad, 5 mice per group), (○) and (▵) denote mice that received 5.0 × 106 total cells, of which 5% or 0.5% were normal, respectively; (□) corresponds to mice that received 0.5 × 106 total cells, of which 5% were normal; (◊) indicates the 3 responding mice that received 0.5 × 106 total cells, of which 0.5% were normal, and (*) shows the 2 mice in this group that did not have significantly increased serum IgG3.
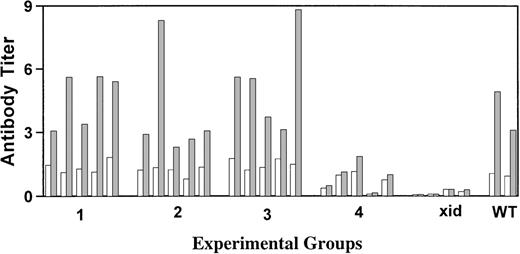
Xid mice are unresponsive to the T-cell–independent antigen NP-Ficoll; therefore, this antigen was chosen to evaluate the correlation of serum Ig concentrations with the capacity to make antibody after antigen challenge. Mice were injected intraperitoneally with NP-Ficoll 18 weeks posttransplant, and 10 days later, NP-specific antibodies were measured by ELISA. As shown in Fig 2, all transplanted mice that achieved IgM and IgG3 concentrations within the normal range made IgM anti-NP titers equivalent to those seen in the CBA/J control mice. Of the mice that received 2,500 wild-type cells, the 3 with the highest concentrations of serum IgM and IgG3 had anti-NP titers that were above those seen in the xid control but below those seen in the wild-type mice.
Measurement of the serum IgM response against the T-cell–independent antigen NP-Ficoll. Results are for individual mice with (□) the preimmunization titer and (▩) the postimmunization titer. All titers were normalized to the average preimmunization titer obtained for the CBA/J control mice. Groups 1 and 2 received 5.0 × 106 total cells, of which 5% or 0.5% were normal, respectively, whereas groups 3 and 4 received 0.5 × 106total cells, of which 5% or 0.5% were normal, respectively. Responses seen in xid (xid) and wild-type (WT) control mice are indicated.
Measurement of the serum IgM response against the T-cell–independent antigen NP-Ficoll. Results are for individual mice with (□) the preimmunization titer and (▩) the postimmunization titer. All titers were normalized to the average preimmunization titer obtained for the CBA/J control mice. Groups 1 and 2 received 5.0 × 106 total cells, of which 5% or 0.5% were normal, respectively, whereas groups 3 and 4 received 0.5 × 106total cells, of which 5% or 0.5% were normal, respectively. Responses seen in xid (xid) and wild-type (WT) control mice are indicated.
Lethal irradiation is not required for successful engraftment of donor cells.
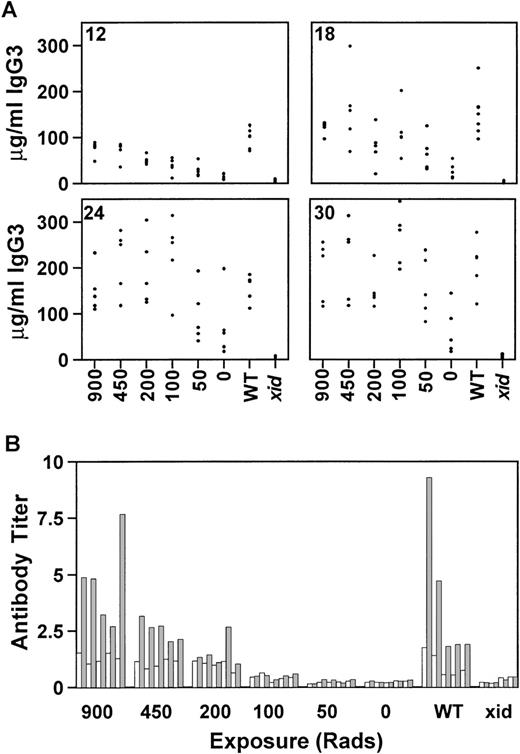
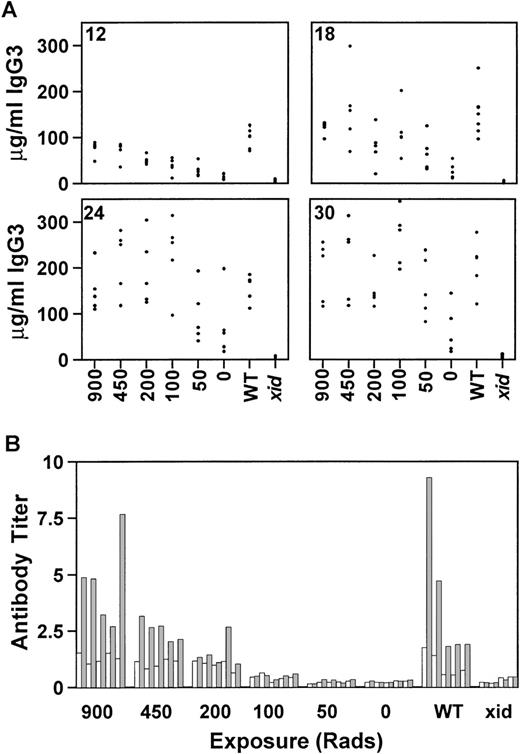
There are significant medical risks associated with the intense chemotherapy or lethal irradiation required for most types of bone marrow transplantation. Gene therapy is a more realistic goal if gene correction can occur in the absence of life-threatening ablative therapy. To evaluate the requirements for lethal irradiation, recipientxid mice were exposed to 900, 450, 200, 100, 50, or 0 rad and reconstitution by competitive B-cell repopulation was monitored as before. All mice received a total of 0.5 × 106 cells, of which 25,000 (5%) were from wild-type CBA/J donors. By 12 weeks postreconstitution, mice that were exposed to 450 or 200 rad had serum IgG3 concentrations equivalent to the control group that had received 900 rad (Fig 3A). However, mice exposed to 100, 50, or 0 rad were not significantly different from xidcontrols. By 18 weeks postreconstitution, some of the mice in the groups that had received either 100 or 50 rad had achieved normal concentrations of IgG3. This trend continued until 30 weeks posttransplant, when 3 mice in the group that had received 50 rad and 1 mouse in the group that had received 0 rad demonstrated IgG3 concentrations within the normal range. A subset of mice, ie, the mice that were exposed to 450, 100, or 50 rad, were maintained for 42 weeks after transplant to permit evaluation of the persistence of the reconstitution. At that time, all of the mice, including the mice that received 50 rad, had IgG3 concentrations within the normal range. As in the previous experiments, the concentrations of IgM correlated with the concentrations of IgG3 and the increase in IgM generally preceded the increase in IgG3 (data not shown).
The effect of reduced radiation on serum IgG3 reconstitution at 12, 18, 24, and 30 weeks posttransplant (A) and on the response to the T-cell–independent antigen NP-Ficoll (B) is shown. Concentrations are given in micrograms per milliliter and the radiation doses are indicated on the x-axis. All mice received 0.5 × 106 total cells, of which 5% were normal. WT, normal CBA/J mice; xid, CBA/N mice. Each group contained 5 mice, except for the xid controls, which contained 4 mice.
The effect of reduced radiation on serum IgG3 reconstitution at 12, 18, 24, and 30 weeks posttransplant (A) and on the response to the T-cell–independent antigen NP-Ficoll (B) is shown. Concentrations are given in micrograms per milliliter and the radiation doses are indicated on the x-axis. All mice received 0.5 × 106 total cells, of which 5% were normal. WT, normal CBA/J mice; xid, CBA/N mice. Each group contained 5 mice, except for the xid controls, which contained 4 mice.
The lethally and sublethally irradiated mice were immunized with NP-Ficoll 18 weeks after bone marrow reconstitution. The mice exposed to 450 rad developed NP-specific antibody titers comparable to both the lethally irradiated recipient control group and to the wild-type CBA/J control group (Fig 3B). Only 1 of the 5 mice exposed to 200 rad had a normal response to NP-Ficoll. This was not the mouse with the highest concentration of serum IgG3. None of the mice that received lower doses of irradiation developed antibody to NP-Ficoll.
Long-term B-cell repopulation requires an undifferentiated precursor.
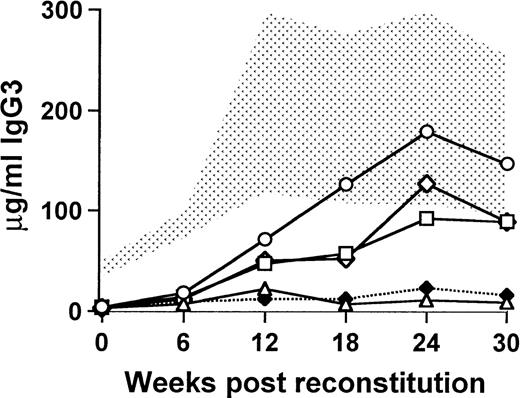
The delay in the onset of IgG3 production and the persistence of normal concentrations of Igs for 30 to 40 weeks after transplantation suggested that reconstitution was due to the engraftment of undifferentiated B-cell precursors. To examine this question, sublethally irradiated (450 rad) xid recipients were transplanted as before with a total of 0.5 × 106 bone marrow cells, of which 5% or fewer were derived from CBA/J mice and the remaining cells were from CBA/N xid mice. The wild-type cells were sorted into B220+/CD19+ cells and B220−/CD19− cells, and the sorted cells were added to the xid bone marrow in numbers proportionate to the percentage of those cells in 25,000 unsorted cells. In 1 group of mice, the sorted cells were pooled to mimic the original unsorted population of cells. The mice that received the B220−/CD19− cells developed an increase in the concentration of serum IgG3 (89.4 ± 44.9 μg/mL at 30 weeks) that was almost identical to that seen in the mice receiving the pooled cells (88.8 ± 25.1 μg/mL at 30 weeks) and similar to those that received unsorted cells (147.0 ± 53.4 μg/mL at 30 weeks; Fig 4). By contrast, the mice that received B220+/CD19+ cells were indistinguishable from xid controls (9.4 ± 2.1 μg/mL and 11.0 ± 9.9 μg/mL, respectively). The relative decrease in the concentration of serum IgG3 in the mice that received the B220+/CD19+ cells or the pooled sorted cells compared with the unsorted cells may be attributed to the effects of the manipulation required for the sort.
Functional B-cell reconstitution requires engraftment of an undifferentiated precursor. The stippled area represents the normal range for 9 wild-type CBA/J mice, and xid controls (5 mice) are denoted by (⧫). All mice were exposed to a sublethal dose of 450 rad and then transplanted with 0.5 × 106 cells, of which 5% were normal. (○) Mice that received unsorted wild-type cells; (□) mice that received the uncommitted, B220−/CD19−, wild-type cells; (▵) mice that received committed B220+/CD19+wild-type B cells; and (◊) mice that received the pooled fractions of committed and uncommitted wild-type cells. Each experimental group contained 5 mice.
Functional B-cell reconstitution requires engraftment of an undifferentiated precursor. The stippled area represents the normal range for 9 wild-type CBA/J mice, and xid controls (5 mice) are denoted by (⧫). All mice were exposed to a sublethal dose of 450 rad and then transplanted with 0.5 × 106 cells, of which 5% were normal. (○) Mice that received unsorted wild-type cells; (□) mice that received the uncommitted, B220−/CD19−, wild-type cells; (▵) mice that received committed B220+/CD19+wild-type B cells; and (◊) mice that received the pooled fractions of committed and uncommitted wild-type cells. Each experimental group contained 5 mice.
Functional B-cell reconstitution can be achieved when as few as 10% of peripheral B cells express normal Btk.
In the lethally irradiated mice, one might expect the percentage of cells in the bone marrow with normal Btk to be similar to the percentage of wild-type cells in the reconstituting bone marrow infusion; however, it was not clear whether all of the peripheral blood B cells would have normal Btk in the mice that had demonstrated complete reconstitution of IgM and IgG3 concentrations as well as normal antibody responses to NP-Ficoll. To address this question, genomic DNA was extracted from bone marrow cells and B220+splenic B cells 30 weeks after transplantation. A semiquantitative PCR was used to distinguish wild-type Btk from Btk with the xidmutation. The xid mutation, a C to T transition in exon 2 of Btk, results in the loss of a Hha I restriction site; therefore, this region of the gene was amplified by PCR and the 558-bp product was digested with Hha I. The xid allele demonstrated 222- and 336-bp fragments, whereas the wild-type allele gave fragments of 222, 45, and 291 bp. By comparing the intensity of the wild-type specific 291-bp fragment with that of the common 222-bp fragment, the degree of chimerism was calculated in the reconstituted mice.
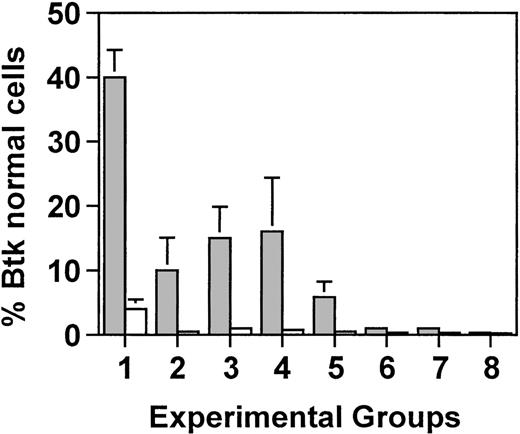
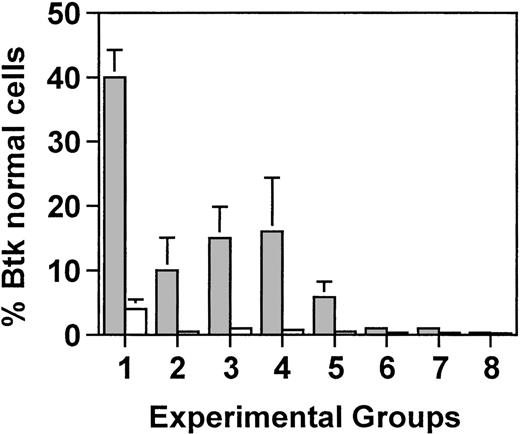
As expected, in lethally irradiated mice, the percentage of bone marrow cells with normal Btk mirrored the percentage of CBA/J cells in the initial bone marrow infusion. In xid mice that received 5 × 106 cells, of which 5% were from the Btk normal donor, 4% of the cells had normal Btk (Fig5). Similarly, in mice that received the same total number of cells but of which only 0.5% were from the wild-type CBA/J mice, approximately 1% of the cells had normal Btk. However, in splenic B cells, there was a marked increase in the percentage of cells with wild-type Btk, ranging from 40% in mice that received 250,000 wild-type cells to 10% in mice that had received 25,000 wild-type cells. This corresponds to an approximate 10-fold enrichment for B cells with normal Btk in the spleen compared with the bone marrow (ie, an increase from 4% to 40% and an increase from 1% to 10%, respectively).
Semiquantitative PCR analysis was used to determine the proportion of wild-type Btk-containing cells in the bone marrow (□) and in purified B cells from the spleen (▩). Groups 1 through 3 were lethally irradiated (900 rad) and received either 5.0 × 106 total cells (groups 1 and 2) or 0.5 × 106total cells (group 3), of which either 5% (groups 1 and 3) or 0.5% (group 2) were wild-type. Groups 4 through 8 received reducing amounts of radiation of 450, 200, 100, 50, or 0 rad, respectively, and all mice received 0.5 × 106 total cells, of which 5% were normal. Five mice were included in each experimental group. Values represent the average for each group ± standard deviation. In groups 2 through 8, all of the mice had between 0.1% and 1% normal Btk in the bone marrow.
Semiquantitative PCR analysis was used to determine the proportion of wild-type Btk-containing cells in the bone marrow (□) and in purified B cells from the spleen (▩). Groups 1 through 3 were lethally irradiated (900 rad) and received either 5.0 × 106 total cells (groups 1 and 2) or 0.5 × 106total cells (group 3), of which either 5% (groups 1 and 3) or 0.5% (group 2) were wild-type. Groups 4 through 8 received reducing amounts of radiation of 450, 200, 100, 50, or 0 rad, respectively, and all mice received 0.5 × 106 total cells, of which 5% were normal. Five mice were included in each experimental group. Values represent the average for each group ± standard deviation. In groups 2 through 8, all of the mice had between 0.1% and 1% normal Btk in the bone marrow.
In mice that were sublethally irradiated and received 0.5 × 106 total cells of which 5% were wild-type, the percentage of bone marrow cells with normal Btk was approximately 0.5% in mice that were exposed to 450 or 200 rad and at the level of detection in mice that were exposed to less than 200 rad. By contrast, B cells with normal Btk constituted 16% of the splenic B cells in mice that received 450 rad, 6% of the B cells in mice that received 200 rad, and 3% in mice that received 100 rad. Between 0.1% and 0.5% wild-type Btk was seen in the splenic B cells from mice exposed to less than 100 rad. These results demonstrate that normal concentrations of IgM and IgG3 and normal responses to NP-Ficoll could be achieved in mice that had as few as 10% of the B cells expressing normal Btk.
DISCUSSION
The results of this report provide strong support for the hypothesis that the selective advantage of B-cell precursors with normal Btk over those with mutant Btk can be used to provide clinical benefit to patients with mutations in Btk, patients with XLA. Lethally and sublethally irradiated Btk-deficient xid mice predictably developed normal concentrations of IgG3 and the ability to make antibodies to NP-Ficoll when they received 25,000 unmanipulated wild-type bone marrow cells along with 475,000 syngeneic bone marrow cells to provide hematopoietic recovery. As few as 2,500 wild-type cells altered B-cell function in 3 of 5 lethally irradiated xidmice. An increase in serum Igs was first detected 6 weeks after marrow infusion and was persistent until the end of the experiment, 30 to 40 weeks postinfusion. In unirradiated mice and mice that received very low doses of irradiation, there was a slow increase in the concentration of serum Igs after the infusion of 25,000 wild-type bone marrow cells, such that by 30 weeks after the infusion, all of the mice that were exposed to 50 rad and half of the unirradiated mice demonstrated IgG3 concentrations higher than those seen in thexid controls.
The identity of the cells responsible for reconstitution in this model was addressed by separating bone marrow cells into those expressing cell surface markers characteristic of precursors committed to the B-cell lineage, CD19 and B220, and those that were negative for these markers. The mice that received cells that did not express the B-cell–specific markers demonstrated an increase in IgG3 concentration that was similar to that seen in mice that received unmanipulated cells, whereas the mice that received committed B cells had IgG3 levels comparable to the untreated xid controls. This demonstrates that the reconstituting cells are derived from immature precursors, but it does not clarify whether the reconstituting cells are multipotent stem cells that could give rise to all hematopoietic lineages or whether there might be self-renewing precursors that are restricted to the B-cell lineage. Studies that have examined the phenotype and frequency of bone marrow stem cells with long-term multilineage potential have estimated that these cells occur with a frequency of less than 1 in 10,000 to 20,000.43,44 Our ability to provide long-term increases in IgM and IgG3 in xid mice with as few as 2,500 cells argues for a progenitor that occurs with a higher frequency but a more restricted potential.45 46
Others have shown reconstitution of B-cell function in xidmice; however, these studies have usually evaluated immune function shortly after reconstitution or they examined mice receiving large numbers of wild-type cells. Quintans et al47 treated unirradiated mice carrying the xid mutation with 20 × 106 normal splenic B cells and demonstrated the acquisition of the ability to make an antiphosphocholine antibody 2 weeks later. In another set of experiments, Quan et al48 demonstrated that 20 × 106 neonatal liver cells could reconstitute unirradiatated xid males, whereas mice that were exposed to 300 rad could be reconstituted with 106 normal neonatal liver cells. These investigators noted that reconstitution of xidmice was influenced by 4 variables: (1) the number of normal cells infused, (2) the irradiation dose, (3) the time after transplantation, and (4) the specific antigen used to evaluate the immune response. Our results emphasize the importance of the first 3 variables and suggest that the fourth variable might be expanded to indicate that reconstitution may vary depending on the method used to evaluate alterations in B-cell function. Serum Ig concentrations were a sensitive indicator of improved B-cell function, but an increase in serum IgM and IgG3 was not always associated with the ability to make antibody to NP-Ficoll.
The delay in production of IgG3 in this model may be explained, at least in part, by the absence of a selective advantage for precursors with normal Btk at the earliest stages of B-cell differentiation in the mouse.7 The expansion of the B cells or B-cell precursors with normal Btk may occur at varying stages of B-cell differentiation depending on the circumstances. This may help explain why mice that were lethally irradiated and received very low numbers (2,500) of normal donor bone marrow cells had low concentrations of IgG3 but detectable antibody to NP-Ficoll, whereas the animals that received 25,000 donor cells but low doses of irradiation (<200 rad) had low concentrations of IgG3 but no antibody to NP-Ficoll. In the lethally irradiated mice, there may have been expansion of very early precursors that may have permitted a broad antibody repertoire even in animals with low concentrations of IgM and IgG3. In contrast, the B-cell expansion may have occurred at a later stage of differentiation in the mice that received little or no irradiation. As a result, these mice may have a more limited antibody repertoire.
Because the block in B-cell differentiation in humans with defects in Btk is earlier and more severe than the block seen in mice with defects in the same gene, the selective advantage for B-cell precursors with normal Btk would presumably be stronger in the human compared with the mouse. Our studies suggest that improvements in B-cell function may be slow, but those improvements are likely to be long-lasting. The degree of B-cell reconstitution required to provide clinical benefit to patients with XLA is not clear. Some patients with XLA who have a milder disease as characterized by slightly higher concentrations of serum Igs or more B cells than expected usually have fewer life-threatening infections, and some of these patients do well in the absence of consistent gammaglobulin therapy.49-51
When compared with other genetic disorders for which gene therapy might be considered, XLA appears to have several advantages. Tight regulation of gene expression is not an absolute requirement, although it is likely that maximum benefit will be achieved by strategies that mimic endogenous expression.33-36 In contrast to patients with adenosine deaminase deficiency or other forms of severe combined immunodeficiency, patients with XLA could be maintained on routine therapy, intravenous gammaglobulin, and prophylactic antibiotics during reconstitution without compromising the success of treatment. Finally, the strong selective advantage of gene corrected cells may permit successful therapy even when gene correction is inefficient and the patient has not received ablative therapy.
Supported in part by National Institutes of Health Grant No. AI25129, March of Dimes Grant No. FY97-0384, National Cancer Institute CORE Grant No. P30 CA 21765, the Assisi foundation, the American Lebanese Syrian Associated Charities, and funds from the Federal Express Chair of Excellence.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mary Ellen Conley, MD, St Jude Children’s Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail:maryellen.conley@stjude.org.