Bone marrow transplantation (BMT) from an HLA-identical donor is an established therapy to cure homozygous β-thalassemia. Approximately 10% of thalassemic patients developed a persistent mixed chimerism (PMC) after BMT characterized by stable coexistence of host and donor cells in all hematopoietic compartments. Interestingly, in the erythrocytic lineage, close to normal levels of hemoglobin can be observed in the absence of complete donor engraftment. In the lymphocytic lineage, the striking feature is the coexistence of immune cells. This implies a state of tolerance or anergy, raising the issue of immunocompetence of the host. To understand the state of the T cells in PMC, repertoire analysis and functional studies were performed on cells from 3 ex-thalassemics. Repertoire analysis showed a profound skewing. This was due to an expansion of some T cells and not to a collapse of the repertoire, because phytohemagglutinin stimulation showed the presence of a complex repertoire. The immunocompetence of the chimeric immune systems was further established by showing responses to alloantigens and recall antigens in vitro. Both host and donor lymphocytes were observed in the cultures. These data suggest that the expanded T cells play a role in specific tolerance while allowing a normal immune status in these patients.
BONE MARROW transplantation (BMT) from an HLA-identical donor is a rational therapeutic modality for the cure of β-thalassemia major as well as for other hemoglobinopathies.1-5 In the great majority of transplanted thalassemic patients, a full donor engraftment is observed and the donor’s type pattern of β globin chain synthesis is established.1-6 In some cases, there is the presence of mixed chimerism after BMT. This normally resolves into complete engraftment or leads to graft loss. Mixed chimerism has also been described after BMT for other diseases.7-9
We have previously reported that approximately 10% of thalassemic patients develop a persistent mixed chimerism (PMC).4,5,10,11 PMC is defined as the coexistence of donor and recipient cells in the marrow of the host for a period longer than 24 months. Patients showing PMC have a functioning graft, normal levels of hemoglobin (Hb), and are cured from thalassemia.10 It still remains unclear how cells of different origin may last without adverse immune response. If this long-term coexistance is due to a tolerance/suppresion mechanism, it remains unclear to what extent this would affect overall immune function.
To better understand the mechanisms underlying the establishment and maintenance of the immune system in PMC, we analyzed the T-cell repertoire of 3 well-characterized PMC patients by measuring the CDR3 length of TCR BV families.12-16 This showed a profound skewed repertoire composed of a small number of specific T-cell clonotypes. After in vitro stimulation by phytohemagglutinin (PHA), the normal gaussian distribution TCR CDR3 sizes were reestablished. This shows that the skewing in the unmanipulated peripheral blood mononuclear cell (PBMC) is associated with an expansion of certain T cells but not with a collapse of the whole repertoire.
The immune functions of these patients were tested by measuring the response to recall antigens and third-party allogeneic cells in mixed lymphocyte reaction (MLR). All 3 patients showed an active immune response. Fluorescence in situ hybridization (FISH) or restriction fragment length polymorphism of variable number tandem repeats (RFLP-VNTR) analysis showed the presence of cells of both host and donor origin after culture. These data suggest that the skewed immune profile of these PMC patients plays a role in maintaining a specific status of tolerance allowing the coexistence of immune cells with different origin. However, this T-cell repertoire is still functional, resulting in a state of normal immunocompetence.
MATERIALS AND METHODS
Patients.
Patients described in these studies were chosen among a cohort of more than 800 patients affected by homozygous β-thalassemia and were subjected to allogenic BMT from an HLA-identical sibling. Clinical data concerning these patients are summarized in Table 1. Briefly, during periodical follow-ups, we chose 3 patients who fullfilled the following criteria: BMT performed at least 3 years before; persistence of at least 30% RHCs; minimum Hb level of 12 g/dL; and absence of any therapy. Two patients who developed rejection of the graft and 2 patients with full donor engraftment were included in the study as controls. Three normal donors volunteered their blood to perform mixed lymphocyte reactions. HLA typing on PBMC of normal donors was performed using a standard lymphotoxicity assay. All of the individuals who donated blood for this study were fully informed about the aims of the study before giving their consent.
Cells and cultures.
PBMCs were obtained from whole blood by gradient density separation using Ficoll according to a standard procedure, washed, and viable-stored in liquid nitrogen until their use. Cell cultures to test responses to PHA were prepared in flat-bottom 96-well multiplates (Costar, Boston, MA). Cells were resuspended at 2 × 105 in 200 μL of complete medium (RPMI 1640, 10% heat-inactivated fetal calf serum [FCS], penicillin [100 U/mL], and streptomycin sulfate [100 μg/mL]) and then stimulated with PHA at a final concentration of 5 μg/mL. After 3 days of culture in an incubator at 37°C and 5% CO2, 0.5 μCi of [3H]thymidine (Amersham-Pharmacia, Milano, Italy) was added to each well; 16 hours later, the cells were harvested and counted for thymidine incorporation following standard protocols. To test the response to recall antigens, cells were resuspended as described before and then stimulated with purified protein derivative (PPD) or tetnus toxoid (TT) at a final concentration of 5 μg/mL. After 6 days of culture, 0.5 μCi of [3H]thymidine was added to the culture and incorporated radioactivity was measured as described above. For MLR cultures, PBMC were irradiated (3,000 rad) using a 137Cs source and were used to stimulate the PBMCs of PMC patients at a 1:2 ratio of responders/stimulators. After 6 days of culture performed as described above, a standard [3H]thymidine incorporation test was performed. For T-cell repertoire analysis, cells were recovered after cultures; we then prepared RNA and cDNA for spectratype analysis.
FISH analysis.
FISH was performed on cells recovered from cultures when the host and donor were sex-mismatched. A biotinylated pY3.4 probe complementary to a part of the Y chromosome was used as reported10,11; positive and negative controls were included in each test. After separation by means of Dynabeads, cells were analyzed on the membrane surface directly.11
RFLP-VNTR analysis.
This analysis was performed as described.10 11 Briefly, DNA was extracted from cells recovered from the cultures after a Ficoll separation, and the highly polymorphic VNTR genes were amplified. We screened locus D1S80 and locus D17S30 as described. Conditions for polymerase chain reaction (PCR) and gel electrophoresis have been described as well. Southern blot analysis was performed with hypervariable VNTR probes as reported.
RNA extraction and cDNA preparation.
PCR amplification and samples normalization for spectratype analysis.
Amplification of cDNA was performed as previously reported.12,13 Briefly, we used a fluoresceinated primer with specificity for the β-chain TCR constant region and 2 primers bearing the specificity for the V region, as published.14,15 The amplification conditions have been described as well.12,13 To normalize the results, the amounts of templates from different samples were titrated at different dilution points of starting material by amplifying the TCR β-chain constant cDNA; the level of expression was then quantified by analyzing the fluoresceinated signal.12 A coamplification, using 1 μL of a 1:1,000 dilution starting from 20 μmol/L solution of primer pair specific for β actin was performed in each PCR tube to check the efficiency of the single PCR reaction.
Generation of spectratypes.
PCR product (0.5 μL) was diluted 1:1 with distilled water. The sample was boiled and loaded on sequencing gel and then run in a fluorescence-based DNA sequencer (Applied Biosystem 377 model; Applied Biosystems, Foster City, CA) in the presence of Rox-labeled size markers (Applied Biosystems).14 The data were analyzed by means of Applied Byosistems Genescan software that allows us to assign size and peak areas to the different PCR products. The data for each TCR-Vβ family were then visualized as chromatograms.
Single-strand conformational polymorphism (SSCP).
This analysis was performed according to standard procedures with minor modifications.14 Briefly, PCR products of given TCR BV families, having a predominant peak when analyzed by spectratyping, were serially diluted until only the predominant peak was visible by Genescan analysis. PCR product (0.5 μL) was then further diluted in 0.1% sodium dodecyl sulfate (SDS)/10 mmol/L EDTA. Finally, 2 μL of the obtained solution was mixed to loading buffer and run on a nondenaturing gel (6% [wt/vol] acrylamide/10% [vol/vol] glycerol). The results were analyzed, as reported for spectratype analysis, by means of Genescan software and expressed as a gel file image.
RESULTS
Three patients were analyzed as part of this study. The patient data are given in Table 1. All 3 were defined as showing PMC with at least 30% residual host cells at 3 or more years posttransplant. They have normal lymphocyte counts with normal CD4/CD8 ratios and normal levels of Hb. Leukocyte subpopulation distributions, determined by staining with monoclonal antibodies and subsequent fluorescence-activated cell sorting (FACS) analysis, were normal in all the patients (data not shown). None of the 3 patients was receiving any therapeutic treatment, and there were no reports of clinical complications during the periodic posttransplant follow-ups.
TCR repertoire analysis of PMC patients.
To better understand the immunological phenomena underlying the unexpected status found in these patients, we analyzed the T-cell repertoire by means of TCR β-chain CDR3 spectratyping. This technique visualizes the T-cell repertoire for each TCR BV family as a series of bands having a gaussian distribution.12-16 Any modification in the normal gaussian profile and/or intensity of the bands represents an alteration of the TCR repertoire. Because only 1 TCR BV gene is productively rearranged for each T cell, the study of TCR-BV repertoires allows us to follow the behavior of T-cell populations under different conditions, both in vivo and in vitro.12-16Repertoire analysis of the PBMCs from the 3 PMC patients showed severe alterations. A representative part of these findings is shown in Fig 1. The data for each of the TCR BV families showed a normal gaussian profile in the donor PBMCs (top panel) and a skewed repertoire in the recipient (bottom panel). Such a skewed repertoire was observed in almost all of the TCR BV families from all 3 patients, with the presence of predominant peaks and the loss of the characteristic gaussian pattern. Such a profound skewing in the T-cell repertoire is compatible with a collapse of the repertoire and has been associated with severely compromised immune systems posttransplant.13 In contrast, the 3 patients were healthy and did not show any sign of a compromised immune system. Because there was a disparity between the repertoire of these patients and their immune status, we decided to investigate these patients further.
Spectratype analysis of 1 representative donor and recipient pair (UPN688). The donor data are from a pretransplant PBMC sample. The recipient data are from PBMC obtained 7 years posttransplant. The histograms show the TCR profile of 9 different BV families (indicated on top of each pair of spectratypes). The donor or recipient origin of each spectratype is identified on the right.
Spectratype analysis of 1 representative donor and recipient pair (UPN688). The donor data are from a pretransplant PBMC sample. The recipient data are from PBMC obtained 7 years posttransplant. The histograms show the TCR profile of 9 different BV families (indicated on top of each pair of spectratypes). The donor or recipient origin of each spectratype is identified on the right.
T-cell repertoire analysis of PBMCs from patients who developed a full donor engraftment or rejection.
As a control, we analyzed TCR on PBMCs derived from 2 patients with full donor engraftment and 2 patients who rejected the transplant. Patients with full donor engraftment showed TCR Vβ spectratype profiles comparable to those found in normal donors (data not shown). Patients with rejection showed an hyperexpression of most of the TCR Vβ families’ clonotypes (data not shown), reflecting a massive activation of T cells. Similar results were previously reported by several investigators.13,17 18
Evaluation of the complexity of the predominant clonotypes.
In spectratype analysis, each peak represents TCR having the same BV-CDR3 length; therefore, each peak may consist of a variable number of TCR sequences. To determine the complexity underlying of the predominant peaks observed in the peripheral blood, we performed SSCP on the DNA. This technique allows detection of single basepair differences in 2 DNA strands of the same size. We selected the prominent peaks present in different TCR BV families from each of the 3 patients for the SSCP analysis: BV 20 in UPN 855, BV2 in UPN 688, and BV 5.3 in UPN1074. In all cases, the predominant spectratype peaks could be resolved into 1 or 2 bands (Fig 2, lanes 1 through 4, 7, and 8). Lanes 5 and 6 show the SSCP profile of single spectratype peak known to be polyclonal. Rather than distinct bands, a smear is observed. These results indicate that the repertoire skewing is caused by a limited number of T-cell clonotypes. Although it is possible that this may be the result of repertoire contraction, it is more likely that this reflects an expansion of these cells in response to some stimulus associated with the PMC state.
SSCP analysis of predominant spectratype bands. Each pair of lanes represents data derived from different samples. Lanes 1 and 2, BV2 from UPN688; lanes 3 and 4, BV20 from UPN855; and lanes 7 and 8, BV5.3 from UPN1074. Lanes 5 and 6 show results from the analysis of a normal, polyclonal spectratype band. In each case, the first lane represents PCR in which twice the amount of cDNA was used.
SSCP analysis of predominant spectratype bands. Each pair of lanes represents data derived from different samples. Lanes 1 and 2, BV2 from UPN688; lanes 3 and 4, BV20 from UPN855; and lanes 7 and 8, BV5.3 from UPN1074. Lanes 5 and 6 show results from the analysis of a normal, polyclonal spectratype band. In each case, the first lane represents PCR in which twice the amount of cDNA was used.
PHA stimulation of the PBMC reestablishes a complex T-cell repertoire.
As part of the investigation of the ability of the T cells from these patients to mount an immune response to common stimuli, the proliferative responses to the mitogen, PHA, was tested. It was observed that the response to this mitogen induced the proliferation of a large number of T cells. Spectratype analysis of the cultures showed that, despite starting out with a heavily skewed repertoire, a normal repertoire pattern was restored (Fig 3). The starting spectratype pattern is shown at the top of each panel, and the resulting spectratype after PHA stimulation is shown at the bottom. These results indicate that the T cells giving rise to the skewed starting repertoire do not have the same proliferative potential to PHA as do the other T cells in the repertoire. These data also clearly point out the existence of a complex repertoire in these individuals that is masked in the PBMCs by the expansion of a select number of T-cell clonotypes. Because a fixed amount of cDNA is used in these analyses, the resulting patterns are always relative. It requires the preferred expansion of the underlying repertoire by the PHA for it to be visualized by the spectratype analysis.
Spectratype analysis of in vitro culture with PHA. PBMCs from UPN688 (A), UPN885 (B), and UPN1074 (C). For each patient, the TCR profiles of 3 representative BV families (indicated on top of each pair of spectratypes) are shown. The spectratype of the PBMC before culture (pre) is shown at the top of each panel and that after the culture (post) is shown on the bottom.
Spectratype analysis of in vitro culture with PHA. PBMCs from UPN688 (A), UPN885 (B), and UPN1074 (C). For each patient, the TCR profiles of 3 representative BV families (indicated on top of each pair of spectratypes) are shown. The spectratype of the PBMC before culture (pre) is shown at the top of each panel and that after the culture (post) is shown on the bottom.
Analysis of the in vitro response of T cells to antigens.
The immune status of the PMC individuals appears to be normal in that they do not show any of the clinical signs associated with compromised immune function. Although the results from the PHA stimulation showed the existence of a complex repertoire in these individuals, it was necessary to show that this repertoire was capable of standard responses to recall antigens (PPD and TT) and unrelated third-party alloantigens in culture. The results of these stimulation as assayed by proliferation are shown in Fig 4. All of the stimuli elicited a response from the T cells, and the proliferation values were comparable to those obtained from cells of a normal donor. The results for the stimulation with PHA are also included for comparison.
Proliferative analysis of the in vitro response to different antigens. [3H]thymidine uptake, expressed in cpm, of PBMCs of UPN 688, UPN855, and UPN1074 after in vitro culture with PPD, TT, PHA, and an unrelated third-party alloantigen (see bar legend) is shown. The same assay was made using a normal donor (ND) as a control. One representative experiment of 3 performed is shown.
Proliferative analysis of the in vitro response to different antigens. [3H]thymidine uptake, expressed in cpm, of PBMCs of UPN 688, UPN855, and UPN1074 after in vitro culture with PPD, TT, PHA, and an unrelated third-party alloantigen (see bar legend) is shown. The same assay was made using a normal donor (ND) as a control. One representative experiment of 3 performed is shown.
In addition to the proliferation studies, T-cell repertoire analysis was performed using spectratyping after stimulation with PPD or TT. Several new peaks in a number of TCR-BV families were observed after the cells were stimulated (data not shown). Others using this technique have described similar results in response to nominal antigens.12,14 16 It is interesting to point out that the responding T cells did not correspond in CDR3 length to the skewed cells present in the beginning of the culture. These experiments indicate that standard immune responses can be observed in these individuals and that these responses originate from the normal underlying T-cell repertoire and not from the expanded T cells that make up a large portion of the circulating lymphocytes.
Both donor and host cells are observed after in vitro responses to different stimuli.
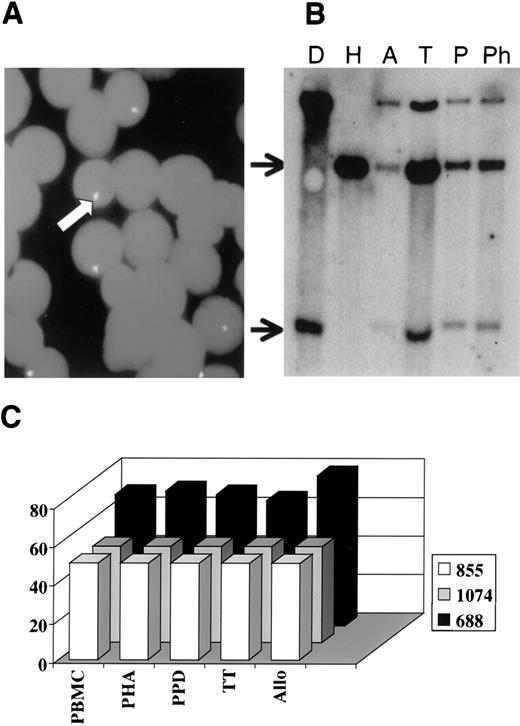
Because PMC is characterized by the presence of both host and donor T cells,8 an important issue in understanding this system is to determine if cells of both origins can contribute to the observed immune response. Although it is normally the donor cells that are responsible for responses in cases of complete engraftment, it is possible that host cells may also contribute. This would show the nature of the tolerance phenomenon. FISH or RFLP-VNTR analysis were used to determine the origin of the cells after culture. FISH was used for UPN688, because it depends on the presence of a sex-mismatch. We show an example of the FISH analysis (Fig5A) and of an RFLP-VNTR analysis (Fig 5B). The data are summarized in Fig 5C, which plots the percentage of donor cells for each of the cultures and each of the individual unstimulated PBMC. This shows that the ratio of donor to host cells did not change appreciably during the culture and that both were present after the culture period. This is compatible with both donor and host cell responses in the cultures.
Detection of donor and host cells after in vitro responses to different stimuli. (A) A representative FISH analysis performed on PBMCs of UPN688 after culture with PPD. The arrow indicates the biotinylated pY3.4 probe complementary to a part of Y chromosome, showing the presence of some recipient cells. (B) RFLP-VNTR analysis performed on PBMCs of the donor (D) and the respective recipient UPN855 (H) pretransplant, on PBMCs of the host after culture with cells of a third party (A), and on the PBMCs of the same transplanted recipient after in vitro culture with TT (T), PPD (P), and PHA (Ph). The top arrow indicates the presence of recipient cells and the bottom arrow indicated the presence of donor cells. (C) Data summary in which the y-axis shows the percentage of donor cells evaluated by FISH or RFLP-VNTR and the x-axis shows the different samples analyzed before (PBMC) and after (PHA, PPD, TT, and Allo) in vitro culture. Different color bars refer to the 3 different patients (see legend).
Detection of donor and host cells after in vitro responses to different stimuli. (A) A representative FISH analysis performed on PBMCs of UPN688 after culture with PPD. The arrow indicates the biotinylated pY3.4 probe complementary to a part of Y chromosome, showing the presence of some recipient cells. (B) RFLP-VNTR analysis performed on PBMCs of the donor (D) and the respective recipient UPN855 (H) pretransplant, on PBMCs of the host after culture with cells of a third party (A), and on the PBMCs of the same transplanted recipient after in vitro culture with TT (T), PPD (P), and PHA (Ph). The top arrow indicates the presence of recipient cells and the bottom arrow indicated the presence of donor cells. (C) Data summary in which the y-axis shows the percentage of donor cells evaluated by FISH or RFLP-VNTR and the x-axis shows the different samples analyzed before (PBMC) and after (PHA, PPD, TT, and Allo) in vitro culture. Different color bars refer to the 3 different patients (see legend).
DISCUSSION
It is not a rare event to observe host hematopoietic cells after marrow transplantation; this is referred to as mixed chimerism (MC). There is evidence that, in some settings, MC is associated with an increased risk of graft failure and/or disease recurrence. T-cell repertoires with extensive gaps and contractions have been observed in cases of MC and were associated with an impairment of immune response and recurrent infections.17-21 These studies were mainly performed in patients who underwent BMT for malignancies and before 1 year from the transplant. In this report, we study T-cell responses to mitogens, recall antigens, and T-cell repertoires in patients who, transplanted years before, are cured from the disease and have developed a stable MC.
Our analysis showed a very skewed T-cell repertoire in PMC patients. Such a finding is usually associated with a poor immune system function,13,17,18 21 whereas these 3 patients had a normal immune function, because they were not receiving any therapeutic treatment and they did not suffer from recurrent infections. It should also be pointed out that the skewing of the T-cell repertoire was stable over time, because the repertoire analysis, which was performed at different times after BMT, was found to be similar (Andreani et al, unpublished data). Additional evidence for normal immune system function is that the cells of PMC patients were able to mount a normal immune response in culture to different stimuli, such as mitogens, recall antigens, and alloantigens. We also determined that both donor and recipient cells were contributing to the immune response, as shown by RFLP-VNTR analysis and FISH.
A very striking observation came from the analysis of the T-cell repertoire after culture with PHA. In these cultured cells, a gaussian pattern of spectratype bands that is found in normal individuals was reestablished. This result indicates the presence of a normal repertoire in the peripheral blood of these patients that can be shown by culturing with PHA. Thus, the skewing observed in the absence of PHA results from the expansion of some T cells to the extent that they mask the normal repertoire. The nature of this skewing was further analyzed by SSCP analysis of the prominent peaks found by TCR repertoire analysis in PMC individuals. This analysis demonstrated that those peaks were oligoclonal or pauciclonal, supporting the idea that an expansion of a few T-cell clonotypes was responsible for the spectratype pattern found in the PMC patients.
Taken together, these data showed that preferential expansions of given T-cell clonotypes were present in the peripheral blood of PMC patients and suggest that these expansions could correlate with the establishing of specific tolerance/anergy. A recent report has demonstrated that mice spontaneously developing autoimmune disease may be cured by BMT that establishes PMC. Unfortunately, there are no data concerning the T-cell repertoire in this animal model.22 The existence in mice of T cells that specifically inhibit a proliferation towards specific antigens in presence of interleukin-10 has been reported.23 It is possible that the cell populations found expanded in our patients are similar to those present in the PMC patients. They may expand specifically to maintain an equilibrium that is needed for the coexistence of 2 different immune systems in the host after BMT. Only in the presence of a specific trigger (eg, infections) is this status altered and other T-cell populations start to proliferate, as demonstrated by the experiment with PHA. More experiments are needed to further clarify the origin of the expanded cells found in the blood of these individuals and to assess if they are from donor, recipient, or both or if they have specific phenotype features. Moreover, the dynamics regulating the interactions between host and donor repertoire after different stimuli need to be investigated.
The generalization of these findings to more cases of PMC is needed. It should be pointed out that, in all 3 cases studied, the donor:host engraftment was approximately equal. It will be interesting to determine if the same phenomenon of repertoire skewing is observed in patients with low host or donor engraftment. The mechanism of maintianing more skewed chimerism may not be the same as that described here.
Our data support the idea that a condition of PMC is sustained by the expansion of specific regulatory T cells able to induce a status of tolerance or anergy, but the driving elements that select and expand these cells as well as the functions of these cells remain to be detailed. The understanding of the steps required for establishing the PMC is of great importance and may contribute to improve greatly the handling of allogeneic BMT. For example, the ability to generate PMC could lead to a reduction of the total amount of drugs used for conditioning regimens and a lowering of the doses of immunosuppressive drugs administered before BMT, thus reducing the transplant-related mortality. Furthermore, the analysis of the PMC phenomenon may shed new light on the way the induction of tolerance takes place after transplantation and, more generally, in the course of an immune response.
ACKNOWLEDGMENT
The authors thank Dr Emanuele Angelucci for several helpful suggestions and comments and Dr Franco Locatelli for stimulating discussions.
M.B. and M.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Guido Lucarelli, MD, Divisione di Ematologia e CentroTrapianto di Midollo Osseo, Ospedale di Muraglia, Pesaro, Italy, Via Lombroso 19, 61100 Pesaro, Italia; e-mail: g.lucarelli@wnt.it.




![Fig. 4. Proliferative analysis of the in vitro response to different antigens. [3H]thymidine uptake, expressed in cpm, of PBMCs of UPN 688, UPN855, and UPN1074 after in vitro culture with PPD, TT, PHA, and an unrelated third-party alloantigen (see bar legend) is shown. The same assay was made using a normal donor (ND) as a control. One representative experiment of 3 performed is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/10/10.1182_blood.v94.10.3432.422k17_3432_3438/6/m_blod42217004y.jpeg?Expires=1767840303&Signature=c~kCYG3jOYDxC2vLrm-BZ7zsOWagwt4xuwY6Iaq4lMV1pvhzbhD~qAgKb-5q20If2feRxY3FT~6t~28Img10LkbW~hZkGVhl6DZDFHnBqXyaEJ-4t0sAeJ7jpX470ImEJvI2rNdIZtpRQQplao1bTbuLlzPzNDpRQJ-FAYVsj4UN6uaSgFa3UH12qRN~Dnn0RQeZYazDDGEUUtUGLEyT3~dbuoUYN5H4roKqpoSwVA4-bs2CfqLBI99ReujtTNHicO0vaM0kzxrwC9PcaO0wbOhO6OTDiH-SpT04PLZTrR9EKmhS8qcpCHybZalMDeL-wpzWepBJgF1JEDpJxYFpxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)