Abstract
Early B lymphopoiesis is marked by plasticity between the myeloid and B lineages. An attractive model for B-lineage development is that commitment to this lineage is partly determined by the ordered expression of genes that prohibit switching to the myeloid lineage. In this regard, whereas the role of the B-cell–specific transcription factor BSAP/Pax5A in regulating B-lymphoid–restricted gene expression has been well-established, its role in maintaining B-lineage commitment is unclear. Thus, BSAP/Pax5A was constitutively expressed in the multipotent EML cell line, which can be directed toward the myeloid lineage by culture with interleukin-3 (IL-3) and retinoic acid. EML cells expressing BSAP/Pax5A successfully acquired the myeloid lineage markers CD11b and F4/80 in response to IL-3 and retinoic acid, indicating differentiation to the myeloid lineage. However, these early myeloid cells failed to expand in culture with granulocyte-macrophage colony-stimulating factor and were directed instead toward an apoptotic pathway. In parallel, primary bone marrow stem cells transduced with retrovirus constitutively expressing BSAP/Pax5A began myeloid cell differentiation, but like the transformed EML model failed to expand in response to myeloid growth factors. These studies identify a role for BSAP/Pax5A in suppressing the response to myeloid growth factors, which may be a component of the regulatory processes that limit plasticity of early B-lymphoid progenitors.
A FUNDAMENTAL QUESTION in hematopoiesis is how cells once directed to a particular lineage stay committed to that lineage. Lineage-determinant genes such as mafB/ets-1, GATA-1, E2A, and PU.1 are thought to play a role in these processes.1-5 These genes encode transcriptional regulators that direct or enhance expression of lineage-specific genes. In addition, these transcriptional regulators also repress expression of genes associated with closely related lineages. For example, whereas ectopic PU.1 expression in a chicken thromboblast progenitor was shown to activate genes associated with the myeloid lineage, enabling these cells to respond to myeloid growth factors, PU.1 also repressed expression of GATA-1, which is a determinant gene for erythroblast, thromboblast, and eosinophil commitment.1 2
This question of how cells maintain lineage commitment is particularly important to the B-cell lineage as many transformed pre-B cell lines spontaneously differentiate or can be induced to differentiate into myeloid cells.6-14 These observations suggest that the B-cell determinant protein that limit lineage divergence are weak or can be disrupted by transformation. To be effective in initiating B-lineage commitment, these B-cell–determinant genes must be expressed and functional early in B-cell development. In this regard, evidence for commitment to the B lineage is found within the earliest identifiable B cells.15 This stage (fraction A1according to the Hardy nomenclature16,17) is defined by the coexpression of B220, CD43, and low levels of CD4.16,17 B cells at this stage begin to express low levels of the transcription factor E2A, the earliest appearing gene that is thought to be responsible for commitment to the B-cell lineage.17
Recent work implicates E12, a product of the E2A gene, as a B-lineage–determinant protein, because it upregulates B-cell–specific genes and represses myeloid-associated genes.5 In these studies, ectopic expression of E12 in the macrophage cell line 70Z/3 caused a loss of macrophage morphology, downregulation of the myeloid cell genes CD11b and c-fms, inability to adhere to plastic, activation of the κ light chain in response to lipopolysaccharide (LPS), and upregulation of the B-cell genesIL-7Rα, RAG-1, λ5, BSAP/Pax5A, andEBF.5 Thus, E12 stripped a cell of its myeloid characteristics and reprogrammed it with B-cell characteristics, which led to the hypothesis that the endogenous expression of E12 in B cells normally prohibits them from switching to the myeloid lineage in vivo.5
Target genes for E12 include the genes that encode the B-cell–restricted transcription factors EBF and BSAP/Pax5A.5,18 The role of these molecules in B-lineage determination is only partially defined. Ectopic expression of EBF in the 70Z/3 macrophage cell line resulted in a limited expression of B-cell–associated genes, including upregulation of BSAP/Pax5A and λ5 expression and the activation of κ chain expression in response to LPS.5 However, the expression of the myeloid-lineage–associated genes covered in this study was unaffected by ectopic EBF expression.5 Thus, only a subset of the E12-linked processes that determine B-cell gene expression and none of the E12-linked myeloid-suppressing processes can be attributed to EBF or BSAP/Pax5A by extension, because it was upregulated by EBF.5 These data do not necessarily exclude a role for EBF or BSAP/Pax5A in the suppression of myeloid gene expression, because this study did not cover several myeloid genes.
BSAP/Pax5A is the major alternatively spliced isoform of Pax5, a member of the Pax family of transcription factors.19,20 Pax5 is expressed in B cells developing neural tissue and testis and is abnormally expressed in certain B-cell lymphomas and medulloblastoma.21,22 Within the B-lineage, BSAP/Pax5A is first expressed immediately after commitment to the B-lineage in the bone marrow17,23 and continues to be expressed throughout B-cell development, except in plasma cells.17 Besides its regulation by E12 and EBF, other evidence suggests that BSAP/Pax5A may have B-cell–determinant properties. Similar to E2A−/− mice,Pax5−/− mice completely lack B220+cells in the fetal liver, suggesting that BSAP/Pax5A is required for B-cell commitment.24,25 However, in the bone marrow,Pax5−/− mice display a complete block later in B-cell development.26 In this case B-cell development is blocked at the pro-B–cell stage after immunoglobulin D to J rearrangement, but before immunoglobulin V to DJ rearrangement of the heavy chain locus.24 Like E12, BSAP/Pax5A is also thought to upregulate several B-cell–specific genes that are first expressed during the early pro-B stage. These genes, which includeVpreB1, blk, mb-1 (Igα), andCD19, encode both markers of early B-lineage commitment as well as proteins that are necessary for the transition through the initial stages of B-cell development in both the bone marrow and the fetal liver.27-31
The combined evidence that BSAP/Pax5A plays a role in B-cell commitment, developmental progression, and B-cell marker expression throughout most of B-cell development strongly suggests that BSAP/Pax5A, like E12, may have its own B-cell–determinant properties. To test this possibility, BSAP/Pax5A was ectopically expressed in bone marrow stem cells. Because EBF/BSAP-expressing 70Z/3 cells failed to suppress the myeloid phenotype,5 it was expected that the B-cell–determinant properties of BSAP/Pax5A would be limited to upregulation of B-cell gene expression. Surprisingly, contrary to these expectations, whereas BSAP/Pax5A lacked the ability to alone mediate B-lineage differentiation, it nonetheless was found to limit myeloid-lineage potential by suppressing expansion and survival of early myeloid cells. By doing so, these results argue that one of the functions of BSAP/Pax5A in B-lymphoid commitment is to limit the ability of lineage-divergent cells to proceed through development.
MATERIALS AND METHODS
Plasmid construction.
pGEM7(KJ1)SalI contains the neomycin resistance gene downstream of the phosphoglycerokinase (PGK-1) gene.32 The PGK-1 promoter was removed from pGEM7(KJ1)SalI after digestion with Xba I andPst I and was ligated into pBluescript II SK (Stratagene, La Jolla, CA) digested with Xba I and PstI to form pBSPGK. The BSAP/Pax5A cDNA and SV40 poly A signal was removed from pmBSAP-233 (a kind gift from Dr Meinrad Busslinger, Research Institute of Molecular Pathology, Vienna, Austria) after digestion with Cla I and Apa I and was ligated into pBSPGK digested with Cla I and Apa I to form pPGKPax5A. The Mig R1 plasmid, when transiently transfected into the Bosc23 packaging cell line, produces the MIGR retrovirus that expresses the green fluorescence protein (GFP) marker at an internal ribosomal entry site.34 BSAP/Pax5A cDNA was removed from pmBSAP-2 with Cla I and HindIII, blunt-ended at theHindIII site, and ligated into the Cla I andEcoRV sites of pBluescriptII SK to form pBSBSAP. BSAP/Pax5A cDNA was then removed from pBSBSAP with EcoRI digestion and then ligated into the EcoRI site of Mig R1 to produce Mig R1BSAP.
Hematopoietic growth factors.
Conditioned supernatant from the J558-IL-7 cell line (a kind gift from Dr Fritz Melchers, Basel Institute for Immunology, Basel, Switzerland) was used as a source of murine interleukin-7 (IL-7). Human flt3L and murine granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Genzyme Diagnostics (Cambridge, MA). Conditioned supernatant from the WEHI-3B cell line was used as a source of IL-3. Erythropoietin was obtained from Amgen (Thousand Oaks, CA). Murine IL-6, stem cell factor (SCF), and IL-3 were purchased from R&D Systems (Minneapolis, MN).
Antibodies.
To identify primitive stem cells, monoclonal antibodies RA3-6B2 (B220), RM4-5 (CD4), 53-6.7 (CD8a), RB6-8C5 (gr-1), M1/70 (CD11b/Mac-1), and Ter119 (erythroid lineage marker) were used coupled to phycoerythrin (PE) as lineage markers. Stem cell markers include 2B8 (c-kit) coupled to allophycocyanin (APC) and E13-161.7 coupled to biotin (sca-1) and streptavidin red-670 as a secondary reagent. These antibodies were handled according to the instructions of the supplier (Pharmingen, San Diego, CA). The PE-coupled F4/80 antibody was handled according to the instructions of the supplier (Caltag, Burlingame, CA). Polyclonal antibodies directed against the paired domain of Pax5A were a kind gift from Dr Meinrad Busslinger, who generated them as previously described.33 All flow cytometry was performed using a Becton Dickinson (Franklin Lakes, NJ) FACScan at the University of Pennsylvania Flow Cytometry Facility (Philadelphia, PA) and Cellquest software.
Cell lines.
EML cells35 (a kind gift from Dr Schickwann Tsai, Mount Sinai School of Medicine, New York, NY) were maintained in Iscove’s Modified Dulbecco’s Media (IMDM), supplemented with 20% heat-inactivated horse serum, and 12% to 15% BHK/MKL (a kind gift from Dr Schickwann Tsai, Mount Sinai School of Medicine, New York, NY) conditioned media. OP9 cells36 (a kind gift from Dr Tasuko Honjo, Kyoto University Yoshida, Kyoto, Japan) were maintained in minimum essential medium α medium supplemented with 20% fetal calf serum.
Electromobility shift analysis (EMSA).
Nuclear extracts were prepared as described elsewhere.37The CD19Ains probe33 38 consisted of the following 2 oligonucleotides that were annealed to form a double-stranded oligonucleotide: 5′CTGGAGAATGGGGCACTGAGGCGTGACCACCGCCT3′ and 5′AGGCGGTGGTCACGCCTCAGTGCCCCATTCTCCAG3′ (Nucleic Acid Facility, University of Pennsylvania Cancer Center). Fifty nanograms of probe was end-labeled with 10 μCi [γ32P]ATP using T4 kinase and purified over a G-25 spin column. Binding reactions consisted of 20,000 DPM probe, 2 μg poly dI-dC, 10 mmol/L HEPES, pH 7.9, 100 mmol/L NaCl, 10% glycerol, 0.5 mmol/L MgCl2, and 1 mmol/L dithiothreitol for 15 minutes at room temperature. Nuclear extracts were then added to the probe mixture for an additional 15 minutes at room temperature. The reaction was then subjected to electrophoresis on a native 4% polyacrylamide gel in 1× Tris-borate/EDTA (TBE) buffer to separate protein-DNA complexes. Quantitation was performed with a Personal Densitometer (Molecular Dynamics, Sunnyvale, CA).
In vitro generation of myeloid precursors from EML cells.
EML cells were induced to form myeloid cells, as previously described.35 After 3 days of induction in media A (IMDM supplemented with 10% WEHI-3B conditioned supernatant, 10 μmol/L all-trans retinoic acid (ATRA), and 20% heat-inactivated horse serum), the cells were harvested, washed, and replated at 1.5 × 105 cells per 3.5-cm bacterial plate in media B (IMDM supplemented with 250 U/mL murine GM-CSF [muGM-CSF] and 20% heat-inactivated horse serum) and 1.0% methylcellulose. Colonies were counted after 7 days of growth. The entire plate of cells was harvested after 9 days, and the number of CD11b+/Gr-1+ cells was determined by flow cytometry.
EML proliferation assays.
Two hundred microliters of undifferentiated EML cells at 2.5 × 105 cells/mL were plated in triplicate in 96 flat well plates. Twenty-four hours later, 0.5 μCi of [3H]TdR was added to each well, and 17 hours later, the cells were harvested. EML cells that were induced for 3 days in media A to generate colony-forming units–granulocyte-macrophage (CFU-GM) were then plated in 200 μL at 5 × 105 cells/mL in triplicate in 96 flat well plates in media B. Forty-eight hours later, 0.5 μCi of [3H]TdR was added to each well, and 12 hours later, the cells were harvested. Cells were harvested using a PHD cell harvester (Cambridge Technology, Inc, Watertown, MA) on glass fiber discs. Incorporated 3H radioactivity was measured in a scintillation counter (1209 Rackbeta; EG&G Wallace, Gaithersburg, MD).
EML apoptosis assays.
EML cells induced to differentiate in media A as described above were replated at 5 × 105 cells/mL in 400 μL media containing 250 U/mL muGM-CSF. Two days later, the cells were washed with fluorescence-activated cell sorting (FACS) buffer (1× phosphate-buffered saline [PBS], 0.2% bovine serum albumin [BSA], and 0.01% sodium azide) and incubated with ice-cold 70% ethanol for several hours. Cells were then washed with FACS buffer and then stained with 1× PBS/50 μg/mL RNAase/10 μg/mL propidium iodide/0.01% sodium azide overnight at 4°C. The percentage of cells with a subdiploid DNA content was determined using flow cytometry.
Retroviral transduction of bone marrow stem cells.
Retroviral supernatants were prepared by transiently transfecting the Mig R1 or Mig R1BSAP plasmids into the Bosc23 packaging cell line.34,39,40 For infection of stem cells, 8- to 12-week-old female BALB/C mice were injected with 200 μL of 25 mg/mL 5-fluorouracil. Four days later, bone marrow was harvested and then cultured at 2.5 × 106 cells/mL in 1 mL Dulbecco’s modified Eagle’s medium (DMEM) in the presence of 15% heat-inactivated fetal calf serum, 5% WEHI-3B conditioned supernatant, 40 ng/mL flt3 ligand, 200 ng/mL SCF, 12 ng/mL IL-3, and 20 ng/mL IL-6. Forty-eight hours later, the cells were transduced with 1 mL of MIGR or MIGRPax5A retroviral supernatant as described.34,39 40Twenty-four hours later, the cells were plated on γ-irradiated OP9 stromal cells in IMDM plus 10% heat-inactivated fetal calf serum and 50 μmol/L β-mercaptoethanol. Five days later, the cells were washed and recultured in media A. Three days later, the cells were washed and recultured in media B. Three days later, the cells were harvested by gentle pipetting and then analyzed by flow cytometry.
RESULTS
Establishment of EML cells that ectopically express BSAP/Pax5A.
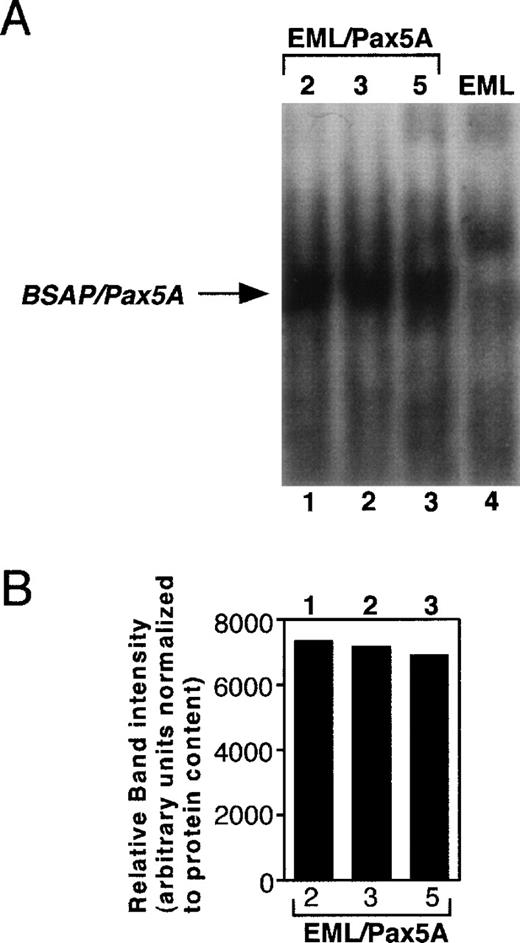
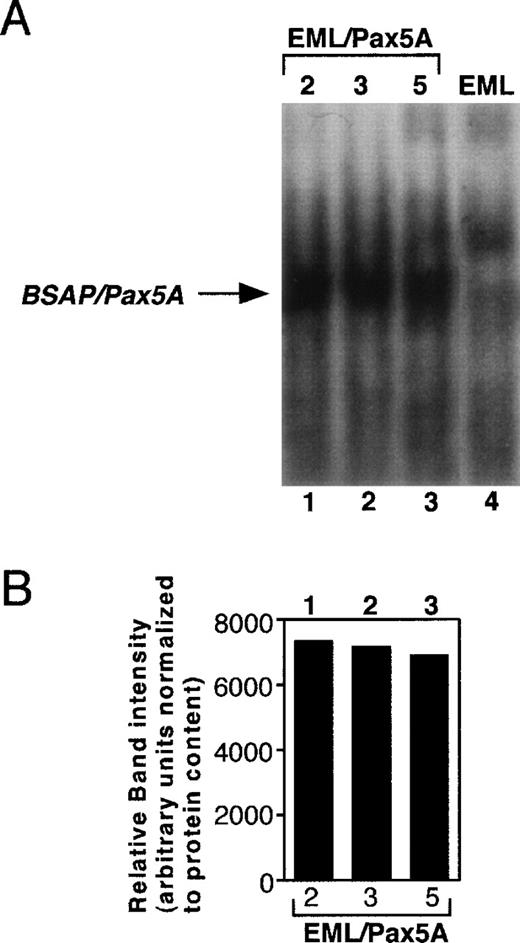
To test whether BSAP/Pax5A affects the differentiation of stem cells to myeloid cells, murine BSAP/Pax5A under the control of the constitutive PGK promoter (pPGKPax5A) was stably transfected into the EML cell line, a bone marrow-derived multipotent progenitor that expresses some markers associated with the B-lymphoid lineage as well as a dominant-negative retinoic acid receptor that blocks spontaneous differentiation to the myeloid lineage at low levels of ATRA.35 In the presence of IL-3 and high levels of ATRA, these cells undergo myeloid lineage differentiation, which can be followed by the de novo acquisition of the myeloid-restricted markers CD11b and F4/80 (previous studies41-44 and M. Chiang and J. Monroe, unpublished observations). The addition of GM-CSF causes further maturation to CD11b+/Gr-1+ granulocytes (M. Chiang and J. Monroe, unpublished observations). Three EML cell clones (EML/Pax5A-2, EML/Pax5A-3, and EML/Pax5A-5) were subcloned and shown to express BSAP/Pax5A by EMSA (Fig 1A and B). BSAP/Pax5A was observed as a single band in the stable transfectants but not in the parental EML cells. Furthermore, this specific complex was disrupted by preincubation of the nuclear extracts with an antibody directed against the paired domain of murine BSAP/Pax5A33 and was expressed at approximately one fifth to one fourth the level in pre-B–cell lines (M. Chiang and J. Monroe, unpublished observations). Parental EML cells and 4 EML cells stably transfected with only the hygromycin resistance plasmid (EML/Hyg-1, EML/Hyg-2, EML/Hyg-3, and EML/Hyg-4) were used as negative controls to study the effects of BSAP/Pax5A on lineage potential and differentiation.
Establishment of BSAP/Pax5A-expressing EML cells. Parental EML cells (EML) were stably transfected with pPGKHygro and pPGKPax5A (the cDNA of Pax5A downstream of the PGK-1 promoter). Hygromycin-resistant cells were subcloned by limiting dilution and 5 μg of nuclear extract of individual clones containing pPGKPax5A detected by genomic PCR (M. Chiang and J. Monroe, unpublished observations) were subjected to EMSA with a32P-end-labeled probe containing the high-affinity BSAP/Pax5A binding site isolated from the huCD19 promoter. (A) EML, parental wild-type EML; EML/Pax5A-2, -3, and -5, BSAP/Pax5A-expressing EML clones. (B) The intensity of the BSAP/Pax5A:probe complexes were quantitated using a densitometer.
Establishment of BSAP/Pax5A-expressing EML cells. Parental EML cells (EML) were stably transfected with pPGKHygro and pPGKPax5A (the cDNA of Pax5A downstream of the PGK-1 promoter). Hygromycin-resistant cells were subcloned by limiting dilution and 5 μg of nuclear extract of individual clones containing pPGKPax5A detected by genomic PCR (M. Chiang and J. Monroe, unpublished observations) were subjected to EMSA with a32P-end-labeled probe containing the high-affinity BSAP/Pax5A binding site isolated from the huCD19 promoter. (A) EML, parental wild-type EML; EML/Pax5A-2, -3, and -5, BSAP/Pax5A-expressing EML clones. (B) The intensity of the BSAP/Pax5A:probe complexes were quantitated using a densitometer.
Enforced expression of BSAP/Pax5A does not suppress myeloid cell differentiation of EML cells.
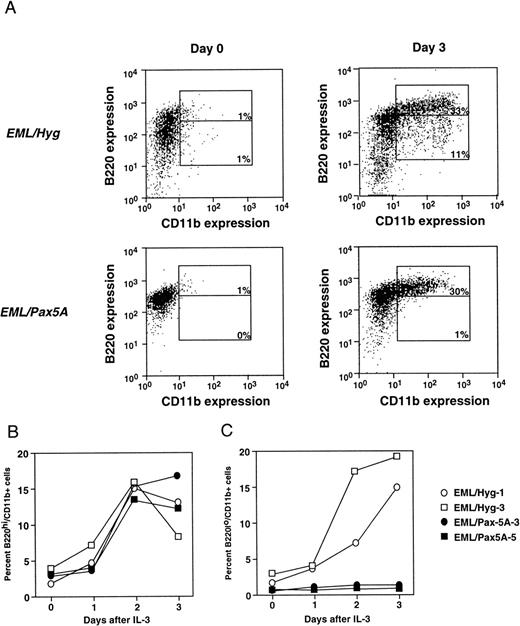
To test whether BSAP/Pax5A affects myeloid lineage potential, BSAP/Pax5A-expressing EML cells were induced to differentiate to the myeloid lineage with the addition of IL-3 and ATRA. Undifferentiated EML cells are B220+ and lack markers associated with myeloid commitment such as CD11b and F4/80. Therefore, differentiation to the myeloid lineage was followed by analyzing for the acquisition of CD11b and F4/80. On day 3 after induction, in a typical experiment, BSAP/Pax5A-expressing EML cells generated approximately the same percentage of B220hi/CD11b+ cells compared with controls (33% for EML/Pax5A v 30% for EML/Hyg; Fig 2A). These percentages reflect absolute numbers of cells, because the average difference in total cell number between the EML/Hyg and EML/Pax5A cultures over 6 independent experiments was only 5.7% and ranged up to 20.3%. There was also no difference in the kinetics of the accumulation of the percentage of B220hi/CD11b+ cells by EML/Pax5A-3 and EML/Pax5A-5 compared with EML/Hyg-1 and EML/Hyg-3 over the 3-day period (Fig 2B).
BSAP/Pax5A-expressing EML cells successfully acquire the myeloid lineage commitment marker CD11b. (A) B220+/CD11b− EML cells were placed in culture at 3 × 105 cells/mL and then induced to differentiate into B220hi/CD11b+ and B220lo/CD11b+ cells with 10% WEHI-3B conditioned supernatant (contains murine IL-3) and 10 μmol/L ATRA. Flow cytometry was performed on days 0 and 3. Control EML clones EML/Hyg-1 and EML/Hyg-3 and Pax5A/BSAP-expressing EML clones EML/Pax5A-3 and EML/Pax5A-5 were induced to differentiate as described in (A). On days 1, 2, and 3 of differentiation, a small sample of cells was removed from the culture, and differentiation to B220hi/CD11b+ (B) and B220lo/CD11b+ (C) cells was determined by flow cytometry.
BSAP/Pax5A-expressing EML cells successfully acquire the myeloid lineage commitment marker CD11b. (A) B220+/CD11b− EML cells were placed in culture at 3 × 105 cells/mL and then induced to differentiate into B220hi/CD11b+ and B220lo/CD11b+ cells with 10% WEHI-3B conditioned supernatant (contains murine IL-3) and 10 μmol/L ATRA. Flow cytometry was performed on days 0 and 3. Control EML clones EML/Hyg-1 and EML/Hyg-3 and Pax5A/BSAP-expressing EML clones EML/Pax5A-3 and EML/Pax5A-5 were induced to differentiate as described in (A). On days 1, 2, and 3 of differentiation, a small sample of cells was removed from the culture, and differentiation to B220hi/CD11b+ (B) and B220lo/CD11b+ (C) cells was determined by flow cytometry.
In contrast, on day 3 after induction, EML/Pax5A cells generated a lower percentage of B220lo/CD11b+ cells (1% for EML/Pax5A v 11% for EML/Hyg), which represent a more mature stage of myeloid development than the B220hi/CD11b+ cells because nearly 100% of the B220lo/CD11b+ cells express the mature myeloid marker F4/80, in contrast to only 68% of the B220hi/CD1lb+ cells (Fig 2A and M. Chiang and J. Monroe, unpublished observations). The accumulation of the percentage of B220lo/CD11b+ by EML/Pax5A-3 and EML/Pax5A-5 compared with EML/Hyg-1 and EML/Hyg-3 was suppressed over the 3-day period (Fig 2C).
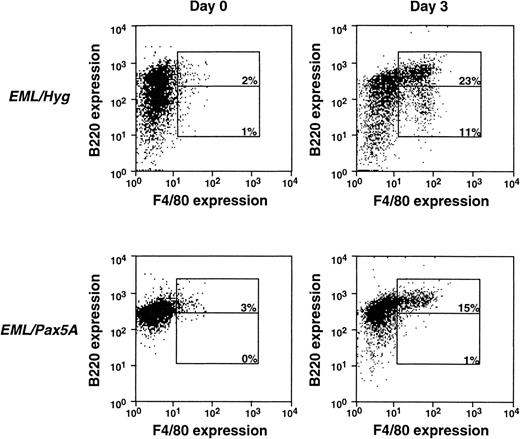
Although CD11b is an early appearing myeloid-restricted marker, it has been known to be expressed at a low level in other cell types, such as the fetal liver stem cells,45 activated/memory CD8+ T cells,46 and B1 B cells.47Thus, we repeated the experiments shown in Fig 2A with the F4/80 antigen, a marker that appears later in myeloid differentiation than CD11b, but is more tightly restricted to the myeloid lineage (Fig 3).41,42 44 In a typical experiment, the EML/Hyg cells generated approximately 23% B220hi/F4/80+ cells and 11% B220lo/F4/80+ cells, whereas EML/Pax5A cells generated 15% B220hi/F4/80+ cells and 1% B220lo/F4/80+ cells. These data using the F4/80 marker confirm the experiments using the CD11b marker in Fig 2 and reinforce the point that BSAP/Pax5A does not prohibit myeloid differentiation, as evidenced by the successful de novo acquisition of the CD11b and F4/80 markers during differentiation of the EML/Pax5A cells. However, the negative effect of BSAP/Pax5A on myeloid cell maturation/expansion subsequent to the initiation of myeloid cell differentiation, as reflected in the diminished generation of B220lo/CD11b+ cells and B220lo/F4/80+ cells, was of particular interest.
BSAP/Pax5A-expressing EML cells successfully acquire the myeloid lineage commitment marker F4/80. B220+/F4/80− EML cells were placed in culture at 3 × 105 cells/mL and then induced to differentiate into B220hi/F4/80+ and B220lo/F4/80+ cells with 10% WEHI-3B conditioned supernatant (contains murine IL-3) and 10 μmol/L ATRA. Flow cytometry was performed on days 0 and 3.
BSAP/Pax5A-expressing EML cells successfully acquire the myeloid lineage commitment marker F4/80. B220+/F4/80− EML cells were placed in culture at 3 × 105 cells/mL and then induced to differentiate into B220hi/F4/80+ and B220lo/F4/80+ cells with 10% WEHI-3B conditioned supernatant (contains murine IL-3) and 10 μmol/L ATRA. Flow cytometry was performed on days 0 and 3.
BSAP/Pax5A inhibits the expansion of EML-derived myeloid progenitors.
To test whether BSAP/Pax5A affected further maturation/expansion of the EML cells subsequent to the initial stages of myeloid lineage differentiation, BSAP/Pax5A-expressing EML cells were induced with IL-3 and ATRA, washed, and recultured in methylcellulose media containing GM-CSF. GM-CSF supports the further maturation and expansion of myeloid precursors from progenitors that are dependent on IL-3.35The colonies derived from BSAP/Pax5A-expressing EML cells appeared to be unusually small compared with those derived from EML/Hyg cells (M. Chiang and J. Monroe, unpublished observations). To quantitate this observation, the colonies were counted, aspirated, and analyzed by flow cytometry. BSAP/Pax5A-expressing colonies on average contained 60% to 95% fewer Gr-1+/CD11b+ cells than did the negative control colonies (Fig4A). These data suggest that BSAP/Pax5A inhibits the ability of myeloid cells to expand in response to myeloid growth factors.
BSAP/Pax5A-expressing EML cells fail to expand to myeloid growth factors. (A) EML cells were induced to differentiate into myeloid cells as described in Fig 2A and then replated in 1% methylcellulose with 250 U/mL muGM-CSF at 1.5 × 105 cells per 3.5-cm plate. On day 9, the colonies were aspirated and analyzed by flow cytometry to determine the mean number of CD11b+/Gr-1+ cells per colony. The average among 3 control EML/Hyg clones and the average among 3 BSAP/Pax5A-expressing EML clones are shown and were found to be significantly different from each other using the heteroscededastic 1-tailed t-test (P < .001). (B) EML cells induced to differentiate as described in Fig 2A were replated in suspension culture in the presence of 250 U/mL muGM-CSF. After 48 hours of culture, the cells were pulsed with 0.5 μCi 3H-thymidine for 12 hours. The average 3H-thymidine incorporation among 3 control EML/Hyg clones and the average 3H-thymidine incorporation among 3 EML/Pax5A clones are shown and were found to be significantly different from each other using the heteroscededastic 1-tailed t-test (P < .001). (C) Three EML/Pax5A clones and 3 control EML/Hyg clones were split into SCF-containing growth media at 2.5 × 105 cells/mL and pulsed with3H-thymidine after 24 hours of culture for 17 hours. The average 3H-thymidine incorporation among 3 control EML/Hyg clones and the average 3H-thymidine incorporation among 3 EML/Pax5A clones are shown and were found to be significantly different from each other using the homoscededastic 2-tailed t-test (P < .004). (D) EML cells induced to differentiate as described in Fig 2A were replated at 5 × 105 cells/mL in 400 μL media containing 250 U/mL muGM-CSF. Two days later, the cells were stained with propidium iodide and then analyzed for apoptotic cells by flow cytometry. The average percentage of 3 EML/Hyg clones with subdiploid content of DNA and the average percentage of 3 EML/Pax5A clones with subdiploid content of DNA are shown and were found to be significantly different from each other using the homoscededastic 1-tailed t-test (P < .002).
BSAP/Pax5A-expressing EML cells fail to expand to myeloid growth factors. (A) EML cells were induced to differentiate into myeloid cells as described in Fig 2A and then replated in 1% methylcellulose with 250 U/mL muGM-CSF at 1.5 × 105 cells per 3.5-cm plate. On day 9, the colonies were aspirated and analyzed by flow cytometry to determine the mean number of CD11b+/Gr-1+ cells per colony. The average among 3 control EML/Hyg clones and the average among 3 BSAP/Pax5A-expressing EML clones are shown and were found to be significantly different from each other using the heteroscededastic 1-tailed t-test (P < .001). (B) EML cells induced to differentiate as described in Fig 2A were replated in suspension culture in the presence of 250 U/mL muGM-CSF. After 48 hours of culture, the cells were pulsed with 0.5 μCi 3H-thymidine for 12 hours. The average 3H-thymidine incorporation among 3 control EML/Hyg clones and the average 3H-thymidine incorporation among 3 EML/Pax5A clones are shown and were found to be significantly different from each other using the heteroscededastic 1-tailed t-test (P < .001). (C) Three EML/Pax5A clones and 3 control EML/Hyg clones were split into SCF-containing growth media at 2.5 × 105 cells/mL and pulsed with3H-thymidine after 24 hours of culture for 17 hours. The average 3H-thymidine incorporation among 3 control EML/Hyg clones and the average 3H-thymidine incorporation among 3 EML/Pax5A clones are shown and were found to be significantly different from each other using the homoscededastic 2-tailed t-test (P < .004). (D) EML cells induced to differentiate as described in Fig 2A were replated at 5 × 105 cells/mL in 400 μL media containing 250 U/mL muGM-CSF. Two days later, the cells were stained with propidium iodide and then analyzed for apoptotic cells by flow cytometry. The average percentage of 3 EML/Hyg clones with subdiploid content of DNA and the average percentage of 3 EML/Pax5A clones with subdiploid content of DNA are shown and were found to be significantly different from each other using the homoscededastic 1-tailed t-test (P < .002).
To confirm that BSAP/Pax5A was suppressing myeloid cell expansion, the BSAP/Pax5A-expressing EML cells were induced with IL-3 and ATRA, washed, recultured in media containing GM-CSF, and then subjected to proliferation and apoptosis assays. Proliferation of BSAP/Pax5A-expressing EML cells in response to GM-CSF was markedly reduced relative to non–BSAP/Pax5-expressing controls (Fig 4B). This failure to expand is unlikely to result from a nonspecific suppression by BSAP/Pax5A on the expansion of EML cells, because undifferentiated BSAP/Pax5A-expressing EML cells have a slightly but significantly higher expansion rate than control cells in response to SCF (Fig 4C). Instead of expanding in response to GM-CSF, the BSAP/Pax5A-expressing EML cells underwent a 15-fold increase in the rate of apoptosis as compared with controls (Fig 4D). In combination with the colony size data, these data strongly suggest that BSAP/Pax5A inhibits the ability of myeloid cells to expand specifically in response to myeloid growth factors. This reduced proliferative capacity results in a marked reduction in the generation of more mature myeloid cells, an effect that is then exacerbated by the high apoptotic frequency in the BSAP/Pax5A-expressing population.
BSAP/Pax5A was introduced retrovirally into primary bone marrow stem cells to verify the BSAP/Pax5A-mediated suppression of myeloid cell expansion in a nontransformed cell.
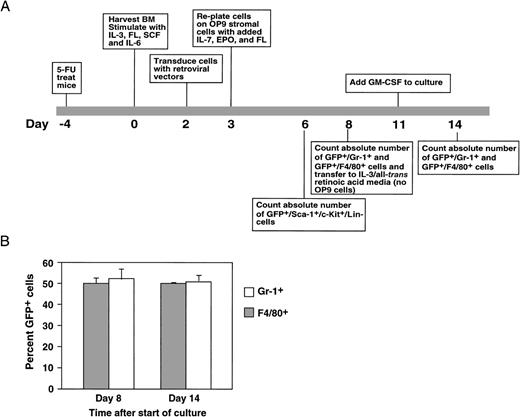
Stem cells transduced with BSAP/Pax5A-expressing retrovirus were tested for the ability to differentiate to the myeloid lineage and expand during 5 days of culture on OP9 stromal cells, followed by 3 days of IL-3/ATRA and 3 days of GM-CSF (Fig 5A). The last 6 days of culture were designed to replicate the conditions used in the experiments described in Fig 4, which demonstrated that EML-derived myeloid precursors failed to expand in response to GM-CSF. OP9 stromal cells were derived from the op/op mouse, which lacks functional macrophage colony-stimulating factor (M-CSF).36 Because of this mutation, hematopoietic cultures are not artificially skewed predominantly toward macrophages, as has been seen with other stromal cell lines (M. Chiang and J. Monroe, unpublished observations).36 Rather, normal bone marrow hematopoiesis is more closely approximated, including the efficient generation of early CFU-GM myeloid precursors, erythroid cells, nonmacrophage lineage myeloid cells such as granulocytes, B lymphocytes, and mature macrophages.36 Thus, the OP9 system offers the unique advantage of a more physiologically relevant in vitro bone marrow culture system and more efficient generation of GM-CSF/IL-3 responsive precursors and nonmacrophage myeloid cells.
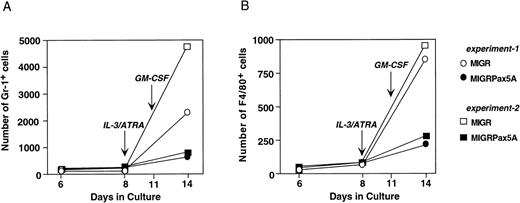
Retroviral transduction of bone marrow stem cells with MIGRPax5A. (A) Bone marrow stem cells from 5-fluorouracil–treated mice were transduced with MIGRPax5A, a retrovirus that coexpresses BSAP/Pax5A with the GFP marker as a bicistronic message, and then analyzed by flow cytometry for GFP+ stem cells (c-Kit+/Sca-1+/Lin−). These stem cells were then differentiated as indicated and on days 6, 8, and 14. A small sample of cells was then removed from the culture and the numbers of myeloid cells were determined by flow cytometric analysis for granulocytes (Gr-1+) and macrophages (F4/80+). MIGR is the parental retrovirus of MIGRPax5A and expresses only the GFP marker. (B) Bone marrow stem cells were transduced with the MIGR retrovirus, cultured as described in (A), and then analyzed for the percentage of GFP+/Gr-1+ cells and GFP+/F4/80+ cells on days 8 and 14 by flow cytometry.
Retroviral transduction of bone marrow stem cells with MIGRPax5A. (A) Bone marrow stem cells from 5-fluorouracil–treated mice were transduced with MIGRPax5A, a retrovirus that coexpresses BSAP/Pax5A with the GFP marker as a bicistronic message, and then analyzed by flow cytometry for GFP+ stem cells (c-Kit+/Sca-1+/Lin−). These stem cells were then differentiated as indicated and on days 6, 8, and 14. A small sample of cells was then removed from the culture and the numbers of myeloid cells were determined by flow cytometric analysis for granulocytes (Gr-1+) and macrophages (F4/80+). MIGR is the parental retrovirus of MIGRPax5A and expresses only the GFP marker. (B) Bone marrow stem cells were transduced with the MIGR retrovirus, cultured as described in (A), and then analyzed for the percentage of GFP+/Gr-1+ cells and GFP+/F4/80+ cells on days 8 and 14 by flow cytometry.
Retrovirus containing BSAP/Pax5A cDNA was called MIGRPax5A and was derived from the parental retrovirus MIGR.34 To differentiate transduced cells from nontransduced cells, these retroviruses express the GFP marker from an internal ribosomal entry site. Thus, cells that express GFP also coexpress BSAP/Pax5A as a bicistronic message. To correct for differences in the retroviral transduction efficiencies of hematopoietic stem cells from experiment to experiment, the GFP+ myeloid cells were normalized to the number of GFP+c-Kit+/Sca-1+/Lin− cells 72 hours after transduction (which allows time for GFP to be fully expressed). The c-Kit+/Sca-1+/Lin− bone marrow subset is highly enriched for the most primitive hematopoietic stem cells, which have long-term multilineage reconstitution potential.48 49 Normalization to this population ensures that any reduction in the number of GFP+ myeloid cells generated is not due to a lower number of stem cells that were successfully transduced but is rather due to the myelosuppressive effect of BSAP/Pax5A.
In all experiments, the level of GFP expression in MIGRPax5A-transduced hematopoietic stem cells (identified by sca-1+/c-kit+/lin−) was generally lower than that in MIGR-transduced hematopoietic stem cells. In a typical experiment, the mean fluorescence intensity of GFP+ hematopoietic stem cells transduced with MIGRPax5A was 41.37 ± 3.83, whereas the mean fluorescence intensity of GFP+ hematopoietic stem cells transduced with MIGR was 141.68 ± 0.02. This difference unlikely reflects uneven promoter activity between the 2 populations, but rather is likely the result of the integration of a large cDNA insert upstream of the GFP marker in the MIGRPax5A retrovirus but not in the MIGR retrovirus (Chiang et al, unpublished observations).
Because the expression levels of GFP were different in the control and experimental cells, we determined the effect of GFP and retroviral transduction on myeloid differentiation. MIGR-transduced stem cells were cocultured with nontransduced stem cells and found to generate approximately 50% of the Gr-1+ cells and 50% of the F4/80+ cells present in the cultures on day 8 and on day 14 (Fig 5B). Thus, the frequency of transduced myeloid cells did not change over time, verifying that neither expression of GFP nor retroviral transduction had any measurable effects on myelopoiesis.
BSAP/Pax5A does not inhibit myeloid differentiation of primary stem cells but does inhibit the expansion of myeloid progenitors.
Primary bone marrow stem cells were transduced with either MIGR or MIGRPax5A retrovirus and then cultured in vitro according to the scheme in Fig 5A to generate myeloid cells. When cultured without myeloid growth factors during the first 8 days, MIGRPax5A-transduced stem cells and control MIGR-transduced stem cells generated approximately equal numbers of Gr-1+ and F4/80+ cells (days 6 and 8 of culture, Fig 6A and B). However, upon addition of IL-3 and ATRA for the next 3 days, followed by GM-CSF for another 3 days, which simulated the experimental conditions used to differentiate EML cells in Fig 4, MIGRPax5A-transduced stem cells generated 60% to 85% fewer Gr-1+ cells (Fig 6A) and 60% to 75% fewer F4/80+ cells (Fig 6B) than did MIGR-transduced controls when analyzed on day 14 of culture. This suppression of myeloid cell generation was not observed with stem cells that were cultured without growth factors for the entire 2 weeks of culture (Fig 6A and B and M. Chiang and J. Monroe, unpublished observations), but rather was observed only when the stem cells were cultured in the presence of myeloid growth factors. These data suggest that BSAP/Pax5A does not completely suppress myeloid cell differentiation, but does suppress myeloid cell expansion to myeloid growth factors, which is consistent with our interpretation of the EML studies.
MIGRPax5A-transduced bone marrow cells successfully committed to the myeloid lineage but failed to expand to myeloid growth factors. Bone marrow stem cells were transduced with MIGR or MIGRPax5A and then cultured on OP9 stromal cells in 2 experiments according to the protocol described in Fig 5A. The absolute number of GFP+ gr-1+ cells (A) or F4/80+ cells (B) was normalized to the absolute number of Sca-1+/c-Kit+/Lin− stem cells on day 6 to estimate the number of granulocytes or macrophages produced by a retrovirally transduced stem cell. Ten percent WEHI-3B conditioned media and 10 μmol/L ATRA were added on day 8 and 250 U/mL of muGM-CSF was added on day 11 to replicate the conditions of Fig 4 in which EML/Pax5A cells generated myeloid precursors that failed to expand.
MIGRPax5A-transduced bone marrow cells successfully committed to the myeloid lineage but failed to expand to myeloid growth factors. Bone marrow stem cells were transduced with MIGR or MIGRPax5A and then cultured on OP9 stromal cells in 2 experiments according to the protocol described in Fig 5A. The absolute number of GFP+ gr-1+ cells (A) or F4/80+ cells (B) was normalized to the absolute number of Sca-1+/c-Kit+/Lin− stem cells on day 6 to estimate the number of granulocytes or macrophages produced by a retrovirally transduced stem cell. Ten percent WEHI-3B conditioned media and 10 μmol/L ATRA were added on day 8 and 250 U/mL of muGM-CSF was added on day 11 to replicate the conditions of Fig 4 in which EML/Pax5A cells generated myeloid precursors that failed to expand.
DISCUSSION
During hematopoiesis, developing cells increasingly become more restricted with regard to their potential to develop along multiple lineages. Lineage-determinant transcription factors are thought to drive this commitment process by upregulating the genes of a specific lineage and downregulating genes specific to other lineages. For example, the transcription factor E12 is thought to prevent B cells from acquiring myeloid characteristics, because ectopic expression of E12 in a macrophage cell line upregulated B-cell genes such as λ5, BSAP/Pax5A, EBF, andIL-7Rα and downregulated myeloid-specific genesCD11b and c-fms.5 Because BSAP/Pax5A is upregulated by E12,5 is itself a transcription factor that is thought to upregulate B-cell–associated genes,23,24,27-31,50-61 and is required for B-cell commitment, as determined by gene deletion studies,24 it was of particular interest to determine if BSAP/Pax5A like E12 had B-cell–determinant properties.
To test whether BSAP/Pax5A had B-cell–determinant properties, BSAP/Pax5A was constitutively expressed in the EML cell. Surprisingly, BSAP/Pax5A-expressing EML cells did not upregulate the B-cell–specific genes CD19 or mb-1 (M. Chiang and J. Monroe, unpublished observations). This finding suggested that BSAP/Pax5A, although required for the expression of CD19 and mb-1 in pre-B cells, as demonstrated by loss of function analysis,31 is not sufficient for their expression, presumably because of the absence of requisite cofactors or presence of suppressive factors in the EML cell line. This finding is consistent with the E12 study discussed earlier, which found that, although BSAP/Pax5A was upregulated in EBF-expressing 70Z/3 macrophages, only one B-cell gene, λ5, was also upregulated.5 Furthermore, BSAP/Pax5A is unlikely to be responsible for this λ5 upregulation, because gain-of-function/loss-of-function genetic studies have shown that λ5 is a target gene of EBF, but not of BSAP/Pax5A.24,31 62 Therefore, in EML cells and 70Z/3 macrophages, BSAP/Pax5A is not sufficient to upregulate the B-cell genes that were examined.
BSAP/Pax5A-expressing EML cells differentiated to the myeloid lineage in response to IL-3 by successfully acquiring de novo the myeloid-restricted markers CD11b and F4/80. However, these early myeloid cells then failed to expand in response to GM-CSF. To confirm these results in a nontransformed cell, BSAP/Pax5A was retrovirally transduced into primary bone marrow stem cells. When cultured in vitro, BSAP/Pax5A-transduced stem cells successfully differentiated to the myeloid lineage. However, the addition of myeloid growth factors to these cultures showed a defect in cell expansion. Taken together, the EML cell studies and primary bone marrow studies share consistent results and thus provide strong evidence that BSAP/Pax5A, although unable to completely suppress myeloid lineage differentiation, does suppress the expansion response to myeloid growth factors, a characteristic myeloid trait acquired shortly after the initiation of myeloid commitment.
In this study, BSAP/Pax5A was ectopically expressed in stem cells to demonstrate that BSAP/Pax5A can suppress myeloid cell expansion. However, in vivo, BSAP/Pax5A is not expressed in multipotential stem cells, but later in the fraction A1 stage of B-cell development after B-cell commitment has already occurred.15,17,23 It is formally possible that BSAP/Pax5A is expressed in rare multipotential progenitors at low levels undetectable by reverse transcriptase-polymerase chain reaction (RT-PCR) and that a higher level of BSAP/Pax5A expression in these cells would prevent them from responding to myeloid growth factors. However, because this possibility seems remote due to the high sensitivity of PCR, the finding of BSAP/Pax5A-mediated suppression of myeloid expansion from stem cells more likely supports a role for BSAP/Pax5A in preventing B cells from responding to myeloid growth factors. This effect may in part maintain commitment to the B lineage as some myeloid growth factors, notably IL-3 and GM-CSF,63 have been shown to convert certain pre-B cell lines to the myeloid lineage.
Although early B cells in vivo seem to acquire some myeloid traits, such as low levels of CD11b,15 they are prevented from expanding in response to myeloid growth factors and further maturation,15 perhaps in part due to BSAP/Pax5A expression. Because BSAP/Pax5A is not sufficient to completely prevent maturation down the myeloid lineage, especially in the absence of growth factors, there probably exists additional lineage-determinant genes that act singly or together with BSAP/Pax5A to suppress myeloid differentiation. These genes are likely to be target genes of E12 in view of previous studies already discussed in which ectopic expression of E12 in a macrophage cell line completely suppressed all myeloid characteristics that were reported.5
Whether BSAP/Pax5A is sufficient to suppress myeloid expansion in vivo is currently being studied by generating mice transgenic for BSAP/Pax5A. This question is particularly interesting, because studies involving myeloid growth factor-deficient mice have indicated the existence of multiple redundant mechanisms that can drive myelopoiesis.64 These mice display either normal or reduced (but never abrogated) myelopoiesis. For example, IL-3/GM-CSFR/IL-5R–deficient mice,65 IL-3–deficient mice,65 and GM-CSF–deficient mice66 all display normal steady-state myelopoiesis. Granulocyte colony-stimulating factor (G-CSF)–deficient mice display neutropenia,67 which is somewhat exacerbated when the GM-CSF gene is additionally deleted.68 M-CSF–deficient mice have fewer macrophages and osteoclasts, but these cells accumulate to near normal levels in older mice.69-71 Thus, the number of growth factor responses that can be suppressed by BSAP/Pax5A will dictate the degree to which enforced expression of BSAP/Pax5A will suppress myelopoiesis in vivo. We predict, based on our studies reported here, that IL-3–mediated responses may remain largely unaffected in the presence of BSAP/Pax5A expression.
How BSAP/Pax5A suppresses myelopoiesis has proven elusive in part because the effect of BSAP/Pax5A on the function and expression of the downstream mediators of myeloid growth factor signal transduction cascade is unknown. Because the paired domain of BSAP/Pax5A can bind the transcription factor Ets-1, which is itself involved in the regulation of many myeloid genes,72 it was postulated that BSAP/Pax5A could suppress Ets-1–mediated transactivation. However, transient transfection of BSAP/Pax5A failed to inhibit Ets-1–mediated transactivation of the CD18 promoter (M. Chiang and J. Monroe, unpublished observations). Because BSAP/Pax5A suppresses the expression of c-Myc in pre-B cells,31 it was also postulated that, in myeloid cells, BSAP/Pax5A would similarly suppress c-Myc, which is upregulated during GM-CSF stimulation.73-76 However, transcripts of c-Myc in BSAP/Pax5A-expressing EML cells were comparable to that in controls after induction with IL-3 followed by stimulation with GM-CSF (M. Chiang and J. Monroe, unpublished observations). Currently, the effect of BSAP/Pax5A on the expression of the various myeloid growth factor receptors, such as IL-3Rα, β-common, IL-3Rβ, and GM-CSFRα, is being studied.
These studies expand on the results of the earlier E12 study by using cells that are growth-factor dependent, early in development, and nontransformed. Using growth-factor–dependent cells instead of the growth factor-independent macrophage cell line 70Z/35 has shown that BSAP/Pax5A affects the growth response of cells to myeloid growth factors. Using progenitor stem cells instead of mature macrophages has shown that BSAP/Pax5A can act in a model of early development to prevent the acquisition of the myeloid growth factor response, one of the earliest appearing myeloid-specific characteristics after myeloid commitment. This aspect of our studies is relevant, because anecdotal reports of lineage switching to myeloid cells in vitro have almost always involved cells early in development when plasticity is thought to be greatest.6-9 In this regard, our studies provide additional evidence that suppressive factors, which prohibit the acquisition of lineage-specific traits, may be integral to maintaining B-lineage commitment as developing cells chose between the B-cell and myeloid fates.
ACKNOWLEDGMENT
The authors thank Dr M. Busslinger for providing the pmBSAP-2 plasmid and the anti-paired domain antisera, Dr S. Tsai for providing the EML and BHK/MKL cell lines, Dr F. Melchers and A. Groenewegen for providing the J558-IL-7 cell line, Dr W. Pear for providing the Mig R1 plasmid and Bosc23 cell line, P. Sandel and Dr M. Atchison for critical reading of the manuscript, and Dr L. King and Dr L. Xu for assistance in designing the experimental protocols.
Supported in part by Grants No. AI23568, AI 32592, and AI43620 from the National Institutes of Health and the Medical Scientist Training Program.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John G. Monroe, PhD, Room 311, Biomedical Research Bldg II/III, 421 Curie Blvd, Philadelphia, PA 19104-6142.