Abstract
The generation of autoantibodies and deposition of immune complexes (ICs) in tissue play a primary role in autoimmune diseases. However, the IC-triggered response consists of complex mechanisms that make it difficult to identify the pathogenesis and develop specific therapy. We clarified here a sequential mechanism for the induction of hypersensitivity angiitis by analyzing the responsible Fc receptor (FcR), effector cells, and mediators in an animal model using FcR-deficient mice. In this model, rheumatoid factor-mediated skin vasculitis was induced in wild-type mice, whereas FcRγ-deficient mice did not develop the vasculitis. Adoptive transfer of various FcR+ cells into FcRγ-deficient mice showed that mast cells but not macrophages derived from wild-type mice triggered skin vasculitis. Mast cells derived from either FcγRIII-deficient or tumor necrosis factor (TNF)-deficient mice did not possess the inducibility of skin vasculitis. These results indicate that triggering of vascular inflammation was induced by mast cells through IC binding on FcγRIII. TNF produced by such activated mast cells was mainly responsible for the pathogenesis of autoantibody-mediated vasculitis. These findings illustrate the clinical significance of mast cells, Fcγ receptors, and TNF in IC-induced vasculitis syndrome.
VASCULITIS IS A clinicopathologic process characterized by inflammation and necrosis of blood vessels that leads to vessel occlusion and ischemia of tissues. Vasculitis may occur as a primary process or as a component of other underlying diseases. The pathogenesis of vasculitis involves a variety of mechanisms acting in concert to bring about necrotizing inflammation of vessel walls. Although most types of vasculitis are associated with immunologic abnormalities, the primary immunopathogenic events for initiating the vascular inflammation are still unknown.
In some human vasculitis syndromes, elevated levels of circulating immune complexes (ICs) and deposits of complement and Ig in vasculitic lesions are frequently observed, implying that ICs might play a primary role in their pathogenesis. These syndromes include Henoch-Schönlein purpura, essential cryoglobulinemia, hepatitis B-associated polyarteritis nodosa (PAN), and vasculitis associated with collagen diseases and are collectively called hypersensitivity angiitis. Although these syndromes have long been suspected of being mediated mainly by complement activation, just how the pathogenic IC formation initiates the process of inflammation is still not fully understood.
IgG Fc receptors (FcγRs) are expressed primarily on immune effector cells and link cellular and humoral immunity and trigger inflammatory activities of effector cells. There are 3 classes of FcγR: FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16). Whereas FcγRI is a high-affinity receptor, FcγRII and FcγRIII are low-affinity receptors, which bind only polymeric IgG or IC. Whereas FcγRII is a single-chain receptor, both FcγRI and FcγRIII require the association of γ chains (FcRγ) with a ligand-binding α chain for surface expression and signal transduction. FcRγ is also associated with high-affinity IgE receptor (FcεRI). Indeed, FcRγ-deficient mice lack the functional expressions of FcγRI, FcγRIII, and FcεRI.1,2 Recent analyses of various FcR-deficient mice have shown that FcR plays crucial roles in IC-triggered inflammation.1-6 FcRγ-deficient mice were unable to induce IgG-mediated phagocytosis by macrophages and IgE-mediated anaphylaxis by mast cells.1 More importantly, it was shown that Arthus reaction induced by specific ICs was severely reduced in both FcRγ- and FcγRIII-deficient mice.4,5 FcR-deficient mice also exhibited reduced experimental hemolytic anemia and thrombocytopenia,3 Ab-dependent antitumor immunity,7 and anti-glomerular basement membrane (GBM) Ab-induced or genetically occurring glomerulonephritis.2,8These results of experimental inflammatory type II and III responses suggest the possibility that a wide range of inflammatory diseases and related autoimmune diseases may be initiated by FcR-dependent mechanisms. Therefore, we analyzed the contribution of FcR to the pathogenesis of vasculitis as a model of human hypersensitivity angiitis by using FcRγ-deficient mice2 and then analyzed the sequential mechanisms, including identification of the responsible effector cells, receptors, and mediators using various gene-knockout mice.
MATERIALS AND METHODS
Mouse.
C57BL/6 and Balb/c mice were purchased from the Shizuoka Laboratory Animal Corp (Hamamatsu, Japan). The establishment and characteristics of FcRγ-, FcγRIII-, and tumor necrosis factor (TNF) αβ-deficient mice have been described previously.2,4,9,10 FcRγ−/− mice were established with C57BL/6 background by the use of C57BL/6 ES cell line, and FcγRIII- and TNFαβ-deficient mice derived from ES cells with 129-origin were backcrossed several generations with C57BL/6 mice. For the present experiments, these knockout mice were crossed again with BALB/c mice to obtain Igha allotype background to be recognized by 6-19 monoclonal antibody (MoAb).11 The offsprings were intercrossed to obtain FcRγ+/− and FcRγ−/− mice. The FcRγ alleles were analyzed with tail DNA by polymerase chain reaction (PCR) using pairs of primers: the pair of 5′-GGAATTCGCTGCCTTTCGGACCTGGAT-3′ (exon 3-specific) and 5′-GCCAACGCTATGTCCTGATAG-3′ (neor-specific) for the targeted allele, and the other pair of the former one (exon 3-specific) and 5′-GAAAATCGATGCTGTCCT GTTTTTGTA-3′ (exon 4-specific) for the wild-type allele. All mice were bred and maintained in our own animal facility under SPF conditions and used for experiments at 10 to 15 weeks of age.
Antibodies.
Hybridoma cells producing 6-19 MoAb were established from MRL-lpr/lpr mice11; their characteristics have been extensively analyzed.12-14 Hybridomas producing anti-CD4 (GK-1.5) and anti-CD8 (53-6.7) MoAbs were obtained from ATCC (Rockville, MD) and MoAbs were obtained from ascites and purified over protein A-beads column. Biotinylated IgE (anti-dinitrophenol [DNP] IgE; Sigma, St Louis, MO), anti–c-Kit MoAb (ACK4; kindly provided by Dr Shin-ichi Nishikawa, Department of Molecular Genetics, Kyoto University Graduate School of Medicine, Kyoto, Japan), anti-FcγRII/III (2.4G2), fluorescein isothiocyanate (FITC) anti–Mac-1 MoAb, phycoerythrin (PE) anti–Gr-1 MoAb, and anti-rat IgG (PharMingen, San Diego, CA) were used for cytometric analysis. Anti-TNP MoAb (PharMingen) was used for stimulation of mast cells and macrophages through FcγR as immobilized IgG1.
Cell preparation.
For mast cell preparation, bone marrow cells from wild-type and knockout mice were cultured by 10% fetal calf serum (FCS) supplemented with 15% WEHI-3 conditioned medium as a source of interleukin-3 (IL-3). Nonadherent cells were harvested and resuspended in the same medium weekly. After 4 weeks of culture, greater than 90% of the cells were c-kit+. These bone marrow-derived mast cells were harvested and resuspended in phosphate-buffered saline (PBS) at a density 1 × 106 per 30 μL or 1 × 107 per 300 μL for subcutaneous (SC) and intravenous (IV) injections, respectively. The mast cells were immediately injected into FcRγ−/− mice with or without 6-19 inoculation 7 days before. For macrophage preparation, 3 mL of thioglycolate medium (DIFCO, Detroit, MI) was injected intraperitoneally (IP) into FcRγ+/− littermates. Seven days after injection, the peritoneal cavity was washed with PBS and the fluid was collected. To obtain activated macrophages, 20 μg of lipopolysaccharide (LPS; serotype 0127:B8; Sigma) was injected 2 days before cell harvest. Adherent cells in 2.5 mmol/L EDTA/PBS were harvested and resuspended in PBS at the same densities as for the SC and IV injections described above. Approximately 90% to 95% of the recovered cells were Mac-1+.
Induction of skin vasculitis and glomerulonephritis.
Pristane-treated mice were injected with a mixture of anti-CD4 MoAb (GK1.5) and anti-CD8 MoAb (53-6.7; 500 μg of each MoAb) 1 day before and 1 day after the hybridoma injection. Hybridoma cells instead of 6-19 MoAb were inoculated because both skin vasculitis and glomerulonephritis can be induced only after the implantation of hybridoma cells. Mice were inoculated IP with 107 6-19 hybridoma cells and killed when moribund (generally 7 to 12 days after the implantation of hybridomas). The macroscopic appearance of skin lesions was carefully noted. The ear skin and kidneys were subjected to histological analysis. Positive vasculitis was evaluated by macroscopic observation of skin purpura of the ear and the extravasation of erythrocytes by histological analysis. Leukocyte invasion in the ear lesion was always associated with positive vasculitis, in conjunction with extravasation of erythrocytes.
Histological analysis.
Blocks of ear skin and kidneys were obtained at autopsy and sections were stained with hematoxylin-eosin (HE) and periodic-acid Schiff (PAS) for histopathological examination. Sections were also studied for deposition of IgG3 by direct staining with FITC-conjugated antimouse IgG3 Ab (PharMingen). For staining of mast cells in the skin, ears from wild-type and TNFαβ−/− mice were fixed in formalin, and sections were stained with naphthol AS-D chloroacetate, N,N′-dimethylformaide, and hexazotized new fuchsin, pH 6.3.
Serum titration of Ab and blood urea nitrogen (BUN).
Blood was collected from mice at death for IgG3 and BUN determinations. After removal of fibrin clot at 37°C, sera were placed in conical tubes at 4°C for 3 days to precipitate cryoglobulin. The supernatant was used for BUN determination, whereas the precipitates were used for the measurement of the serum 6-19 MoAb level. The precipitates were washed and resolubilized in 4 mol/L urea in the same volume as the original sera. The solubilized samples were subjected to enzyme-linked immunosorbent assay (ELISA) using plates coated with sheep antimouse Ig Ab. After washing, horseradish peroxidase (HRP)-conjugated sheep antimouse IgG3 (Serotec, Oxford, UK) was added. Then, after incubation with o-Phenylenediamine as a substrate (Wako, Osaka, Japan), the samples were read spectrophotometrically with an ELISA plate reader. J606, the IgG3 class myeloma protein, was used to generate a reference standard curve.
Flow cytometric analysis.
Mast cells were stained with nonlabeled antibody or with biotinylated IgE for 60 minutes on ice, followed by PE anti-rat IgG or PE streptavidine for 40 minutes. Mast cells were also stained with directly labeled antibodies for 60 minutes. After washing, cells were analyzed on a FACScan (Becton Dickinson, Mountain View, CA) using CELL Quest software (Becton Dickinson).
Degranulation assay of mast cells.
Mast cells were incubated with or without 10 μg/mL of anti-DNP mouse IgE for 2 hours at 37°C. The cells were then challenged with 15 ng/mL of DNP-bovine serum albumin (DNP-BSA; Sigma) for 30 minutes. Nonsensitized mast cells stimulated with 200 ng/mL of A23187 (Wako) were used for positive control. The supernatants were collected for measurement of β-hexosaminidase. The cell pellets were lysed with 0.5% Triton X in tyrode buffer (130 mmol/L NaCl, 5 mmol/L KCl, 1.4 mmol/L CaCl2, 1 mmol/L MgCl2, 5.6 mmol/L glucose, 10 mmol/L HEPES, and 0.1% BSA, pH 7.4). Supernatants and cell lysates were incubated with substrate (1.3 mg/mL p-nitrophenyl-N-acetyl β-D-glucosamine [Sigma] in 0.1 mol/L sodium citrate, pH 4.5) for 2 hours at 37°C. The reaction was stopped by 0.2 mol/L glycine and the enzyme reactivity was evaluated by measuring optical density at 405 nm. The percentage of specific β-hexosaminidase release was calculated as follows: percentage re- lease = 100 × supernatant activity/(supernatant activity + cell lysate activity).
Measurement of TNFα.
Mast cells and macrophages were stimulated with 50 μg/mL immobilized mouse IgG1 or 12 μg/mL immobilized 2.4G2 for 30 minutes. Culture supernatants were collected and TNFα levels in supernatant were measured using an ELISA kit (Endogen, Woburn, MA) according to the manufacturer’s protocol.
RESULTS
We have previously shown that a fraction of murine IgG3 anti-IgG2a rheumatoid factor (RF) MoAbs with cryoglobulin activity, typically represented by 6-19 MoAb derived from lupus-prone MRL/MpJ-lpr/lpr (MRL-lpr) mice, induces skin vasculitis in association with IC formation and glomerulonephritis resembling wire-loop glomerular lesions in normal mice.11 12 We used this animal model of hypersensitivity angiitis associated with cryoglobulinemia to analyze the molecular basis of the induction of skin vasculitis.
FcRγ-deficient mice did not develop skin vasculitis.
To determine whether FcγR is involved in the induction of skin vasculitis and glomerulonephritis in vivo, we injected 6-19 hybridoma cells into FcRγ+/− and FcRγ−/− mice and analyzed the development of inflammatory responses. A protocol of injecting purified 6-19 MoAb cannot be used because of its cryoprecipitability. Seven to 12 days after the IP injection of 6-19 hybridoma, FcRγ+/− mice developed vascular purpura on the skin of ears, tails, and footpads. In contrast, none of the FcRγ−/− mice injected with 6-19 hybridoma developed the characteristic purpura (Table 1 and Fig 1A). Histological examination confirmed these results (Fig 2). 6-19–treated FcRγ+/− mice exhibited leukocytoclastic vasculitis in the skin, characterized by infiltration of polymorphonuclear leukocytes (PMNs) and extravasation of erythrocytes (Fig 2B). In contrast, treated FcRγ−/− mice did not show any indication of inflammatory responses (Fig 2C), remaining the same as untreated normal mice (Fig2A). Serum levels of IgG3 (Table 1) and the presence of intracapillary PAS-positive materials, likely ICs with cryoglobulins, in the skin were comparable in FcRγ+/− and FcRγ−/− mice. Indeed, immunofluorescence staining of sections of affected skin by anti-IgG3 MoAb demonstrated that IgG3 deposits corresponding to the PAS-positive material in the vascular lumens were present in both groups of mice (Fig 3A and B, see page 3858). These results indicate that skin vasculitis is completely inhibited in FcRγ−/− mice despite the fact that the depositions of ICs containing IgG3 were similar to those in wild-type mice.
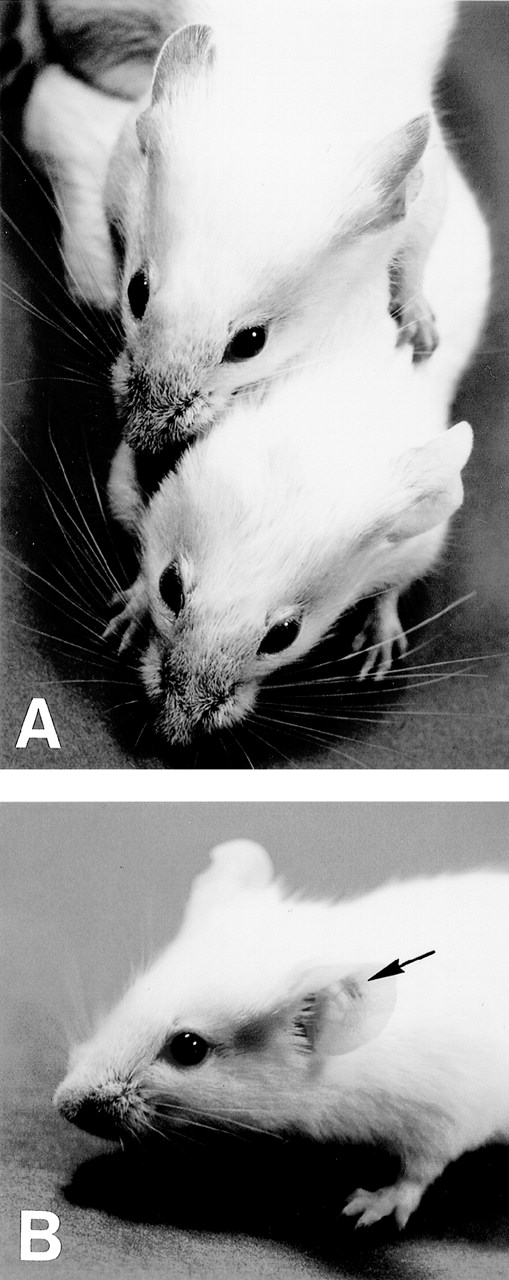
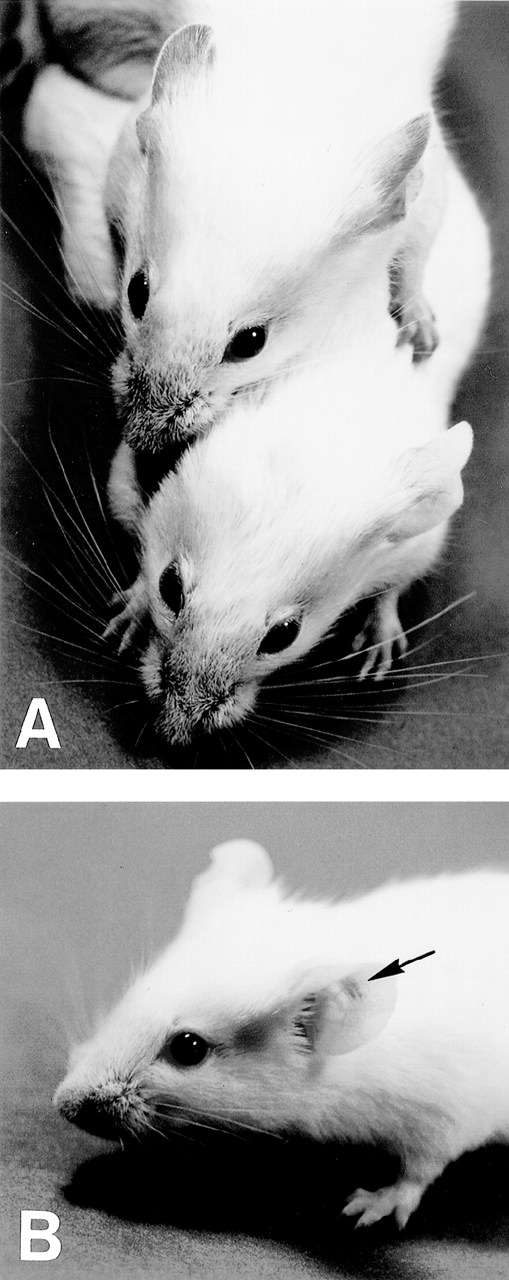
Macroscopic appearance of skin vasculitis. (A) 6-19–treated FcRγ+/− mice developed purpura in the ear, tail, and footpads (upper mouse), whereas 6-19–treated FcRγ−/− mice failed to develop vascular purpura (lower mouse). (B) 6-19–treated FcRγ−/− mice were SC inoculated with 1 × 106 BMMC in the left ear and with PBS in the right ear as a control. After 1 to 2 days, vascular purpura appeared in the restricted site of the BMMC injection (arrow).
Macroscopic appearance of skin vasculitis. (A) 6-19–treated FcRγ+/− mice developed purpura in the ear, tail, and footpads (upper mouse), whereas 6-19–treated FcRγ−/− mice failed to develop vascular purpura (lower mouse). (B) 6-19–treated FcRγ−/− mice were SC inoculated with 1 × 106 BMMC in the left ear and with PBS in the right ear as a control. After 1 to 2 days, vascular purpura appeared in the restricted site of the BMMC injection (arrow).
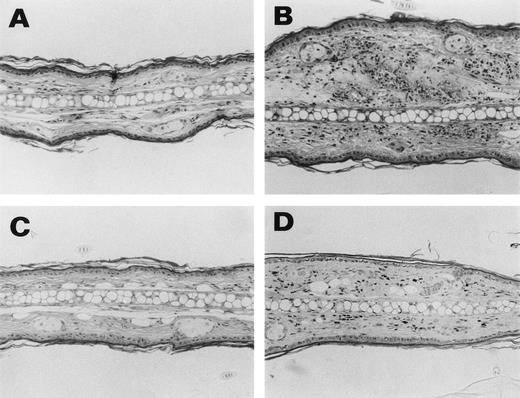
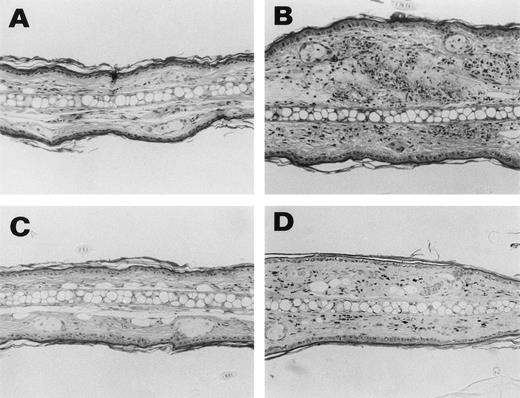
Representative histological appearance of 6-19–induced skin lesions. The sections of the ear from untreated normal mice (A) and 6-19–treated FcRγ+/− (B), FcRγ−/− (C), and TNFβ−/− (D) mice were prepared 12 days after IP injection of 6-19 hybridoma cells. Tissues sections were stained with HE (original magnification × 100). FcRγ+/− mice developed leukocytoclastic vasculitis characterized by the infiltration of PMNs and the extravasation of erythrocytes (B). FcRγ−/− and TNF−/− mice did not develop leukocytoclastic vascular lesions (C and D). Note that in (D), darkly stained cells were melanocytes due to 129 background and that small numbers of PMNs had infiltrated the skin of TNF−/− mice.
Representative histological appearance of 6-19–induced skin lesions. The sections of the ear from untreated normal mice (A) and 6-19–treated FcRγ+/− (B), FcRγ−/− (C), and TNFβ−/− (D) mice were prepared 12 days after IP injection of 6-19 hybridoma cells. Tissues sections were stained with HE (original magnification × 100). FcRγ+/− mice developed leukocytoclastic vasculitis characterized by the infiltration of PMNs and the extravasation of erythrocytes (B). FcRγ−/− and TNF−/− mice did not develop leukocytoclastic vascular lesions (C and D). Note that in (D), darkly stained cells were melanocytes due to 129 background and that small numbers of PMNs had infiltrated the skin of TNF−/− mice.
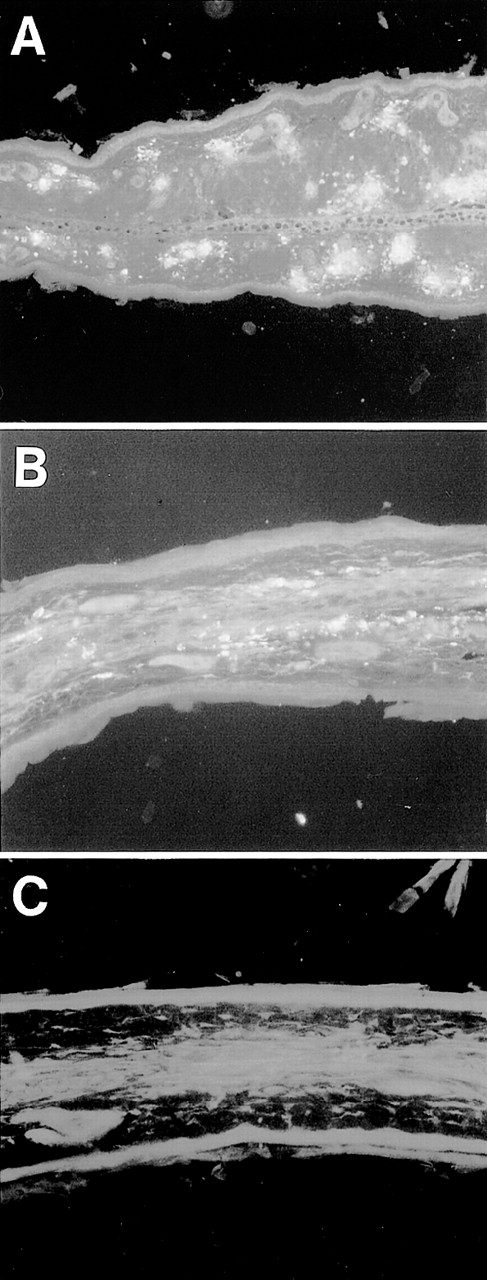
Deposition of IgG3 RF on the vessel wall of ear skin. Ear sections from 6-19–treated FcRγ+/− (A) and FcRγ−/− (B) mice and nontreated FcRγ+/−mice (C) were stained with FITC–anti-IgG3 Ab (original magnification × 200).
Deposition of IgG3 RF on the vessel wall of ear skin. Ear sections from 6-19–treated FcRγ+/− (A) and FcRγ−/− (B) mice and nontreated FcRγ+/−mice (C) were stained with FITC–anti-IgG3 Ab (original magnification × 200).
Differential effects of FcR on induction of vasculitis and glomerulonephritis.
In contrast to the results with skin vasculitis, both FcRγ+/− and FcRγ−/− mice developed typical glomerulonephritis by 6-19 treatment: exudation of PMNs, disseminated plugging of glomerular capillaries by voluminous PAS-positive hyaline thrombi, an increase in glomerular cellularity (Fig 4B and C), and electron-dense subendothelial deposits resembling wire-loop lesions (data not shown), as previously described15 (Fig 4A). All mice became sick and showed muscle weakness and low mobility from 7 to 12 days after the 6-19 injection, and they finally died. The serum levels of BUN from FcRγ+/− and FcRγ−/− mice were equally elevated at the moribund stage (Table 1). These results indicate that, unlike skin vasculitis, deposition of 6-19 cryoglobulin induces glomerulonephritis in an FcγR-independent manner. This is compatible with earlier reports that 6-19 MoAb possesses different pathogenic potential for vasculitis and glomerulonephritis.11,13 14 Because there was no difference in the induction of this type of nephritis between FcRγ+/− and−/− mice, we focused on the analysis of vasculitis to try to identify the effector cells and effector mediators involved in this disease.
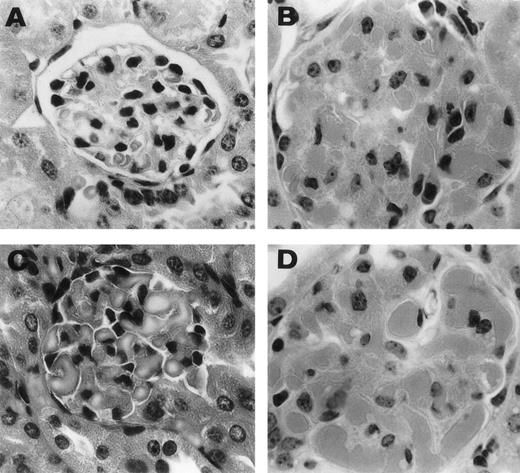
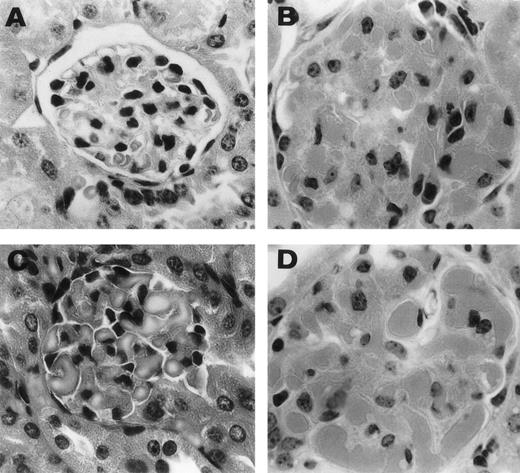
Representative histological appearance of 6-19–induced glomerular lesions. The sections of the ear from untreated normal mice (A) and 6-19–treated FcRγ+/− (B), FcRγ−/− (C), and TNFβ−/− (D) mice were prepared 12 days after the IP injection of 6-19 hybridoma cells. Tissues sections were stained with HE (original magnification × 400). 6-19–treated FcRγ+/− mice developed glomerulonephritis with global proliferative and exudative changes (B). FcRγ−/− and TNF−/− mice developed similar glomerular lesions (C and D).
Representative histological appearance of 6-19–induced glomerular lesions. The sections of the ear from untreated normal mice (A) and 6-19–treated FcRγ+/− (B), FcRγ−/− (C), and TNFβ−/− (D) mice were prepared 12 days after the IP injection of 6-19 hybridoma cells. Tissues sections were stained with HE (original magnification × 400). 6-19–treated FcRγ+/− mice developed glomerulonephritis with global proliferative and exudative changes (B). FcRγ−/− and TNF−/− mice developed similar glomerular lesions (C and D).
Mast cells as the responsible effector cells of vasculitis.
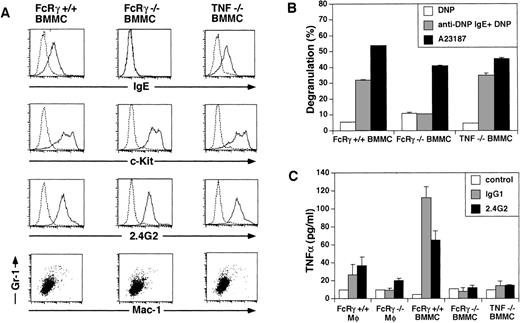
To determine the pathogenic effector cells that trigger vasculitis, we devised a cell transfer system in which various FcR+ cells from wild-type mice were transferred SC or IV into 6-19–injected FcRγ−/− mice that had the depositions of IC but did not develop vasculitis. As shown in Table 2, the IV transfer of 1 × 107 bone marrow-derived mast cells (BMMC) induced typical skin vasculitis, whereas the injection of peritoneal macrophages did not. To assess the possibility that the capability of macrophages to induce the disease was dependent on the stage of activation, LPS-activated macrophages were also IV injected. However, these cells failed to trigger vasculitis (Table 2). To exclude the possibility that the macrophages might be trapped in tissues and/or could not migrate into the ear, we performed a similar experiment by SC injection of these FcR+ cells into the ear (1 × 106 cells per site). The results were the same; only mast cells induced skin vasculitis in 6-19–injected FcRγ−/− mice (Table 2). In this case, vascular purpura developed on the restricted site, where mast cells were transferred 1 to 4 days (2 days in most cases) after the SC injection of BMMC, and the rest of the skin remained intact (Fig1B). These results indicate that, among FcγR-expressing cells, mast cells possess the specific ability to induce FcR-mediated skin vasculitis. To confirm the phenotype and function of the mast cells that were used for the transfer experiment, we characterized the BMMC by the staining of cell surface molecules and degranulation assay (Fig 5A and B). BMMC cultured in vitro for 4 weeks became almost a homogeneous population that coexpressed c-Kit and FcγRII/III and did not express Mac-1 and Gr-1. BMMC from wild-type mice but not from FcRγ−/− mice expressed FcεRI (Fig 5A). These BMMC from wild-type mice released β-hexosaminidase, a granule-associated enzyme of the murine mast cells, in response to cross-linking of FcεRI, whereas BMMC from FcRγ−/− mice showed no such release. FcRγ−/− BMMC released β-hexosaminidase upon stimulation with Ca2+ ionophore, indicating that these cells contained the enzyme but failed to release it (Fig 5B). These data indicate that the BMMC used in the analysis represented functional mast cells.
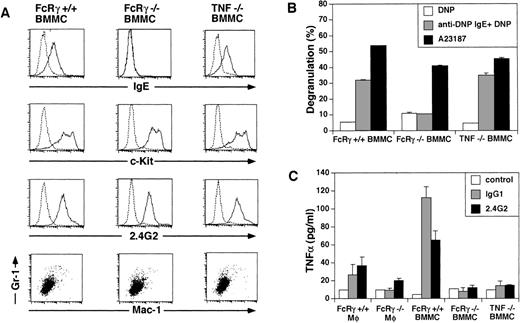
Characterization of the BMMC and macrophages used for transfer experiment. (A) Surface expression of FcɛR, c-Kit, FcγRII/III, Mac-1, and Gr-1 on BMMC. BMMC from FcRγ +/+, FcγR−/−, and TNFβ−/− mice were stained with biotinylated IgE plus avidin-PE, anti–c-Kit, 2.4G2, anti–Mac-1 MoAb, and anti–Gr-1 MoAb. The dotted line indicates control staining. (B) β-hexosaminidase assay of BMMC. BMMC from FcRγ +/+, FcγR−/−, and TNFβ−/− mice were stimulated by FcɛRI cross-linking by biotin-IgE and avidin or by Ca2+-ionophore for 30 minutes. β-hexosaminidase activity in the supernatants and the lysate of the cell pellets was quantified by spectrophotometric analysis as described in Materials and Methods. Total β-hexosaminidase content (supernatant activity plus lysate activity) was similar in these cells (data not shown). (C) FcγR-induced release of TNF. Macrophages and BMMC from FcRγ +/+, FcRγ−/−, and TNFβ−/− mice were stimulated with or without 50 μg/mL immobilized IgG1 or 12 μg/mL immobilized 2.4G2 for 30 minutes. TNF released into the media was measured by ELISA.
Characterization of the BMMC and macrophages used for transfer experiment. (A) Surface expression of FcɛR, c-Kit, FcγRII/III, Mac-1, and Gr-1 on BMMC. BMMC from FcRγ +/+, FcγR−/−, and TNFβ−/− mice were stained with biotinylated IgE plus avidin-PE, anti–c-Kit, 2.4G2, anti–Mac-1 MoAb, and anti–Gr-1 MoAb. The dotted line indicates control staining. (B) β-hexosaminidase assay of BMMC. BMMC from FcRγ +/+, FcγR−/−, and TNFβ−/− mice were stimulated by FcɛRI cross-linking by biotin-IgE and avidin or by Ca2+-ionophore for 30 minutes. β-hexosaminidase activity in the supernatants and the lysate of the cell pellets was quantified by spectrophotometric analysis as described in Materials and Methods. Total β-hexosaminidase content (supernatant activity plus lysate activity) was similar in these cells (data not shown). (C) FcγR-induced release of TNF. Macrophages and BMMC from FcRγ +/+, FcRγ−/−, and TNFβ−/− mice were stimulated with or without 50 μg/mL immobilized IgG1 or 12 μg/mL immobilized 2.4G2 for 30 minutes. TNF released into the media was measured by ELISA.
FcγRIII as the receptor responsible for induction of vasculitis.
BMMC developed in the presence of IL-3 are thought to represent the in vitro equivalent of mucosal mast cells16 and coexpress FcγRII, FcγRIII, and FcεRI.17 To examine the specific contribution of the Fc receptors for the recognition of ICs and induction of vasculitis, BMMC from FcRγ+/−, FcRγ−/−, and FcγRIII−/− mice that express FcγRII/FcγRIII/FcεRI, FcγRII, and FcγRII/FcεRI, respectively, were transferred into 6-19–injected FcRγ−/− mice. FcγRIII−/− mice lacking the α chain of FcγRIII have been shown to be deficient in IgG-mediated mast cell degranulation, to be resistant to IgG-dependent passive cutaneous anaphylaxis, and to exhibit an impaired Arthus reaction.4 Transfer of BMMC from either FcRγ−/− mice or FcγRIII−/− mice could not induce skin vasculitis in 6-19–injected FcRγ−/−, but those from FcRγ+/− mice caused vasculitis (Table 3). IV transfer of BMMC from FcRγ−/− and FcγRIII−/− mice, similar to that shown in Table 3 for SC transfer, failed to induce skin vasculitis (data not shown). This result clearly demonstrates that FcγRIII, but not other FcRs on mast cells, is responsible for the initiation of vascular inflammation. This was confirmed by the finding that the injection of 6-19 cells failed to induce skin vasculitis in FcγRIII−/− mice (data not shown).
TNF as the primary mediator for induction of vasculitis.
Aggregation of FcεR or FcγR on mast cells leads to the rapid release of various mediators, including histamine, proteoglycans, and proteases from cytoplasmic granules, and to the induction of the synthesis and secretion of other mediators such as leukotrienes, prostaglandins, and platelet-activating factor (PAF) as well as a variety of cytokines, including TNF. Among these, leukotrienes, PAF, and TNF are possible candidates for mediators of leukocytoclastic angiitis, because they strongly induce PMN recruitment and the destruction of vessel walls. Among them, TNF turned out to be a crucial mediator, based on our finding that TNFαβ-deficient mice10 failed to develop skin vasculitis in this system despite the fact that some PMNs were observed in the ear section (Fig2D). On the other hand, these mice still developed glomerulonephritis similar to wild-type and FcRγ−/− mice (Fig 4D and Table 4). Staining of ear skin from wild-type and TNFαβ−/− mice with naphthol AS-D chloroacetate esterase exhibited similar distribution and number of mast cells in the tissue (Fig 6, see page3858). These results suggest that TNF is important for the effector phase of mast cells, but not for maturation or migration. To further establish the critical role of TNF in the induction of vasculitis, particularly in relationship with mast cells, BMMC from TNFαβ-deficient mice were transferred subcutaneously into6-19–injected FcRγ−/− mice. We found that the transfer of BMMC from TNFαβ−/− mice also failed to induce skin vasculitis in 6-19–treated FcRγ−/− mice (Table 3). BMMC from TNFαβ−/− mice show a similar surface phenotype (FcεR+c-Kit+FcγRII/III+Mac-1−Gr-1−) to wild-type BMMC and also function similarly in terms of the release of β-hexosaminidase in response to FcεR cross-linking (Fig 5A and B). Furthermore, we found that BMMC release a higher level of TNF than macrophages in the early phase of stimulation through FcγR (Fig 5C). These data suggest that TNF released from mast cells upon stimulation via FcγRIII is the main mediator responsible for the pathogenesis of skin vasculitis.
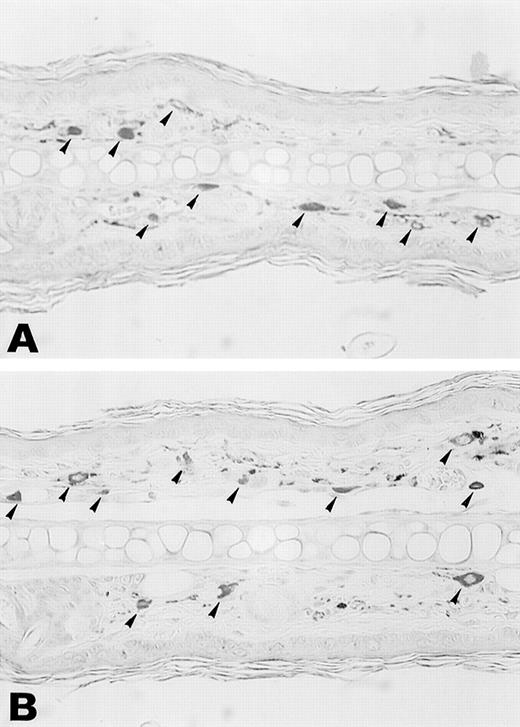
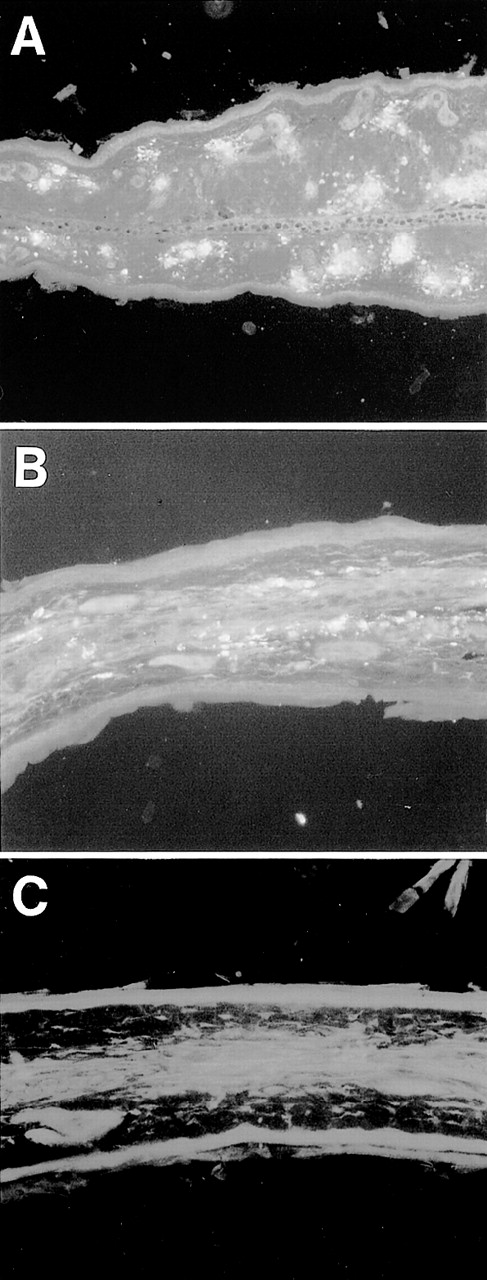
Esterase staining of skin of the ear. Ear skin tissues from wild-type and TNFβ−/− mice were fixed in formalin and sections were stained with naphthol AS-D chloroacetate, N,N′-dimethylformamide, and hexazotized new fuchsin, pH 6.3. Arrowheads indicate esterase-positive cells. Estimated numbers of mast cells were 16.4 ± 1.4/field and 18.1 ± 3.2/field, respectively.
Esterase staining of skin of the ear. Ear skin tissues from wild-type and TNFβ−/− mice were fixed in formalin and sections were stained with naphthol AS-D chloroacetate, N,N′-dimethylformamide, and hexazotized new fuchsin, pH 6.3. Arrowheads indicate esterase-positive cells. Estimated numbers of mast cells were 16.4 ± 1.4/field and 18.1 ± 3.2/field, respectively.
DISCUSSION
When organ damage becomes clinically evident, various bystander cells and effector molecules present in the tissues make it difficult to understand the pathogenesis. However, in the present study, we succeeded in unraveling this complex pathogenesis by identifying the effector cells, receptors, and mediators responsible for the induction of skin vasculitis by the use of various knockout mice. To the best of our knowledge, this is the first analysis of such sequential mechanisms of the cells, receptors, and mediators responsible for the pathogenesis of a particular disease. We developed a transfer system in which various FcR+ cells from several knockout mice, such as FcRγ-, FcγRIII-, and TNF-deficient mice, were transferred into 6-19 MoAb-treated FcRγ-deficient mice to identify the responsible cells and molecules. This system was fundamentally based on the observation that there were similar levels of IC deposition on the vessels of FcRγ+/− and FcRγ−/− mice, although only the former developed vasculitis.
Because 6-19 MoAb-induced vasculitis was induced neither in B-cell–depleted mice that had markedly reduced serum levels of IgG2a12 nor by a chimeric 6-19 MoAb whose κ chain was replaced by λ light chain,12 the formation of IC of host IgG2a and IgG3 anti-IgG2a RF must be crucial for the induction of the disease. The fact that non-IgG3 RFs13 were unable to induce vasculitis despite their reactivity to IgG2a further suggests that the pathogenicity of ICs arises from a self-associating property of IgG3. Although such ICs were precipitated at a similar level on the vessels of FcRγ−/− mice, as shown by histochemical staining for IgG3, the deposition of these auto-ICs per se could not lead to the development of vasculitis. Apparently, complement activation is not essential in the pathogenesis of skin vasculitis.15Instead, the triggering of the disease is mediated by recognition of IC by FcR+ cells, which activates these cells and induces the secretion of several inflammatory mediators that recruit PMNs and induce damage to blood vessels.
It was surprising to find that the cells responsible for triggering skin vasculitis are mast cells. Mast cells have been known to be the main effector cells in IgE- and IgG-mediated type I hypersensitivity. However, considering the recent report by Sylvestre and Ravetch5 that mast cells trigger Arthus reaction, it could easily be supposed that mast cells also play a critical role in type III hypersensitivity diseases. Indeed, our observation that vasculitis in FcRγ−/− mice was induced as soon as 1 to 3 days after IV transfer of mast cells suggests that mast cells recognize IC on the vessel walls and trigger vasculitis within a short period. This suggests an additional function of mast cells, ie, they not only reside in the cutaneous or mucosal surface sensitized by antigen-specific IgE, but also survey ICs and direct their clearance. Although the inducibility of vasculitis was not detected in macrophages in our system, the possibility that small populations of macrophages or other types of FcR+ cells also possess the capacity to induce vasculitis cannot be ruled out. However, because a subcutaneous cell transfer system was used, we could exclude the possibility that the inability of macrophages to induce vasculitis was due to the failure to migrate into the tissues.
We determined that FcγRIII on mast cells and TNF from mast cells are the responsible receptor and mediator, respectively, for triggering this type of vasculitis. Because FcγRIII apparently lacks a significant affinity to the complexes of the IgG3 isotype,18 an efficient interaction of host IgG2a present in the IgG3 cryoglobulin complexes with FcγRIII may be responsible for the activation of mast cells. This idea is consistent with the fact that non-IgG3 anti-IgG2a RF lacking cryoglobulin activity or IgG3 cryoglobulins lacking anti-IgG2a RF activity fail to induce skin vasculitis.13,14 TNFα can be released rapidly from preformed granules of mast cells, and then its transcripts are induced at a later stage of activation.19 TNF production by mast cells was induced upon stimulation through FcγR, particularly FcγRIII.20 Furthermore, several studies have demonstrated that TNFα is an important mediator of mast cell-dependent leukocyte recruitment both in response to IgE and antigen21 or ICs22 and also is strongly involved in the damaging of vessel walls.23 These previous results are consistent with our present observation that TNF released from mast cells upon cross-linking of FcγRIII with ICs plays an essential role in the induction of vasculitis. In fact, the BMMC from FcRγ+/+ mice used in our transfer experiments released TNFα in response to FcγR cross-linking within a short period. BMMC release more TNFα than macrophages do, probably due to the immediate degranulation of preformed TNFα within the granules in mast cells, which macrophages do not possess. Such degranulation may induce a rapid increase of TNFα concentration in local areas and induce the expression of adhesion molecules in endothelial cells and leukocytes. Over a longer time course (>6 hours), TNFα released from FcRγ+/+ macrophages reached to the similar or even higher level of that of BMMC in vitro (data not shown). However, such late secretion of TNFα from macrophages appears to be less effective in inducing vasculitis.
There are several possibilities for the selective inducibility of inflammation by mast cells in vivo, such as differences in the release of inhibitory molecules, diversion by FcγRI or FcγRII, different requirements of cofactors, and so on. Among them, TNFα released from mast cells appears to be the most upstream key mediator for the initiation of skin vasculitis. TNFα promotes the recruitment of inflammatory leukocytes into tissues, which release lysosomal enzymes, oxygen radicals, and arachidonic acid metabolites. These mediators directly damage the endothelium and result in the generation of other toxic mediators, such as eicosanoids, and the production of inflammatory cytokines (IL-1, TNFα, etc) from infiltrating lymphocytes. These factors are chemotactic for leukocytes, thereby promoting further migration of leukocytes and the perpetuation of this process. It was noted that small numbers of PMNs aggregated at the ear of 6-19–treated TNF-deficient mice, although vasculitis did not develop. In contrast, infiltrating cells were not observed at all in FcRγ−/− mice. This difference may suggest the possibility that some other mediators TNF are also involved cooperatively with TNF to induce vasculitis.
Unlike the case of vasculitis, 6-19 MoAb-induced glomerulonephritis was not inhibited in FcRγ−/− mice. It has been shown that, whereas vasculitis requires IC with cryoglobulin, glomerulonephritis was induced by the deposition of cryoglobulin alone, independently of IC formation.12 We also observed that the anti-GBM Ab inducible glomerulonephritis was not induced at all in FcRγ−/− mice.2 FcR-dependent development of glomerulonephritis has also been demonstrated in (NZBxNZW)F1 mice.8 Thus, in terms of the requirement of FcγR for the induction of diseases, it is indeed essential for the induction of skin vasculitis as well as the glomerulonephritis inducible by anti-GBM Ab and also for that in BWF1 mice, whereas 6-19–induced glomerulonephritis is induced independently of FcγR and IC formation. In contrast, preliminary results showed that FcRγ−/− mice backcrossed with MRL/lpr mice developed lethal glomerulonephritis (GN) as severe as conventional MRL/lpr mice. Considering that MRL/lpr mice spontaneously generate extremely large amounts of IgG3 cryoglobulins,24 25 these results suggest that lupus-like nephritis in MRL/lpr mice might be more dependent on the production of IgG3 cryoglobulins, unlike the BWF1 mice. Thus, this illustrates the heterogeneity of the induction mechanisms of GN in different murine models of systemic lupus erythematosus. GN in BWF1 mice and anti-GBM Ab-induced glomerulonephritis may be triggered by the recognition of IC and the release of specific mediators by specific FcR+ cells such as mast cells for vasculitis and mesangial cells for glomerulonephritis. The distinct mechanism of 6-19 MoAb-induced glomerulonephritis still has to be determined.
The position of FcγRIII as the gateway to the immune cascade leading to skin vasculitis makes it a potentially attractive candidate as a target for directed immunotherapy. Instead of the corticosteroids often used as the first line of treatment for hypersensitivity angiitis, a functional FcγR blocker that can inhibit the function of and/or signaling through FcγRs and then impede mast cell activation should be developed as a pathogenesis-specific treatment. For example, soluble FcγR,26 anti-FcγR antibodies, or FcγR binding peptides could be used. Furthermore, considering the fact that TNF production upon degranulation of mast cells induces vasculitis, the administration of antiallergic drug (degranulation inhibitor) or anti-TNF Ab may also function as a potent treatment for the vasculitis syndrome. The frequency of IgG3 anti-IgG2a cryoglobulins in human cryoglobulinemia has not been precisely analyzed. It may not be very frequent, because Gorevic et al27 reported that 22% of the cryoglobulins from their 40 cryoglobulinemia patients were found to be monoclonal IgG, whereas 62% were mixed, containing 2 or more Ig classes. In these cases, all of the sera tested showed RF activity and 93% of the patients presented cutaneous involvement (purpura). Accordingly, a certain proportion of human hypersensitivity angiitis cases might be induced by mechanisms similar to those described here, suggesting the possible application of the treatment discussed above for the diseases.
ACKNOWLEDGMENT
The authors are grateful to M. Sakuma and R. Shiina for preparing MoAbs, Dr K. Arase for experimental help, and H. Yamaguchi for secretarial assistance.
Supported by Grants-in-Aids for Scientific Research from the Ministry of Education, Science and Culture and from the Ministry of Welfare, a grant from the Swiss National Foundation for Scientific Research, and a grant from the Deutsche Forschungsgemeinschaft DFG Ge 892/5-1 and SFB 244.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Takashi Saito, PhD, Department of Molecular Genetics, Chiba University Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan; e-mail: saito@med.m.chiba-u.ac.jp.