Abstract
The human Lin−CD34− cell population contains a newly defined class of hematopoietic stem cells that reconstitute hematopoiesis in xenogeneic transplantation systems. We therefore developed a culture condition in which these cells were maintained and then acquired CD34 expression and the ability to produce colony-forming cells (CFC) and SCID-repopulating cells (SRCs). A murine bone marrow stromal cell line, HESS-5, supports the survival and proliferation of Lin−CD34− cells in the presence of fetal calf serum and human cytokines thrombopoietin, Flk-2/Flt-3 ligand, stem cell factor, granulocyte colony-stimulating factor, interleukin-3, and interleukin-6. Although Lin−CD34− cells do not initially form any hematopoietic colonies in methylcellulose, they do acquire the colony-forming ability during 7 days of culture, which coincides with their conversion to a CD34+ phenotype. From 2.2% to 12.1% of the cells became positive for CD34 after culture. The long-term multilineage repopulating ability of these cultured cells was also confirmed by transplantation into irradiated NOD/SCID mice. These results represent the first in vitro demonstration of the precursor of CD34+ cells in the human CD34− cell population. Furthermore, the in vitro system we reported here is expected to open the way to the precise characterization and ex vivo manipulation of Lin−CD34− hematopoietic stem cells.
THE MEMBRANE phosphoglycoprotein CD34 has been proven to be a useful marker of human hematopoietic stem/progenitor cells, and various types of colony-forming activity in human bone marrow (BM), cord blood (CB), and mobilized peripheral blood (PB) are contained in the CD34+ population.1,2Clinical transplantation studies using enriched CD34+ BM cells have also indicated the presence of hematopoietic stem cells (HSCs) with long-term BM reconstitution ability within this fraction.3,4 Based on such evidence, the current acceptance of experimental and clinical strategies for the enrichment of human HSCs relies on the positive selection of the CD34 antigen. However, studies on the murine system showed that HSCs with a long-term reconstitution capacity were present in the lineage markers-negative (Lin−) CD34− cell population rather than in CD34+ cells.5,6 Osawa et al5 reported that single murine Lin−c-kit+Sca-1+CD34low/−cells transplanted into lethally irradiated mice were able to sustain long-term multilineage engraftment. As a result, the presence of another class of HSCs other than CD34+ cells has also been presumed in the human hematopoietic system.7
Recently, 2 groups demonstrated the possibility of human CD34− HSCs in xenogenic transplantation experiments. Zanjani et al8 showed that the transplantation of human CD34− populations resulted in long-term, multilineage human cell engraftment in a human/sheep model, and Bhatia et al9 reported an ex vivo culture system that induced SCID-repopulating cells' (SRCs) expansion. Although they showed CD34+ cells to be derived from Lin−CD34− cells using in vivo models, there has yet to be any direct evidence that CD34+cells are generated from CD34− cells. An in vitro culture system is thus required for a precise analysis of Lin−CD34− cells that produce CD34+ cells and CFC.
Some murine stromal cell lines have demonstrated a remarkable ability to support the survival and proliferation of human primitive progenitors in vitro.10-13 We established a murine BM stromal cell line, HESS-5,14 and showed that it dramatically and synergistically supported the expansion of human CD34+38− cells with human cytokines thrombopoietin (TPO) and Flk-2/Flt-3 ligand (FL).13
The current studies were thus designed to assess whether the coculture system with HESS-5 cells can maintain human Lin−CD34− cells. The results obtained in this study showed this system to be useful in analyzing the behavior of these cell populations and also demonstrated direct evidence of CD34+ multilineage stem cells from Lin−CD34− cells in vitro.
MATERIALS AND METHODS
Cell purification.
Umbilical cord blood was obtained from normal full-term deliveries according to the guidelines approved by the Tokai University Committee on Clinical Investigation. The cells were processed within 24 hours of collection. Mononuclear cells (MNCs) were isolated using Ficoll-Hypaque (specific gravity = 1.077 g/dL) density gradient centrifugation and put through the red blood cell (RBC)-depletion filter (Asahi Medical Co Ltd, Oita, Japan), which made it possible to pass small-size cells for further enrichment.15 The cells were stained with phycoerythrin (PE)-conjugated antihuman CD2 (39C1.5), CD3 (UCHT1), CD4 (SK3), CD14 (LeuM3), CD16 (3G8), CD19 (4G7), CD20 (2H7), CD33 (WM53), CD34 (QBEnd10: class II), CD41 (P2), CD56 (N901), glycophorin A (KC16), and reacted antimouse IgG-conjugated magnetic beads (Dynabeads; NIHON DYNAL, Tokyo, Japan) and then were depleted according to the manufacturer's manual. The cells were again stained with fluorescein isocyanate (FITC)-conjugated antihuman CD45 (T-200), PE-CD34 (class I:Immu133), and PE-lineage-specific antigens and sorted as positive for CD45 but negative for 11 lineage-specific antigens (CD2, CD3, CD4, CD14, CD16, CD19, CD20, CD33, CD41, CD56, and glycophorin A) and CD34. The cells were sorted on FACSVantage (Becton Dickinson, Mountain View, CA) equipped with an argon laser tuned to 488 nm.
In vitro culture system.
The hematopoietic-supportive stromal cell line HESS-5 was previously established from murine BM.14 HESS-5 cells were maintained in minimum essential medium (MEM)-α supplemented with 10% horse serum at 37°C under 5% CO2 in humidified air. The Lin−CD34− cells were plated at 4 × 104 to 30 × 104 cells per 35-mm2 plate onto pre-established irradiated HESS-5 layers in StemProTM-34SFM (GIBCO BRL, Grand Island, NY) supplemented with StemProTM-34 Nutrient Supplement, 5% fetal calf serum (FCS), and cytokines. The final concentrations of cytokines were as follows: TPO, 300 ng/mL; FL, 300 ng/mL; stem cell factor (SCF), 300 ng/mL; granulocyte colony-stimulating factor (G-CSF), 10 ng/mL; interleukin-3 (IL-3), 10 ng/mL; and IL-6, 10 ng/mL. Human IL-3, G-CSF, SCF, and TPO were a generous gift from the KIRIN Brewery Co Ltd (Tokyo, Japan). All cells were harvested by vigorous pipetting, washed in phosphate-buffered saline (PBS), and then used for further analyses.
Flow cytometric immunophenotyping.
Aliquots of cells or cultured cells were suspended in EDTA-BSA-PBS and incubated with mouse IgG (Inter-Cell Technologies, Hopewell, NJ) to block any nonspecific binding. The cells were then reacted for 15 minutes with antihuman CD34 (class I:Immu133, class II:QBEnd10, and classIII:581). Unbound antibodies were removed by 2 washes, and then the cells were resuspended in EDTA-BSA-PBS. Stained cells were then passed though a nylon mesh filter and subjected to a 2-color flow cytometric analysis. Gating on the lymphoid region was used to exclude stromal cells by size and granularity. The cells labeled with FITC- and PE-conjugated mouse isotype-matched antibodies were used as a control. The surface markers of the cells were analyzed by FACSCalibur using Cell Quest software (Becton Dickinson).
Polymerase chain reaction (PCR) and reverse transcriptase-PCR (RT-PCR).
To detect whether any CD34+ cells were contaminated in the sorted samples, the total RNA was prepared from 1 × 105 freshly sorted cells using ISOGEN (Nippon Gene, Toyama, Japan) and reverse transcribed using oligo dT primer and RAV-2 reverse transcriptase (Takara, Otsu, Japan). To detect human cells in BM from NOD/SCID mice, high molecular DNA was prepared from whole BM cells by a series of phenol-chloroform extractions and ethanol precipitation. The PCR conditions were optimized for each primer set to maintain amplification in the linear range. The primer pairs were as follows: CD3416, 5′ sequence (5′) 5-TAGATTTCACTGAGCAAGAT-3 and 3′ sequence (3′) 5-CTTGCCCCACCTAGCCGAGT-3; HLA-DPB1,17 (5′) 5-GTGAAGCTTTCCCCGCAGAGAATTAC-3 and (3′) 5-CACCTGCAGTCACTCACCTCGGCGCTG-3; and glyceraldehyde phosphate dehydrogenase (GAPDH), (5′) 5-GATGACATCAAGAAGGTGGTG-3 and (3′) 5-GCTGTAGCCAAATTCGTTGTC-3. The samples were denatured at 94°C for 3 minutes, followed by amplification rounds consisting of 94°C for 1 minute (denaturing), 55°C to 65°C for 2 minutes (annealing), and 72°C for 3 minutes (extension) for 30 cycles. The products were separated on a 1.0% agarose gel, transferred to the membranes, and then hybridized with probes labeled by using ECL labeling and a Detection System (Amersham, Buckinghamshire, UK). The sensitivities of RT-PCR and PCR were 10−5 and 10−4, respectively.
Clonogenic assay.
The colony-forming unit-cells (CFU-C) assay was performed as described previously.13 All cultures were performed in triplicate, and the number of CFU-C was scored at day 14 of culture. The colony types were determined by in situ observations using an inverted microscope.
NOD/SCID repopulating cell (SRC) assay.
An SRC assay was performed as described by Hogan et al.18Eight-week-old NOD/shi-scid/scid (NOD/SCID) mice were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan) and were maintained in the germ-free animal facility located at the Tokai University School of Medicine. Purified cell populations at the indicated dose were transplanted by tail-vein injections into 350 cGy irradiated mice. The mice were killed 4 to 6 weeks after transplantation, and the BM from the 2 femurs and 2 tibias of each mouse were flushed into RPMI1640 containing 10% FCS. The presence of human hematopoietic cells was determined by detecting the number of cells positively stained with FITC-conjugated antihuman CD45 in flow cytometric analyses. The specific subsets of human hematopoietic cells were quantified by gating on human CD45+ cells and then assessing staining with antihuman CD14-PE, CD19-PE, CD33-PE, and CD34-PE. Human hematopoietic cells were also determined by detecting the human HLA-DPB1 sequence in DNA isolated from BM cells by a PCR analysis as described above.
Statistical analysis.
A comparison of the mean values of the parameters was performed using the 2-group paired t-test. The strength of the correlation was estimated by Pearson's coefficient of correlation.
RESULTS
Purification of Lin−CD34− cells.
Although the definition of Lin−CD34− cells is an important issue, no consensus about the best combination of surface antigens or clones of monoclonal antibodies has yet been made. We herein defined Lin− as 11 different lineage-specific antigens including CD2, CD3, CD4, CD14, CD16, CD19, CD20, CD33, CD41, CD56, and glycophorin A−.
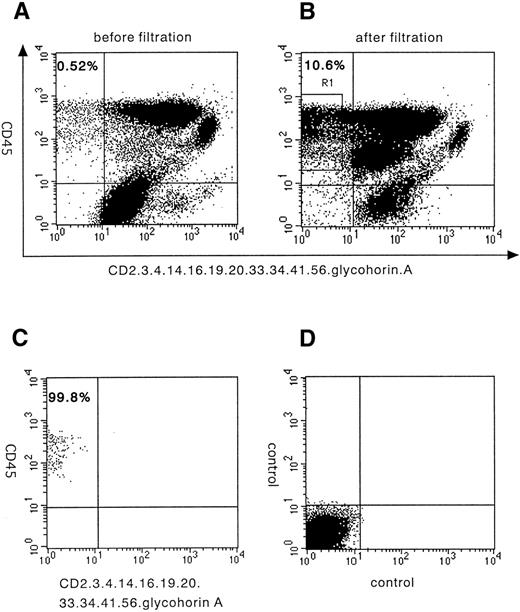
The filtration of MNCs by newly developed RBC-depletion filter enriched Lin− small-size cells from 0.52% to 10.6% (∼20-fold) in this particular sample15(Fig 1A and B). We then depleted human cord blood of MNCs that express CD34 (class II) and 11 different lineage-specific antigens, CD2, CD3, CD4, CD14, CD16, CD19, CD20, CD33, CD41, CD56, and glycophorin A by using immunomagnetic beads. The cells were again stained with FITC-CD45, PE-CD34 (class I), and PE-lineage-specific antigens and sorted as positive for CD45 and negative for 11 lineage-specific antigens. More than a 99.8% pure fraction of Lin−CD34− cells was sorted, as shown in Fig1C. The frequency of Lin−CD34− cells among all nucleated CB cells was 0.58% ± 0.36% (mean ± SD, n = 11). In 3 experiments, the sorted Lin−CD34−cells were relabeled with CD34 class III antibodies that recognize the different epitopes of the CD34 molecule, and they also showed no positive events and confirm the lack of CD34 surface expression (data not shown). In the 11 separate experiments, each involving a different normal donor, the number of Lin−CD34− cells obtained ranged from 0.4 × 105 to 3.7 × 105 (mean, 1.7±1.2 × 105).
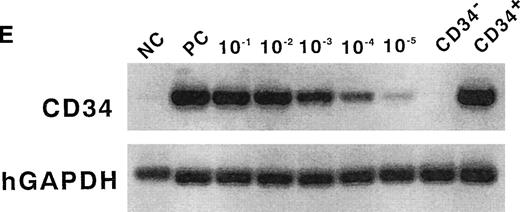
A flow cytometric analysis of lineage-specific antigens and CD34 expression on human cord blood cells and their purification. Staining profile of lineage markers and CD34 versus CD45 expression on human cord blood MNCs before (A) and after filtration (B). (C) Sorted Lin−CD34− cells show a more than 99% pure fraction. (D) Isotype antibody was used as a negative control. (E) Semiquantitative RT-PCR analysis from CD34−Lin− cells. Top lane, human CD34 cDNA amplification; bottom lane, GAPDH cDNA amplification used to normalize RT-RNAs; PC, RT-PCR products from KG-1 cells; NC, water; CD34−, sorted CD34− cells; CD34+, sorted CD34+ cells; 10−1, 10−2, 10−3, 10−4, and 10−5, ratio of positive and negative cell; 10−5, mean positive and negative cell ratio is 1:105.
A flow cytometric analysis of lineage-specific antigens and CD34 expression on human cord blood cells and their purification. Staining profile of lineage markers and CD34 versus CD45 expression on human cord blood MNCs before (A) and after filtration (B). (C) Sorted Lin−CD34− cells show a more than 99% pure fraction. (D) Isotype antibody was used as a negative control. (E) Semiquantitative RT-PCR analysis from CD34−Lin− cells. Top lane, human CD34 cDNA amplification; bottom lane, GAPDH cDNA amplification used to normalize RT-RNAs; PC, RT-PCR products from KG-1 cells; NC, water; CD34−, sorted CD34− cells; CD34+, sorted CD34+ cells; 10−1, 10−2, 10−3, 10−4, and 10−5, ratio of positive and negative cell; 10−5, mean positive and negative cell ratio is 1:105.
These fractions of cells were further analyzed to determine their mRNA expression of CD34 by using a very sensitive semiquantitative RT-PCR, and it was confirmed that no CD34 mRNA were expressed (Fig 1E). These results indicate both that the sorted fractions are highly purified Lin−CD34− cells that do not express mRNA of CD34 and that the possibility of CD34+ cell contamination is impossible.
Induction of CD34+ cells from Lin−CD34− cells in vitro.
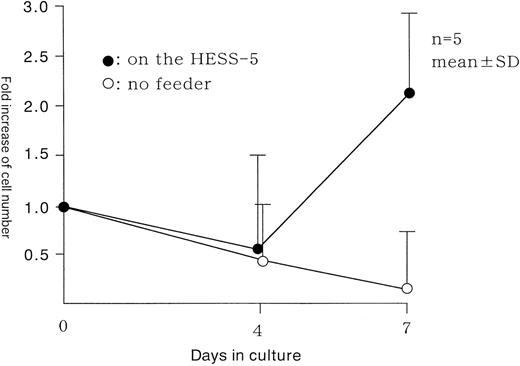
The viability of the cultured Lin−CD34− cells in the stroma-free condition was reduced by 50% within 4 days of culture (Fig 2). The proliferation of these cells was impossible, even in the presence of a variety of human cytokines reported to support the proliferation of human CD34+38− stem cells: TPO, FL, SCF, G-CSF, IL-3, and IL-6.13 This observation also excludes the possibility of CD34+ cell contamination.
Cell kinetics in stroma-free and stromal culture system. Lin−CD34− cells were plated onto pre-established irradiated HESS-5 stromal cells or no stroma cells in medium containing 10% FCS and human cytokines: TPO, FL, SCF, G-CSF, IL-3, and IL-6. The cell number was determined on days 4 and 7.
Cell kinetics in stroma-free and stromal culture system. Lin−CD34− cells were plated onto pre-established irradiated HESS-5 stromal cells or no stroma cells in medium containing 10% FCS and human cytokines: TPO, FL, SCF, G-CSF, IL-3, and IL-6. The cell number was determined on days 4 and 7.
Some murine stromal cell lines were reported to support the survival and proliferation of human stem/progenitor cells.10-13 We have recently demonstrated that the murine BM stroma cell line, HESS-5, dramatically supported the in vitro expansion of human CD34+38− cells in combination with human cytokines.13 We therefore plated Lin−CD34− cells on HESS-5 cells in combination with human TPO, FL, SCF, G-CSF, IL-3, and IL-6. After plating, we observed that some cells were attached to HESS-5 cells. Therefore, the viability and number of the nonattached cells were measured. The viability of cells was maintained, and the number of cells was increased by 2.2- ± 0.8-fold (n = 5) after 7 days in culture (Fig 2).
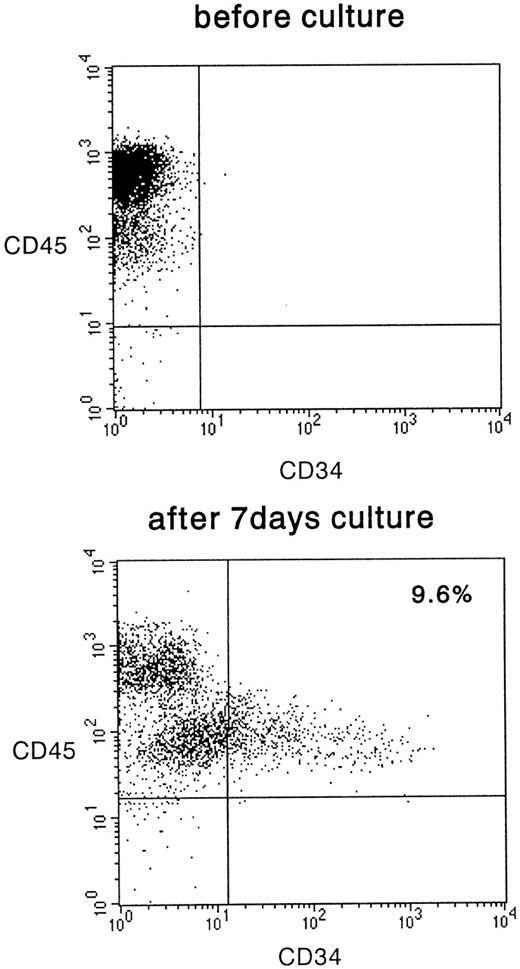
We then analyzed the surface expression of CD34 in these cultured cells. Although they represented only a small proportion of the total number of cultured cells, a definite expression of CD34 was detected and is shown in Fig 3. The mean frequency was 6.0% ± 3.7% (n = 11). The cells expressed CD45, confirming that they were of human hematopoietic and not of stromal origin. The CD34+ cells were derived from CD45med but not from CD45high fraction. These results indicate that the Lin−CD34− cells can be induced to differentiate into cells that express CD34 after in vitro cytokine stimulation.
FACS analysis of CD34− cells after 7 days of culture on irradiated HESS-5 stroma cells. The cells were evaluated for their expression of CD34 and CD45. The percentage of CD34+ cells is indicated in the upper right of the panel.
FACS analysis of CD34− cells after 7 days of culture on irradiated HESS-5 stroma cells. The cells were evaluated for their expression of CD34 and CD45. The percentage of CD34+ cells is indicated in the upper right of the panel.
Clonogenic ability of Lin−CD34−cells before and after culture.
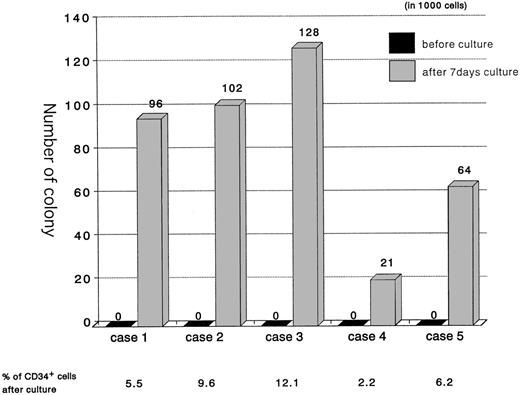
To evaluate the coincidence of CD34 induction on the cell surface with a generative capacity of Lin−CD34−cells, CFU-C output was determined before and after culture. As shown in Fig 4, the number of CFU-C per 1,000 cells was 82.2 ± 41.1 (n = 5) after 7 days of culture, although they did not initially possess a hematopoietic colony-forming capability. In some experiments, cultures of up to 10,000 cells before culture in methylcellulose supplemented with IL-3, SCF, and EPO showed no colonies, even after 28 days of culture. The colony-forming activity was positively correlated with the extent of CD34 expression among Lin−CD34− cells (r = .91). These data suggest that there is a strong relationship between the expression of CD34 and the in vitro response to cytokines that drive myeloid and erythroid differentiation.
The induction of CFUs from Lin−CD34− cells in vitro. Lin−CD34− cells were isolated from 5 individual samples and cultured for 7 days on HESS-5 stroma cells. One thousand cells were plated in methylcellulose medium before and after culture. The colonies were counted 14 days after plating. *The percentages of CD34+ cells in the Lin−CD34− cultured cells at day 7, when the 1,000 cells were plated in the methylcellulose.
The induction of CFUs from Lin−CD34− cells in vitro. Lin−CD34− cells were isolated from 5 individual samples and cultured for 7 days on HESS-5 stroma cells. One thousand cells were plated in methylcellulose medium before and after culture. The colonies were counted 14 days after plating. *The percentages of CD34+ cells in the Lin−CD34− cultured cells at day 7, when the 1,000 cells were plated in the methylcellulose.
Long-term multilineage reconstitution ability of Lin−CD34− cells before and after culture.
To compare the relative ability of the long-term repopulation of Lin−CD34− cells before and after culture, we examined this cell population regarding its engraftment ability in NOD/SCID mice. As summarized in Table 1, 0.4 to 4.6 × 105cells after 4 days of culture derived from 6 individuals were transplanted into 6 NOD/SCID mice. Mouse BM was analyzed for the presence of human cells after 4 to 6 weeks of transplantation. Four of 6 mice transplanted with Lin−CD34−cells after 4 days of culture were chimeric. The presence of human hematopoietic cells in the recipient BM was also confirmed by the detection of human HLA-DPB1 sequences in PCR analysis of the BM cells' DNA (data not shown). The presence of 0.8% to 65.4% CD45+cells represents a total number of at least 8 × 105to 65.4 × 106 human cells, indicating that SRCs derived from Lin−CD34− cells after 4 days of culture have an extensive proliferative capacity. Both the degree of engraftment and the calculated total number of CD34+ cells induced during the culture period showed a positive correlation. On the other hand, when 1 × 105cells before culture were transplanted into NOD/SCID mice (n = 5), human CD45+ cells were found in only 1 recipient mouse with 1.3% of chimerism in BM cells, as determined by flow cytometry. A more sensitive PCR analysis of the HLA-DPB1 gene confirmed the result. These data indicated that SRCs were induced from the Lin−CD34− population after 4 days of in vitro culture.
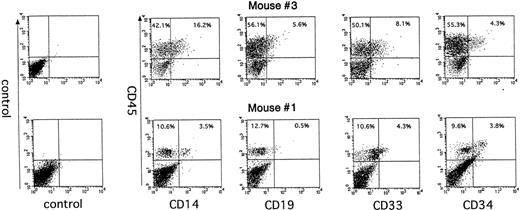
Human CD45+ cells in mice that were engrafted with cultured Lin−CD34−cells were further analyzed to determine the presence of multilineage reconstitution. In BM cells, 16.2% of CD14+ cells, 8.1% of CD33+cells, 5.6% of CD19+ cells, and 4.3% of CD34+cells were detected on mouse no. 1, and 3.5%, 4.3%, 0.5%, and 3.8% of each lineage of cells were detected on mouse no. 3 in Table 1(Fig 5). The presence of multiple lineage of myeloid and lymphoid cells in the engrafted mice indicates that the SRCs derived from the Lin−CD34−cell fraction during in vitro culture have an extensive differentiation capacity in vivo and that they are developmentally earlier than CD34+ stem cells in the hierarchy of human hematopoiesis.
The multilineage reconstitution in NOD/SCID mice 6 weeks after transplantation of cultured Lin−CD34−cells. Human Lin−CD34− cells were cultured on HESS-5 cells for 4 days and transplanted into irradiated NOD/SCID mice. Mice were killed 6 weeks after transplantation and BMC were analyzed for human CD45-FITC and lineage markers labeled with PE.
The multilineage reconstitution in NOD/SCID mice 6 weeks after transplantation of cultured Lin−CD34−cells. Human Lin−CD34− cells were cultured on HESS-5 cells for 4 days and transplanted into irradiated NOD/SCID mice. Mice were killed 6 weeks after transplantation and BMC were analyzed for human CD45-FITC and lineage markers labeled with PE.
DISCUSSION
The presence and characteristics of human CD34− HSCs have gained attention from both biological and clinical points of view7-9 since murine HSCs with long-term reconstitution capacity have been demonstrated to be present in the Lin−c-kit+Sca-1+CD34low/−cell population rather than in CD34+ cells.5Our studies demonstrated the generation of CD34+ cells, CFC, and SRCs from Lin−CD34− cells in vitro. These in vitro data indicate that CD34−cells contain precursor cells of CD34+ HSCs. This experiment is, therefore, the first demonstration of human CD34+ induction ex vivo from CD34− HSCs.
When comparing the data of Lin−CD34− cells, it is first necessary to define Lin−. However, the consensus about the best combination of surface antigens or clones of monoclonal antibodies has not yet been made. Bhatia et al9 used the combination of CD2, CD3, CD14, CD16, CD19, CD24, CD41, CD56, CD66b, and glycophorin A, whereas Zanjani et al8 used T-cell lineage-depleted (CD3, CD4, and CD8) fractions of CD34− cells. We defined Lin− as 11 different lineage-specific antigens, including CD2, CD3, CD4, CD14, CD16, CD19, CD20, CD33, CD41, CD56, and glycophorin A−, which were slightly modified from the former combination. We used CD20 instead of CD24 as a B-cell lineage marker and CD33 instead of CD66b as a myeloid lineage marker. We added CD4 to the lineage markers, because it was reported to function as a marker of primitive hematopoietic cells.19
The next issue involves the possible contamination of our CD34− cell preparation by CD34+ cells, because it would compromise the interpretation of the results of experiments presented herein. We have to be very careful about contamination below the threshold level when using flow cytometry. To avoid this, 2-step depletion with magnetic beads and sorting by fluorescence-activated cell sorting (FACS) was performed and resulted in a very pure fraction of lin−CD34− cells. Although the cells expressing CD34 cytoplasmically could not be eliminated by the methods used in this study, the results from the RT-PCR exclude this possibility. Finally, the inability to proliferate in a stroma-free condition in the presence of human cytokines, and the absence of CFU-Cs detected in the Lin−CD34− cells provide independent confirmation that they are not contaminated with CD34+ cells. It also indicates that Lin−CD34−cells are not able to respond to the cytokines that support differentiation of CFC in methylcellulose, as has been previously reported for subpopulations of CD34− cells.7 9 These data strongly excluded the possible contamination of our sorted samples by CD34+ cells.
It is widely accepted that the microenvironment plays an important role in hematopoiesis in vivo and that stromal cells are the principal components of the microenvironment.20,21 Indeed, several cloned stromal cell lines can promote the survival, proliferation, and differentiation of hematopoietic cells in vitro. We, along with others, demonstrated the successful maintenance and proliferation of CD34+CD38− stem/progenitor cells in a xenogeneic stromal cell coculture system combined with human cytokines.10-13 Murine stromal cell line, HESS-5, was also shown to support the survival and differentiation of the Lin−CD34− cell population. The proliferative and cell survival effects have been attributed to the many cytokines and extracellular matrix molecules produced by the stroma. Development of the stroma-dependent culture condition of Lin−CD34− cells will contribute to the elucidation of the molecular mechanisms associated with the commitment and the differentiation of those newly defined stem cells.
To further assess the long-term repopulating ability of cultured cells, we performed an SRC assay. We detected 1 engraftment of 5 transplantations when 105Lin−CD34− cells before culture were transplanted in NOD/SCID mice, whereas Bhatia et al9reported more than 80% engraftment in NOD/SCID mice. There are several possible reasons to explain the difference. We did not administer any human cytokines to mice after transplantation, whereas they regularly administered human SCF, IL-3, and GM-CSF. The sorted population of Lin−CD34− cells was slightly different, as mentioned previously. In either case, we detected human cell engraftment when the cultured cells were transplanted under the same conditions as the transplantation of cells before culture, which means that SRC expanded after 4 days of culture. NOD/SCID mice are known to support high levels of B-lymphocyte production when CD34+ cells are transplanted.13 However, in our results shown in Fig 5, only low levels of B lymphocytes were detected. It is possible that Lin−CD34− cells in mice should require longer time than 6 weeks before producing mature B lymphocytes.
Our present study raises several important questions that need to be solved to understand further the nature of the Lin−CD34− population. We showed that the Lin−CD34− population subdivided into CD45high and CD45med fraction, and CD34+ cells derived from CD45med fraction in Figs 1 and 3. This indicates that the Lin−CD34− population might be still heterogeneous; a further fractionation study is now in progress. Although the coincidence of the CD34 induction and acquisition of the capability to form CFC and SRCs was shown, a direct relationship between generation of CFC or SRCs and CD34 induction should thus be demonstrated by comparing the sorted fraction of CD34+ and CD34−. These issues are now being investigated in our laboratory (K.A., manuscript submitted).
The findings presented in this study are expected to help the study and manipulation of these newly defined HSCs. The further establishment of optimal conditions that can maintain CD34− cells will thus be useful as a valuable model to study the mechanism of stem cell differentiation and self-renewal and as a gene transduction system.
ACKNOWLEDGMENT
The authors thank Hideyuki Matsuzawa for technical assistance, Shizuko Imai for secretarial work, and the members of Tokai CBSC Study Group for their assistance. The authors also thank the KIRIN Brewery Co Ltd for supplying various growth factors.
Supported in part by the Japan Society for the Promotion of Science (JSPS) Grant No. JSPS-RFTF97 I 00201 and by a Research Grant of the Science Frontier Program from the Ministry of Education, Science, Sports, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kiyoshi Ando, MD, Department of Hematology, Tokai University School of Medicine, Bohseidai, Isehara, Kanagawa 259-1183, Japan.