Abstract
The pathogenesis of Langerhans cell histiocytosis (LCH) remains poorly understood. To further elucidate LCH pathogenesis, we analyzed the expression of 10 cytokines relevant to cellular recruitment and activation at the protein level in 14 patients and identified the lesional cells responsible for cytokine production in situ by immunohistochemistry. The cytokines investigated included the hematopoietic growth factors interleukin-3 (IL-3), IL-7, and granulocyte-macrophage colony-stimulating factor (GM-CSF); the lymphocyte regulatory cytokines IL-2, IL-4, and IL-10; the inflammatory regulators IL-1 and tumor necrosis factor- (TNF-); and the effector cell-activating cytokines IL-5 and interferon-γ (IFN-γ). In all specimens, CD1a+ histiocytes (LCH cells) and CD3+ T cells produced large amounts of cytokines, creating a true cytokine storm. IL-2, IL-4, IL-5, and TNF- were produced exclusively by T cells, whereas only IL-1 was produced by LCH cells. Equal numbers of LCH cells, T cells, and macrophages produced GM-CSF and IFN-γ. Equal numbers of LCH cells and macrophages produced IL-10, whereas IL-3 was produced by T cells and macrophages. IL-7 was only produced by macrophages. Eosinophils, present in some specimens, were partially responsible for the production of IL-5, IFN-γ, GM-CSF, IL-10, IL-3, and IL-7. Expression of all cytokines, abundant in most biopsies, was irrespective of age, gender, or site of biopsy. These findings emphasize the role of T cells in LCH. The juxtaposition of T cells and LCH cells suggests that both cells interact in a cytokine amplification cascade, resulting from stimulation of autocrine and paracrine stimulatory loops. This cascade can be linked directly to the development of LCH through recruitment, maturation, and proliferation of LCH cells. The cytokines studied are known to be involved in the development of other characteristic features of LCH, such as fibrosis, necrosis, and osteolysis.
LANGERHANS CELL histiocytosis (LCH), a rare disorder, mainly of children, is of unknown pathogenesis with variable course.1 The broad clinical spectrum of disease ranges from a single lytic lesion of bone that is usually cured by simple curettage to a disseminated leukemia-like illness with significant mortality. Intermediate forms of disease are chronic, often with multiorgan involvement and diabetes insipidus.2Lesions of LCH are polymorphous, featuring a monoclonal population of CD1a+ histiocytes with a phenotype akin to that of cells of the antigen-presenting Langerhans cell family. T cells, macrophages, and eosinophils are variably present. The key histiocyte of LCH and its normal counterpart, the Langerhans cell, express CD1a and S-100 and contain Birbeck subcellular organelles.3 In contrast to normal Langerhans cells, the principal histiocytes of LCH (LCH cells) are actively proliferating, have a round rather than dendritic shape, and express several contrasting antigenic markers.4
The morphology of LCH lesion and the clinical signs and symptoms of disease suggest that cytokines may be important in the pathogenesis of the disorder. Using immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR), upregulation of the following cytokines in LCH lesions has previously been shown: interleukin-1 (IL-1), IL-3, IL-4, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and leukemia inhibitory factor (LIF).5,6 These studies did not identify the cellular source of these cytokines in the LCH lesion. We have previously developed methods to analyze cytokine expression at the protein level in situ so as to establish the identity of cells producing an array of cytokines for both human and mouse tissues.7 8 Using validated antibody-based immunohistochemistry, intracellular cytokine present in cytokine-producing cells can be reliably visualized, and double-staining techniques for CD markers allow unequivocal identification of the cell type producing the cytokine. We used these methods here to analyze the expression of an extended panel of cytokines relevant to LCH pathogenesis and to identify their cellular origin in 14 LCH lesions. We demonstrate that T cells and LCH cells are the major local sources of cytokines, which are involved in recruitment and survival of Langerhans cells, as well as in their maturation into effector cells contributing to LCH pathogenesis.
MATERIALS AND METHODS
A definitive diagnosis of LCH and the representative nature of 14 specimens from 14 cases were confirmed by BEF. Eleven of the specimens were from bone in cases of monoostotic LCH and 3 were from excisional lymph node biopsies from patients with disseminated LCH. In all cases, specimens were from initial diagnostic biopsies, with the duration of symptoms and signs being less than 1 month before biopsy. All LCH lesions were in the cellular phase of evolution.
Specimens were promptly snap-frozen and kept at −80°C until sectioned and stained using an immuno-enzyme histochemical staining method.7,8 Briefly, frozen sections were cut at 8 μm in an environment of −20°C. A series of 7 specimen sections together with appropriate controls were placed on individual glass slides and were kept overnight at room temperature in a closed container with high humidity. After air-drying for 1 hour, sections were fixed in acetone containing 0.01% hydrogen peroxide to block endogenous peroxidase activity. Sections were then air-dried for 10 minutes and incubated with optimally diluted primary antibodies (Table 1) overnight at 4°C in the dark. Sections were washed twice in phosphate-buffered saline (PBS), and second- and third-step incubations of 2 hours per RT each were performed. After a PBS wash, horseradish peroxidase (HRP) activity was shown with 3-aminocarbazole (AEC; Sigma, St Louis, MO) in bright primary red.7,8 Alkaline phosphate was shown with naphtol-AS-MX-phosphate (Sigma) and fast blue (BB base; Sigma) as brilliant blue.7,8 Antibody 1/34 (DAKO Corp, Carpinteria, CA) was used for CD1a and CD3 DAKO-EPOS, a rabbit polyclonal antibody coupled to an inert polymer backbone and HRP, was used for CD3. Double stainings were performed, titrated, and evaluated (red/blue and violet colors) as described before.7 8
The specificity of antibodies was determined as noted earlier,8 but isotype-matched control antibodies were always included (Fig 1A and B) to assess nonspecific background staining (eg, by binding to Fc receptors). When positive control stains were weak or negative, study results were discarded. Negative controls uniformly showed no staining. Positive control stainings on sections of human tonsil, spleen, and skin that featured an allergic reaction and negative control stains on human liver and skin were performed with each batch of study material on the same individual slide (ie, on all slides). All staining was performed in duplicate on separate slides on the same day and duplicate stains were performed on different days. As in earlier studies,8cells staining positive for cytokines were scored only when the cytoplasm was brightly colored and the nucleus was clear and transparent. When stains could not be reproduced because of lack of tissue or lack of tissue integrity and/or lack of clearly discernible cytology, they were not included in the data and were marked as not determinate (Table 2). Two observers (M.v.M. and E.C.) scored all slides independently and reconciled differences in scoring by studying the slide(s) together. Slides were scored for estimates of the number of positively staining lesional cells. The frequency of cytokine expression/staining is indicated in Table 2 as +++ reaction, meaning that the majority of cells in the specimen produces that certain cytokine. This high level of expression is illustrated in Fig 1C and D. Double staining for cell type and cytokine was performed in at least 7 specimens for each cytokine profile. We defined LCH cells as being CD1a+ and T cells as CD3+. Eosinophils were identified by morphology, and CD1a− cells with morphological features of histiocytes were called macrophages.
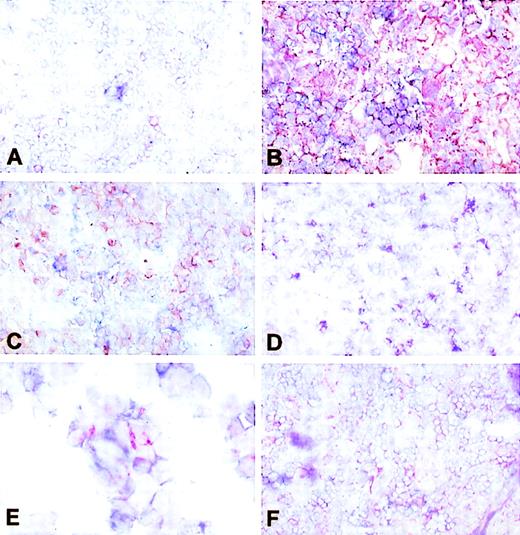
In situ cytokine expression in LCH biopsies. Single immunohistochemical labeling was used to assess cytokine production in frozen sections of LCH biopsies. (A) IL-1 demonstrated in a positive control tissue sample from human tonsil. Such control tissue was included on each glass slide to internally validate different cytokine stainings (original magnification × 100). (B) Human tonsil: negative control staining using an isotype-matched primary antibody of irrelevant specificity to exclude nonspecific binding of secondary and tertiary step reagents (original magnification × 100).8(C) IL-1–producing cells in a LCH lesion in bone (patient no. S926704). Note the high density of cytokine-expressing cells and the very intense staining of most of the cytoplasm, compared with (B) (original magnification × 100). (D) IL-3–producing cells in a monoostotic lesion (patient no. S9411100; original magnification × 100). (E) IL-10–producing cells in a affected lymph node in disseminated LCH (patient no. S93292). Note that no clustering occurs and that isolated cells display only partially cytokine-filled cytoplasm (original magnification × 100). (F) GM-CSF–producing cells in a monoostotic lesion (patient no. 9408P025; original magnification × 50).
In situ cytokine expression in LCH biopsies. Single immunohistochemical labeling was used to assess cytokine production in frozen sections of LCH biopsies. (A) IL-1 demonstrated in a positive control tissue sample from human tonsil. Such control tissue was included on each glass slide to internally validate different cytokine stainings (original magnification × 100). (B) Human tonsil: negative control staining using an isotype-matched primary antibody of irrelevant specificity to exclude nonspecific binding of secondary and tertiary step reagents (original magnification × 100).8(C) IL-1–producing cells in a LCH lesion in bone (patient no. S926704). Note the high density of cytokine-expressing cells and the very intense staining of most of the cytoplasm, compared with (B) (original magnification × 100). (D) IL-3–producing cells in a monoostotic lesion (patient no. S9411100; original magnification × 100). (E) IL-10–producing cells in a affected lymph node in disseminated LCH (patient no. S93292). Note that no clustering occurs and that isolated cells display only partially cytokine-filled cytoplasm (original magnification × 100). (F) GM-CSF–producing cells in a monoostotic lesion (patient no. 9408P025; original magnification × 50).
RESULTS
The cytokine profile of the 14 LCH specimens is shown in Table 2. Some cytokines were expressed so abundantly that staining resulted in the blending of the images of individual cells. With the exception of IL-7, IL-10, and GM-CSF, which were expressed by relatively few cells in most specimens, all cytokines were consistently expressed at high levels. The lowest levels of cytokine expression were seen in an isolated skull lesion from an 11-year-old boy and a lymph node from a 2-year-old boy with disseminated LCH. However, the cytokine profile on the latter was limited. Eosinophils were obvious in 8 of the 14 specimens (Table 2), and they were associated with expression of IL-3, IL-5, IL-7, IL-10, interferon-γ (IFN-γ), and GM-CSF.
Under physiological conditions, IL-3 can stimulate the generation and differentiation of progenitor cells of every lineage derived from the pluripotential hematopoietic stem cells, including macrophages, neutrophils, eosinophils, basophils, mast cells, megakaryocytes, and erythroid cells. There was abundant expression of IL-3 in 6 of the 11 LCH specimens (Fig 1D), with 1 demonstrating no staining. Half of the IL-3–producing cells were T cells and half were macrophages. In 5 specimens, IL-3 was also expressed by eosinophils. No IL-3 was produced by LCH cells.
IL-7 is normally a major growth and differentiation factor for T cells and B cells. It promotes immune effector functions in T cells, natural killer (NK) cells, and monocytes-macrophages. There was less IL-7 expression than any other cytokine in the 12 specimens studied, and none was demonstrated in 4 specimens. The source of IL-7 in these lesions appeared to be macrophages. None was associated with T cells or LCH cells. Eosinophils produced IL-7 in the 4 specimens.
The main activities of GM-CSF include the promotion of differentiation and proliferation of hematologic progenitors. Modest numbers of cells expressed GM-CSF (Fig 1F) in all but 1 (no expression) of the 13 specimens studied. All types of lesional cells, including eosinophils in 4 specimens, produced GM-CSF.
IL-2 is the T-cell cytokine that stimulates ongoing specific immune responses, T-cell differentiation, and synthesis of IFN-γ. IL-2 is almost exclusively produced by T cells, but perhaps also by B cells. IL-2, the most abundantly expressed cytokine in these specimens, was absent in 1 specimen and was uniformly associated with the marker for T cells.
IL-4 can act on many cell types and at various stages of maturation as it induces B cells to proliferate and to secrete IgG1. It also induces T-cell proliferation. IL-4 modulates cyto- kine production by B cells, T cells, NK cells, monocytes-macrophages, and several other cells and, therefore, plays an important role. Generally IL-4 is very difficult to demonstrate in mouse and human tissue, possibly due to low levels of expres- sion and/or to the use of low-affinity antibodies. In contrast, staining for IL-4, which was uniformly associated with T cells, was seen in all but 1 of the 8 LCH specimens studied (Fig 2D).
Identification of cellular origin of cytokines in LCH biopsies. Double immunohistochemical labeling was used to determine cytokine profiles of T cells and LCH cells in frozen sections of LCH biopsies. IL-1 (red) is not produced by CD3+ T cells (blue), as shown by the absence of intermediate (violet) staining (original magnification × 100). (B) In contrast to (A), all CD1a+ LCH cells (blue) also stain for IL-1 (red), resulting in violet double staining (original magnification × 100). (C) IL-5 (red) is not coexpressed with CD1a (blue) and is therefore not produced by LCH cells (original magnification × 100). (D) IL-4 (red)–producing cells stain violet with anti-CD3 (blue) and hence are T cells (original magnification × 100). (E) IFN-γ–producing cells stain in part double (note red, blue, and violet) with CD1a. Because the same was shown in double stainings with anti-CD3, this cytokine is produced by both T cells and LCH cells (original magnification × 200). (F) TNF-–producing cells coexpress CD3 and are therefore identified as T cells (original magnification × 100).
Identification of cellular origin of cytokines in LCH biopsies. Double immunohistochemical labeling was used to determine cytokine profiles of T cells and LCH cells in frozen sections of LCH biopsies. IL-1 (red) is not produced by CD3+ T cells (blue), as shown by the absence of intermediate (violet) staining (original magnification × 100). (B) In contrast to (A), all CD1a+ LCH cells (blue) also stain for IL-1 (red), resulting in violet double staining (original magnification × 100). (C) IL-5 (red) is not coexpressed with CD1a (blue) and is therefore not produced by LCH cells (original magnification × 100). (D) IL-4 (red)–producing cells stain violet with anti-CD3 (blue) and hence are T cells (original magnification × 100). (E) IFN-γ–producing cells stain in part double (note red, blue, and violet) with CD1a. Because the same was shown in double stainings with anti-CD3, this cytokine is produced by both T cells and LCH cells (original magnification × 200). (F) TNF-–producing cells coexpress CD3 and are therefore identified as T cells (original magnification × 100).
IL-10 exhibits important functions in immune regulation, particularly in controlling inflammatory responses via inhibition of monocyte-macrophage activation. Very important as well are the potent deactivating effects of IL-10 on monocytes-macrophages, granulocytes, and dendritic cells. Modest expression of this cytokine was seen in 9 of 11 specimens (Fig 1E), and no staining was seen in 2. Staining was associated equally with LCH cells and macrophages. Eosinophils also expressed IL-10 in 3 specimens.
Cytokine IL-1 is a highly inflammatory cytokine and affects nearly every cell type, often in concert with other cytokines. There was expression by large numbers of cells in 8 of 14 specimens, with LCH cells appearing to be the singular source of the cytokine (Fig 2A and B).
TNF-α is produced during immune and host defense responses as a primary mediator of immune regulation and the inflammatory response. In 9 of the 13 specimens tested, large numbers of T cells, the sole source, expressed TNF-α (Fig 2F).
Cytokine IL-5 is the main controling cytokine for eosinophilia. Only T cells and eosinophils in 3 specimens produced IL-5 (Fig 2C).
IFN-γ is, in addition to its antiviral activities, an important modulator of the immune system because of its ability to activate T cells. Six specimens showed prominent expression with equal contributions by LCH cells and T cells (Fig 2E). Faint expression was associated with macrophages in a few specimens, and eosinophils produced IFN-γ in 1.
There was a paucity of multinucleated giant histiocytes in the sections from these specimens and, thus, too few were observed to establish the cytokine profile of these lesional elements.
DISCUSSION
In this study, we demonstrate high expression of a large panel of cytokines in LCH lesions at the protein level and, for the first time, we show that T cells and LCH cells are the predominant sources of this cytokine storm. LCH cells were the only source of IL-1α and a major source of IL-10. Most of the cytokines were of T-cell origin, suggesting a prominent role for this cell in the disease. The pattern of cytokine expression forming this cytokine storm favors recruitment of Langerhans cell progenitors as well as their maturation and rescue from apoptosis, thereby explaining the pathologic accumulation of LCH cells. The large number of cytokine-producing cells in LCH lesions supports local amplification cascades of cellular proliferation and activation, involving autocrine and paracrine stimulatory loops. Several of the cytokines produced in LCH lesions directly contribute to pathological sequelae of LCH, including fibrosis, bone resorption, and necrosis. These findings are interpreted in an integrated view of the pathology of LCH and contrasted with physiological Langerhans cell reactivity.
Langerhans Cells
The diagnostic lesional cell in LCH is most akin to the normal Langerhans cell, with comparative data having been summarized recently.9 These cells, and only they, are monoclonal in LCH lesions.10 The Langerhans cell, an immune cell of bone marrow origin, is the sentinel of the human skin with respect to foreign antigens. Strategically located in the epidermis, Langerhans cells capture antigens upon entry, a process that is facilitated by their dendritic morphology. Antigen uptake, processing, and expression of antigenic peptides on the cell surface in the context of major histocompatibility complex (MHC)/HLA molecules leads to differentiation and activation, including increased longevity of MHC-peptide complexes at the cell surface.11 Langerhans cells carrying antigen migrate through the lymph as veiled cells and present antigen to peptide-specific T cells in the T-cell areas of draining lymph nodes and spleen, where they are called interdigitating dendritic cells.12 The interaction between Langerhans cells and T cells needs to be bidirectional, because MHC plus antigenic peptide, adhesion molecules, costimulatory molecules (eg, CD40-CD40L, CD80/CD86), and cytokines are all required for complete activation of both the antigen-presenting cell and the T cell.13
Langerhans Cells and Cytokines
The full range of cytokines produced by Langerhans cells and their function needs to be further elucidated, but reviews on the characteristics of Langerhans cells14 indicate that GM-CSF, IL-1, and TNF-α are important in the development and trafficking of Langerhans cells. Furthermore, TNF-α, GM-CSF, and IL-3 enhance proliferation of Langerhans cells generated from CD34+ cord blood or bone marrow cells.15 These in vitro-generated Langerhans cells, as well as the dendritic cells generated from peripheral blood, produce IL-1 and, when stimulated through CD40, also secrete TNF-α.16 Dermal Langerhans cell evolution to lymph node interdigitating dendritic cells is enhanced by GM-CSF and IL-1.17,18 GM-CSF has a role in the recruitment of Langerhans cells to various tissue sites.19 Expression of relevant cytokine receptors on Langerhans cells has been confirmed, including those for GM-CSF, IL-1, TNF-α, and IFN-γ for humans,20 whereas IL-2R is expressed by murine cells21 and can be induced by CD40 ligation.16
Langerhans Cells Versus LCH Cells
LCH cells probably originate from the bone marrow and are very sensitive to local factors such as cytokines and growth factors for replication, maturation, and differentiation, as well as functional antigen processing and presentation. The issue as to which of the many immunologically important cytokines are expressed in LCH lesions remains a somewhat vexed one, because a limited number of biopsies have been studied. Furthermore, in situ analysis is technically challenging, and in vitro studies of both LCH cells and Langerhans cells are confounded by contaminating cell populations producing cytokines. Therefore, the goal in studying the production of cytokines in LCH is to improve our understanding of disease pathogenesis and ultimately to explore the potential of novel therapeutics such as recombinant cytokines and antagonist molecules targeting costimulatory events. To date, most studies on cytokine production in LCH lesions have focused on descriptive analysis often based on mRNA detection and ignoring which cells produce the different cytokines. We therefore analyzed cytokine expression in LCH lesions at the protein level in a large series of biopsies, including some cytokines studied in LCH previously, but expanding the panel to a total of 10, with additional relevant cytokines not evaluated before. Biopsy material of LCH is extremely limited, so only confining studies are possible. This limited factor forced us to use a restrictive panel of markers for the double staining studies. However, the double labeling in situ with CD3 for T cells and CD1a for LCH cells enabled us to provide new insights into the identity of cytokine-producing cells as well.
Pathogenic Roles of Cytokines in LCH Lesions
Indirect evidence for cytokine involvement in LCH.
Indirect evidence of a role for cytokines in LCH lies in the finding that peripheral blood T-helper:suppressor ratios are high.22 This finding may be related to early studies establishing that major Ig isotypes are elevated in 75% of patients with LCH,23 because Th-cell cytokines are differential Ig isotype switch factors and also stimulate B-cell proliferation and antibody production. The positive clinical effects of cyclosporin,24 thymic hormone,22 and bone marrow transplantation25 further attest to a prominent role of T cells and cytokines in LCH. Prostaglandin E2 and IL-1 have been found in unseparated cell preparations of LCH lesions in vitro. IFN-γ increases IL-1 secretion by LCH cells in vitro, indicating a role of T cells in regulating the production of IL-1 in bone lesions.26 T cells may stimulate Langerhans cells by IL-2 production, as evidenced by IL-2 receptor expression on murine epidermal Langerhans cells. In our study (Table 2), T cells and LCH cells in most specimens produced IFN-γ, but in less than half did large numbers of cells stain for this cytokine. However, more cells producing IFN-γ were seen in bone lesions than in lesions of lymph nodes.
Recruitment and differentiation of cells.
Previously, lesions of LCH have been shown to contain mRNA transcripts for IL-1, IL-3, IL-4, IL-8, GM-CSF, TNF-α, TGF-β, and LIF. No transcripts for IL-2, IL-5, IL-6, or IFN-γ were found.6Using immunohistochemistry, De Graaf et al5showed that cells in LCH lesions and those from normal and allergic skin expressed IL-1α, IL-1β, GM-CSF, TGF-α, TGF-β, TNF-α, and IFN-γ, whereas LCH cells alone did not express basic fibroblast growth factor. The cytokine IL-2 was not studied.5 Our data complete these findings in showing that crucial immunologic active cytokines, such as IL-2, IL-5, and IFN-γ, are, indeed, produced by lesional T cells and that LCH cells produce IFN-γ, but they do not produce IL-2 and IL-5. Because under physiological conditions IL-3 can stimulate the generation and differentiation of progenitor cells of every lineage derived from the pluripotential hematopoietic stem cells, IL-3 might contribute to the differentiation of LCH cell progenitors.
The distribution of LCH cells in the affected patient may be influenced by GM-CSF27 (Table 2) and by adhesion molecules defined by De Graaf et al.28 There are few studies of cytokine receptors in LCH, but Emile et al29 demonstrated the expression of the GM-CSF receptor on LCH cells. Under physiological conditions, GM-CSF increases differentiation and proliferation of hematologic progenitors. Kaplan et al19 have shown that GM-CSF is important in recruitment of Langerhans cells into different tissues. Likewise, in LCH lesions, GM-CSF probably promotes growth and differentiation of LCH cell progenitors. Elevated levels of GM-CSF were found in the sera of patients with disseminated disease, but not in that of patients with localized LCH, suggesting that serum GM-CSF may be a marker of the extent of the disease in LCH.30 Serum levels of IL-2 receptor show similar correlations.31GM-CSF, IL-3, TNF-α, and a number of other cytokines function as chemoattractants for eosinophils, neutrophils, macrophages, and CD34+ Langerhans cell precursors.32Importantly, TNF-α has been shown to inhibit spontaneous apoptosis of Langerhans cells, promoting their survival,33,34,35 which would explain the accumulation of LCH cells in lesions. Furthermore, IL-1α promotes this effect of TNF-α,33 as do GM-CSF and IL-3,36 both of which we demonstrated in the lesions. Accumulation of LCH cells may be further enhanced by stimulation of Langerhans cell formation from their CD34+ progenitors by GM-CSF and TNF-α.36
Osteolysis and fibrosis.
IL-1α and TNF-α, both amply expressed in LCH lesions (Table 2), and IL-1α may synergistically enhance osteoclastic activity with resultant osteolysis, the hallmark of the LCH lesion of bone. The evolution of fibrosis in LCH lesions of bone, liver, and lungs4 may be related to the lesional production of TGF-β (not studied here), which has been implicated as a potent sclerosing agent.37 TNF-α can recruit leukocytes and promote angiogenesis as well as fibroblast proliferation in wound healing, and similar activities might occur in the LCH lesions. Sclerosis has also been implicated in the evolution of diabetes insipidus, the most common endocrinopathy in LCH, secondary to obstructive involvement of the hypothalamic/pituitary axis.
Rationale for 2-Chlorodeoxyadenosine (2-Cda) Treatment in LCH
This evidence of cross-talk between the LCH cell and the T cell in LCH lesions raises prospects of a better understanding of the mechanism of action of promising therapeutic agents such as 2-Cda in this disorder.38,39 2-Cda is a purine analogue resistant to degradation by adenosine deaminase. Phosphorylated derivates of 2-Cda accumulate in cells with high deoxycytidine kinase activity, such as lymphocytes, inducing changes in lymphocyte viability, resulting in DNA strand breaks. The DNA damage is followed by a progressive decrease in lymphocyte RNA synthesis, resulting in cell death.40 The cytotoxic properties of 2-Cda are independent of cell division, making it a powerful agent in the treatment of lymphoid neoplasms with low-growth fractions, such as T-cell lymphomas.41 In vitro and in vivo human monocytes are as sensitive as lymphocytes to these effects of 2-Cda, following the same pathway.42 Until now, the rationale for using 2-Cda in LCH was based on this latter finding.38 39 Because Langerhans cells and monocytes/macrophages are thought to originate from the same stem cell, it was hypothesized that, because 2-Cda is toxic to monocytes, it might be toxic to LCH cells. To confirm this, in vitro and in vivo studies concerning the sensitivity of Langerhans cells or, even better, LCH cells, should be undertaken. Until these studies are performed, the results presented here showing that the LCH lesion is the result of cross-talk between the LCH-cell and the activated T cell form a rationale for using 2-Cda in LCH.
Perspective
The fact that clonality has been found in all LCH lesions reported to date argues that LCH is a neoplastic disorder with varied biological behavior, which could result from a genetic defect, an abnormal response to infection, or an autoimmune phenomenon, but the true significance of this clonality remains to be clarified. We have demonstrated that a cytokine storm consisting of at least 10 major cytokines occurs in LCH lesions. CD3+ T cells are pivotal, because they produce 7 of 10 analyzed cytokines, whereas LCH cells produce 4 of 10. We conclude that T cells are an important driving force for the accumulation, proliferation, and differentiation of cells in LCH lesions. The observed juxtaposition of T cells and LCH cells in all tissues analyzed suggests intimate, if not cognate, interactions of these 2 cell types, contributing to bidirectional stimulation and cytokine production. Associated pathogenic effects of these cytokines are likely to include chemotaxis of additional inflammatory cells, overexpression of adhesion molecules, and fibrosis, necrosis, and osteolysis. Systemic manifestations of LCH, such as macrophage activation and fever, may reflect more far-reaching effects of these cytokines. The current findings should prove useful in further rational development of experimental LCH therapy.
ACKNOWLEDGMENT
The authors thank Louis Ribbens (TNO-Prevention and Health, Leiden, The Netherlands) for performing the initial immunohistochemical analyses. Some of the specimens were provided through the Pediatric Division of the Cooperative Human Tissue Network, Children's Hospital (Columbus, OH).
These studies were initiated through the Nikolas Symposium (organizers Paul and Elizabeth Kontoyannis). The Histiocytosis Association of America and the Histiocytosis Stichting in The Netherlands provided additional financial support.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R. Maarten Egeler, MD, PhD, Southern Alberta Children's Cancer Program, Alberta Children's Hospital, 1820 Richmond Rd SW, Calgary, Alberta, Canada T2T 5C7; e-mail:Maarten.Egeler@CRHA-Health.ab.ca.