Abstract
It has been known for a long time that IgG–anti-F(ab′)2 antibodies (Abs) are able to suppress the B-cell response. We showed that natural IgG-anti–F(ab′)2 autoantibodies appear in the serum of patients with cold agglutination. If the anti-F(ab′)2 Ab suppresses cold agglutinin (CA)-producing B cells, one would expect an inverse correlation between the titers of these two Abs. Our study confirmed this correlation. Subsequent experiments showed that some anti-F(ab′)2 Abs bind to the hinge region of IgG. It was difficult to explain how this Ab suppresses CA-producing B cells, which are of IgM isotype. Here we show that patients with cold agglutination have an IgG–anti-κ light chain autoantibody in their serum. This is another member of the anti-F(ab′)2 Ab group. Because the vast majority of CAs are IgM-κ Abs, the anti-κ Ab might suppress CA-producing B cells. If this is the case, there should be an inverse association between the titer of anti-κ Ab and CA. In a group of 302 patients, we found that high titers of the anti-κ Ab correlate with low titers of CA and vice versa (P= .009). Interestingly, this association is found only in patients whose disease is caused by noninfectious agents, including mainly B-cell proliferations (P = .0058). Our data show that the inverse correlation is not confined to a particular CA autoantibody specificity. The results are discussed in the light of recent findings showing that anti-IgM Abs may either inactivate or kill tumoral B cells by apoptosis.
IgG–anti-F(ab′)2AUTOANTIBODIES were shown to strongly suppress the B-cell response including that of autoreactive cells.1-3 If these antibodies (Abs) were also involved in the suppression of antierythrocyte autoantibody producing B cells, there should be an inverse correlation between anti-F(ab′)2 and antierythrocyte autoantibody titer in patients with autoimmune hemolytic anemia. We studied a group of patients with cold agglutination, a disease caused by cold reactive antierythrocyte autoantibodies (cold agglutinins [CA]),4 and found an inverse association between the 2 Abs.5 The vast majority of previously described human anti-F(ab′)2autoantibodies were antiidiotypes, ie, they bound to epitopes located in the variable region of the immunoglobulin molecule. In previous studies, we showed that some anti-F(ab′)2autoantibodies recognize a constant region epitope located in the hinge region of the IgG molecule. Subsequent clinical studies showed that the previously defined inverse correlation between CA and anti-F(ab′)2 Abs also applies to the anti-hinge Ab.6 However, it was difficult to explain how an Ab directed against the hinge region of IgG regulates CA-producing B cells, which belong to the IgM subclass. We speculated that the anti-hinge Ab might cross-react with an homologous epitope on IgM, that IgM-producing B cells coexpress a small number of IgG molecules, or that other mechanisms are involved. All of those explanations, however, were not satisfactory.
Meanwhile, our studies have shown that anti-F(ab′)2autoantibodies contain, in addition to the anti-hinge, other specificities. One of them is an Ab binding to κ light chains. It is known that the vast majority of CAs are IgM Abs with κ light chains.4 Therefore, anti-κ Abs interact with CAs and thus might regulate their activity. If they had an inhibitory effect on CA-producing B cells, there should be an inverse association between IgG–anti-κ and CA titer. In the present study, we analyze the association between the anti-κ and the CA autoantibodies in a group of 302 patients with cold agglutination.
MATERIALS AND METHODS
Detection of CA.
Sera of 302 patients with cold agglutination were tested. CA were detected and their specificities defined by titration of patients' sera against untreated, protease-, and sialidase-treated human adult red blood cells, as well as newborn cells in the standard tube technique.
Detection of IgM antibodies against mycoplasma pneumoniae, Epstein-Barr virus (EBV), and rubella virus.
IgM Abs against infectious agents were determined to show fresh infections. Anti-I containing sera were tested for antimycoplasma, anti-i sera for anti-EBV, and anti-Pr sera for antirubella virus Abs using standard kits (Virotech, Rüsselsheim and Abbott GmbH Diagnostika, Wiesbaden, Germany, respectively).
Detection of IgG–anti-κ antibody.
Microtiter plates were coated with 0.25 μg/well of human κ light chains (ARP, Belmont, MA). Remaining active groups were blocked with phosphate-buffered saline (PBS) + 0.4% gelatine. The test serum was diluted 1:20 and applied to precoated plates (50 μL/well, duplicates). A reference serum with known anti-κ activity was used as positive and a serum without anti-κ activity, as well as PBS, as negative controls. After incubation, 50 μL of alkaline phosphatase-conjugated goat antihuman IgG(Fc) (Jackson Immunoresearch Lab, West Grove, PA) was added. Each step was followed by extensive washing with PBS + 0.05% Tween. Substrate (250 μg p-nitrophenyl phosphate disodium/well) (Sigma Chemical Co, St Louis, MO) was added and the extinction was measured at 405 nm every minute up to 90 minutes and values were multiplied by 103. The test was stopped at an extinction of 1,000 optical density (OD) 405 nm in the positive control.
Statistical analysis.
Data are expressed as the median ± MAD (median absolute deviation). Patients with identical CA titer were included in the same group. Correlations were calculated according to the Spearman rank correlation test corrected for ties (rho) and corresponding P values determined.
RESULTS
Sera of patients with cold agglutination contain an IgG–anti-κ light chain autoantibody.
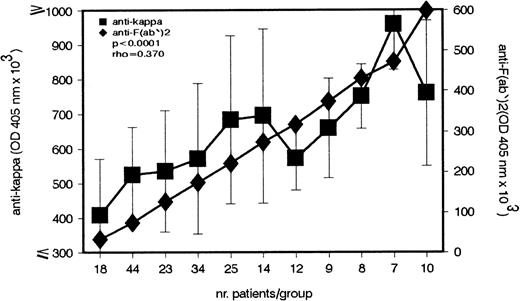
Our previous studies showed that sera of patients with cold agglutination contain IgG–anti-F(ab′)2 Abs whose concentrations show an inverse correlation with CA titers.5This study has identified an IgG–anti-κ light chain autoantibody in the same group of patients. Because κ light chains are expressed in most F(ab′)2 fragments, anti-κ Abs bind to F(ab′)2 and therefore belong to the anti-F(ab′)2 Ab group. The first experiment addressed the question of whether the titer of anti-κ Ab parallels that of anti-F(ab′)2 Ab. As shown in Fig 1, a strong positive correlation between the 2 Ab titers is found in CA patients (P < .0001).
Direct association between the IgG–anti-κ and IgG–anti-F(ab′)2 autoantibody in patients with cold agglutination. In the serum of 204 patients with cold agglutination, the IgG–anti-κ and IgG–anti-F(ab′)2 autoantibody titers were measured by enzyme-linked immunosorbent assay (ELISA). Antibody titers (median ± MAD) are given in OD 405 nm (ordinate).
Direct association between the IgG–anti-κ and IgG–anti-F(ab′)2 autoantibody in patients with cold agglutination. In the serum of 204 patients with cold agglutination, the IgG–anti-κ and IgG–anti-F(ab′)2 autoantibody titers were measured by enzyme-linked immunosorbent assay (ELISA). Antibody titers (median ± MAD) are given in OD 405 nm (ordinate).
IgG–anti-κ antibody titers show an inverse correlation with CA antibody titers.
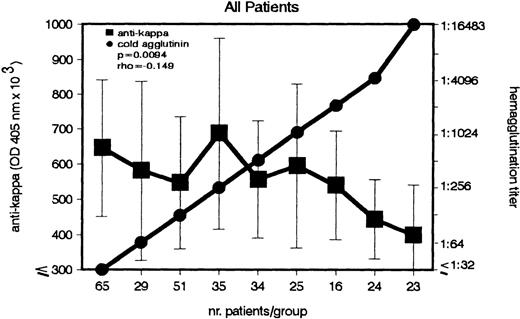
The question arose whether similar to anti-F(ab′)2, the anti-κ autoantibody correlates with the CA titers. In a group of 302 patients, both anti-κ and CA titers were measured and statistically analyzed (Fig 2). A weak, but significant (P = .009), inverse association between the 2 Abs was detected. Increasing CA titers were associated with decreasing anti-κ titers.
Inverse association between IgG–anti-κ autoantibody and CA in patients with cold agglutination. In a group of 302 patients, the titers of IgG–anti-κ and CA were determined (left and right ordinate). Sera with identical CA titers were included into the same group (abscissa). Anti-κ titers are given in OD 405 nm (median ± MAD).
Inverse association between IgG–anti-κ autoantibody and CA in patients with cold agglutination. In a group of 302 patients, the titers of IgG–anti-κ and CA were determined (left and right ordinate). Sera with identical CA titers were included into the same group (abscissa). Anti-κ titers are given in OD 405 nm (median ± MAD).
The inverse correlation appears only in cold agglutination of noninfectious etiology.
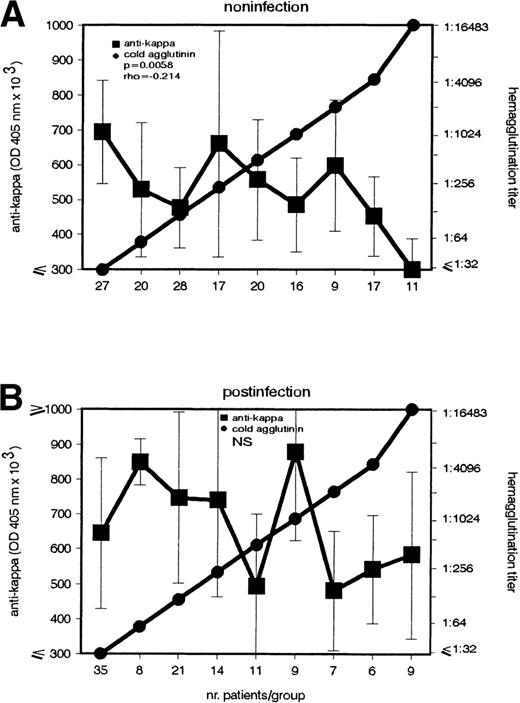
According to its etiology, the cold agglutination can be subdivided into a postinfection and noninfection group. The latter is a heterogenous entity including mainly patients with lymphoproliferative diseases. Because previous data5 showed that the anti-F(ab′)2 Ab's role is related to the disease's etiology, in the present study, the patients were subdivided into 2 groups, 1 with recent infections (IgM Abs against mycoplasma pneumonia, EBV, or rubella virus) and 1 without demonstrable infections. Interestingly, the inverse association between CA and anti-κ Ab appeared only in the noninfection group (P = .0058) (Fig 3).
Inverse association between IgG–anti-κ autoantibody and CA in cold agglutination caused by noninfectious agents. In patients (A) without (n = 165) and (B) with (n = 120) recent infections, the anti-κ and CA autoantibody were determined (left and right ordinate). Sera with identical CA titers were included in the same group (abscissa). Anti-κ titers are given in OD 405 nm (median ± MAD).
Inverse association between IgG–anti-κ autoantibody and CA in cold agglutination caused by noninfectious agents. In patients (A) without (n = 165) and (B) with (n = 120) recent infections, the anti-κ and CA autoantibody were determined (left and right ordinate). Sera with identical CA titers were included in the same group (abscissa). Anti-κ titers are given in OD 405 nm (median ± MAD).
The inverse correlation appears in all noninfection patients regardless of the CA's specificity.
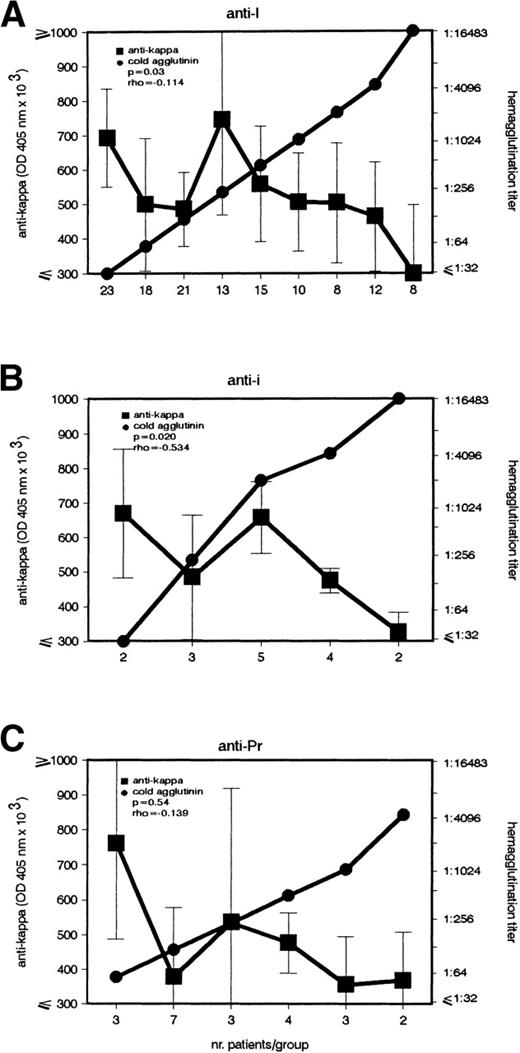
Noninfection patients were further subdivided according to the specificity of their CA (Fig 4). Both anti-I and anti-i patients presented the above-described inverse association (P = .03 and P = .02). Although the same correlation also appeared in the anti-Pr group, it was not statistically significant.
Inverse association between IgG–anti-κ autoantibody and CA in noninfection patients with anti-I and anti-i autoantibodies. In patients with cold agglutination of noninfectious origin caused by (A) anti-I (n = 128), (B) anti-i (n = 16), and (C) anti-Pr (n = 22) autoantibody, the titers of IgG–anti-κ and CA were determined (left and right ordinate). Sera with identical CA titers were included into the same group (abscissa). Anti-κ titers are given in OD 405 nm (median ± MAD).
Inverse association between IgG–anti-κ autoantibody and CA in noninfection patients with anti-I and anti-i autoantibodies. In patients with cold agglutination of noninfectious origin caused by (A) anti-I (n = 128), (B) anti-i (n = 16), and (C) anti-Pr (n = 22) autoantibody, the titers of IgG–anti-κ and CA were determined (left and right ordinate). Sera with identical CA titers were included into the same group (abscissa). Anti-κ titers are given in OD 405 nm (median ± MAD).
DISCUSSION
The data analyzed in this study rely on the largest group of cold agglutination patients described so far. Therefore, they offer an excellent opportunity to study the role of various factors involved in the pathogenesis of this disease.
Our statistical analyses showed an inverse correlation between the anti-κ and CA autoantibody titer. As in the case of anti-F(ab′)2 autoantibody,5 this association applied only to the subgroup of patients whose disease was caused by noninfectious agents, mainly by B-cell proliferation. Of course, the described association does not allow us to conclude that the anti-κ Ab suppresses CA production. However, this finding becomes interesting in the light of many previous observations showing that anti-F(ab′)2 Abs strongly suppress the B-cell response, including that of autoreactive cells.1-3 7-14
Although it was not the objective of this report to elucidate the mechanism by which the anti-κ Ab might suppress CA-producing B cells, it is tempting to speculate on it. Previous experiments showed that murine B-cell lymphoma cells undergo apoptosis in response to cross-linking of membrane immunogobulin (mIg) with anti-Ig reagents.7 A similar effect was described with regard to human tumoral B cells. Cross-linking of surface IgM by Abs induced apoptosis in Burkitt lymphoma, an effect enhanced by transforming growth factor (TGF)-beta 18 or the FcγIIB receptor.9 Cold agglutination of noninfectious origin is mainly caused by B-cell proliferative disorders. We speculate that the anti-κ autoantibody induces apoptosis of tumoral B cells and thus suppresses the production of the deleterious antierythrocyte autoantibody. This would be one mechanism of suppression.
In a series of experiments in mice, it was shown that Abs to IgM induce a state of dormancy in B-cell lymphoma.10 11 Tumor dormancy is an operational term used to describe a prolonged quiescent state in which tumor cells are present but do not proliferate. If this mechanism is also operative in humans, one would expect that high titers of anti-κ autoantibodies render CA-producing tumoral B cells dormant. This could be another control mechanism of CA-producing tumor cells.
It has been known for a long time that anti-Ig Abs are able to suppress the B-cell response by cross-linking the mIg with the FcγII receptor on the B-cell membrane.12-14 The first model of FcR-mediated suppression of B-cell response was advanced by Chan and Sinclair.15 The biochemical basis of the FcR-inhibitory effect has been partially elucidated. It is known that an influx of extracellular Ca+2 is necessary for B-cell proliferation and differentiation. Receptor cross-linking prevents the influx of extracellular Ca+2 by closing the plasma membrane Ca+2 channels.16,17 A 13-amino acid motif in the cytoplasmatic tail of the FcRγII receptor is necessary and sufficient for this effect.16,18 Tyrosine at residue 309 is phosphorylated on cross-linking, and mutation of this residue aborts the inhibitory effect.16 Our previous experiments in rats showed that IgG–anti-Ig autoantibodies exert a B-cell suppressive effect by mIg-FcγII cross-linking, and that suppression is induced only if the B cells' mIg is occupied by its ligand.1 2That means that only antigen-occupied B cells are susceptible to suppression. One lymphocyte subpopulation whose antigen is always available is autoreactive B cells. Therefore, they might be preferentially downregulated by anti-Ig autoantibodies. Suppression of antierythrocyte autoantibody-producing B cells by the anti-κ Ab through mIg-FcR cross-linking might be another immunosuppressive mechanism in cold agglutination. This latter possibility, however, does not provide an explanation for the preferential suppression of tumoral B cells as indicated by our results.
In conclusion, our data show a highly significant inverse association between CA and anti-κ autoantibody, a biomolecule with a potential B-cell suppressive function, in patients with cold agglutination caused by noninfectious agents.
ACKNOWLEDGMENT
D.N. is deeply indebted to Prof Dr Irinel Popescu, Prof Dr Ştefan Drãgulescu, and Dr Francise Bárányi for their continuous support that enabled him to participate in this project.
P.T. and D.N. contributed equally to this work and should both be regarded as the first author.
Supported in part by the Ministry of Education (Bucharest, Romania) by an award to D.N.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter Terness, MD, Department of Transplantation Immunology, Institute of Immunology, University of Heidelberg, INF-305, 69120 Heidelberg, Germany.