To the Editor:
Disparities in the polymorphic minor histocompatibility antigens (mHag) between donor and recipient are associated with the development of graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) reactions after allogeneic stem cell transplantation. The mHag HA-1 has a restricted tissue distribution with an exclusive expression in human hematopoietic cells. HA-1 is recognized by alloreactive donor-derived major histocompatibility complex (MHC) class I-restricted cytotoxic T cells (CTL), making this epitope an interesting target for the development of specific immunotherapies against leukemia after allogeneic stem cell transplantation with a low risk of GVHD.1 Recently, the peptide sequence of the HLA-A*0201–restricted HA-1 mHag (HA-1H) has been identified and shown to differ in only 1 amino acid in position 3 from the HA-1–negative allelic counterpart (HA-1R).2 Moreover, Mutis et al3have demonstrated that HA-1H peptide-pulsed dendritic cells (DC) generated from bone marrow CD34+ cells induce HA-1–specific CTL in vitro that efficiently lyse leukemic cells without lytic activity against nonhematopoietic cells, confirming the restricted expression of this epitope. The in vitro expansion kinetics of the HA-1–specific CTL were slow, although it was estimated that 109 HA-1–specific CTL can be obtained after 5 weeks of culture for adoptive immunotherapy purposes.3
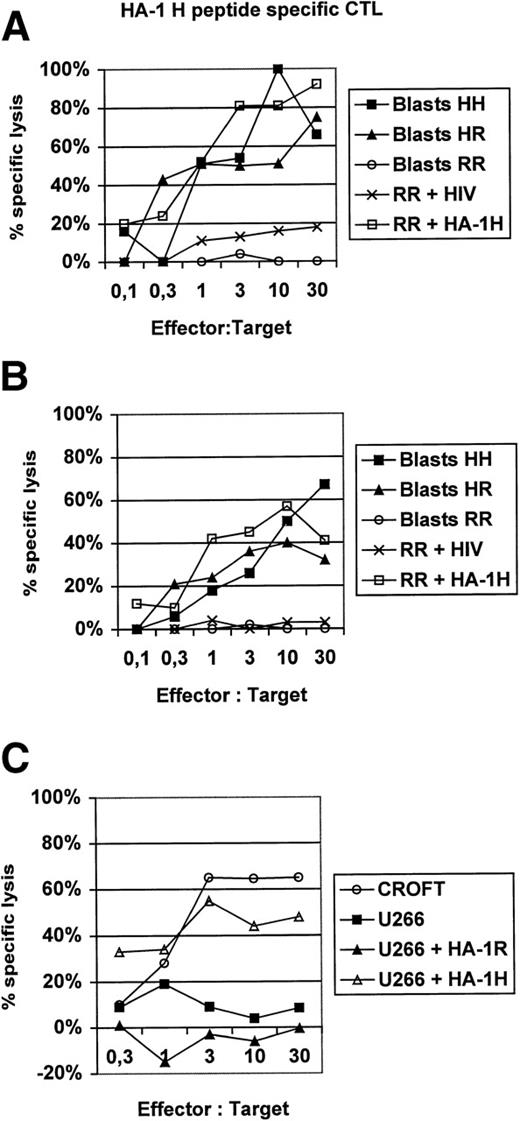
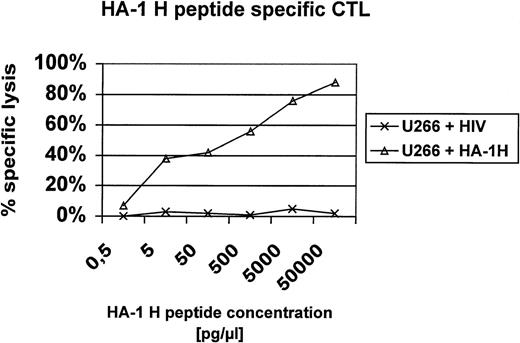
In this report, we present additional data providing evidence that HA-1H peptide-specific CTL induced in vitro using HA-1H peptide-pulsed monocyte-derived DC as antigen-presenting cells from unprimed HA-1–negative healthy donors are not only able to lyse primary leukemic blasts or immortalized B cells naturally expressing the HA-1H/H phenotype (Fig1), but also recognize heterozygous leukemic cells with the HA-1H/R phenotype, showing that these HA-1–specific CTL are of high affinity to the peptide/MHC complex. This finding was also confirmed in peptide titration assays (Fig 2). Furthermore, the HA-1H peptide-specific CTL did not cross-react with the HA-1R peptide (Fig 1C), because HA-1–negative (HA-1R/R) U266 cells or HA-1R peptide-pulsed U266 cells were not lysed by HA-1H–specific CTL, whereas U266 cells pulsed with the cognate HA-1H peptide were efficiently lysed by HA-1H–specific CTL. Interestingly, we were also able to induce T cells specific for the HA-1Rpeptide in 2 individuals; however, these cells were not able to lyse targets with the HA-1R/R phenotype, demonstrating either that this epitope is not presented or that the CTL are of low affinity (data not shown).
(A and B) Lysis of homozygous or heterozygous HA-1–positive primary leukemic blasts by HA-1–specific CTL. HA-1H peptide-specific CTL were induced in vitro from 2 different healthy HLA-A2–positive, HA-1–negative blood donors by monocyte-derived DC pulsed with synthetic HA-1Hpeptide.2 DC were generated from adherent blood monocytes in the presence of GM-CSF, IL-4, and tumor necrosis factor (TNF), as described.6 Leukemic HA-1–positive acute myelogenous leukemia (AML) or acute lymphoblastic leukemia (ALL) blasts from 6 different patients were used as target cells in a classical51Cr release assay.6 The HA-1–negative cell line “RR” pulsed with an irrelevant human immunodeficiency virus (HIV)-peptide was used as a negative control. Cell line “RR” (line 6574803) was kindly provided by Dr E. Goulmy (University of Leiden, Leiden, The Netherlands). (C) HA-1Hpeptide-specific CTL do not cross-react with the HA-1Rpeptide. CROFT is an HLA-A2+, HA-1H/H–positive immortalized B-cell line, whereas U266 is an HLA-A2+, HA-1–negative (HA-1R/R) myeloma cell line.
(A and B) Lysis of homozygous or heterozygous HA-1–positive primary leukemic blasts by HA-1–specific CTL. HA-1H peptide-specific CTL were induced in vitro from 2 different healthy HLA-A2–positive, HA-1–negative blood donors by monocyte-derived DC pulsed with synthetic HA-1Hpeptide.2 DC were generated from adherent blood monocytes in the presence of GM-CSF, IL-4, and tumor necrosis factor (TNF), as described.6 Leukemic HA-1–positive acute myelogenous leukemia (AML) or acute lymphoblastic leukemia (ALL) blasts from 6 different patients were used as target cells in a classical51Cr release assay.6 The HA-1–negative cell line “RR” pulsed with an irrelevant human immunodeficiency virus (HIV)-peptide was used as a negative control. Cell line “RR” (line 6574803) was kindly provided by Dr E. Goulmy (University of Leiden, Leiden, The Netherlands). (C) HA-1Hpeptide-specific CTL do not cross-react with the HA-1Rpeptide. CROFT is an HLA-A2+, HA-1H/H–positive immortalized B-cell line, whereas U266 is an HLA-A2+, HA-1–negative (HA-1R/R) myeloma cell line.
Peptide titration assay. U266 cells were pulsed with titrating amounts of the HA-1H peptide (P2) or an irrelevant HLA-A2 binding peptide derived from HIV and used as targets in a 51Cr release assay with HA-1H–specific CTL.
Peptide titration assay. U266 cells were pulsed with titrating amounts of the HA-1H peptide (P2) or an irrelevant HLA-A2 binding peptide derived from HIV and used as targets in a 51Cr release assay with HA-1H–specific CTL.
To estimate the applicability of HA-1–specific CTL for the treatment of relapsed HA-1H/H– or HA-1H/R–positive leukemias after allogeneic transplantation, we analyzed the frequency of HA-1 gene expression in 65 HLA-A*0201–positive healthy individuals using reverse transcription-polymerase chain reaction (RT-PCR) with HA-1 allele specific primers (Fig3). We found that 16% of this population is homozygous for HA-1 (HA-1H/H) and that 54% of this population is heterozygous (HA-1H/R), whereas 30% is HA-1–negative (HA-1R/R), suggesting that approximately 70% of the HLA-A2–positive patient population could be candidates for such a treatment if they have an HA-1R/R–negative stem cell donor. Taken together, these results open new possibilities for the treatment of HA-1–positive leukemia patients after allogeneic stem cell transplantation from HA-1R/R–negative donors by adoptive transfer of HA-1–specific CTL, as suggested by Mutis et al,3 or by immunizing the donor with HA-1H peptide-pulsed autologous DC before transplantation. The quantitation and isolation of in vivo-induced HA-1–specific CTL in HA-1–negative stem cell donors after vaccination would be performed using tetrameric HLA-peptide complexes4 5 and thus would probably facilitate the clinical application of donor-derived mHag-specific CTL.
HA-1 gene expression in 65 HLA-A2–positive healthy blood donors, hematopoietic cell lines, and primary leukemic blasts by allele-specific RT-PCR. PCR using HA-1 allele-specific primers was performed as previously described,2 resulting in a 255-bp fragment. Results from representative samples from homozygous (HA-1H/H) (including CROFT and cell line HH [6574805; kindly provided by Dr E. Goulmy]) and heterozygous (HA-1H/R) HA-1–positive cell lines (OPM2 and KG1) or primary leukemic blasts (1, 2, and 3) as well as HA-1–negative cell lines (U266, RR [line 6574803]) are demonstrated.
HA-1 gene expression in 65 HLA-A2–positive healthy blood donors, hematopoietic cell lines, and primary leukemic blasts by allele-specific RT-PCR. PCR using HA-1 allele-specific primers was performed as previously described,2 resulting in a 255-bp fragment. Results from representative samples from homozygous (HA-1H/H) (including CROFT and cell line HH [6574805; kindly provided by Dr E. Goulmy]) and heterozygous (HA-1H/R) HA-1–positive cell lines (OPM2 and KG1) or primary leukemic blasts (1, 2, and 3) as well as HA-1–negative cell lines (U266, RR [line 6574803]) are demonstrated.
Response
We appreciate the letter of Brossart et al confirming our recent results of ex vivo generation of cytotoxic T cells specific for HA-1 minor histocompatibility antigen (mHag) for adoptive immunotherapy of relapsed leukemia. Brossart et al have generated HA-1–specific CTL from unprimed, HLA-A2–positive healthy donors using monocyte-derived dendritic cells (moDC) as antigen-presenting cells (APC), whereas we used peripheral blood dendritic cells (PBDC) or DC cultured from CD34+ progenitor cells (BMDC).1-1 The usage of moDC as primary APC is convenient and generally applicable. The drawback in the protocol of Brossart et al is the addition of fetal calf serum (FCS) in their moDC culture.1-2Xenogenic material should be avoided in the clinical work, but FCS removal from the protocol may affect the outcome. For instance, xeno-antigens of FCS may activate helper T cells, which in turn can provide nonspecific help to HA-1–specific CTL. We have also observed that maturation of DC is easier if they are cultured in FCS as compared with autologous plasma (unpublished observations). We have recently addressed this latter issue and developed a clinically applicable moDC culture protocol in which we show the stable and full maturation of moDC cultured in autologous plasma by the usage of clinically applicable poly I:C, a double-stranded RNA that can be safely used in vivo.1-3
Identical to our results, Brossart et al show that ex vivo-generated HA-1–specific CTLs exhibit potent and antigen-specific lytic activity against HA-1–positive leukemic cells. Unfortunately, Brossart et al did not show the discrimination of hematopoietic cells from nonhematopoietic cells by HA-1–specific CTL, which is crucial for clinical application. Yet, we expect the hematopoietic specificity based on our results in which ex vivo-generated HA-1–specific CTL do not lyse nonhematopoietic cells.1-1
Regarding the number of candidates who are eligible for the therapy, we find the remark of Brossart et al that 70% of the HLA-A2–positive individuals would be candidates if they have HA-1–negative donors misleading. Based on population frequency studies, combined with segregation analyses, we and others have calculated that only 13% of HA-1–positive patients will have an HA-1–negative sibling.1-4-1-6 Evidently, the number of candidates for the therapy will increase if HA-1R–specific CTL can also be generated. We therefore appreciate the attempts of Brossart et al to generate HA-1R–specific CTL.
REFERENCES
ACKNOWLEDGMENT
Supported in part by grants from Deutsche Forschungsgemeinschaft (SFB 510) and Deutsche Krebshilfe.

![Fig. 3. HA-1 gene expression in 65 HLA-A2–positive healthy blood donors, hematopoietic cell lines, and primary leukemic blasts by allele-specific RT-PCR. PCR using HA-1 allele-specific primers was performed as previously described,2 resulting in a 255-bp fragment. Results from representative samples from homozygous (HA-1H/H) (including CROFT and cell line HH [6574805; kindly provided by Dr E. Goulmy]) and heterozygous (HA-1H/R) HA-1–positive cell lines (OPM2 and KG1) or primary leukemic blasts (1, 2, and 3) as well as HA-1–negative cell lines (U266, RR [line 6574803]) are demonstrated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4374/5/m_blod42446003w.jpeg?Expires=1766186845&Signature=h8Q4co5f4xBUbI-2xK6Y21TX71SmX7tBklStgrJ2gjoaPr~FEDNhnzTw9-~qvkecOEndcpvWCZVXickwBWyR3VpUkJTv6c~dp2B35GHt3OUPJ026VKG0ZfRHOHel66ZNiZPMc9SSyG0oYUNCcG5JLfeCNQH-sETBRfS0rT086opH6Kc95Vik5k~LyYX26Jlrffe5GWNu7ClBiCygoE4zG1BuQCsrQZwGwy6uFE7b-pYgz4VrJm4vhDhTVteqRFAyoI1Un8DPrY9S5p~T7wG7L~5XQ7A0zz2q~0-Obt3yrUY-z8EJFxSkDgsKmVwEj5P92nFRVIJs~~nPYjiJJ2bg8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
