Abstract
Integrin-associated protein (IAP; or CD47) is a receptor for the cell binding domain (CBD) of thrombospondin-1 (TS1). In platelets, IAP associates with and regulates the function of IIbβ3 integrin (Chung et al, J Biol Chem 272:14740, 1997). We test here the possibility that CD47 may also modulate the function of platelet integrin 2β1, a collagen receptor. The CD47 agonist peptide, 4N1K (KRFYVVMWKK), derived from the CBD, synergizes with soluble collagen in aggregating platelet-rich plasma. 4N1K and intact TS1 also induce the aggregation of washed, unstirred platelets on immobilized collagen with a rapid increase in tyrosine phosphorylation. The effects of TS1 and 4N1K on platelet aggregation are absolutely dependent on IAP, as shown by the use of platelets from IAP−/− mice. Prostaglandin E1 (PGE1) prevents 4N1K-dependent aggregation on immobilized collagen but does not inhibit the 4N1K peptide stimulation of 2β1-dependent platelet spreading. Finally, a detergent-stable, physical association of IAP and 2β1 integrin is detected by coimmunoprecipitation. These results imply a role for IAP and TS1 in the early activation of platelets upon adhesion to collagen.
THROMBOSPONDINS comprise a family of multidomain, secreted glycoproteins that regulate cell adhesion, migration, inflammation, and platelet aggregation.1,2 The COOH-terminal cell binding domains (CBD) of thrombospondins share a higher degree of homology than other domains of thrombospondin family members.1 The CBD of thrombospondin-1 (TS1) and the CBD peptide 4N1K bind to a widely expressed 50-kD membrane protein that we have identified as integrin-associated protein (IAP) or CD47.3,4 IAP is a member of the IgG superfamily of receptors with a single IgGv extracellular domain, five transmembrane segments, and a short COOH-terminal cytoplasmic tail.5 IAP is involved in host defense6 and transendothelial migration of neutrophils (PMNs).7 In C32 melanoma cells, we observed that recombinant CBD or the IAP agonist peptide 4N1K (KRFYVVMWKK) derived from the CBD, but not the mutant peptide 4NGG (KRFYGGMWKK), could stimulate αvβ3-mediated spreading of C32 cells on a sparse vitronectin coating and is markedly enhanced by 4N1K.8The peptide can also activate washed platelets, stimulate their spreading on immobilized fibrinogen, and increase the binding of the ligand mimetic monoclonal antibody (MoAb) PAC-1 to αIIbβ3.9,10 Activation of these αIIbβ3- and αvβ3-dependent functions by 4N1K is specifically mediated by IAP as judged by the effects of a function blocking MoAb versus IAP.8 The stimulatory effects of 4N1K are inhibited by pertussis toxin treatment, indicating the participation of heterotrimeric Gi proteins in the IAP signaling pathway.8 10 The activation of αIIbβ3 by IAP appears to be quite analogous to the activation of this integrin by thrombin, ADP, epinephrine, and thromboxane A2, all of which act via serpentine or 7-transmembrane receptors and heterotrimeric G proteins.
An additional, physiologically critical mechanism for platelet activation involves the interaction of platelets with subendothelial interstitial collagens.11-13 Several candidates exist for the receptors that mediate this pathway of activation12-18; however, it is clear that genetic deficiency or MoAb blockade of either α2β1 integrin11-13 or glycoprotein VI (GpVI)14,15 cause a severe deficit in collagen-stimulated platelet activation/aggregation.14,15,17 Recently, a direct collagen binding study suggested that an initial interaction of fibrillar collagen with platelet GpVI (Mg2+ independent) could activate α2β1 to bind collagen in a Mg2+-dependent fashion.16 Although the role of α2β1 as a Mg2+-dependent collagen receptor has been well established,11 the precise role of α2β1 in platelet activation and its mechanism of signaling remain to be defined.19
We have recently demonstrated that IAP associates with α2β1 integrin in smooth muscle cells in which 4N1K specifically stimulates α2β1-dependent activities such as chemotaxis to soluble collagen.20 Thus, we tested the possibility that IAP could augment α2β1-dependent functions in platelets. We find that 4N1K synergizes with suboptimal levels of soluble collagen to stimulate aggregation of platelet-rich plasma (PRP). 4N1K and TS1 also induce aggregation and spreading of washed platelets on immobilized collagen only in platelets expressing IAP. 4N1K treatment also accelerates tyrosine phosphorylation of platelet proteins. Finally, we demonstrate a physical association of IAP with platelet α2β1 integrin.
MATERIALS AND METHODS
Materials.
Apyrase, indomethacin, bovine serum albumin (BSA), type I calf skin collagen (for aggregation), and prostaglandin E1 (PGE1) were obtained from Sigma (St Louis, MO). Rat tail type I collagen (for platelet adhesion) was from Collaborative Biochemical Products (Bedford, MA). Antiphosphotyrosine MoAb, PY20, was from Transduction Laboratories (Lexington, KY) and antiphosphotyrosine MoAb, 4G10, was from Upstate Biotechnology Inc (Lake Placid, NY). Antihuman IAP MoAb, B6H12, has been described.3-7 MoAbs P4C10 (anti-β1) and P1E6 (anti-α2) were purchased from GIBCO BRL (Grand Island, NY) and Western blotting polyclonal antibodies against α2 and β1 were from Chemicon International Inc (Temecula, CA). Goat antirabbit F(ab)2 conjugated with horseradish peroxidase (HRP) was from Jackson Labs (West Grove, PA). ECL Western blotting detection kits were obtained from Amersham (Arlington Heights, IL). All peptides used were synthesized, purified, and verified by mass spectrometry by the Protein and Nucleic Acid Chemistry Laboratory of Washington University (St Louis, MO). TS1 was prepared from human platelets as described.8
Platelet preparation and assays.
Fresh human platelets were prepared from 3% sodium-citrate anticoagulated blood. For assays with mouse platelets, blood was collected from 30 wild-type and 30 IAP-deficient (IAP−/−) mice6 by cardiac puncture using heparin as an anticoagulant. Human blood samples were centrifuged at 1,000 rpm in a Beckman GP table top centrifuge for 15 minutes to obtain PRP. PRP was used for aggregation assays performed in a dual channel Chrono-Log aggregometer.9 To prepare washed human and mouse platelets, 1 μmol/L PGE1 was added to PRP and platelets were pelleted by centrifugation at 2,000 rpm for 15 minutes. Pelleted platelets were resuspended in buffer A (138 mmol/L NaCl, 2.9 mmol/L KCl, 0.5 mmol/L MgCl2, 12 mmol/L NaHCO3, 0.3 mmol/L NaH2PO4, 5.5 mmol/L glucose, 1 mg/mL BSA, and 10 mmol/L HEPES, pH 7.4). Washed platelet adhesion to immobilized collagen was performed on Lab-Tek 8 chamber slides (VWR Scientific, St Louis, MO). Slides were precoated with rat tail type I collagen (5 μg/mL) overnight at 4°C and blocked with 1 mg/mL BSA. Washed platelets (2 × 108/mL [human] and 2 × 107/mL [mouse]) in buffer A were allowed to adhere on collagen-coated slides. After 60 minutes at room temperature, adherent platelets were fixed with 1% paraformaldehyde, permeabilized by 0.1% Triton-X 100, and stained with rhodamine-phalloidin. To detect tyrosine phosphorylation of platelet proteins at various time points, adherent platelets were lysed in 6× precooled modified RIPA buffer. 1× RIPA buffer is 50 mmol/L Tris/HCl, pH 7.4, 0.15 mol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mmol/L EGTA, 1 mmol/L Na3VO4, and protease inhibitor cocktail.8 Lysates were subjected to immunoprecipitation using MoAb PY20 and immunoblotting using MoAb 4G10 as described.8,9 To demonstrate the association of IAP with α2β1 integrin, platelets were lysed in 30 mmol/L octyl-glucoside and lysates were subject to immunoprecipitation and blotting as described.8 9
RESULTS
The IAP agonist peptide, 4N1K, synergizes with soluble collagen in platelet aggregation.
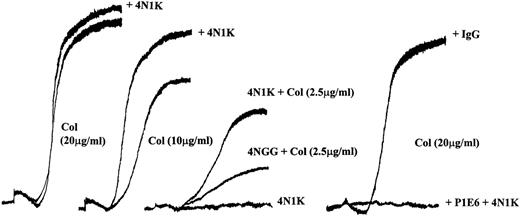
We9 and others10 have reported that the IAP agonist peptide 4N1K activates washed platelets resulting in their aggregation. However, the peptide fails to stimulate aggregation of platelets in PRP (Fig 1, center, and Dorahy et al10). In view of the effects of 4N1K on collagen-stimulated smooth muscle cell migration,20 we tested the ability of 4N1K to augment platelet aggregation in response to soluble collagen. We first determined the response of PRP to increasing concentrations of soluble collagen. As shown in Fig 1, a concentration-dependent aggregation response was observed between 0 and 20 μg/mL of collagen. We found that the threshold for aggregation by collagen varied depending on the donor, but in general, collagen concentrations of 1 to 3 μg/mL gave a detectable response, whereas 20 μg/mL resulted in a maximal aggregation response. The addition of 4N1K along with submaximal amounts of collagen resulted in a significant increase in the rate and extent of aggregation. This synergistic effect was more evident at low concentrations of collagen (2 to 10 μg/mL, depending on the donor; Fig 1, left 3 sets). The inactive control peptide 4NGG did not synergize with soluble collagen at any concentration (Fig 1, center, shown for 2.5 μg/mL of collagen only). The α2 function blocking antibody, P1E6, blocked the aggregation induced by collagen as reported12 19 with or without 4N1K pretreatment, whereas an IgG control antibody had no effect on collagen-induced aggregation of PRP in the presence or absence of 4N1K (Fig 1, right).
4N1K synergizes with soluble collagen in platelet aggregation in PRP. Freshly prepared PRP was equilibrated in the aggregometer cuvette at 37°C for 5 minutes and then stimulated with soluble collagen (2.5, 10, or 20 μg/mL). 4N1K (50 μmol/L) or 4NGG, control peptide (50 μmol/L), was added 2 minutes before the soluble collagen. Anti-2 MoAb P1E6 (10 μg/mL) or mIgG (10 μg/mL) was added 2 minutes before agonist addition in the right set of curves. These experiments were repeated 10 times with platelets from different donors.
4N1K synergizes with soluble collagen in platelet aggregation in PRP. Freshly prepared PRP was equilibrated in the aggregometer cuvette at 37°C for 5 minutes and then stimulated with soluble collagen (2.5, 10, or 20 μg/mL). 4N1K (50 μmol/L) or 4NGG, control peptide (50 μmol/L), was added 2 minutes before the soluble collagen. Anti-2 MoAb P1E6 (10 μg/mL) or mIgG (10 μg/mL) was added 2 minutes before agonist addition in the right set of curves. These experiments were repeated 10 times with platelets from different donors.
TS1 and 4N1K induce the aggregation of platelets adherent to immobilized collagen.
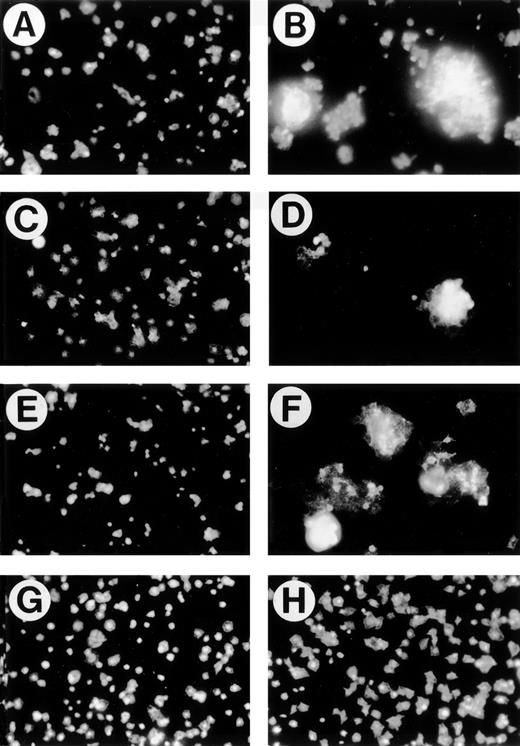
Platelets spread and change shape on immobilized collagen.11,21 The morphology of washed platelets attached and spread on collagen-coated slides was monitored by fixing and staining with rhodamine-phalloidin. Unstimulated platelets spread slowly such that, at 60 minutes, most are unspread (Fig 2A). The presence of 50 μmol/L 4N1K in solution caused platelets to form large aggregates on the immobilized collagen (Fig 2B), whereas only a few small aggregates were seen in the negative control (Fig 2A). We did not observe platelet aggregate formation in the presence of 4N1K on plates coated with fibrinogen or BSA (data not shown). Thus, 4N1K stimulation of IAP together with α2β1 engagement by immobilized collagen leads to aggregation of adherent platelets even without stirring (Fig 2A and B). The biologically inert control peptide, 4NGG, was not able to induce aggregation or enhance spreading (Fig 2C). The intact TS1 molecule was also able to stimulate the aggregation of platelets attaching to immobilized collagen (Fig 2D). To determine if the aggregation of platelets observed upon 4N1K stimulation was due to costimulation by ADP released from the platelets, we included apyrase22 in the spreading experiment. As seen in Fig 2E (control) and F (4N1K), apyrase slightly reduces the size of aggregates present in the 4N1K-treated platelets but does not eliminate aggregation, indicating that release of ADP is not necessary for aggregation in response to 4N1K. A similar partial effect was seen with indomethacin (not shown). PGE1 inhibits platelet aggregation by increasing intracellular cAMP levels and blocking secretion of platelet granule proteins such as fibrinogen required for aggregation.12 In the presence of PGE1 (Fig 2G and H), stimulation of spreading by 4N1K, but not aggregation, was observed (Fig 2H). Aggregation was also blocked by GRGDSP peptide, whereas the GRGESP control peptide was without effect (not shown), further indicating that the aggregation is dependent on αIIbβ3, whereas spreading on collagen requires α2β1 (below). We also tested the effect of thrombin receptor peptide (SFLLRN), a potent costimulator of platelet activation.9 Thrombin receptor peptide 5 μmol/L stimulated the spreading of platelets on collagen (data not shown), but aggregate formation, as seen with 4N1K in Fig 2B, was not observed. Thus, it appears that the simultaneous engagement of α2β1 and IAP represents a unique stimulus for aggregation.
4N1K and TS1 induce the aggregation of platelets adherent to immobilized collagen. Washed platelets were allowed to adhere on immobilized collagen (10 μg/mL). After 60 minutes, platelets were fixed with 1% paraformadehyde, permeabilized with 0.1% Triton X-100, and stained with rhodamine-phalloidin. Treatments added 15 minutes before plating were (A) no treatment; (B) 4N1K (50 μmol/L); (C) 4NGG (50 μmol/L); (D) TS1 (50 μg/mL); (E) apyrase (10 U/mL); (F) apyrase (10 U/mL) plus 4N1K (50 μmol/L); (G) PGE1 (1 μmol/L); (H) PGE1 (1 μmol/L) + 4N1K (50 μmol/L).
4N1K and TS1 induce the aggregation of platelets adherent to immobilized collagen. Washed platelets were allowed to adhere on immobilized collagen (10 μg/mL). After 60 minutes, platelets were fixed with 1% paraformadehyde, permeabilized with 0.1% Triton X-100, and stained with rhodamine-phalloidin. Treatments added 15 minutes before plating were (A) no treatment; (B) 4N1K (50 μmol/L); (C) 4NGG (50 μmol/L); (D) TS1 (50 μg/mL); (E) apyrase (10 U/mL); (F) apyrase (10 U/mL) plus 4N1K (50 μmol/L); (G) PGE1 (1 μmol/L); (H) PGE1 (1 μmol/L) + 4N1K (50 μmol/L).
Specificity of the effect of 4N1K and TS1.
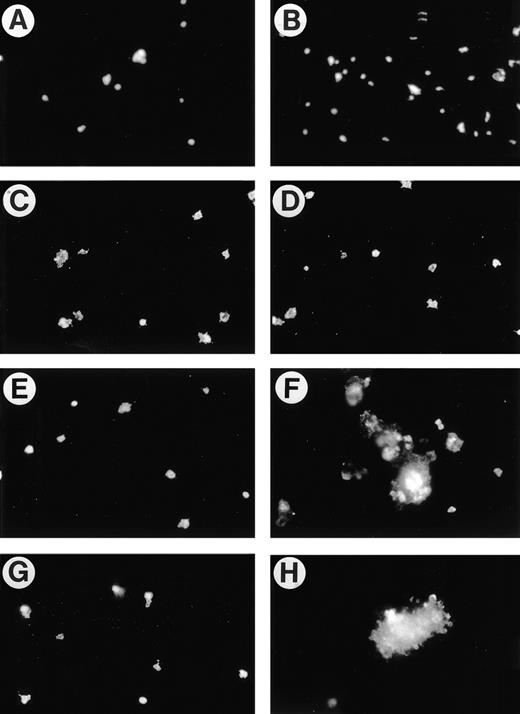
We next tested the specificity of the effect of 4N1K/TS1 on platelet spreading and aggregation using antibodies. We first determined that adhesion of the platelets to collagen was completely dependent on α2β1 function, because P1E6, an anti-α2 specific function-blocking MoAb, virtually obliterated platelet adhesion to collagen regardless of whether platelets were treated with 4N1K (Fig 3A). Pretreatment of the platelets with F(ab)2 fragments of the function-blocking anti-IAP MoAb B6H12 prevented 4N1K-induced aggregate formation and also somewhat reduced the adhesion of platelets to immobilized collagen (Fig 3B, compare with Fig 2A and C).
Specificity of the effect of 4N1K and TS1 on platelet aggregation on collagen. Human platelets prepared as in Fig 2 were allowed to adhere to immobilized collagen after pretreatment for 15 minutes with (A) anti-2 MoAb, P1E6 (10 μg/mL) + 4N1K (50 μmol/L); or (B) B6H12 F(ab)2 (100 μg/mL) + 4N1K (50 μmol/L). For (C) through (H), mouse platelets were processed as for human platelets (see Materials and Methods). (C, E, and G) Platelets from IAP-deficient mice; (D, F, and G) platelets from wild-type mice. (C and D) 4NGG (50 μmol/L); (E and F) 4N1K (50 μmol/L); (G and H) human TS1 (50 μg/mL).
Specificity of the effect of 4N1K and TS1 on platelet aggregation on collagen. Human platelets prepared as in Fig 2 were allowed to adhere to immobilized collagen after pretreatment for 15 minutes with (A) anti-2 MoAb, P1E6 (10 μg/mL) + 4N1K (50 μmol/L); or (B) B6H12 F(ab)2 (100 μg/mL) + 4N1K (50 μmol/L). For (C) through (H), mouse platelets were processed as for human platelets (see Materials and Methods). (C, E, and G) Platelets from IAP-deficient mice; (D, F, and G) platelets from wild-type mice. (C and D) 4NGG (50 μmol/L); (E and F) 4N1K (50 μmol/L); (G and H) human TS1 (50 μg/mL).
The recent availability of IAP-deficient mice6 allows for the most stringent test of the specificity of these effects of 4N1K and TS1 on platelet interactions with collagen. Platelets were harvested from IAP-deficient mice and age-matched wild-type mice of the same strain. They were then processed as the human platelets used in Fig 2and allowed to adhere to collagen-coated slides. The control peptide 4NGG had no effect on the spreading or aggregation of IAP-deficient (Fig 3C) or wild-type (Fig 3D) platelets. The IAP agonist peptide 4N1K was unable to stimulate either spreading or aggregation of IAP-deficient platelets (Fig 3E) while causing massive aggregation of the wild-type mouse platelets (Fig 3F), just as seen with human platelets (Fig 2B). The same results were obtained with intact human TS1, ie, no effect on IAP-deficient platelets (Fig 3G) and strong aggregation of the wild-type mouse platelets (Fig 3H). The requirement for IAP to manifest the effects of both 4N1K and TS1 on platelet spreading and aggregation on collagen rules out several trivial explanations for this effect and supports the idea that the TS1-IAP axis plays a special role in platelet stimulation.
4N1K induces an early increase in tyrosine phosphorylation of platelet proteins.
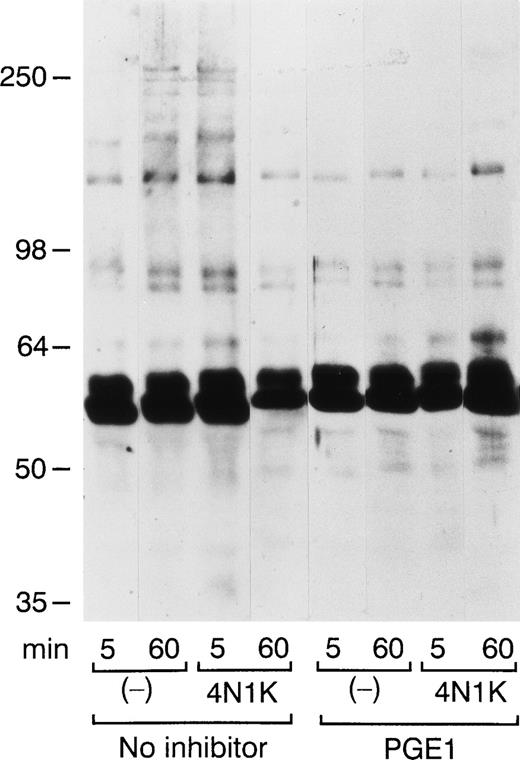
As an index of intraplatelet signaling, we monitored the tyrosine phosphorylation status of platelets adhering to immobilized collagen at different times. As shown in Fig 4, 4N1K induced a rapid increase in tyrosine phosphorylation of platelet proteins. Control platelets adhering to collagen displayed increased tyrosine phosphorylation at 60 minutes. The tyrosine phosphorylation status of 4N1K-treated platelets at 5 minutes was very similar to that of control platelets at 60 minutes, suggesting that IAP stimulation by 4N1K accelerates the tyrosine phosphorylation cascade. PGE1 pretreatment inhibited the early tyrosine phosphorylation induced by 4N1K, suggesting that it was triggered by secretion-dependent aggregation as seen in Fig 2B. In the presence of PGE1, we observed no aggregation and only marginally increased tyrosine phosphorylation at later times up to 60 minutes (Fig 4). The extent of tyrosine phosphorylation of proteins from 4N1K-treated platelets at 60 minutes in the presence of PGE1 was greater than that of control platelets at the same time. Tyrosine phosphorylation of the high molecular weight proteins was preferentially suppressed by PGE1 treatment (Fig 4), indicating that these phosphorylation events probably depend on platelet aggregation, as noted by others.22
4N1K causes an early increase in tyrosine phosphorylation of platelet proteins. Washed platelets adherent to collagen for the indicated times in the presence or absence of 4N1K (as in Fig 2) were lysed in RIPA buffer. Platelets were preincubated without (left) or with PGE1 (right) for 15 minutes. Lysates were subjected to immuno-precipitation with PY20 (antiphosphotyrosine) and 4G10 (antiphosphotyrosine) immunoblot analysis.
4N1K causes an early increase in tyrosine phosphorylation of platelet proteins. Washed platelets adherent to collagen for the indicated times in the presence or absence of 4N1K (as in Fig 2) were lysed in RIPA buffer. Platelets were preincubated without (left) or with PGE1 (right) for 15 minutes. Lysates were subjected to immuno-precipitation with PY20 (antiphosphotyrosine) and 4G10 (antiphosphotyrosine) immunoblot analysis.
IAP physically associates with α2β1 integrin.
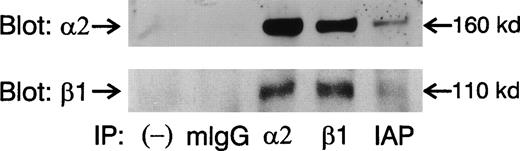
The effects of 4N1K peptide on the spreading of platelets on immobilized collagen (Figs 2 and 3) and the synergy with soluble collagen to induce platelet aggregation (Fig 1) suggest that 4N1K acts through IAP modulation of the affinity/avidity of α2β1 integrin. In the case of β3 integrins such as αvβ3 and αIIbβ3, in which such an effect is well established, IAP has been shown to physically associate with the integrin heterodimer.4-6,8 9 We thus sought to determine if IAP physically associates with α2β1 integrin in platelets. As shown in Fig 5, both α2 and β1 integrin subunits are detected in IAP immunoprecipitates from 30 mmol/L octyl-glucoside extracts of platelets. IgG control antibody and secondary antibody alone did not precipitate α2 and β1 subunits (Fig 5) and neither did an anti-HLA MoAb (data not shown). Thus, the association of IAP with the α2β1 in platelets is specific. Similar results were also obtained when platelets were lysed in Triton X-100 (not shown).
IAP physically associates with 2β1 integrin. Platelets were lysed in 30 mmol/L octyl-β-D-glucoside and lysates were subjected to immunoprecipitation using mIgG and antibodies against 2, β1, and IAP. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), immunoblots were probed with 2 and β1 antibodies.
IAP physically associates with 2β1 integrin. Platelets were lysed in 30 mmol/L octyl-β-D-glucoside and lysates were subjected to immunoprecipitation using mIgG and antibodies against 2, β1, and IAP. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), immunoblots were probed with 2 and β1 antibodies.
DISCUSSION
These studies imply a role for thrombospondin in early platelet activation and aggregation upon platelet adhesion to collagen at sites of vascular injury. Exposure of collagen and the adherence and activation of platelets on the subendothelial matrix is the primary hemostatic response to wounding. Binding to collagen represents a pathway for initiating platelet activation that is distinct from the action of many soluble agents, such as ADP, thrombin, and epinephrine, that engage serpentine or 7TMS receptors. Unlike activation stimulated by these soluble agents, collagen activation of platelets is not antagonized by elevated cyclic AMP levels.23 The loss or blockade of either α2β111-13 or Gp VI14,15,17 prevents the collagen-induced activation of platelets. Recent data suggest that these two receptors act in series, with the initial interaction with collagen occurring via Gp VI, which then in some way activates α2β1.15,16 We recently noted that IAP agonists can functionally activate α2β1 in smooth muscle cells, resulting in enhanced chemotaxis to soluble collagen.20 This provided a precedent for the modulation of α2β1 action by associated membrane proteins and suggested that IAP, which is abundant on platelets,9,24 might also activate platelet α2β1. The data presented here indicate that this is indeed the case. The IAP agonist 4N1K augments the response of platelets to collagen in PRP, even though 4N1K alone cannot stimulate aggregation in PRP (Fig 1 and Dorahy et al10). Aggregate formation by washed platelets stimulated by 4N1K, even without stirring, on immobilized collagen (Fig 2) suggests that α2β1 engagement with collagen together with IAP stimulation generate a potent signal for platelet secretion leading to rapid aggregation even under suboptimal conditions. Thrombin receptor peptide, a potent agonist of platelet aggregation (in stirred conditions), results in spreading on collagen (Fig 2) and fibrinogen,9 but not aggregation. Furthermore, 4N1K treatment of platelets attaching to immobilized fibrinogen results in spreading, but not aggregation.9 This suggests that aggregate formation of washed platelets on collagen, triggered by 4N1K, is a unique result of the simultaneous engagement of α2β1 and IAP that could be physiologically important in augmenting formation of a platelet plug.
The effect of 4N1K and the intact TS1 protein on platelets adherent to collagen is absolutely dependent on the presence of IAP on the platelets. The platelets from IAP-deficient mice fail to respond to either 4N1K or TS1 (Fig 3), with increased spreading or strong aggregation. Thus, these results with IAP-deficient platelets rule out effects mediated by 4N1K or TS1 binding to collagen or other proteins secreted from platelets as well as the interaction of 4N1K and TS1 with other platelet receptors.
Aggregate formation in response to collagen is followed by activation of signaling pathways that include intraplatelet calcium release, formation of phosphoinositides, and phosphorylation of proteins on tyrosine.11,12,15,17,19,21,23 The acceleration of tyrosine phosphorylation in 4N1K-treated platelets on collagen, even in the presence of PGE1 where aggregation is suppressed (Fig 4), suggests that the IAP-CBD interaction dramatically augments one or more primary events upstream of αIIbβ3 activation. The lack of tyrosine phosphorylation of the highest molecular weight proteins in the presence of PGE1 suggests that their phosphorylation occurs subsequent to engagement of αIIbβ3 and aggregation. In our previous studies, we found that 4N1K stimulation of IAP does in fact activate the binding of PAC-1 MoAb, which detects the activated state of αIIbβ3.9 22 Thus, the stimulation of aggregation by 4N1K in the presence of collagen is likely due to activation of αIIbβ3.
TS1 and 4N1K enhance the spreading of platelets on collagen even when aggregation is blocked by PGE1 or RGD peptide. This effect of IAP appears to be mediated soley by α2β1 and may represent activation of this integrin as well.20 The mechanism by which this occurs is not clear at this point. One possibility is a direct regulation of α2β1 integrin function by IAP, as suggested by coimmunoprecipitation of α2, β1, and IAP. Such a direct mechanism might be conformationally mediated and, thus, might not require signaling. Another possibility is that α2β1 or Gp VI and IAP send separate signals, resulting in maximal activation when they combine. Expression of IAP (∼50,000 copies per platelet24) is far more abundant than α2β1 integrin (∼1,500 copies11). Thus, most of the IAP is associated with the highly abundant αIIbβ3 integrin,9 suggesting a more complex role for IAP. It is possible that IAP might only act in combination with αIIbβ3,9 with the synergistic effect of 4N1K with collagen resulting from αIIbβ3/IAP signaling in response to 4N1K. The presence of TS1, which could bind αIIbβ3 and IAP simultaneously,8,25 in the subendothelial matrix26 makes this a viable possibility.
Whatever the mechanism, the synergistic effect on aggregation of collagen and 4N1K/TS1 and the early increase in tyrosine phosphorylation caused by 4N1K suggest that a role of TS1 is the amplification or augmentation of α2β1-mediated signaling, thereby shortening the time required for full activation leading to aggregation. Physiological support for the relevance of this role of thrombospondin is found in the thrombospondin-2–deficient mice.27 In a standard bleeding time tail wound assay, these animals display extremely prolonged bleeding times and often require intervention to stop bleeding. Thrombospondin-2, which is normally present in the vessel wall,1 2 contains a perfect copy of the 4N1K IAP agonist sequence.
ACKNOWLEDGMENT
The authors thank Dr Samuel Santoro for many helpful discussions, advice, and the use of the aggregometer; Dr Eric Brown for helpful advice and anti-IAP F(ab)2 fragments; Drs Thomas Mariani and John McDonald for critical reading of the manuscript; and Dr Sanford Shattil for advice and encouragement at the inception of this work.
Supported by National Institutes of Health Grants No. GM57573 (to F.P.L.) and GM54390 (to W.A.F.) and a grant from Monsanto-Searle (to W.A.F.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to William A. Frazier, PhD, Box 8231, Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, 660 S Euclid Ave, St Louis, MO 63110; e-mail: frazier@biochem.wustl.edu.