Abstract
The major obstacles to successful outcome after allogeneic bone marrow transplantation (BMT) for leukemia remain graft-versus-host disease (GVHD) and leukemic relapse. Improved survival after BMT therefore requires more effective GVHD prophylaxis that does not impair graft-versus-leukemia (GVL) effects. We studied the administration of human recombinant keratinocyte growth factor (KGF) in a well- characterized murine BMT model for its effects on GVHD. KGF administration from day -3 to +7 significantly reduced GVHD mortality and the severity of GVHD in the gastrointestinal (GI) tract, reducing serum lipopolysaccharide (LPS) and tumor necrosis factor (TNF) levels, but preserving donor T-cell responses (cytotoxic T lymphocyte [CTL] activity, proliferation, and interleukin [IL]-2 production) to host antigens. When mice received lethal doses of P815 leukemia cells at the time of BMT, KGF treatment significantly decreased acute GVHD compared with control-treated allogeneic mice and resulted in a significantly improved leukemia-free survival (42%v 4%, P < .001). KGF administration thus offers a novel approach to the separation of GVL effects from GVHD.
ALLOGENEIC BONE MARROW transplantation (BMT) remains the treatment of choice for a number of malignant conditions. Much of the therapeutic potential of this procedure relates to the graft-versus-leukemia (GVL) effect, which eradicates host malignancy after BMT and is mediated by donor T and natural killer (NK) cells.1 GVL effects are closely associated, however, with graft-versus-host disease (GVHD), the major limitation of allogeneic BMT. During GVHD, the skin, gastrointestinal (GI) tract, and liver are damaged by both cellular and inflammatory cytokine effectors.2 The separation of beneficial GVL effects from destructive GVHD remains a prerequisite to improving allogeneic BMT for hematologic malignancies.
Depletion of T cells from the graft effectively prevents GVHD, but it results in the loss of the GVL effect and increases the rate of graft failure.3 An alternative approach to the prevention of acute GVHD is to retain mature T cells in the bone marrow graft, but to disrupt the amplification of inflammatory cytokine effectors. GI tract injury is critical in this regard to subsequent systemic GVHD,4,5 and a pharmacological agent such as keratinocyte growth factor (KGF), which can shield the GI tract, offers an attractive approach to GVHD prophylaxis. KGF is a fibroblast growth factor family member (FGF-7) with a specificity for epithelial tissues expressing its receptors including gut epithelial cells, hepatocytes,6 skin keratinocytes,7 alveolar type II cells,8 mammary epithelium,9 and urothelium.10 In previous studies, KGF administration before autologous BMT dramatically protected the gut epithelium from injury by lethal chemoradiotherapy.11 This protection appears to be due to a potent trophic effect on intestinal epithelium6 and an improved survival of crypt stem cells,12 perhaps through the regulation of genes that reduce oxidative damage (nonselenium glutathione peroxidase)13 and enhance DNA repair (DNA polymerases-α, -δ, and -ε).14
MATERIALS AND METHODS
Mice.
Female C57BL/6 (B6,H-2b, Ly-5.2+) and B6D2F1 (H-2b/d, Ly-5.2+) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). B6 Ly-5a(H-2b, Ly-5.1+) mice were purchased from Frederick Cancer Research Facility (Frederick, MD) and used as donors to document engraftment. The age of recipients ranged between 12 to 14 weeks. Mice were housed in sterilized microisolator cages and received filtered water and normal chow, or autoclaved hyperchlorinated drinking water for the first 2 weeks after BMT.
BMT.
Mice were transplanted according to standard protocol as has been described previously.15 Briefly, on day 0, mice received 1,550 cGy or 1,300 cGy total body irradiation (TBI) (137Cs source), split into two doses separated by 3 hours to minimize GI toxicity. A total of 5 × 106 bone marrow cells and 0.5 × 106 or 2 × 106 nylon wool purified splenic donor T cells were resuspended in 0.25 mL of Leibovitz’s L-15 media, (GIBCO BRL, Gaithersburg, MD) and injected intravenously into recipients after TBI. Studies of T-cell function (see Table 3) were performed after 1,300 cGy of TBI to ensure sufficient number of surviving control mice. In some experiments, Ly-5.2a (H-2b, Ly-5.1+) animals were used as donors (see below). Survival was monitored daily, recipient’s body weight and GVHD clinical score were measured weekly. The degree of systemic GVHD was assessed by a scoring system, which sums changes on a scale from 0 to 2 in five clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10).16
KGF treatment.
Recombinant human KGF was supplied by Amgen (Thousand Oaks, CA). KGF was reconstituted in supplied carrier (Amgen) and diluted in 0.1% bovine serum albumin/phosphate-buffered saline (BSA/PBS) before injection. Mice were injected subcutaneously with KGF (5 mg/kg/dose) once daily from either day -3 to 0 or day -3 to day +7 after BMT. Mice from the control groups received injection of diluent only.
Fluorescence-activated cell sorting (FACS) analysis.
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MoAb) to mouse Ly 5.1 and Ly 5.2 antigens, FITC-conjugated CD4 and phycoerythrin (PE)-conjugated CD8 were purchased from PharmMingen (San Diego, CA). Cells were stained and analyzed as previously described.15 Donor cell engraftment was determined by examining a percentage of Ly-5.1+ cells in peripheral blood at day 56 after transplantation.
Cell cultures.
All culture media and incubation conditions were as previously described.4,15 Splenocytes were removed from animals 14 days after transplant and three to six spleens combined from each group. Mononuclear cells were isolated by ficoll separation as previously described.15 The percentage of CD4+and CD8+ T cells was estimated and cells were plated at a concentration 105 CD4+ plus CD8+ T cells/well with 105 irradiated (2,000 rad) peritoneal macrophages lavaged from naive B6D2F1 (allogeneic) or B6 (syngeneic) animals in 96-flat bottomed plates (Falcon Labware, Lincoln Park, NJ). The ratio of CD4 to CD8 was similar in respective allogeneic splenocyte populations. At 40 hours, cultures were pulsed with 3[H] thymidine (1 μCi per well), and proliferation was determined 20 hours later on a 1205 Betaplate reader (Wallac, Turku, Finland). Cytotoxic T lymphocyte (CTL) assays were performed using the same splenic T-cell populations for effector:target ratios as described above in standard51Cr release assays.15 P815, a mouse mastocytoma cell line (H-2d, American Type Culture Collection, Rockville, MD), was carried in RPMI/10% fetal calf serum (FCS) at 37°C, 5% CO2 and was used for both GVL experiments in vivo and as a target for CTL assays.
Cytokine enzyme-linked immunosorbent assay (ELISA) and lipopolysaccharide (LPS) assays.
The antibodies and standards used in tumor necrosis factor (TNF)α and interleukin (IL)-2 ELISAs were purchased from Genzyme (Cambridge, MA) and PharMingen, respectively. All assays were performed according to the manufacturer’s protocol. LPS concentrations in serum were detected using the Limulus Amebocyte Lysate (LAL) assay (Bio Whittaker, Walkersville, MD) according to the manufacturer’s protocol.
Histology.
Formalin-preserved distal small and transverse large bowel were embedded in paraffin, and 5-μm thick sections were stained with hematoxylin and eosin for histologic examination. Slides were coded and examined in a blinded fashion by one individual (J.M.C) using a semiquantitative scoring system for abnormalities known to be associated with GVHD.15 In the small bowel, the eight parameters scored were crypt loss, villus blunting, lamina propria inflammatory cell infiltrate, mucosal atrophy, enterocyte vacuolization, loss of microvillus brush border, epithelial attenuation, and lymphocytic infiltrate. In the large bowel, the parameters scored were mucosal atrophy, goblet cell mucus depletion, and lymphocytic infiltration. The scoring system for each parameter denoted 0 as normal; 0.5 as focal and rare; 1 as focal and mild; 3 as diffuse and moderate; and 4 as diffuse and severe, as previously described in human17 and experimental4 GVHD histology. Scores were added to provide a total maximum score of 32 in the small bowel and 12 in the large bowel.
Statistical analysis.
Survival curves were plotted using Kaplan-Meier estimates and compared with the Mantel-Cox log-rank test. The Mann-Whitney U test was used for the statistical analysis of cytokine data, LPS levels, clinical scores, weight loss, and histology. P < .05 was considered statistically significant.
RESULTS
KGF reduces intestinal injury, serum LPS, and TNFα levels after allogeneic BMT.
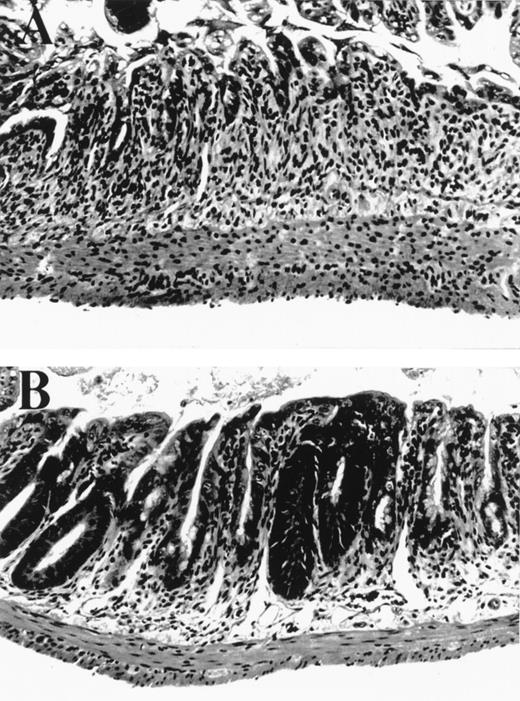
We tested the effects of KGF in a well established murine BMT system where GVHD is induced by both minor and major histocompatibility antigens (B6→ B6D2F1). We hypothesized that KGF may protect the GI tract epithelium from damage inflicted by cellular and inflammatory cytokine effectors of GVHD that occur in addition to that from radiation and chemotherapy. BMT recipients were conditioned with 1,550 cGy of TBI and transplanted with 2 × 106splenic T cells and 5 × 106 bone marrow cells from B6 (allo) or B6D2F1 (syn) mice. KGF was given to allogeneic BMT recipients from day -3 to day +5 at a dose 5 mg/kg/day subcutaneously. The severity of GI histopathology was examined on day 5 after BMT, a time of maximal GI damage in this model, according to a standard scoring system.15 Macroscopic evaluation of control-treated allogeneic recipients showed dilated and edematous bowel, while the intestine in both syngeneic and KGF-treated allogeneic mice appeared normal (data not shown). Microscopically, the small bowel in allogeneic BMT controls was more severely damaged than in syngeneic controls, with significant differences in several features of mucosal architecture and epithelial cytology (Table 1). The large majority of these parameters was reduced by KGF treatment (Table 1 and Fig 1) to the level found in syngeneic BMT recipients, completely abrogating the damage specific to GVHD. Interestingly, KGF treatment given before BMT only did not significantly protect the GI tract from GVHD (Table 1). KGF administration from day -3 to +5 also significantly reduced damage to colonic mucosa in allogeneic animals as determined by semiquantitative scoring of histologic features, which were specific for GVHD as described in Materials and Methods (KGF allo v control allo: 1.8 ± 0.5 v 9.0 ± 0.8, P < .001).
Small bowel histology 5 days after BMT. (A) Allogeneic control mouse, exhibiting severe villus blunting, extensive crypt destruction with no appreciable regenerative response, and a moderate lamina propria inflammatory infiltrate. (B) Allogeneic mouse treated with KGF from day -3 to +5 exhibiting moderate villus blunting, prominent crypt regenerative features, and minimal lamina propria inflammatory infiltrate. Features in syngeneic control mice (not shown) were identical to those in KGF allogeneic animals.
Small bowel histology 5 days after BMT. (A) Allogeneic control mouse, exhibiting severe villus blunting, extensive crypt destruction with no appreciable regenerative response, and a moderate lamina propria inflammatory infiltrate. (B) Allogeneic mouse treated with KGF from day -3 to +5 exhibiting moderate villus blunting, prominent crypt regenerative features, and minimal lamina propria inflammatory infiltrate. Features in syngeneic control mice (not shown) were identical to those in KGF allogeneic animals.
Damage to gut mucosa is believed to be critical for the translocation of LPS into the systemic circulation.4,5,18 Consistent with the anatomic evidence of GI protection, KGF dramatically reduced serum LPS levels in allogeneic BMT recipients to levels detected in syngeneic controls (Table 2). It is interesting to note that allogeneic damage in controls accounted for nearly 90% of the LPS translocated to the systemic circulation, and thus this entire increase was prevented by KGF, correlating with the histologic improvement. LPS plays an important role in GVHD pathophysiology by inducing the production of inflammatory cytokines such as TNFα,18 which is secreted by both host4 and donor5 macrophages. Consistent with reduced serum LPS levels, KGF administration reduced serum TNFα levels by 60% compared with allogeneic BMT controls (Table 2).
KGF treatment reduces GVHD mortality and morbidity.
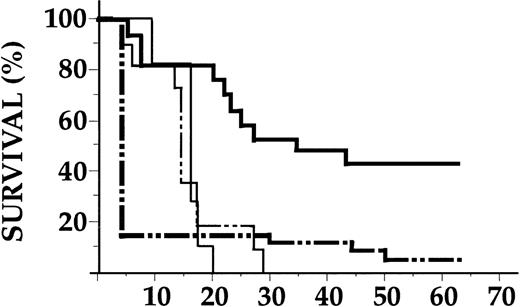
The ability of KGF to protect the GI tract from GVHD-mediated damage and to reduce systemic TNFα levels led us to hypothesize that KGF administration would improve survival and decrease the morbidity of acute GVHD. KGF or control diluent was injected from day -3 to day +7 after allogeneic BMT (0.5 × 106 splenic T cells and 5 × 106 bone marrow cells) and GVHD was assessed (Fig 2A). A total of 81% of allogeneic BMT controls died of GVHD by day 50, whereas mice identically transplanted and treated with KGF had only 22% mortality. KGF also protected animals from lethal GVHD when the donor T-cell dose was escalated fourfold to 2 × 106 per animal (100% v 25% mortality at day 50 in control-v KGF-treated groups, P< .01). Surviving animals were evaluated weekly for clinical GVHD severity using a standard scoring system of five parameters (weight loss, skin integrity, fur texture, mobility, and posture) as detailed in Materials and Methods. As shown in Fig 2B, surviving allogeneic BMT controls developed significantly more severe clinical GVHD from day 21 onwards than KGF-treated animals, although mild GVHD was clearly evident. In pilot studies, recipients of KGF both before and after BMT had significantly less GVHD compared with cohorts treated before BMT only (Fig 2B). Among the clinical parameters of GVHD, weight loss was the most dramatically improved by KGF treatment and was similar to syngeneic controls (data not shown). Analysis of Ly 5 alleles on peripheral blood cells at day 56 after BMT showed complete (100%) donor hematopoietic engraftment in all allogeneic animals, excluding mixed chimerism as a cause of tolerance and reduced GVHD in KGF-treated recipients.
KGF reduces GVHD mortality and morbidity in allogeneic BMT. Recipients were transplanted with 5 × 106 bone marrow cells and 0.5 × 106 splenic T cells from allogeneic (B6) or syngeneic (B6D2F1) donors after 1,550 cGy of TBI. KGF (Amgen) or control diluent was given subcutaneously from either day -3 to day 0 or day -3 to +7. Syngeneic BMT (n = 22, - - - ), control diluent-treated allogeneic BMT (n = 26, - · · - ), KGF-treated (day - 3 to 0) allogeneic BMT (n = 16, ), KGF-treated (day -3 to +7) allogeneic BMT (n = 18, ). (A) Survival. Control-treated allogeneic BMT recipients versus KGF-treated (both treatment schedules) and syngeneic BMT recipients (P < .01 by Mantel Cox logrank test). (B) GVHD clinical score. Animals were scored for clinical GVHD by five parameters as described in Materials and Methods. GVHD severity (mean ± standard error [SE]) was significantly less in animals receiving KGF from day -3 to +7 than those receiving KGF from day -3 to 0 and control-treated animals from day 21 onwards (P < .05) and significantly higher than in syngeneic BMT recipients (P < .05). Data represent results combined from two similar experiments.
KGF reduces GVHD mortality and morbidity in allogeneic BMT. Recipients were transplanted with 5 × 106 bone marrow cells and 0.5 × 106 splenic T cells from allogeneic (B6) or syngeneic (B6D2F1) donors after 1,550 cGy of TBI. KGF (Amgen) or control diluent was given subcutaneously from either day -3 to day 0 or day -3 to +7. Syngeneic BMT (n = 22, - - - ), control diluent-treated allogeneic BMT (n = 26, - · · - ), KGF-treated (day - 3 to 0) allogeneic BMT (n = 16, ), KGF-treated (day -3 to +7) allogeneic BMT (n = 18, ). (A) Survival. Control-treated allogeneic BMT recipients versus KGF-treated (both treatment schedules) and syngeneic BMT recipients (P < .01 by Mantel Cox logrank test). (B) GVHD clinical score. Animals were scored for clinical GVHD by five parameters as described in Materials and Methods. GVHD severity (mean ± standard error [SE]) was significantly less in animals receiving KGF from day -3 to +7 than those receiving KGF from day -3 to 0 and control-treated animals from day 21 onwards (P < .05) and significantly higher than in syngeneic BMT recipients (P < .05). Data represent results combined from two similar experiments.
KGF preserves donor T-cell responses to host alloantigens.
Alloreactive donor T cells are critical mediators of acute GVHD.2 3 We therefore investigated whether the inhibition of GVHD by KGF treatment was associated with decreased donor T-cell responses to host alloantigens. Serum interferon (IFN)-γ levels were measured 5 days after BMT and were similarly increased in KGF and control-treated allogeneic animals (79 ± 8 U/mL v 87 ± 9 U/mL, P = .75), suggesting equivalent T-cell expansion early after BMT. On day 14 after allogeneic BMT as described in Materials and Methods, donor splenic T cells from KGF- and control-treated allogeneic BMT recipients showed equivalent proliferation to host alloantigens in mixed lymphocyte culture (MLC), although the differences in proliferation to syngeneic stimulators resulted in a twofold reduction of the stimulation index in the KGF-treated group (Table 3). IL-2 production and CTL activity to host antigens was similar in control- and KGF-treated recipients. Splenocytes from both groups showed equivalent lysis of P815 (H-2d) leukemia cells (syngeneic to host) and only background lysis of EL-4 (H-2b) leukemia targets (syngeneic to donor). Thus, KGF treatment in vivo produced little or no alteration of critical T-cell responses to host antigens despite dramatic attenuation of GI tract GVHD and improved survival after allogeneic BMT.
KGF administration preserves GVL effects.
The vigorous donor T-cell activity against host antigens in vitro suggested that KGF treatment might preserve GVL effects of allogeneic BMT. We therefore evaluated the effects of KGF treatment in this BMT model when 5,000 host type P815 leukemia cells were injected with the donor inoculum on the day of BMT. Injection of 2,000 cells of this aggressive leukemia produces 100% mortality by day 30 after syngeneic BMT (data not shown). KGF treatment dramatically improved leukemia-free survival in allogeneic BMT recipients compared with T-cell depleted (TCD) BMT recipients treated either with KGF or control diluent (Fig 3). Mortality due to leukemia began to occur from day 10 onwards in all groups and all animals that died after this time point had gross evidence of tumor on necropsies. Eradication of leukemia was confirmed in all surviving animals by macroscopic examination of the spleen and liver at day 65 after BMT. The incidence of death from leukemia after day 10 was similar in both KGF- and control-treated allogeneic groups (6 from 14 v 2 from 4). In addition, KGF- and control diluent-treated TCD recipients died at similar rates, confirming that KGF did not alter the kinetics of leukemic growth after BMT.
Preservation of allogeneic GVL effects in KGF-treated mice. B6D2F1 recipients were conditioned and transplanted as in Fig 1with the addition of 5,000 P815 tumor cells to the bone marrow inoculum at day 0. Recipients of TCD bone marrow or bone marrow plus T cells from allogeneic B6 donors were treated with KGF or control diluent from day -3 to +7 as described in Materials and Methods. Results are represented as Kaplan-Meier cumulative survival estimates from two similar experiments. Control diluent-treated TCD recipients (, n = 11), KGF-treated TCD recipients ( - - - , n = 6), control allogeneic BMT recipients ( – · · – , n = 28), KGF allogeneic BMT recipients (, n = 17). KGF versus control (allogeneic BMT groups), P < .0001.
Preservation of allogeneic GVL effects in KGF-treated mice. B6D2F1 recipients were conditioned and transplanted as in Fig 1with the addition of 5,000 P815 tumor cells to the bone marrow inoculum at day 0. Recipients of TCD bone marrow or bone marrow plus T cells from allogeneic B6 donors were treated with KGF or control diluent from day -3 to +7 as described in Materials and Methods. Results are represented as Kaplan-Meier cumulative survival estimates from two similar experiments. Control diluent-treated TCD recipients (, n = 11), KGF-treated TCD recipients ( - - - , n = 6), control allogeneic BMT recipients ( – · · – , n = 28), KGF allogeneic BMT recipients (, n = 17). KGF versus control (allogeneic BMT groups), P < .0001.
DISCUSSION
We have shown that KGF administration reduces GVHD mortality and long-term morbidity in an experimental BMT model, primarily by protecting the GI tract from GVHD damage and subsequent inhibition of LPS translocation and TNFα generation. KGF did not suppress T-cell responses to host tissue and preserved a GVL response, resulting in a significant increase in leukemia-free survival.
The ability of KGF to protect the GI tract from injury due to chemotherapy and radiation is maximal when it is administered before chemoradiotherapy.11 The protective mechanisms include enhanced crypt survival11 due to increased intestinal stem cell survival,12 goblet cell hyperplasia, and enhanced mucin secretion.6 KGF also promotes TGFα secretion from intestinal epithelial cells,19 which is an important mediator of mucosal healing.20 In the current study, the maximal protection from GVHD morbidity occurred when KGF was given both before and after BMT rather than before BMT only. This observation confirms and extends a recent study in which KGF, given before BMT only, did not protect the large bowel from GVHD damage.21Limiting the administration of KGF before BMT did delay GVHD mortality, but it produced significantly less protection than observed in this study. The importance of continuing KGF administration after allogeneic BMT suggests that KGF may promote recovery of the GI tract after injury, a phenomenon demonstrated in models of colitis.22
Damage to GVHD target organs is mediated by both inflammatory cytokines (IL-1, TNFα, IFNγ) and cellular effectors (CTL and NK cells).23 Injury to the GI tract is mediated predominantly by TNFα, while the Fas-dependent CTL pathway is important in the development of hepatic GVHD.24 GVHD histopathology in the GI tract has been described in three phases,25 and we have included discriminatory parameters from each phase in our histological scoring system. The initial proliferative phase results in increased crypt cell mitotic activity, crypt lengthening, and increased intraepithelial lymphocytes. However, the histological features of GI tract GVHD in this model are consistent with the destructive and atrophic phases, characterized by villus blunting, lamina propria inflammation, crypt destruction (with crypt stem cell loss), and mucosal atrophy (Table 1). These features together with epithelial vacuolization and attenuation are induced by inflammatory cytokines such as TNFα26 and IL-1.27 The dramatic amelioration of these histological features in KGF-treated recipients is therefore consistent with the reduction in serum TNFα levels in these animals. Disruption of the GI mucosal barrier facilitates the translocation of LPS, a normal constituent of endogenous bowel flora, into the systemic circulation.4 LPS is a potent stimulus for inflammatory cytokine production18 and augments donor T-cell activation,28 thereby amplifying both inflammatory and cellular effectors of GVHD. In this study, KGF administration inhibits TNFα generation, most likely by protecting the GI epithelium from GVHD injury, which is mediated by both TBI and alloreactive T cells.4 Disruption of LPS leakage suppresses TNFα generation that mediates ongoing gut injury4 and thus the “indirect” blockade of inflammatory cytokines by KGF provides dramatic protection from GVHD.
It should be noted that KGF did not entirely prevent clinical GVHD, as evidenced by the elevated clinical scores throughout the transplant period (Fig 2B) and the typical findings of atrophy in the thymus and spleen at day 56 (data not shown). This GVHD probably reflects the persistent responses of allospecific donor T cells to host antigens in animals treated with KGF. The KGF receptor has not been described on cells of hematopoietic origin29,30 and the preservation of T-cell function after KGF administration is therefore not surprising. In contrast to other systems where GVL effects appear to depend on CD8+ T cells,31 depletion of either CD4+ or CD8+ populations before BMT compromises GVL in our model, showing that both these subsets play a critical role in the expansion of GVL effector cells (manuscript in preparation). It is important to note that the proportion of mice dying of leukemia was similar in control-and KGF-treated recipients, confirming that mechanisms of alloreactive leukemia eradication are not impaired by KGF administration. Studies, which further escalate the dose of leukemia, are ongoing to quantify the magnitude of GVL effects preserved after KGF treatment. Finally, KGF does not appear to protect malignant cell lines from chemoradiotherapy in models studied to date,11,32,33 suggesting reductions in the leukemic burden by BMT conditioning will not be adversely affected by KGF administration. The favorable clinical toxicity profile of KGF,34 as well as these compelling preclinical data, make KGF an attractive candidate to study in clinical trials as an adjunct to standard GVHD prophylaxis.
O.I.K and G.R.H. contributed equally to this work and should be regarded as cofirst authors.
Supported by Grants No. CA39542 and HL55162 from the National Institutes of Health (to J.L.M.F.). J.L.M.F. is a scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to James L.M. Ferrara, MD, Departments of Internal Medicine and Pediatrics, Division of Hematology and Oncology, University of Michigan Cancer Center, Ann Arbor, MI 48109-0560; e-mail: ferrara@umich.edu.

![Fig. 2. KGF reduces GVHD mortality and morbidity in allogeneic BMT. Recipients were transplanted with 5 × 106 bone marrow cells and 0.5 × 106 splenic T cells from allogeneic (B6) or syngeneic (B6D2F1) donors after 1,550 cGy of TBI. KGF (Amgen) or control diluent was given subcutaneously from either day -3 to day 0 or day -3 to +7. Syngeneic BMT (n = 22, - - - ), control diluent-treated allogeneic BMT (n = 26, - · · - ), KGF-treated (day - 3 to 0) allogeneic BMT (n = 16, ), KGF-treated (day -3 to +7) allogeneic BMT (n = 18, ). (A) Survival. Control-treated allogeneic BMT recipients versus KGF-treated (both treatment schedules) and syngeneic BMT recipients (P < .01 by Mantel Cox logrank test). (B) GVHD clinical score. Animals were scored for clinical GVHD by five parameters as described in Materials and Methods. GVHD severity (mean ± standard error [SE]) was significantly less in animals receiving KGF from day -3 to +7 than those receiving KGF from day -3 to 0 and control-treated animals from day 21 onwards (P < .05) and significantly higher than in syngeneic BMT recipients (P < .05). Data represent results combined from two similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/2/10.1182_blood.v94.2.825/5/m_blod41410002x.jpeg?Expires=1766356386&Signature=Jc-JtkTa1FAWHJWQMsilAXKNqcbEs9plPkBhwZywG6s5RXSCkXeB4VeSvR7LHTVqpCr37rbjQGKVqRaU0aWRajsKTejhU5I9oOdQTkGhj6PKbNV3vyC1OiHwZT2wJ4cqC50~Ck4yS5kwLlTcYQnSzikKSwD3XCypvuVcabzX5xqQwoWjpIu0SRBJD33O7B78sJdFb90ocDbBV5MMzifabTHeLpWgJWv5fK37xL2mfiMAlJTkFD3wWmax-Jmi7tOGSsScD2zAcot~2aSPTddbjhWWjkyGGvYAxBtxIM7vBDrbgx1x4bvZUeYF5uGILPmFmbFbVqlZii8XHgmkbwp2dA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)