Abstract
Delivery of targeted hematopoietic irradiation using radiolabeled monoclonal antibody may improve the outcome of marrow transplantation for advanced acute leukemia by decreasing relapse without increasing toxicity. We conducted a phase I study that examined the biodistribution of 131I-labeled anti-CD45 antibody and determined the toxicity of escalating doses of targeted radiation combined with 120 mg/kg cyclophosphamide (CY) and 12 Gy total body irradiation (TBI) followed by HLA-matched related allogeneic or autologous transplant. Forty-four patients with advanced acute leukemia or myelodysplasia received a biodistribution dose of 0.5 mg/kg131I-BC8 (murine anti-CD45) antibody. The mean ± SEM estimated radiation absorbed dose (centigray per millicurie of 131I) delivered to bone marrow and spleen was 6.5 ± 0.5 and 13.5 ± 1.3, respectively, with liver, lung, kidney, and total body receiving lower amounts of 2.8 ± 0.2, 1.8 ± 0.1, 0.6 ± 0.04, and 0.4 ± 0.02, respectively. Thirty-seven patients (84%) had favorable biodistribution of antibody, with a higher estimated radiation absorbed dose to marrow and spleen than to normal organs. Thirty-four patients received a therapeutic dose of 131I-antibody labeled with 76 to 612 mCi131I to deliver estimated radiation absorbed doses to liver (normal organ receiving the highest dose) of 3.5 Gy (level 1) to 12.25 Gy (level 6) in addition to CY and TBI. The maximum tolerated dose was level 5 (delivering 10.5 Gy to liver), with grade III/IV mucositis in 2 of 2 patients treated at level 6. Of 25 treated patients with acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), 7 survive disease-free 15 to 89 months (median, 65 months) posttransplant. Of 9 treated patients with acute lymphoblastic leukemia (ALL), 3 survive disease-free 19, 54, and 66 months posttransplant. We conclude that 131I-anti-CD45 antibody can safely deliver substantial supplemental doses of radiation to bone marrow (∼24 Gy) and spleen (∼50 Gy) when combined with conventional CY/TBI.
BONE MARROW transplantation offers the best chance of cure for patients with advanced acute leukemia and myelodysplastic syndrome (MDS), but the high doses of total body irradiation (TBI) and/or chemotherapy used to kill leukemic cells cause systemic toxicities in the majority of patients. Despite this high-dose therapy, many patients relapse after transplantation, especially those in relapse at the time of the procedure. Efforts to decrease the incidence of relapse have included intensification of cytoreductive therapy, either by increasing the TBI dose or adding chemotherapy, but most attempts have resulted in higher transplant-related mortality.
Two studies in particular have demonstrated the marked dose effect of radiation for myeloid leukemias. In studies of patients with acute myeloid leukemia (AML) in first remission1 or chronic myeloid leukemia (CML) in chronic phase2 with randomization between 12 or 15.75 Gy TBI combined with 120 mg/kg cyclophosphamide (CY), the higher radiation dose resulted in a lower relapse rate. For AML, the relapse rate was 12% after 15.75 Gy, compared with 35% after 12 Gy. For CML, the relapse rate was 0% after 15.75 Gy, compared with 25% after 12 Gy. However, in both studies, the higher radiation dose was associated with greater regimen-related toxicities and mortality, with no difference in long-term disease-free survival between the 2 radiation doses. These studies led to the hypothesis that, if radiation could be targeted directly to sites of leukemic involvement in hematopoietic tissues, with relative sparing of normal organs, the radiation sensitivity of leukemia could be exploited and survival might be improved without increased toxicity.
Monoclonal antibodies labeled with radioisotopes have been used to deliver targeted radiation in both preclinical3-12 and clinical13-31 studies, with varying success. Hematologic malignancies may provide an optimum setting in which to use this approach, given their relative radiosensitivity and the comparative ease with which circulating antibody can gain access to cells in marrow and spleen. To maximize potential antibody binding sites in these tissues for patients in remission or relapse, we sought a target antigen expressed by as many cells as possible, including those of both myeloid and lymphoid origin whether normal or malignant. When a monoclonal antibody is labeled with 131I, the 0.8-mm path length of the beta energy of this isotope will result in radiation delivery to all cells in a tissue provided most cells distributed throughout the tissue bind the antibody. We therefore chose to target CD45, the most broadly expressed of the known hematopoietic antigens. CD45 is a tyrosine phosphatase expressed in various isoforms between 180 and 220 kD. It is expressed on virtually all leukocytes, including myeloid and lymphoid precursors in bone marrow and mature lymphocytes in lymph nodes.32 More than 90% of AML samples and most acute lymphoblastic leukemia (ALL) samples express this antigen.33,34 Surface expression averages 200,000 copies per cell, and the antigen does not internalize after antibody binding.9
Preclinical experiments in both mice35 and nonhuman primates36 demonstrated that 131I-anti-CD45 antibody could deliver relatively specific radiation to hematopoietic tissues, with 2 to 3 times more radiation delivered to marrow, up to 12 times more to spleen, and 2 to 8 times more to lymph nodes as compared with liver, lung, or kidney. We thus initiated a phase I dose escalation study combining 131I-anti-CD45 antibody with CY and 12 Gy TBI in patients with advanced AML, ALL, and MDS receiving matched related or autologous stem cell rescue. The goals of this study were to determine the biodistribution of 131I-anti-CD45 antibody in patients with leukemia in remission and relapse, to define factors influencing antibody biodistribution, and to determine the toxicity of targeted hematopoietic irradiation when combined with a conventional preparative regimen. Our initial report of the first 23 patients entered on study demonstrated that the majority had successful targeting of radiation to hematopoietic tissues and that up to 7 Gy of radiation delivered by antibody to the normal organ receiving the highest dose (liver) was well tolerated when combined with CY/TBI.29 We now report the completion of the study, demonstrating that 84% of 44 patients undergoing biodistribution studies had good localization of antibody and that a maximum dose of 10.5 Gy delivered by radiolabeled antibody to the liver could be tolerated in addition to CY and 12 Gy TBI.
MATERIALS AND METHODS
Patient selection.
Patients referred to the Fred Hutchinson Cancer Research Center (Seattle, WA) for treatment of AML or ALL beyond first remission or for advanced MDS (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or chronic myelomonocytic leukemia) were eligible for this study. Patients in relapse with peripheral blast counts in excess of 5,000/μL were eligible only if their blast count could be brought below that level by treatment with hydroxyurea. Patients were excluded if they had major organ dysfunction, were seropositive for human immunodeficiency virus (HIV), were allergic to mouse protein or to iodine, or had pre-existing human antimouse antibody (HAMA). Stem cell sources were either bone marrow harvested from HLA-identical family members, cryopreserved autologous marrow, or cryopreserved autologous peripheral blood stem cells. Purging of autologous marrow with 4-HC37 or monoclonal antibodies and complement38 was allowed. Patients were informed of the potential risks and benefits of participating in this phase I study and signed a consent form approved by the Human Subjects Committee of the University of Washington and the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Antibody production, purification, and radiolabeling.
The BC8 hybridoma (developed and provided by Dr Claudio Anasetti of the Fred Hutchinson Cancer Research Center) secretes a murine IgG1 antibody reactive with all CD45 isoforms. The initial lot of antibody was derived from hybridoma culture supernatant produced in hollow fiber bioreactors by Brunswick (San Diego, CA) and was purified by saturated ammonium sulfate precipitation and ion exchange chromatography. Subsequent lots were produced at the Fred Hutchinson Cancer Research Center Biologics Production Facility. Hybridoma supernatant was harvested from an Applikon bioreactor (Applikon Instruments, Shiedam, The Netherlands), filtered, concentrated, pooled, and purified using anion exchange and protein A affinity chromatography. This was followed by virus inactivation using low pH treatment, SP-Sepharose cation exchange chromatography, and diethylaminoethyl (DEAE) anion batch processing. Processed antibody was 0.2-μm sterile filtered and stored at 4°C. Twenty-nine of the first 32 patients also received nonspecific (negative control) antibodies DT or LS (produced and kindly provided by IDEC Pharmaceuticals, San Diego, CA). These antibodies are murine IgG1 antibodies reactive with idiotypes expressed by B-cell lymphomas.
The BC8 antibody was labeled with 131I (specific activity, 8.0 Ci/mg; New England Nuclear, Boston, MA), and DT or LS antibodies were labeled with 125I (specific activity, 17.2 Ci/mg; New England Nuclear) using the chloramine-T method and were purified and tested as previously described.39 40 The immunoreactivity of all 131I-labeled BC8 antibody doses was required to be at least 80% of that of an 125I-trace-labeled control aliquot of BC8 antibody.
Determination of antibody biodistribution and radiation absorbed dose.
Patient serum was first tested for HAMA using an enzyme-linked immunosorbent assay (ELISA) as previously described.39 For patients in relapse, the expression of CD45 on leukemic blasts was determined by BC8 antibody with indirect immunofluorescence assays using a fluorescein isothiocyanate (FITC)-labeled goat-antimouse-IgG+M F(ab′)2 second-stage reagent (Tago, Inc, Burlingame, CA) or by HLe-1 anti-CD45 antibody directly labeled with Peridinin chlorophyll protein (Becton Dickinson, San Jose, CA). Relapse patients were eligible if the CD45 expression on the blast cell population was clearly above that of negative control antibody. Patients in remission did not require leukemic cell phenotyping.
Organ volumes (liver, lungs, spleen, and kidney) were calculated from chest and abdominal computed tomography (CT) or magnetic resonance image (MRI) scans.40 Organ localization scans (technetium-99m liver-spleen, lung, and kidney) were used in the first 16 patients but were subsequently omitted because organs were easily localized (lungs), readily visualized after 131I-BC8 administration (liver and spleen), or demonstrated little uptake of radionuclide (kidney). Thyroid uptake of free 131I was blocked by oral Lugol’s solution (strong iodine solution).
Biodistribution infusions consisted of 0.5 mg/kg BC8 antibody labeled with 5 to 10 mCi 131I. For 29 patients, this was combined with 0.2 mg/kg DT or LS antibody labeled with 2 to 5 mCi125I. Patients were premedicated with diphenhydramine at 25 to 50 mg intravenously (IV), hydrocortisone at 50 to 100 mg IV, and acetaminophen at 650 mg orally. The infusion rate for BC8 antibody varied from 5 to 10 mg/h, as tolerated, with most patients receiving a steady rate of 7.5 mg/h. Diphenhydramine doses were repeated as needed up to every 2 hours, and other medications (meperidine for chills and lorazepam for nausea) were administered as needed for symptoms. If patients developed more severe symptoms, such as throat tightness or shortness of breath, the infusion was slowed or stopped until the symptoms improved.
Blood samples, which were obtained hourly during antibody infusion and 0, 30, 60, 90, and 120 minutes afterwards and then daily for 3 to 5 days, were analyzed for BC8 and nonspecific (where administered) concentrations. Blood clearance curves were fit to determine the long-term clearance half-time and where obvious, the early phase, rapid-clearance half-time. A bone marrow biopsy was obtained the day after infusion (ie, 16 to 24 hours, where hour 0 was the end of infusion). The sample was weighed and counted against a weighed reference aliquot of the expected dose to calculate the percentage of injected dose per gram (% ID/g). For some patients, the biopsy occurred 42 to 48 hours after infusion. The marrow radioactivity clearance curves obtained by gamma camera imaging were scaled for quantitation using the bone marrow biopsy 131I uptake values. For patients receiving 125I-labeled anti-idiotype control antibody, the marrow localization index (LI) was calculated with reference to a concomitant serum sample: LI = (Specific % ID/g [marrow]/Specific % ID/g [serum])/(Nonspecific % ID/g [marrow]/Nonspecific % ID/g [serum]).
Quantitative gamma images were collected with a dedicated GE 400 AT large-field-of-view camera (General Electric Medical Systems, Milwaukee, WI) with a high energy collimator at hour 0 (end of infusion) and then daily for 2 to 3 days (Fig 1). Regions of interest (spleen, liver, lungs, kidneys if visible, and at least 2 marrow sites) were imaged using a 180° opposing view quantitative planar technique.40 Results were compared with an 131I imaging standard for quantitation and were corrected for whole-body thickness attenuation and radioactive decay. The time-activity curves for each source organ were integrated to obtain residence times. Because organ dose is roughly inversely proportional to mass, corrections were made for patient weight and organ mass when actual weights were known from CT or MRI. This correction was made by multiplying the observed source-organ residence time by the ratio of the defined reference man or woman organ mass to the known organ mass. Radiation absorbed doses were then estimated using methods consistent with those recommended by the Society of Nuclear Medicine’s special committee on Medical Internal Radiation Dose,41,42as previously described.43 The marrow clearance curve was scaled by correcting the biopsy-determined % ID/g of131I-BC8 by a multiplication factor of 2, because antibody cannot bind to the trabecular bone and fat that make up approximately half of the total biopsy weight.44,45 For dosimetry purposes, patient marrow volumes were normalized to the MIRD model values of 1,120 grams for an adult male and 1,050 grams for an adult female. For consistency, the same S values42 were used for all marrow dose calculations throughout the study. Statistical comparisons between disease type or stage and between anti-CD45 and control anti-iodiotype antibody used the Student’s t-test (SPSS for Windows 8.0; SPSS Inc, Chicago, IL).
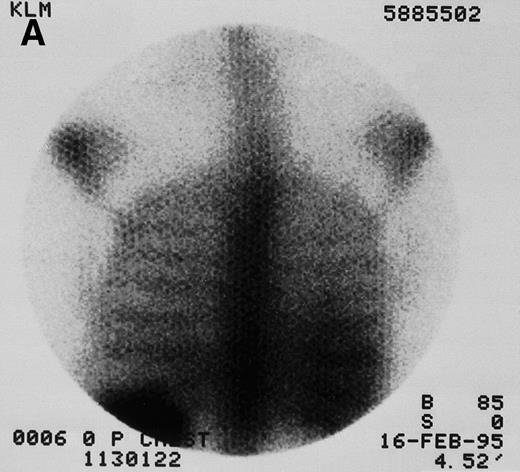
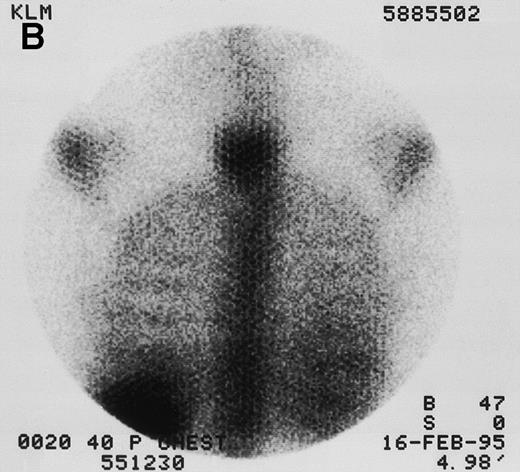
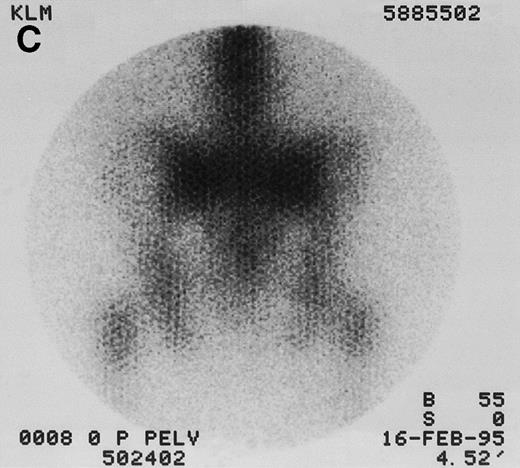
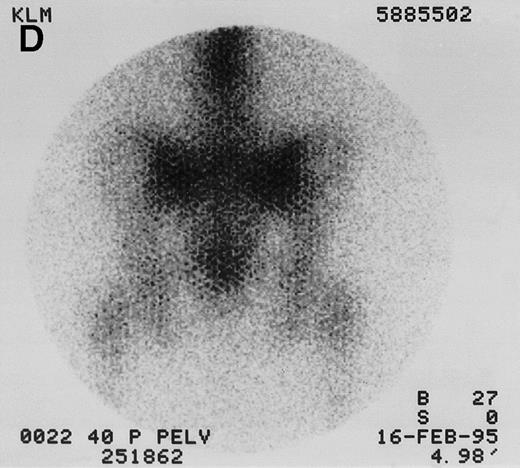
131I-anti-CD45 antibody localization. Posterior 131I-BC8 images of patient with AML in relapse (UPN 9013) immediately after trace-labeled antibody infusion (A and C) and 40 hours after infusion (B and D). Accumulation of labeled antibody is shown in the ribs, vertebral column, ilium, pelvis, and sacrum, all marrow-rich regions of the axial skeleton.
131I-anti-CD45 antibody localization. Posterior 131I-BC8 images of patient with AML in relapse (UPN 9013) immediately after trace-labeled antibody infusion (A and C) and 40 hours after infusion (B and D). Accumulation of labeled antibody is shown in the ribs, vertebral column, ilium, pelvis, and sacrum, all marrow-rich regions of the axial skeleton.
Therapy.
Patients in whom the biodistribution study of 131I-BC8 showed that the target organs of marrow and spleen would receive a greater estimated radiation absorbed dose than liver, lung, or kidney were said to have favorable biodistribution and were eligible for a therapy dose of antibody. Patients were retested for HAMA the day before administration of the therapy dose and were treated only if the test was negative. The therapy dose was labeled with the amount of131I calculated to deliver a specified dose to the normal organ receiving the highest radiation absorbed dose. The starting dose level delivered to this normal organ by 131I-BC8 antibody was 3.5 Gy, and the dose was escalated in groups of not less than 3 patients. The dose was escalated by 1.75 Gy if 0 of 3 or not more than 1 of 6 evaluable patients developed grade III (life-threatening) or IV (fatal) regimen-related toxicity46 at the previous dose level.
The therapy dose, administered at the same rate as the biodistribution dose, was administered on day −14 of the transplant regimen. This dose, generally 9 days after the trace-labeled biodistribution dose, was administered in lead-lined radiation isolation rooms, where patients remained until the total body 131I activity was less than 30 mCi (usually 3 to 6 days). Patients were then admitted to a marrow transplant ward, where they received CY 60 mg/kg IV on days −8 and −7, followed by TBI administered in daily 2 Gy fractions from days −6 to −1. TBI was delivered at a dose rate of 7.36 cGy/min from 2 opposing 60Co sources. Stem cells were infused on day 0. Cyclosporine and methotrexate were used for graft-versus-host disease (GVHD) prophylaxis in allogeneic recipients.47 All patients received routine posttransplant supportive care. Results are current as of September 15, 1998.
RESULTS
Biodistribution studies.
Forty-four patients were entered on study and received a biodistribution dose of trace 131I-labeled BC8 antibody. The median age was 38 years (range, 16 to 55 years). Thirty-one had AML (9 remission and 22 relapse), 10 had ALL (5 remission and 5 relapse), and 3 had MDS (Table 1).
Grade I-III side effects were experienced by 75% of patients receiving the biodistribution dose of 131I-BC8 antibody. Most common were shaking chills and nausea or vomiting. Fever greater than 38.5°C occurred in 4 patients. Grade I-II hypotension developed in 11 patients and generally responded to IV crystalloid administration. Side effects usually developed after the first hour of infusion and responded to slowing of the infusion and treatment with meperidine, diphenhydramine, and lorazepam. Twenty-five percent of patients experienced respiratory symptoms including wheezing or sensations of tightness in the chest or throat. These symptoms usually improved after the antibody infusion was temporarily slowed or halted and diphenhydramine ± hydrocortisone was administered, but in 3 patients, the antibody infusion was stopped after a total dose of 0.2 mg/kg because of these symptoms. In 2 of these patients, antibody infusion rates were greater than 7.5 mg/h when they developed symptoms. After the initial 21 patients, antibody infusion rates were limited to 7.5 mg/h. Biodistribution data from the 3 patients receiving 0.2 mg/kg did not differ from the patients receiving 0.5 mg/kg and are included in the data analysis reported below.
We wished to determine whether a preclearing dose of cold antibody administered before the labeled antibody could decrease hepatic uptake of radiolabeled circulating white blood cells by causing sequestration of cells by unlabeled antibody, as observed in previous preclinical studies.48 We therefore administered the first 7.5 mg of antibody unlabeled or cold antibody in 10 patients after initial experience in the first 9 patients demonstrated that circulating white blood cells were saturated, and the white blood cell count had fallen to near-minimum levels after this antibody dose. However, there was no difference in the hour 0 liver uptake of131I or the biological half-time (t½ of131I in the liver) or in estimated radiation absorbed doses to liver or marrow between the nonprecleared and the precleared group (data not shown). Therefore, these 2 groups were combined for analysis.
The average peak (ie, end of infusion) concentration of BC8 antibody in plasma was 0.0061% ± 0.003% ID/g. In 26 patients in whom comparison between BC8 and nonspecific antibody was possible, the peak concentration of BC8 antibody in plasma was 0.0057% ± 0.003% ID/g, which is significantly lower than the peak concentration of control antibody, 0.021% ± 0.006% ID/g (P < .001). This presumably reflects the rapid antigen-specific binding of the anti-CD45 antibody. Twenty of these patients demonstrated a discernible rapid initial disappearance phase of BC8 antibody (t½ = 1.4 ± 0.8 hours). This was followed by a slower disappearance phase (t½ = 26.7 ± 12.5 hours) that was similar to that of the nonspecific antibody (t½ = 27.3 ± 13.3 hours). There were no differences in peak BC8 antibody concentration or clearance between groups of patients with AML or ALL in remission or relapse.
Pharmacokinetic and biodistribution results for marrow, spleen, and liver are summarized in Table 2. The average peak concentration of 131I-BC8 antibody was 0.028% ± 0.012% ID/g for marrow, 0.060% ± 0.032% ID/g for spleen, and 0.013% ± 0.004% ID/g for liver. For patients receiving125I-anti-idiotype control antibody, the average marrow antibody LI was 42.2 ± 29.0. The average marrow retention half-time of 131I-BC8 antibody was 44.2 ± 14.7 hours. Patients with AML in relapse, including the 3 patients with MDS, had somewhat higher initial antibody uptake in marrow (0.030% ID/g) as compared with those with AML in remission (0.025% ID/g, P = .21) and longer retention of radioiodine in marrow (51.4 v 35.5 hours,P < .001). There was also a trend towards a higher LI for patients with AML in relapse (47.0 ± 41.0) as compared with those with AML in remission (33.1 ± 18.6, P = .34). There were no differences seen in initial uptake, LI (data not shown), or half-life among patients with ALL in remission or relapse or AML in remission. Cells from patients with AML and ALL in relapse did not have appreciable differences in CD45 expression as determined by flow microfluorimetry (data not shown).
Estimated radiation absorbed doses.
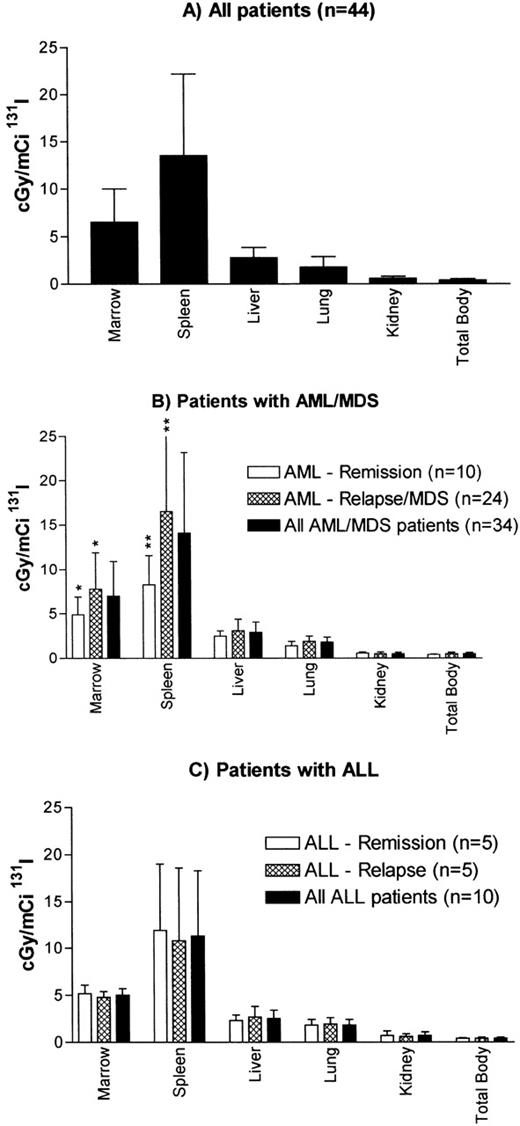
The mean estimated radiation absorbed doses calculated from the biodistribution dose of trace-labeled antibody are shown in Fig 2. The mean radiation absorbed doses (centigray per millicurie of 131I administered ± SEM) were 6.5 ± 0.5 for marrow, 13.5 ± 1.3 for spleen, 2.8 ± 0.2 for liver, 1.8 ± 0.1 for lung, 0.6 ± 0.04 for kidney, and 0.4 ± 0.02 for the total body. Thus, the mean ratio of estimated radiation dose was 2.3 for the marrow as compared with the liver, 3.6 for the marrow as compared with the lung, and 11 for the marrow as compared with the kidney. The liver was the normal organ receiving the highest estimated radiation absorbed dose, except in 1 patient with an unusually long retention of radioiodine in the lungs. Overall, favorable biodistribution was seen in 37 of 44 patients studied (84%).
Estimated radiation absorbed doses per millicurie of131I administered for (A) all patients, (B) patients with AML or MDS, and (C) patients with ALL. Values are the means ± SD. Estimated radiation absorbed doses to marrow were calculated using published methods.41,42 Recently proposed marrow S values incorporated in MIRDOSE3 software60 would result in lower marrow doses than noted here. AML–Remission group includes a patient in marrow remission with leukemia cutis. *P = .04 for difference between marrow remission and relapse in patients with AML/MDS; **P = .001 for difference between marrow remission and relapse in patients with AML/MDS.
Estimated radiation absorbed doses per millicurie of131I administered for (A) all patients, (B) patients with AML or MDS, and (C) patients with ALL. Values are the means ± SD. Estimated radiation absorbed doses to marrow were calculated using published methods.41,42 Recently proposed marrow S values incorporated in MIRDOSE3 software60 would result in lower marrow doses than noted here. AML–Remission group includes a patient in marrow remission with leukemia cutis. *P = .04 for difference between marrow remission and relapse in patients with AML/MDS; **P = .001 for difference between marrow remission and relapse in patients with AML/MDS.
Patients with AML in relapse (including MDS) had higher estimated radiation absorbed doses to marrow (7.8 v 4.9 cGy/mCi,P = .04) and to spleen (16.5 v 8.3 cGy/mCi, P = .001) as compared with patients with AML in remission. There was no significant difference in the estimated radiation absorbed dose to the liver between patients with active disease and those in remission, and therefore the ratio of radiation delivered to target as compared with normal organs was higher for both marrow (2.7 v 2.0, P= .053) and spleen (5.4 v 3.3, P = .012) for patients with AML in relapse. However, even for patients in relapse, there was appreciable variability in estimated radiation dose to marrow and spleen resulting from variation in initial uptake, half-time, or both. For patients with ALL, there were no differences between estimated radiation dose to marrow, spleen, or nontarget organs between patients in remission or relapse (data not shown).
Although the liver was the normal organ receiving the highest radiation dose per millicurie of 131I in all but 1 patient, there was great variability between patients in the estimated radiation absorbed dose to liver, ranging from 1.7 to 7.1 cGy/mCi 131I. No correlations between clinical features such as disease state, type of leukemia, or number of circulating blasts and the estimated liver dose were apparent in this study.
Seven patients did not have favorable biodistribution of131I-BC8 antibody, with a lower estimated radiation dose to marrow or spleen than to liver or lung. Two were in remission and 5 were in relapse at the time of study. Whereas 3 had relatively low marrow cellularity (50% of normal), the other 4 had cellularity ranging from 100% to 200% of normal. The average weight of the 7 patients with unfavorable biodistribution was greater than that of the patients with favorable biodistribution (105.4 v 76.0 kg,P < .001), and the patients with unfavorable biodistribution were overweight (actual body weight divided by ideal body weight) by a higher percentage than those with favorable biodistribution (141%v 107%, P < .02). Of 15 patients weighing 89 kg or more, 7 had unfavorable biodistribution. In contrast, all 29 patients weighing less than 89 kg had favorable biodistribution. Because the antibody dose administered was calculated on actual as opposed to ideal body weight, obese patients received a higher average dose of antibody in relationship to circulating blood volume and to total CD45 antigen. We found a negative correlation (Pearson correlation = −.372,P = .018) between weight and the percentage of injected dose per gram of marrow at biopsy.
An eighth patient had favorable biodistribution of antibody to marrow and spleen but had a low concentration of 131l-BC8 antibody in a skin biopsy from a site of leukemia cutis and thus was not treated on study. The remaining 2 patients that were not treated with radiolabeled antibody included 1 that was positive for HAMA on the day before the planned therapy (8 days after receiving the biodistribution dose) and 1 that received only 0.2 mg/kg BC8 antibody and had an estimated radiation absorbed dose of 1.3 cGy/mCi for liver. This patient, whose antibody dose had been limited by side effects, was not treated because the amount of 131I required to deliver dose level 5 (830 mCi) would have resulted in a specific activity of more than 50 mCi/mg, a level previously shown to damage the immunoreactivity of BC8 antibody. Patients not receiving a therapy dose of131I-BC8 antibody were treated with alternative marrow transplant preparative regimens.
Therapy, toxicities, and engraftment.
Thirty-four patients proceeded to the transplant phase of the study and were treated with the same dose of BC8 antibody received during the biodistribution study labeled with the amount of 131I activity calculated to deliver an estimated radiation dose to the normal organ receiving the highest dose (ie, the liver) of 3.5 (dose level 1) to 12.25 Gy (dose level 6). The 131I activity administered and estimated radiation absorbed doses delivered to marrow and spleen at each dose level are summarized in Table 3 together with regimen-related toxicities at each dose level. Side effects during the therapeutic infusion of antibody were similar to those experienced with the biodistribution dose. Some patients, particularly those treated at higher dose levels, experienced mild to moderate nausea and vomiting during the first few days after administration of the therapeutic dose of antibody. Patients remained in radiation isolation for 3 to 7 days after treatment.
Most regimen-related toxicities were typical for conventional marrow transplant regimens. All patients developed at least grade II mucositis (ie, requiring narcotic therapy). Grade III mucositis occurred in both patients receiving dose level 6 (12.25 Gy to liver) and thus was the dose-limiting toxicity in this study (see below).
A patient treated at dose level 1 (3.5 Gy to liver) developed ileus and hemorrhagic enterocolitis 16 to 18 days posttransplant and thus was considered to have grade III gastrointestinal toxicity. This patient went on to develop gut GVHD that may have been the etiology of his symptoms.
A patient treated at dose level 3 (7 Gy to liver) did not engraft by the time of her death from candida pneumonia 29 days posttransplant, despite the administration of granulocyte colony-stimulating factor (G-CSF). Minimal myeloid and erythroid engraftment was present in a postmortem marrow biopsy, and the presence of macrophages and eosiniphilic debris suggested marrow stromal damage. This patient received an estimated radiation absorbed dose to the bone marrow of 31 Gy from 131I-BC8 antibody, in addition to 12 Gy external beam TBI. It is possible that this high total radiation dose delivered to marrow damaged the marrow microenvironment, and this patient was considered to have grade IV marrow toxicity. In subsequent patients, the estimated marrow dose from 131I-BC8 antibody was limited to 28 Gy. Three subsequent patients with high ratios of radiation delivered to marrow as compared with liver thus received estimated liver radiation doses that were less than those specified by the dose escalation schema to limit the marrow dose to 28 Gy.
One of 6 patients treated at dose level 5 (10.5 Gy to liver) developed grade III hepatic toxicity, with a maximum serum bilirubin concentration of 11.7 mg/dL and severe ascites requiring paracentesis. Two of 2 patients treated at dose level 6 (12.25 Gy to liver) developed grade III mucositis as defined by the need for endotracheal intubation to protect the airway or by the development of aspiration pneumonia. The first of these patients developed significant mucositis by the fifth dose of TBI; thus, the sixth dose was omitted. This patient was intubated because of severe mucositis and oropharyngeal bleeding on day 0 and remained intubated for 3 days. He also developed portal vein thrombosis with ascites and a peak serum bilirubin concentration of 8.0 mg/dL, which was complicated by renal failure secondary to hepatorenal syndrome, but recovered. The second patient developed fever and cough 9 days before transplant and a naso-pharyngeal culture grew respiratory syncytial virus (RSV). He developed both severe mucositis and progressive pulmonary impairment and was intubated on day 6 after transplant. Squamous cells were demonstrated in pulmonary fluid obtained by broncho-alveolar lavage, documenting aspiration. The patient died 8 days posttransplant from progressive RSV pneumonia despite treatment with Ribavirin.
Thus, the maximum tolerated dose estimated by this study was dose level 5 (10.5 Gy delivered to the normal organ estimated to receive the highest dose from radiolabeled antibody in addition to CY and 12 Gy TBI).
Engraftment was analyzed for the patients receiving allogeneic marrow who survived more than 30 days after transplant and did not relapse in the first month. Of 19 such patients, an absolute neutrophil count of 500/μL (first day of 3 sustained days >500/μL) was achieved a median of 21 days (range, 9 to 30 days) posttransplant. A platelet count of 20,000/μL (first day of 7 sustained days >20,000/μL without transfusion) was achieved a median of 23 days (range, 16 to 36 days) posttransplant, with the exception of 1 patient (treated at dose level 1) who developed chronic GHVD and remained platelet transfusion-dependent at the time of discharge from the center at day 113 and a second patient who died from complications of influenza A at day 40.
Methotrexate doses and GVHD.
Among 23 recipients of allogeneic marrow, 18 received all 4 scheduled doses of methotrexate for GVHD prophylaxis. Two patients received 3 doses, and 1 patient each received 2, 1, or no scheduled doses. Twenty-one allogeneic marrow recipients lived at least 30 days and were evaluable for the development of acute GVHD. Twelve patients had no (n = 8) or grade I GVHD (n = 4). Nine patients developed grade II-IV GVHD (grade II, 3; grade III, 5; and grade IV, 1). Sixteen patients were evaluable for the development of chronic GVHD. Eight patients developed clinical extensive chronic GVHD, 6 developed subclinical chronic GVHD, and 2 did not develop chronic GVHD.
Outcome.
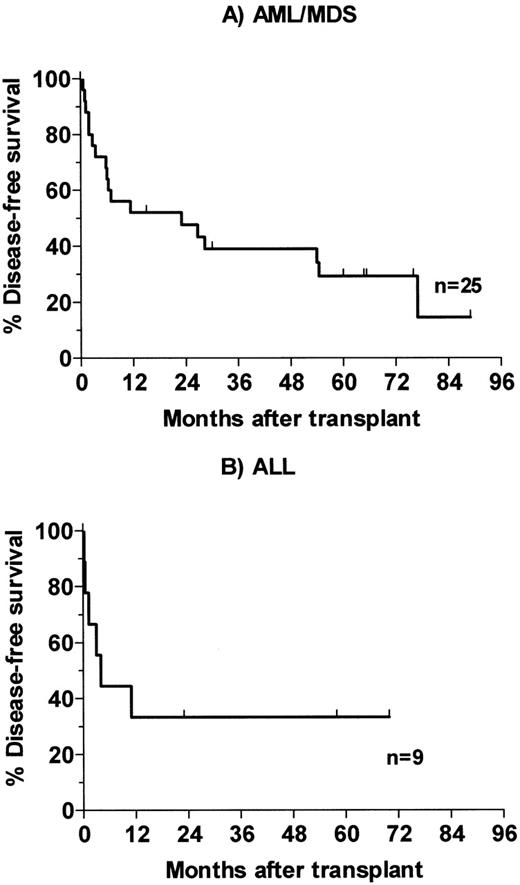
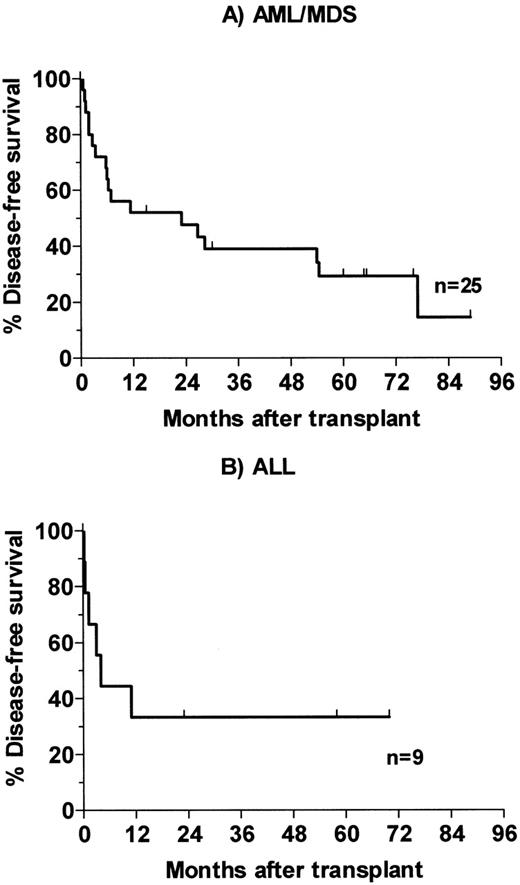
Of 25 patients with advanced AML and MDS, 3 died of infection in the early posttransplant period and a fourth died of infection 4 months posttransplant. One patient who had had poor engraftment after receiving a 4-HC–purged autologous marrow required chronic transfusions and G-CSF support and died with infection 54 months posttransplant. This patient was treated at dose level 2 with an estimated marrow dose of 12 Gy delivered by antibody (24 Gy total). Her course was felt to be consistent with the poor engraftment seen in a minority of AML patients whose autologous marrows have been incubated with 4-HC. Thirteen patients relapsed 2 to 77 months posttransplant, and 7 patients survive disease-free 15 to 89 months (median, 65 months) posttransplant (Fig 3A). One of the 5 patients transplanted for AML in second or third remission and surviving the first 100 days relapsed after transplant, as compared with 11 of 15 patients transplanted in relapse and 1 of 3 patients with MDS. Of the 9 patients with ALL, 2 died of infection and 4 relapsed 0.5 to 11 months posttransplant. Three survive disease-free 23, 58, and 70 months posttransplant (Fig 3B). Two of the survivors were transplanted in second remission and a third had primary refractory disease.
Kaplan-Meier analysis of disease-free survival for patients receiving therapeutic dose of 131I-BC8 antibody followed by CY/TBI. (A) Patients with AML or MDS. (B) Patients with ALL.
Kaplan-Meier analysis of disease-free survival for patients receiving therapeutic dose of 131I-BC8 antibody followed by CY/TBI. (A) Patients with AML or MDS. (B) Patients with ALL.
Fifteen of 18 evaluable patients became hypothyroid, as indicated by an elevated thyroid stimulating hormone, at a median of 13 months (range, 1 to 40 months) posttransplant and were treated with thyroxine.
DISCUSSION
We have demonstrated that 131I-labeled anti-CD45 antibody BC8 can deliver more radiation to marrow and spleen than to any normal organ in most patients with acute leukemia whether in remission or relapse. On average, 131I-BC8 antibody delivered estimated radiation doses to marrow that were 2.3-fold greater than liver and doses to spleen that were 4.8-fold greater than liver. The ratios of radiation delivered to marrow and spleen as compared with lung, kidney, and total body were even greater. The maximum tolerated dose suggested by this phase I study was 10.5 Gy delivered by 131I-BC8 antibody to the normal organ receiving the highest dose. Although the liver was the normal organ receiving the highest dose in all treated patients, the dose-limiting toxicity was mucositis. Based on the average estimates of radiation absorbed dose, this dose level would deliver an average of 24 Gy to marrow and 50 Gy to spleen, combined with CY and 12 Gy TBI.
The ability to deliver such supplemental doses of radiation to sites of leukemic involvement in marrow and spleen may improve the cure rate by decreasing the risk of relapse. As noted, a 3.75 Gy increase of TBI dose (from 12 to 15.75 Gy) decreased the relapse rate after matched related marrow transplant for both AML in first remission and CML in chronic phase. The estimates of radiation dose to marrow were based on marrow biopsy and the measured retention half-times of radioiodine in marrow from serial gamma scans. Because radiolabeled antibody distribution may be heterogeneous in marrow, these may represent overestimates or underestimates of radiation doses delivered at various skeletal marrow sites. Also, the relative biological effectiveness (RBE) of radiation delivered by antibody at low dose rate may be lower than that of external beam TBI, such that we would not anticipate the leukemic cell killing of 24 Gy delivered by antibody to be comparable to the killing provided by the same dose of TBI. Although it is an area of controversy, several studies suggest that hematopoietic progenitor or stem cells possess some capacity for repair of sublethal DNA damage49-52 and that there is apparent heterogeneity in both radiation sensitivity and self-repair capacity in their malignant counterparts.53-55 Although other factors, including accumulation of cells in the radiosensitive G2 stage of the cell cycle,56 can impact radiation sensitivity and dose-rate effects, leukemic cells with such repair ability will generally sustain less cell kill when a given radiation dose is delivered at a low dose rate, as compared with TBI. However, we would predict that in some patients these substantial supplemental doses of hematopoietic irradiation delivered by antibody added to CY/TBI would decrease the risk of relapse as compared with CY/TBI alone.
This phase I study was not designed to determine the efficacy of the preparative regimen of 131I-anti-CD45 antibody combined with CY/TBI. Given the small number of patients treated at each dose level and the variability in disease stage and hematopoietic stem cell source (allogeneic v autologous), it was not possible to correlate the risk of relapse with the amount of radiation delivered by antibody to the normal organ receiving the highest dose or with the estimated radiation dose delivered to marrow.
There was significant variation in the estimated radiation absorbed dose to liver per millicurie of 131I delivered by BC8 antibody. This variation could not be explained by disease stage, disease type, or number of circulating blasts. This observed variability supports the need to determine antibody biodistribution in each patient to avoid delivery of excessive or inadequate radiation dose. Biodistribution studies also identified the minority of patients with unexpectedly low estimated radiation doses to the bone marrow, which generally resulted from unexplained initial low uptake of131I-BC8 antibody in marrow.
Other attempts to deliver targeted radiation to marrow have included131I-labeled anti-CD33 antibody20,21,23,26 and, recently, anti-CD33 antibody labeled with213Bismuth,57 which emits a high-energy α particle of short path length. In our experience with conventionally iodinated p67 (anti-CD33) antibody, only modest ratios of radiation were delivered to marrow as compared with the highest normal organ (average ratio, 1.2:1).21 The use of 131I-p67 antibody was limited by the short retention of 131I in the marrow (t½, 21.4 hours), presumably because of the rapid internalization of the 131I-p67-CD33 complex into the cell with subsequent dehalogenation and excretion of 131I from the cell. Furthermore, because of restricted CD33 antigen expression, CD33 antigen was saturated at relatively low antibody doses (0.05 mg/kg), not allowing attachment of sufficient 131I for treatment of patients at the higher doses used. However, using a different anti-CD33 antibody, 131I-M195, Scheinberg et al20 found longer retention of 131I in the marrow of some patients and thus improved biodistribution. It is uncertain if greater retention was due to different binding properties of the antibodies with subsequent decreased internalization by the cell. The studies by Scheinberg et al20 also demonstrated that relatively low doses of antibody (3 mg/m2) resulted in saturation of CD33 antigen.
Whether the delivery of supplemental hematopoietic irradiation using131I-anti-CD45 antibody will benefit patients with advanced leukemia remains unknown, but the experience reported here demonstrated that the approach is feasible; accordingly, phase II studies are underway. Based on the safety of this approach as detailed in this report, we are also conducting a clinical trial in which131I-BC8 antibody is combined with busulfan and CY for patients with AML in first remission receiving HLA-matched transplants.58 Ninety percent of patients have had favorable biodistribution of 131I-BC8 antibody, and 24 patients in first remission have received 131I-BC8 antibody labeled with the amount of 131I estimated to deliver 3.5 Gy (first 4 patients) to 5.25 Gy to the liver and 6 to 16 Gy to marrow. Eighteen of these 24 patients are surviving disease-free 10 to 63 months (median, 42 months) after transplant, with 4 nonrelapse deaths and 2 relapses (manuscript in preparation). Accrual continues on this study to better define the efficacy and toxicity of this preparative regimen.
In addition to its use for delivery of supplemental antileukemic doses of radiation, radiolabeled anti-CD45 antibody may also provide immunosuppressive effects, because CD45 is expressed by virtually all lymphoid cells. Preclinical studies are underway to determine the marrow ablative and immunosuppressive effects of131I-anti-CD45 antibody. In murine transplant models, we have determined that 131I-anti-CD45 antibody can replace TBI when the donor and recipient differ only with respect to CD45 allotype and can partially replace TBI when transplanting T-cell–depleted H2-mismatched marrow.59 These preclinical studies suggest that it may be possible to increase the proportion of radiation delivered by antibody and decrease the dose of TBI and/or high-dose chemotherapy when transplanting allogeneic marrow without increasing the probability of rejection. Such an approach should allow the delivery of a higher total radiation dose to sites of leukemic involvement with less toxicity.
In summary, substantial supplemental doses of radiation can be delivered to bone marrow and spleen by 131I-anti-CD45 antibody when combined with CY and 12 GY TBI, with acceptable toxicity. Phase II studies of this approach are underway for patients with advanced AML/MDS and with ALL. These studies will include both recipients of matched related as well as matched unrelated marrow and should better define the toxicity and efficacy of this approach. The ability to increase the radiation doses delivered to leukemic cells may decrease the rate of relapse and thus improve the outcome of marrow transplantation for acute leukemia.
ACKNOWLEDGMENT
The authors are indebted to Eileen Sickle and Sharon Bush for their expert nursing assistance and to Minna Zheng and Jennifer Smith for their expert technical assistance. We also acknowledge the excellent care provided to these patients by the physicians and nurses of the marrow transplant teams, as well as the work of the staff in the Long Term Followup office.
Supported by National Institute of Health Grants No. CA44991, CA18029, CA18221, and HL36444.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dana C. Matthews, MD, Fred Hutchinson Cancer Research Center D1-100, 1100 Fairview Ave N, PO Box 19024, Seattle, WA 98109; e-mail: dmatthew@fhcrc.org.