Abstract
Clinical, immunohistological, and molecular biological data suggest the chronic dermatosis small plaque parapsoriasis (SPP) to be a precursor of mycosis fungoides (MF). However, most data are contradictory and confusing due to inexact definition of SPP. Recently, clonal T cells were detected in skin and blood samples of early MF. Because demonstration of identical T-cell clones in skin and blood of SPP patients would indicate a close relationship of SPP to MF, we investigated the clonality of skin and blood specimens from 14 well-defined SPP patients. By a polymerase chain reaction (PCR) amplifying T-cell receptor γ rearrangements and subsequent high-resolution electrophoresis, clonal T cells were detected in 9 of 14 initial and 32 of 49 follow-up blood samples, but in 0 of 14 initial skin specimens. Even a clone-specific PCR showing the persistence of the initial blood T-cell clone in 20 of 20 follow-up samples, failed to detect the T-cell clone in the skin. In 2 patients, the clonal T cells were shown to be CD4+. For the first time, the majority of SPP patients was shown to carry a T-cell clone in the peripheral blood. Although a relation between circulating clonal T cells and SPP cannot directly be proven by the applied techniques, our results indicate blood T-cell clonality to be a characteristic feature of SPP and CTCL because analysis of multiple controls and clinical workup of our SPP patients excluded other factors simulating or causing a clonal T-cell proliferation. A sufficient cutaneous antitumor response but also an extracutaneous origin of the T-cell clones might explain the failure to detect skin infiltrating clonal T cells.
THE TERM PARAPSORIASIS was proposed by Brocq1 in 1902 to describe a group of skin diseases, all of unknown etiology, chronicity, the failure to respond to therapy, and lack of subjective symptoms as pruritus.1 He differentiated three subtypes: parapsoriasis en gouttes, parapsoriasis lichenoide, and parapsoriasis en plaques (PEP). Today, the first subtype is referred as pityriasis lichenoides, the second as parakeratosis variegata, and the last is used analogously to small plaque parapsoriasis (SPP).2 Discrepancies exist regarding the classification of large plaque parapsoriasis (LPP). Most authors refer both, LPP and SPP as PEP,3,4 whereas Lambert and Everett2 group LPP with parakeratosis variegata and differentiate both from SPP. Because the term parapsoriasis is used to name the entire group as well as particular subtypes, data generated in parapsoriatic patients are confusing.5 6 In particular, the relation of PEP and cutaneous T-cell lymphoma (CTCL) is discussed controversially.
According to the clinical course, LPP as well as SPP were found to progress into mycosis fungoides (MF) in 0% to 46% of the cases.2,7-9 Clonal T-cell receptor (TCR) rearrangements were detected by Kikuchi et al10 using Southern blot analysis in 4 of 20 LPP cases and by Haeffner et al11 using a polymerase chain reaction (PCR) assay in two of three SPP patients. In contrast, other authors12,13using the same methods failed to detect clonal rearrangements in LPP and SPP, respectively. Immunohistology showed the skin-infiltrating T cells of 14 MF and 7 LPP patients to be CD4+ and Leu8− in all samples as well as CD7− (Leu9−) in 11 MF and 4 LPP cases, respectively. Because this pattern is uncommon in inflammatory skin diseases, PEP was considered to be an early form of MF.14 This hypothesis is supported by the detection of functionally abnormal blood lymphocytes in both, early MF and LPP.15 Furthermore, G-banding and interphase cytogenetic assays showed circulating chromosomally abnormal T lymphocytes usually found in MF and Sezary syndrome patients also in PEP samples.16
Regarding these data, most authors2,17,18 consider LPP (and SPP) as the most common precursor of CTCL. Ackerman et al6,19 even regard SPP, LPP, and parakeratosis variegata as clinical presentations of MF. In contrast, Burg et al5,20emphasize that differentiation between LPP and SPP is not always clear and that most of the data mentioned above refer to LPP. Therefore, they discuss SPP as a benign process in which an initial DNA defect leads to the generation of a skin homing T-cell clone not undergoing further mutations necessary to develop into overt CTCL and suggest the term abortive CTCL for SPP. The clinical course of the disease should be the criterion to differentiate between SPP and MF simulating SPP. However, differentiation between MF simulating SPP and SPP is advisable at the initial analysis to anticipate the clinical course as well as to treat the patients in an adequate manner. This view might be supported by the observation that early stage MF, appropriately treated with topical mechlorethamine or total skin electron beam therapy, shows survival rates similar to that of a race-, age-, and sex-matched population.21
Recently, we could show clonal T cells not only in skin but also in blood samples of early MF.22 Because SPP might be a precursor of MF, we asked whether T-cell clonality is also detectable in skin and blood specimens of a well-defined group of 14 SPP patients. Demonstration of identical T-cell clones in both compartments would indicate the systemic character of SPP and its close relationship to MF as well as facilitate prediction of the clinical course and selection of therapy modalities.
MATERIALS AND METHODS
Patient samples.
The study included 14 SPP patients that were untreated for at least 6 months before the initial analysis. For each patient, a blood and a skin specimen was collected for the initial analysis, 1 to 8 additional blood samples were taken during the follow-up of 18 to 47 months. Detailed data on medical history and follow-up are given in Tables 1 and 2. Special attention was paid to conditions reported to be risk factors for disease progression in CTCL (lower rate of complete remissions with initial treatment, older age as well as a higher stage of skin lesions at the initial presentation, elevated levels of β2 microglobulin and/or lactic dehydrogenase).21,23,24 The diagnosis of SPP was assessed by two independent investigators according to the following, previously published criteria2,4,18 25: chronic persistence (more than 2 years) of asymptomatic, well defined, round to oval shaped, red to brown colored, nonindurated macules, or thin plaques measuring less than 5 cm in diameter and displaying a fine scaling that gives the surface a wrinkled appearance; symmetrical involvement preferring trunk and proximal extremities, but sparing face and volar surfaces; compact stratum corneum with moderate acanthosis, spongiosis, and foci of laminated parakeratosis; edematous papillary dermis with a sparse, perivascular to band-like infiltrate composed of lymphohistiocytic cells appearing neither enlarged nor atypical or neoplastic; few of the bland-appearing lymphocytes infiltrating the epidermis; size (< 5cm), regular shape, and symmetrical distribution of the lesions discerning SPP from LPP; mild extent of the lymphohistiocytic infiltrate and absence of necrotic keratinocytes and of broad bands of parakeratosis differentiating SPP from pityriasis lichenoides; absence of so-called Pautrier’s microabscesses in the epidermis containing atypical large lymphocytes discriminating SPP from MF. Histological criteria are illustrated in Fig 1. Patients not exactly matching the diagnostic criteria or not equally rated by both investigators were excluded from the study.
Biopsy specimens from SPP patient SF illustrating the diagnostic criteria compact stratum corneum with moderate acanthosis and foci of spongiosis, slightly edematous papillary dermis with a sparse band-like infiltrate composed of bland lymphohistiocytic cells, few of them epidermotropic lymphocytes, and absence of so-called Pautrier’s microabscesses. (A) Hematoxyline-eosine staining (×33); (B) CD3 staining (×50) showing the T-cell nature of the infiltrating lymphocytes.
Biopsy specimens from SPP patient SF illustrating the diagnostic criteria compact stratum corneum with moderate acanthosis and foci of spongiosis, slightly edematous papillary dermis with a sparse band-like infiltrate composed of bland lymphohistiocytic cells, few of them epidermotropic lymphocytes, and absence of so-called Pautrier’s microabscesses. (A) Hematoxyline-eosine staining (×33); (B) CD3 staining (×50) showing the T-cell nature of the infiltrating lymphocytes.
Ten control blood specimens were derived from patients with active contact dermatitis (n = 5), and alopecia areata sensitized with topical diphencyprone to induce a local contact dermatitis (n = 5). Cells of the JM cell line26 (DSMZ, Braunschweig, Germany) served as clonal control.
Sample preparation.
Peripheral blood mononuclear cells (PBMC) were prepared from 10 mL of heparinized blood by density-gradient centrifugation through Ficoll-HyPaque (Pharmacia, Freiburg, Germany). CD4+ and CD8+ T cells were purified by CD4+/CD8+ T cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and Midi MACS. Genomic DNA was prepared from about 1 × 106 cells by a standard procedure using Proteinase K digestion.27 For preparation of genomic DNA from the paraffin-embedded skin specimens, the paraffin of 10 sections per sample (10 μm each) was dissolved with xylene. After centrifugation, the pellet was washed with ethanol and also digested by proteinase K.
TCRγ PCR and determination of clonality.
TCRγ rearrangements were PCR amplified using primers annealing at the V and J segments, respectively.22 Primers VG1 (segments Vγ1 to 8; CTACATCCACTGGTACCT), VG9 (Vγ9; ATTGGTATCGAGAGAGAC), VG2 (Vγ10, 11, B, [A]; CACTGGTACKKGCAGAAAC), and JG12-a (Jγ1, 2; CAACAAGTGTTGTTCCAC) were applied to all specimens (PCR-1), whereas PCR-2 using primers VG1, VG9, VG2, and JGP12-a (JγP1, P2; CTATGAGCYTAGTCCCTT) was performed in those SPP specimens appearing polyclonal in PCR-1 and in all control samples. PCR reaction mixture included 0.5 to 1 μg (5 μL) of genomic DNA, 1.75 U of Taq polymerase, 1.5 mmol/L MgCl2, and 7.5 μL 10 × PCR buffer (Perkin Elmer, Branchburg, NJ), 0.1 mmol/L of each deoxynucleotide triphosphate (dNTP; Pharmacia, Freiburg, Germany), and 0.6 μmol/L of each primer in a final volume of 75 μL. Amplification was performed on a thermal cycler (Varius-V; Vers, Hannover, Germany) by a 4-minute denaturation step at 95°C, followed by 40 cycles including 1 minute denaturation at 94°C, 1 minute annealing at 58°C, and 1 minute extension at 72°C. Finally, an extension step of 5 minutes at 72°C was added. Six microliters of the PCR products were screened for successful amplification on a 2% agarosegel stained by ethidium bromide.
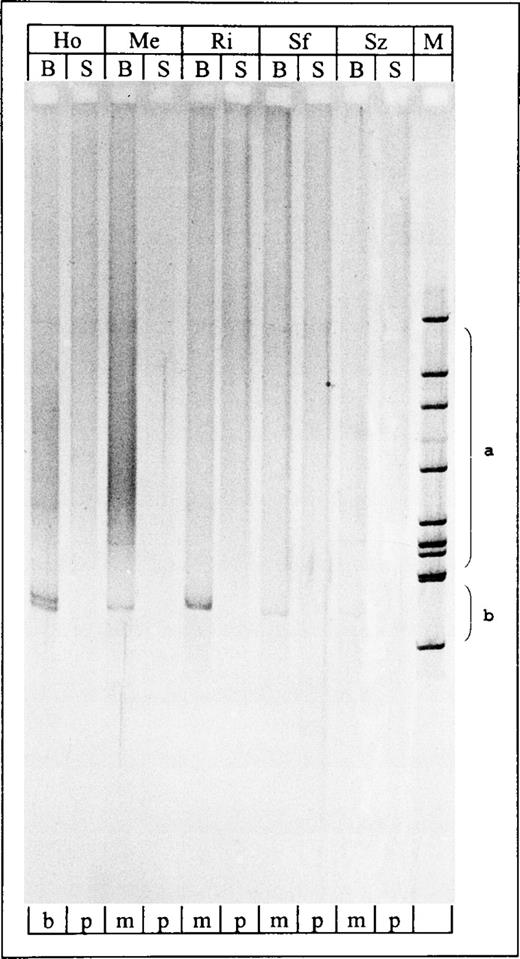
T-cell clonality was established by detection of a dominant TCRγ rearrangement in a heteroduplex loaded temperature gradient gel electrophoresis (HD-TGGE). Eight microliters of the PCR products were prepared to form heteroduplices (5 minutes denaturation at 95°C, gradual cooling to 50°C)28 and separated on the Diagen TGGE-System (Qiagen, Hilden, Germany). Electrophoretic run and subsequent silver staining were performed according to standard protocols.29 Due to the denaturation-renaturation step, polyclonal (ie, not identical) amplification products form heteroduplices that appear as a broad smear on the gel. In contrast, clonal (ie, identical) PCR products form homoduplices migrating as sharp bands into the high temperature range of the gradient gel (Fig 2). PCR products derived from the JM cell line (rearranged Vγ8 and Vγ11) served as positive clonal control.
Temperature-gradient gel of TCRγ PCR products. Lane 1-10, PCR products derived from blood and skin samples of SPP patients HO, ME, RI, SF, and SZ; all blood samples showed clonal PCR products. Lane 11, Hinc II digest of phi X174. S, skin sample; B, blood sample; M, marker (Hinc II digest of phi X 174); b, biallelic (or biclonal); m, monoclonal; p, polyclonal; a, range of polyclonal smears;b, range of clonal bands.
Temperature-gradient gel of TCRγ PCR products. Lane 1-10, PCR products derived from blood and skin samples of SPP patients HO, ME, RI, SF, and SZ; all blood samples showed clonal PCR products. Lane 11, Hinc II digest of phi X174. S, skin sample; B, blood sample; M, marker (Hinc II digest of phi X 174); b, biallelic (or biclonal); m, monoclonal; p, polyclonal; a, range of polyclonal smears;b, range of clonal bands.
Cloning and sequencing of the TCRγ rearrangements.
For direct sequencing, the distinct HD-TGGE band was cut out and dissolved in 40 μL 1 × PCR buffer (Perkin Elmer) overnight. Five microliters of the solution were reamplified under the same conditions described above. Primer JG12-i (TGTTGTTCCACTGCCAAA) or JGP12-i (CCTTYWGCAAAYRTCTTGA) was applied instead of JG12-a or JGP12-a, respectively. The PCR product was purified by the QIAquick PCR purification kit (Qiagen) and sequenced on an automated DNA sequencer (Model 373A, Perkin Elmer Applied Biosystems, Weiterstadt, Germany) by the Taq cycle sequencing method using primers VG, JG12-i, or JGP12-i. Sequences were aligned to the published germline sequences of the TCRγ V and J segments.30-36
Cloning of the PCR products was performed by applying the TA Cloning Kit (Invitrogen, Fleek, The Netherlands). Plasmids were sequenced using the universal forward sequencing primer for M13 as described above.
Clone-specific PCR.
Using the Oligo 5.0 software (National Biosciences, Plymouth, MN), clone-specific primers were designed so that the 3′ end annealed at the N region of the clonal TCRγ rearrangement. After PCR amplification using clone-specific and corresponding VG primer at standard conditions, PCR products were screened on an agarose gel. Primer and PCR conditions were considered specific for the clonal TCRγ sequence if at least 7 randomly chosen clonal or polyclonal DNA samples from CTCL patients and healthy volunteers (tester DNAs) yielded no amplification product (Fig 3A). To achieve this, the annealing temperature was increased by 1°C steps until amplification products of the tester DNA disappeared. In the seminested clone-specific PCR, the clone-specific primer was applied in combination with an inner VG primer corresponding to the rearranged Vγ segment: VGseq (Vγ1-8; AGRCCCCACAGCRTCTTC),22 VG10-i (Vγ10; ATCCGCAGCTCGACGCAGCA), or VG11-i (Vγ11; CTCAAGATTGCTCAGGTGGG).37
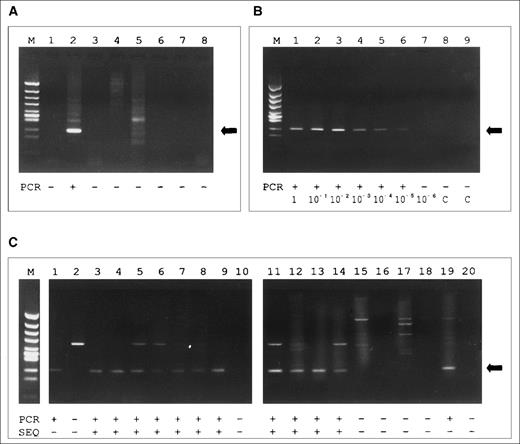
(A) Agarose gel of products of the clone-specific PCR using primer BE-1. Lane 1, skin sample DNA of BE; 2, blood sample DNA of BE; 3-5, skin sample DNA from CTCL patients (tester DNA); 6-8, blood sample DNA from CTCL patients (tester DNA). A specific PCR product (indicated by the arrow) is observed exclusively in lane 2. The additional band in lanes 2 and 5 represents a larger PCR product that, according to the size, is referred to as an unspecific amplificate. (B) Agarose gel of the sensitivity assay using primer JU-5 and 10-fold dilution of JM cell line DNA in PBMC from a healthy donor. A specific PCR product (indicated by the arrow) is found up to the dilution of 10 JM cells in 106 PBMC (10-5) in lane 6. (C) Results of the analysis of the fractionated clones (1-14, established from patient HO; 15-20, from BE): fraction A was sequenced directly, fraction B was amplified by clone-specific PCR using primer HO-2 and separated on an agarose gel. Nonconcordance between PCR and sequencing is observed in lane 1 and 19. M, marker (Hinc II digest of phi X 174); PCR, PCR result; SEQ, result of sequencing; +, PCR product of expected size/sequence identical to the second allel of patient HO; −, no PCR product of expected size/sequence not identical to the second allele of patient HO.
(A) Agarose gel of products of the clone-specific PCR using primer BE-1. Lane 1, skin sample DNA of BE; 2, blood sample DNA of BE; 3-5, skin sample DNA from CTCL patients (tester DNA); 6-8, blood sample DNA from CTCL patients (tester DNA). A specific PCR product (indicated by the arrow) is observed exclusively in lane 2. The additional band in lanes 2 and 5 represents a larger PCR product that, according to the size, is referred to as an unspecific amplificate. (B) Agarose gel of the sensitivity assay using primer JU-5 and 10-fold dilution of JM cell line DNA in PBMC from a healthy donor. A specific PCR product (indicated by the arrow) is found up to the dilution of 10 JM cells in 106 PBMC (10-5) in lane 6. (C) Results of the analysis of the fractionated clones (1-14, established from patient HO; 15-20, from BE): fraction A was sequenced directly, fraction B was amplified by clone-specific PCR using primer HO-2 and separated on an agarose gel. Nonconcordance between PCR and sequencing is observed in lane 1 and 19. M, marker (Hinc II digest of phi X 174); PCR, PCR result; SEQ, result of sequencing; +, PCR product of expected size/sequence identical to the second allel of patient HO; −, no PCR product of expected size/sequence not identical to the second allele of patient HO.
RESULTS
TCRγ PCR analysis.
Previous studies proved specificity and sensitivity of the applied TCRγ PCR/HD-TGGE in cases of CTCL and several benign conditions. Among 60 control specimens derived from peripheral blood of patients with atopic dermatitis (n = 20), psoriasis vulgaris (n = 20), and healthy volunteers (n = 20), all but 1 sample derived from a psoriatic patient showed polyclonal PCR products.22 38 The present study included 10 additional control specimens derived from peripheral blood of patients with active contact dermatitis (n = 5), and with alopecia areata treated by diphencyprone to induce contact dermatitis (n = 5). Clonal T cells circulating in the peripheral blood were detected in none of these control samples.
Among the SPP patients (Table 2), TCRγ PCR/HD-TGGE detected clonally expanded T cells in 9 of the 14 blood samples taken for initial analysis. In patient HO, a biallelic rearrangement was found in this compartment. The clonality of the 9 blood samples was confirmed by directly sequencing the PCR products. The clonal sequences had never been found in our laboratory so far, as excluded by data base searches. Analysis of the follow-up blood specimens showed T-cell clonality in 32 of 34 samples taken from the 9 SPP patients previously shown to carry circulating clonal T cells, whereas all of the 15 samples collected from the remaining 6 SPP cases were shown to be polyclonal.
In contrast to the peripheral blood, no DNA sample from a corresponding SPP skin lesion showed a clonal PCR product (Table 2). Nevertheless, only simultaneous demonstration of the T-cell clone in both, blood and skin specimens of a patient would indicate a direct relation between SPP and the T-cell clonality found in the peripheral blood.
Clone-specific PCR analysis.
To detect the T-cell clone more sensitively than by TCRγPCR/HD-TGGE, a clone-specific PCR was established. Based on the N region of the clonal TCRγ sequences, clone-specific primers were designed and applied in combination with the corresponding VG primer (Table 2). For the biallelic TCRγ rearrangement detected in patient HO, two clone-specific primers were constructed.
Specificity of the clone-specific PCR was achieved by increasing the annealing temperatures until tester DNA amplification products disappeared (Fig 3A). By this procedure, a clone-specific PCR could be established in 6 of the 9 SPP patients with clonal blood samples (Table2). In patients CZ and BC, the N region was too short for the design of specific primers. No clone specificity was achieved with primers HO-1 and HL-1.
Furthermore, specificity of the approach was shown by cloning the PCR products of patient HO and BE. Twenty clones (14 from patient HO, 6 from BE) were picked and each clone was aliquoted into 2 fractions (A and B). Fraction A was sequenced directly, whereas fraction B was reamplified by the clone-specific PCR using primer HO-2. Concordance of sequencing and clone-specific PCR was observed in 18 of the 20 clones (11 clone positive, 7 clone negative). Two samples were positive by the clone-specific PCR of fraction B, but the clonal TCRγ sequence was not obtained by sequencing fraction A (Fig 3C).
The sensitivity of our clone-specific PCR system was determined by dilution of clonal T cells (JM cell line) in polyclonal PBMC of a healthy volunteer. After DNA preparation and PCR by primer VG1 and JU-5, a distinct electrophoretic band was observed down to a dilution of 10 JM cells in 1,000,000 PBMC (0.001%; Fig 3B).
By the clone-specific PCR assays, all of the 20 follow-up blood samples taken from the patients BE, HO, ME, RI, SF, and SZ were shown to carry the T-cell clone detected and sequenced in the corresponding initial blood specimen (Table 2). To confirm the putative relation between SPP and the peripheral blood T-cell clonality, the skin samples of these patients were also reevaluated using the clone-specific PCR. However, in none of the 6 cases, a product was observed after PCR of the skin sample DNA. To further increase the sensitivity, the clone-specific amplification was performed seminested using clone-specific and corresponding VG primer in the first round as well as clone-specific and corresponding VG-i (VGseq) primer in the second round. Again, this procedure failed to detect the peripheral blood T-cell clone in the skin specimens.
In patients SZ and HO, CD4+ and CD8+ T cells were isolated from peripheral blood mononuclear cells (purity 98.26% and 96.79%, respectively). Using the corresponding clone-specific assays, an amplification product was observed only in the CD4+ fraction of both patients.
Clinical investigation.
We analyzed our SPP patients (T-cell clone in the peripheral blood detected v not detected) for the predominant occurrence of conditions previously reported to be risk factors for CTCL progression.21,23 24 Although the mean age (68 v 60 years) and the mean duration of the skin lesions (11.8 v 10.8 years) were found to be somewhat higher in the clone-positive group of SPP patients, no statistical significance of these differences was found (Mann Whitney test, P = .15 and P = .30, respectively). Evaluation of the other markers (course of the disease [remission v relapse rate: 6/3 v 3/2], previous treatment, concomitant disease and medication, laboratory tests) as well showed no differences between the two groups (Table 1).
In summary, clonal T cells were shown by TCRγPCR/HD-TGGE in 9 of 14 blood samples, but surprisingly not in the 14 skin specimens initially taken from the SPP patients. The data obtained from peripheral blood were confirmed in 47 of 49 follow-up samples investigated by this technique. Clone-specific PCR assays could be established in 6 of 9 SPP cases carrying circulating clonal T cells. These assays showed the T-cell clone in all follow-up blood samples of the 6 patients. But, even the use of this high-sensitive technique, including seminested clone-specific PCR failed to detect the peripheral blood T-cell clone in the skin. In 2 patients, the circulating clonal T cells were shown to be CD4+ T cells. The occurrence of T-cell clones in the peripheral blood of the SPP patients was not correlated with changes in clinical markers previously found to be risk factors for disease progression in CTCL.
DISCUSSION
As the classification of parapsoriasis has been controversial, we employed a combination of strict clinical and histological criteria,2,4,18,25 applied independently by 2 investigators, to select the patients evaluated in the present study. To determine the clonality of the samples, a well-established TCRγPCR/HD-TGGE assay was used. The lower detection limit of our test system was estimated at 103 clonal in 106polyclonal T cells (0.1%).22 Such a high sensitivity might enable detection of minor clones of reactive lymphocytes in skin lesions of nonspecific dermatitis and cutaneous lymphoid hyperplasia proposed as the “clonal dermatitis“ concept.39 Clonal PCR products were also found repetitively in skin samples of the benign dermatoses pityriasis lichenoides (chronica and acuta) and angioimmunoblastic lymphadenopathia40 as well as in the peripheral blood of posttransplant41 and immunodefiency patients.42 Some reports described clonal, most notably CD8+ and αβ+ T cells in the blood of healthy elderly donors.43 However, all of these conditions were excluded in our patients (Table 1) and at least in 2 cases, the circulating clonal T cells belong to the CD4+ fraction that was not found to be clonally expanded in healthy volunteers by Fitzgerald et al.43 Moreover, using the applied test system to investigate 60 blood samples obtained from an age-matched cohort of healthy volunteers, psoriatic patients, and atopic patients, we previously found clonal T cells in just 1 blood sample of a patient with psoriasis vulgaris.22 In the present study, circulating clonal T cells were not detected in 10 control blood specimens from patients with active contact dermatitis and with alopecia areata treated by diphencyprone to induce contact dermatitis although both circumstances are expected to carry circulating T-cell clones. Moreover, specificity of the data was also confirmed by direct sequencing of the 9 blood samples showing T-cell clonality.
Validity of the clone-specific PCR was ensured by nondetection of tester DNA in all 6 assays, by fractional analysis of a cloned PCR product of patient HO, and by determination of the sensitivity to 0.001%. The 2 false-positive results in the fractional analysis might be caused by a contamination of the amplified fraction, but also by an unspecific annealing of primer HO-2. This might, at least partly, question the significance of the detection of the initial T-cell clone in all 20 follow-up blood specimens investigated, but concordant results of the TCRγPCR/HD-TGGE in 18 of the 20 cases support the validity of the data achieved by the clone-specific PCR. Because false-negative PCR results were not noticed in the fractional analysis, the failure of the clone-specific PCR to detect the T-cell clone in the skin specimens of the 9 SPP patients showing T-cell clonality in the blood seems not to be caused by technical problems.
Because even the high-sensitive clone-specific techniques failed to detect the T-cell clone in the skin specimens corresponding to the clonal blood samples, this clone is either below 0.001 % of all infiltrating T cells or absent in cutaneous SPP lesions. Due to this nondetection in the skin, a relation of the circulating clonal T cells to the pathogenesis of SPP cannot directly be proven by the applied techniques. Nevertheless, our results indicate the occurrence of T-cell clonality in the peripheral blood to be a characteristic feature of SPP and CTCL because analysis of a large cohort of control specimens and the clinical workup of our SPP patients excluded other factors simulating or causing a clonal T-cell proliferation in the peripheral blood. This is in line with previous reports showing functionally and chromosomally abnormal T lymphocytes in the peripheral blood of both, PEP and CTCL patients.15 16 For explanation, the following hypotheses can be discussed.
One scenario would regard the clonal T cells in the peripheral blood of SPP as already transformed precursors of the malignant skin infiltrating MF. Assuming transformation in the skin, it remains unclear why the untransformed but not the transformed T cells migrate into the skin. Supposing transformation within the peripheral blood, the skin homing should be induced by a secondary event at least in a fraction of the clonal cells. However, because cutaneous lesions already occur in SPP before clonal T cells are detectable in the skin, this scenario is not supported by our current data and knowledge.
In a second hypothesis, the cutaneous lesions of SPP are regarded as a successful antitumor response reducing the skin-infiltrating clonal T cells to an undetectable amount. This would explain the presence of the clonal T cells in the peripheral blood as well as their nondetection in the skin lesions. Antitumor responses are likely to occur, because high frequencies of activated CD8+ T cells, suspected to be cytotoxic T cells, were observed in the majority of early MF patients.44 However, at least at some time points, ie, in some cutaneous specimens, the presence of clonal T cells in the skin should be postulated. To prove this, investigation of numerous lesions per patient is required. Accordingly, Haeffner et al,11also using a PCR assay observed clonal TCR rearrangements even in skin samples of 2/3 SPP patients.
In conclusion, our data for the first time show the majority of SPP patients to carry a clonal T-cell population in the peripheral blood compartment that persists during the course of the disease, and independently from the administered therapy. Significant differences between clone-positive and clone-negative SPP patients were not found for markers previously reported to be risk factors for disease progression in CTCL.21,23 24 Although the T-cell clones were undetectable in the corresponding skin specimens, the blood clonality is most likely associated with the existence of SPP because other conditions previously associated with T-cell clonality were excluded and analysis of multiple control specimens failed to detect circulating clonal T cells. The nondetection of skin-infiltrating clonal T cells might be due to a sufficient cutaneous antitumor response but an extracutaneous origin of the T-cell clones is also conceivable. Extended follow-up of skin and blood specimens from the SPP patients carrying circulating clonal T cells will allow further insight and will indicate a putative correlation of blood clonality and clinical course.
ACKNOWLEDGMENT
We thank U. Heiduk and S. Richter for their excellent technical assistance and P. Zambon for critical review.
Supported by Grant Ste 366/7-1 from the Deutsche Forschungsgemeinschaft and Grant 70-2091-Lu1 from the Deutsche Krebshilfe–Mildred Scheel Stiftung.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A. Lukowsky, PhD, Department of Dermatology, University Hospital Charité, Humboldt University Berlin, Schumannstraβe 20/21, D-10117 Berlin, Germany; email:ansgar.lukowsky@charite.de.