Abstract
Both Rho-kinase and the Ca2+/calmodulin-dependent myosin light chain (MLC) kinase increase the phosphorylation of MLC. We show that upon thrombin receptor stimulation by low-dose thrombin or the peptide ligand YFLLRNP, or upon thromboxane receptor activation by U46619, shape change and MLC phosphorylation in human platelets proceed through a pathway that does not involve an increase in cytosolic Ca2+. Under these conditions, Y-27632, a specific Rho-kinase inhibitor, prevented shape change and reduced the stimulation of MLC-phosphorylation. In contrast, Y-27632 barely affected shape change and MLC-phosphorylation by adenosine diphosphate (ADP), collagen-related peptide, and ionomycin that were associated with an increase in cytosolic Ca2+ and inhibited by BAPTA-AM/EGTA treatment. Furthermore, C3 exoenzyme, which inactivates Rho, inhibited preferentially the shape change induced by YFLLRNP compared with ADP and ionomycin. The results indicate that the Rho/Rho-kinase pathway is pivotal in mediating the MLC phosphorylation and platelet shape change by low concentrations of certain G protein–coupled platelet receptors, independent of an increase in cytosolic Ca2+. Our study defines 2 alternate pathways, Rho/Rho-kinase and Ca2+/calmodulin-regulated MLC-kinase, that lead independently of each other through stimulation of MLC-phosphorylation to the same physiological response in human platelets (ie, shape change).
PLATELET SHAPE CHANGE is the earliest response induced by physiological agonists. It precedes platelet spreading, aggregation, and secretion, and is characterized morphologically by spheration and contraction of the cell, cytoskeletal rearrangements, folding of the surface membrane, and formation of pseudopods.1,2 The dense tubular system of resting platelets is filled with Ca2+, and activated platelets show a large Ca2+-influx through the plasma membrane and pronounced mobilization of Ca2+ from intracellular stores. However, some stimuli such as thrombin, the thrombin receptor ligand YFLLRNP, or the thromboxane receptor agonists U46619 or U44069 induce shape change through a pathway that is apparently independent of an increase in cytosolic Ca2+.3-7 Thus, upon activation of certain receptors, platelets undergo a contractile response that is not regulated by cytosolic Ca2+.
A crucial event in triggering shape change is the stimulation of myosin light chain (MLC) phosphorylation.8 Phosphorylated myosin develops actin-activated adenosine triphosphatase (ATPase) activity, interacts with the cytoskeleton, and assembles into filaments—properties that may lead to the cytoskeletal rearrangements, the folding of the surface membrane, and the contractile wave centralizing the secretory granules observed during shape change.1,2 MLC-phosphorylation is regulated in nonmuscle cells by a Ca2+/calmodulin-dependent MLC-kinase and, as recent studies show, by Rho-kinase, which is activated by the small guanosine triphosphate (GTP)-binding protein RhoA. Rho-kinase directly phosphorylates MLC and phosphorylates the 130-kD myosin-binding subunit (MBS) of myosin phosphatase, thereby inhibiting the catalytic subunit of the enzyme leading to a further increase of MLC-phosphorylation.9,10 Indeed, recent studies show that RhoA, Rho-kinase, and MBS form a complex in platelets, and that in stimulated platelets and in other cells, Rho-kinase phosphorylates MBS, thereby decreasing the activity of myosin phosphatase.10-12 Furthermore, expression of constitutively active Rho-kinase increased the level of MLC-phosphorylation in fibroblasts, and introduction of active Rho-kinase into the cytosol of permeabilized vascular smooth muscle cells provoked MLC-phosphorylation and contraction in the presence and absence of cytosolic Ca2+.13 14
The role of Rho-kinase in platelet function and the contribution of Rho-kinase to the kinetic and degree of MLC-phosphorylation in intact cells stimulated by physiological agonists are not known. By using a newly available, specific inhibitor of Rho-kinase, Y-27632,15 we found that Rho-kinase through stimulation of MLC-phosphorylation and inhibition of MLC-dephosphorylation plays a central role in mediating the pathway of platelet shape change that is independent of an increase in cytosolic Ca2+. The Rho-kinase–dependent pathway was stimulated by the activation of thrombin and thromboxane receptors as opposed to the Ca2+-/MLC kinase-dependent pathway that was stimulated by adenosine diphosphate (ADP) and collagen receptor activation.
MATERIALS AND METHODS
Reagents.
Y-27632 was a gift from Dr Akiko Yoshimura (Yoshitomi Pharmaceutical Industries, Saitama, Japan). Collagen-related-peptide (CRP) was a gift of Dr Michael Barnes (Department of Biochemistry, Cambridge, UK). YFLLRNP peptide was obtained from Bachem Biochemica (Heidelberg, Germany). Human thrombin (catalogue no. T 7009) was from Sigma (St Louis, MO). Recombinant C3-exoenzyme was produced as described.12 All other materials were obtained from sources described previously.6 16-18
Isolation of human platelets, measurement of platelet shape change, and cytosolic [Ca2+].
Human platelets were treated with acetylsalicylate, isolated by centrifugation in the presence of apyrase as described, and resuspended in a buffer (pH 7.4) containing 20 mmol/L HEPES, 138 mmol/L NaCl, 2.9 mmol/L KCl, 1 mmol/L MgCl2, and 0.6 adenosine diphosphatase (ADPase) U/mL apyrase.6 Some experiments with CRP and thrombin were also performed in the presence of indomethacin (10 μmol/L) and EGTA (1 mmol/L) in the resuspension buffer.17 Both methods gave similar results. Platelet shape change was measured by recording the light transmission in an aggregometer as described6 at a cell density of (2 to 4) × 108/mL. Y-27632 and BAPTA-AM were added at the given concentrations 30 minutes before stimulation followed by incubation at 37°C. EGTA (2 mmol/L) was added 2 minutes before the addition of stimulus. In experiments using C3 exoenzyme, platelets were resuspended in buffer exactly as described and incubated with C3 exoenzyme (400 μg/mL) for 4 hours at 37°C.19 This method has been shown to ADP-ribosylate and inactivate approximately 90% of RhoA.19 After incubation with C3 exoenzyme or vehicle, platelets were diluted to 2 × 108/mL for measurement of shape change.
For measurement of the cytosolic Ca2+-concentration, washed platelets were loaded with 4 μmol/L Fura2-AM (Sigma) as described5 and resuspended at a cell density of 2 × 108/mL. Ca2+ was measured in a thermostatically controlled (37°C) chamber while stirring using a Delta-Scan-1 double-beam fluorescence spectrophotometer (Photon Technology International, South Brunswick, NJ) or an LS 5OB spectrofluorimeter (Perkin-Elmer, Beaconsfield, UK). Fluorescence measurement and calculation of cytosolic Ca2+-concentrations were performed as described.5
Fluorescence microscopy of F-actin–stained platelets.
Samples of washed platelets were taken from platelet suspensions in the aggregometer and fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 minutes at room temperature. The fixed platelets were centrifuged on coverslips coated with poly-L-lysine (Sigma no. 6282) at 250g for 5 minutes at room temperature. After washing 3 times with PBS, platelets were incubated with 0.2% Triton X-100 in PBS for 10 minutes at room temperature, and, after further washing, stained for F-actin by incubation with 8 U/mL rhodamine phalloidin (Molecular Probes, Eugene, OR) for 1 hour at 37°C. After washing, the coverslips were mounted on glass slides with mounting medium (Immuno Fluore Mounting Medium; ICN Biochemicals, Inc, Aurora, OH). Fluorescence microscopy was performed using a Leika DM IRB confocal fluorescence microscope and Leika software TCSNT version 1.5.451 (Leika, Bensheim, Germany).
32P-labeling of human platelets and measurement of protein phosphorylation.
Platelets were isolated and incubated with 1 to 5 mCi/mL H332PO4 as described previously except that albumin (0.3 mg/mL) was used instead of 10% plasma during labeling.18 Preincubation with inhibitors and measurement of shape change were performed as described above. Portions of 50 μL were taken at the given time points, transferred to an equal amount of sample buffer (final concentration: 62.5 mmol/L Tris, 3% sodium dodecyl sulfate [SDS], 5% glycerol, 5% 2-mercaptoethanol, 1 mmol/L EDTA; pH 6.8), and boiled for 5 minutes. 32P-labeled proteins were separated by SDS/polyacrylamide-gel electrophoresis, stained with Coomassie Blue, and dried as described previously.18 Autoradiography was performed by exposure of the dried gels to Kodak X-OMAT film (Eastman Kodak, Rochester, NY) for 16 to 24 hours. The bands of phosphorylated MLC were analyzed by densitometry using a JX-325 film scanner (Sharp, Hamburg, Germany) and ImageMaster-1D software (Pharmacia, Uppsala, Sweden). The level of MLC phosphorylation of resting platelets was subtracted, and the results of the individual experiments were expressed as percent of maximal MLC phosphorylation induced by the respective agonists.
Analysis of results.
Results are shown as mean ± SD of individual experiments from different blood donors.
RESULTS
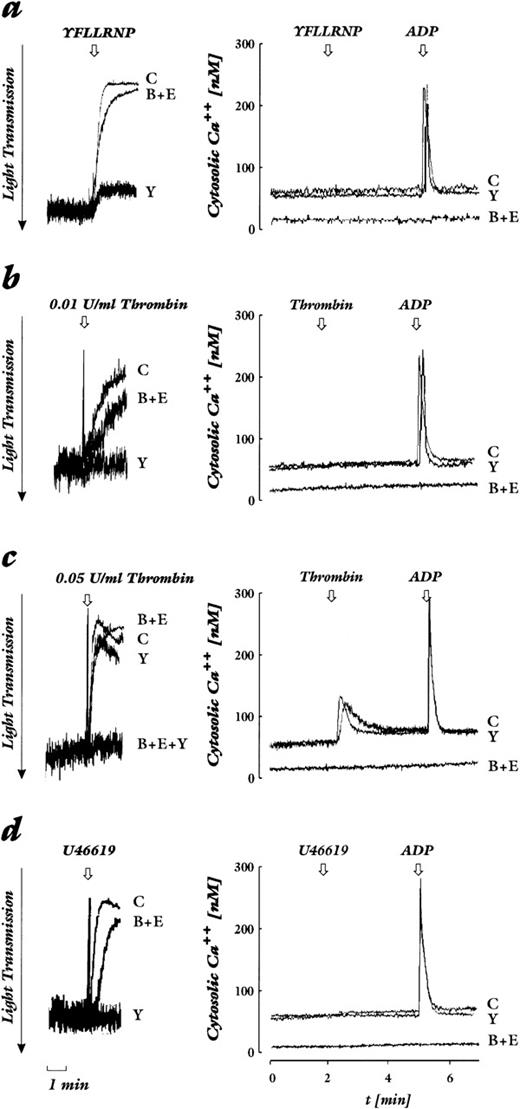
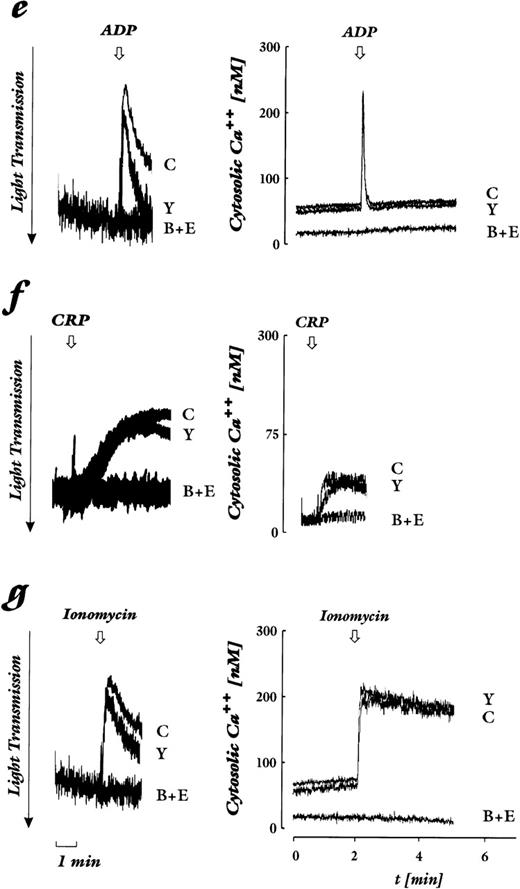
We studied various platelet stimuli that mediate their action through distinct receptors, and chose concentrations that induced a similar level of platelet shape change. Beside several stimuli that activate G protein–coupled receptors, we used CRP as potent agonist of the collagen activatory receptor GP VI.20-22 By measuring in parallel in the same preparation of Fura2-loaded platelets cytosolic Ca2+ concentration and shape change, we show that there are two pathways that lead to platelet shape change: one independent of and without an increase in cytosolic Ca2+, the other dependent of and with an increase in cytosolic Ca2+. The importance of each pathway is agonist- and dose-dependent. Thrombin receptor activation by the heptapeptide ligand YFLLRNP as well as activation of the PG-endoperoxide/thromboxane receptor by low concentrations of U46619 induced shape change in the absence of an increase of cytosolic Ca2+ (Fig 1a and d). The threshold for shape change by thrombin varied depending on the donor but, in general, concentrations of 0.01 to 0.04 U/mL lead to shape change that occurred in the absence of an increase of cytosolic Ca2+ (Fig 1b). In contrast, thrombin at higher concentrations (0.05 to 0.2 U/mL, dependent on the platelet preparation), ADP, CRP, and ionomycin induced shape change that was associated with an increase of cytosolic Ca2+ (Fig 1c, e through g).
Effect of Rho-kinase inhibition by Y-27632 and chelation of cytosolic Ca2+ by BAPTA plus EGTA on shape change (left) and cytosolic calcium levels (right). Platelets loaded with Fura2-AM were stimulated by various agonists. Shown are untreated control samples (C), samples preincubated with 20 μmol/L Y-27632 (Y), 20 μmol/L BAPTA-AM + 2 mmol/L EGTA (B+E), or both (B+E+Y). The arrow indicates addition of the agonist. Decrease of light transmission together with the disappearance of oscillations are indicative of shape change. (a) YFLLRNP (300 μmol/L). (b) Lower concentration of thrombin (0.01 U/mL). (c) Higher concentration of thrombin (0.05 U/mL). (d) U46619 (50 nmol/L). (e) ADP (2 μmol/L). (f) CRP (0.05 μg/mL). (g) Ionomycin (100 nmol/L). Results are representative of 4 to 6 independent experiments.
Effect of Rho-kinase inhibition by Y-27632 and chelation of cytosolic Ca2+ by BAPTA plus EGTA on shape change (left) and cytosolic calcium levels (right). Platelets loaded with Fura2-AM were stimulated by various agonists. Shown are untreated control samples (C), samples preincubated with 20 μmol/L Y-27632 (Y), 20 μmol/L BAPTA-AM + 2 mmol/L EGTA (B+E), or both (B+E+Y). The arrow indicates addition of the agonist. Decrease of light transmission together with the disappearance of oscillations are indicative of shape change. (a) YFLLRNP (300 μmol/L). (b) Lower concentration of thrombin (0.01 U/mL). (c) Higher concentration of thrombin (0.05 U/mL). (d) U46619 (50 nmol/L). (e) ADP (2 μmol/L). (f) CRP (0.05 μg/mL). (g) Ionomycin (100 nmol/L). Results are representative of 4 to 6 independent experiments.
Next we used EGTA and BAPTA-AM treatment of platelets to inhibit any cytosolic Ca2+-increase due to Ca2+-influx through the plasma membrane and Ca2+-mobilization from intracellular stores. We found that shape change through the pathway that was independent of an increase in cytosolic Ca2+ was delayed, but that the maximal response was not reduced (Fig 1a, b, d and Fig 2). The slower kinetic of shape change after BAPTA-AM/EGTA treatment might be due to the lower Ca2+-concentration in resting platelets that was 10 nmol/L in the presence as compared to 50 to 70 nmol/L in the absence of BAPTA-AM/EGTA (Fig 1, right panel). In contrast, shape change through the Ca2+-dependent pathway stimulated by the ADP, CRP, and ionomycin was completely inhibited by BAPTA-AM/EGTA treatment (Fig 1c, e through g; Fig 2). Interestingly, shape change induced by the higher concentration of thrombin that was associated with a cytosolic Ca2+-increase was barely inhibited by BAPTA-AM/EGTA (Fig1c).
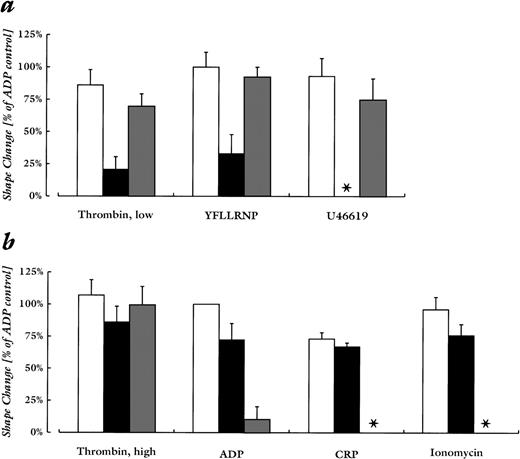
Quantitative comparison of the effect of Rho-kinase inhibition and chelation of cytosolic Ca2+ on platelet shape change induced by various agonists. Shown are untreated controls (□), samples incubated with Y-27632 (20 μmol/L; ▪), and samples pretreated with BAPTA-AM (20 μmol/L) and EGTA (2 mmol/L; ▩). (a) Stimulation conditions that were not associated with an increase in cytosolic Ca2+ (see Fig 1): lower concentrations of thrombin (0.01 to 0.04 U/mL), YFLLRNP (300 μmol/L), U46619 (50 nmol/L). (b) Stimulation conditions that induced a significant increase in cytosolic Ca2+: higher concentrations of thrombin (0.05 to 0.2 U/mL), ADP (2 μmol/L), CRP (0.05 μg/mL), ionomycin (100 nmol/L). Values are mean ± SD of 4 to 6 independent experiments. Asterisk (*) indicates absence of shape change in all experiments.
Quantitative comparison of the effect of Rho-kinase inhibition and chelation of cytosolic Ca2+ on platelet shape change induced by various agonists. Shown are untreated controls (□), samples incubated with Y-27632 (20 μmol/L; ▪), and samples pretreated with BAPTA-AM (20 μmol/L) and EGTA (2 mmol/L; ▩). (a) Stimulation conditions that were not associated with an increase in cytosolic Ca2+ (see Fig 1): lower concentrations of thrombin (0.01 to 0.04 U/mL), YFLLRNP (300 μmol/L), U46619 (50 nmol/L). (b) Stimulation conditions that induced a significant increase in cytosolic Ca2+: higher concentrations of thrombin (0.05 to 0.2 U/mL), ADP (2 μmol/L), CRP (0.05 μg/mL), ionomycin (100 nmol/L). Values are mean ± SD of 4 to 6 independent experiments. Asterisk (*) indicates absence of shape change in all experiments.
The role of Rho-kinase in mediating shape change was investigated using Y-27632, which has been shown to specifically inhibit the kinase activity of p160ROCK purified from platelets.15Pretreatment of platelets with the Rho-kinase inhibitor Y-27632 (20 μmol/L) inhibited selectively the shape change that proceeded through the pathway that was independent of an increase in cytosolic Ca2+: the responses to YFLLRNP, low-dose thrombin, and U46619 were drastically reduced or abolished. Shape change upon stimulation with YFLLRNP was inhibited by 67% ± 7%, with 0.01 to 0.04 U/mL thrombin by 76% ± 6%, and with U46619 by 100% ± 0% (mean ± SEM). However, shape change induced by the higher thrombin concentration, ADP, CRP, and ionomycin was only slightly affected (Figs 1 and 2). Y-27632 (20 μmol/L) reduced the shape change upon stimulation with 0.05 to 0.02 U/mL thrombin by 20% ± 6%, with ADP by 28% ± 5%, CRP by 9% ± 12%, and with ionomycin by 21% ± 5% (mean ± SEM). Also, responses to lower ADP concentrations (100 to 250 nmol/L), which induced 50% of the maximal level of shape change, were associated with an increase of cytosolic Ca2+ and were only marginally inhibited by Y-27632 (data not shown). Y-27632 did not affect the Ca2+-increase observed after addition of these agonists (Fig 1, right panel).
The shape change induced by the higher concentrations of thrombin was delayed, but only weakly inhibited, by either Y-27632 or BAPTA-AM/EGTA treatment, showing that thrombin at this concentration can induce shape change through the separate activation of two pathways (Figs 1c and2b). Treatment with both Y-27632 and BAPTA-AM/EGTA completely inhibited shape change, indicating that thrombin at this concentration stimulated the two pathways separately (Fig 1c). Interestingly, the increase of cytosolic Ca2+ was smaller after the higher concentrations of thrombin than after ADP, although the extent of the shape change after the two stimuli was similar (Fig 1c and e). This was also observed after inhibition of Rho-kinase, indicating that thrombin required a smaller increase of cytosolic Ca2+ to produce shape change than did ADP.
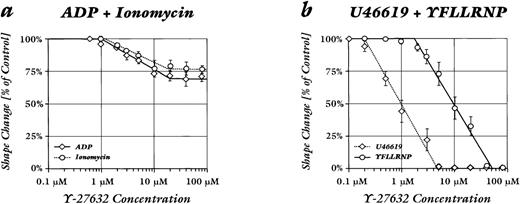
Concentration-response curves of Y-27632 (Fig 3) showed that lower drug concentrations were sufficient for the inhibition of shape change by U46619 as compared with YFLLRNP. The IC50 value (concentration that inhibits 50% of the response) of Y-27632 was 1 ± 0.2 μmol/L for the inhibition of shape change by U46619 as compared with an IC50 value of 9.6 ± 1.1 μmol/L for the inhibition of shape change by YFLLRNP (mean ± SEM). The IC50 values for the 28% and 21% reduction of shape change by ADP and ionomycin were 3.9 ± 0.2 μmol/L and 4.6 ± 0.3 μmol/L, respectively (mean ± SEM). Y-27632 did not induce a significant inhibition of shape change by CRP.
Concentration-response curves of Y-27632 effect on platelet shape change induced by different agonists. Samples were preincubated with the given concentrations of inhibitor for 30 minutes at 37°C. Values are the percent maximum induced by the agonist in the absence of Y-27632. (a) ADP (2 μmol/L; ○) and ionomycin (100 nmol/L; ◊). (b) YFLLRNP (300 μmol/L; ○) and U46619 (100 nmol/L; ◊). Data are mean ± SE of 4 to 6 independent experiments.
Concentration-response curves of Y-27632 effect on platelet shape change induced by different agonists. Samples were preincubated with the given concentrations of inhibitor for 30 minutes at 37°C. Values are the percent maximum induced by the agonist in the absence of Y-27632. (a) ADP (2 μmol/L; ○) and ionomycin (100 nmol/L; ◊). (b) YFLLRNP (300 μmol/L; ○) and U46619 (100 nmol/L; ◊). Data are mean ± SE of 4 to 6 independent experiments.
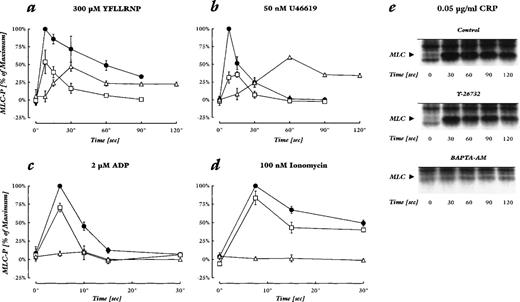
We measured MLC-phosphorylation during the two pathways of shape change, and determined the action of Y-27632. Rho-kinase inhibition by Y-27632 dramatically reduced the early peak of MLC phosphorylation after YFLLRNP and U46619. After YFLLRNP, but not after U46619 stimulation, Y-27632 also accelerated the subsequent dephosphorylation of phosphorylated MLC, possibly due to higher myosin phosphatase activity (Fig 4a and b). In contrast, the effects of Y-27632 on MLC-phosphorylation by ADP, ionomycin, and CRP were much less pronounced. ADP induced a rapid, transient MLC-phosphorylation with a peak at 5 seconds after addition. Y-27632 reduced the maximum level by 28% ± 4%, and subsequently the amplitude of MLC-phosphorylation (Fig 4c). Stimulation of MLC-phosphorylation after addition of CRP and ionomycin was more sustained, and Y-27632 also slightly reduced the maximum level and the amplitude of MLC-phosphorylation (Fig 4d and e). These results indicate that Rho-kinase plays a major role in the phosphorylation of MLC during shape change induced by stimuli that do not produce a detectable increase in cytosolic Ca2+, and only a minor role in phosphorylating MLC upon induction of shape change by the Ca2+-dependent stimuli.
Effect of Rho-kinase inhibition and chelation of cytosolic Ca2+ on MLC-phosphorylation induced by different agonists. (a through d) MLC-phosphorylation levels in percent of the maximal increase upon stimulation of untreated controls. The maximal increase of MLC-phosphorylation stimulated by the different agonists was similar. Shown are controls (•), samples pretreated with 20 μmol/L Y-27632 (□), or 20 μmol/L BAPTA-AM and 2 mmol/L EGTA (▵). Values are mean ± SD of 3 to 4 independent experiments. (e) Autoradiographs of phosphorylated MLC after stimulation with 0.05 μg/mL CRP in untreated control samples (top), samples pretreated with 30 μmol/L Y-27632 (middle), or 50 μmol/L BAPTA-AM and 2 mmol/L EGTA (bottom). The results shown are representative of 2 experiments.
Effect of Rho-kinase inhibition and chelation of cytosolic Ca2+ on MLC-phosphorylation induced by different agonists. (a through d) MLC-phosphorylation levels in percent of the maximal increase upon stimulation of untreated controls. The maximal increase of MLC-phosphorylation stimulated by the different agonists was similar. Shown are controls (•), samples pretreated with 20 μmol/L Y-27632 (□), or 20 μmol/L BAPTA-AM and 2 mmol/L EGTA (▵). Values are mean ± SD of 3 to 4 independent experiments. (e) Autoradiographs of phosphorylated MLC after stimulation with 0.05 μg/mL CRP in untreated control samples (top), samples pretreated with 30 μmol/L Y-27632 (middle), or 50 μmol/L BAPTA-AM and 2 mmol/L EGTA (bottom). The results shown are representative of 2 experiments.
Reduction of basal Ca2+ and inhibition of the increase in cytosolic Ca2+ by BAPTA/EGTA prevented MLC-phosphorylation by ADP, CRP, and ionomycin. BAPTA/EGTA treatment also delayed and reduced the maximum level of MLC phosphorylation by YFLLRNP and U46619. The delayed stimulation of MLC phosphorylation corresponded to the slow shape change response of the [32P]-prelabeled platelets; importantly, the stimulation of MLC-phosphorylation preceded shape change (data not shown), further strengthening the cause/effect relationship of MLC-phosphorylation and shape change.8
The role of Rho kinase in the response to agonists that induced shape change without an increase in cytosolic Ca2+ was also characterized by fluorescence microscopy of platelets stained for F-actin. Treatment with Y-27632 did not have an effect on the discoid shape and the diffuse weak fluorescence of resting platelets, but inhibited the enhanced fluorescence of F-actin and change in platelet morphology (pseudopod formation, irregular surface) after stimulation with YFLLRNP. Shape change after stimulation with ADP, in contrast, was barely affected (data not shown).
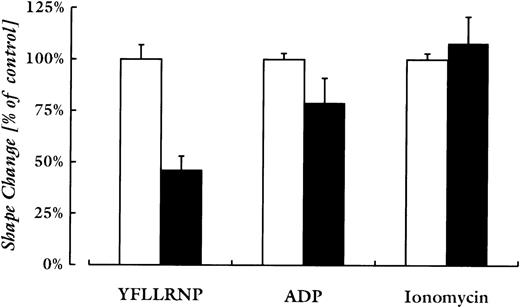
To evaluate the role of Rho, the activator of Rho-kinase, in the 2 pathways of shape change, platelets were preincubated with C3 exoenzyme (400 μg/mL), which has been shown to ADP-ribosylate and inactivate 90% of RhoA in intact human platelets.19 This incubation, which did not result in preactivation of platelets (data not shown), reduced the shape change upon stimulation with YFLLRNP by 54% ± 4%, and with ADP by 21% ± 7% (mean ± SEM). Shape change induced by ionomycin was not inhibited (Fig5).
Quantitative comparison of C3-exoenzyme treatment on platelet shape change induced by various agonists. Platelets were incubated with vehicle (□) or C3-exoenzyme (400 μg/mL; ▪) before stimulation with YFLLRNP (300 μmol/L), ADP (2 μmol/L), or ionomycin (200 nmol/L). The shape change responses (measured by the maximal decrease in light-transmission) induced by the different agonists were similar. Values are mean ± SD of three experiments with different platelet donors.
Quantitative comparison of C3-exoenzyme treatment on platelet shape change induced by various agonists. Platelets were incubated with vehicle (□) or C3-exoenzyme (400 μg/mL; ▪) before stimulation with YFLLRNP (300 μmol/L), ADP (2 μmol/L), or ionomycin (200 nmol/L). The shape change responses (measured by the maximal decrease in light-transmission) induced by the different agonists were similar. Values are mean ± SD of three experiments with different platelet donors.
DISCUSSION
Shape change in human, rabbit, and mice platelets by certain agonists can be induced through a pathway that is independent of an increase of cytosolic Ca2+. Agonists that have been shown to induce this “Ca2+-independent pathway” of shape change in human platelets include the heptapeptide ligand of the thrombin receptor, YFLLRNP, and low concentrations of thrombin3,5,6 (and present study); in human and rabbit platelets, the endoperoxide analogs U46619 and U440694,7(and present study); and in Gαq-deficient mice platelets, thrombin and U46619.23 We report in the present study that Rho-kinase mediates the pathway of shape change that is independent of an increase in cytosolic Ca2+, but plays only a minor role in the Ca2+-dependent pathway of shape change. Upon platelet stimulation by YFLLRNP and U-46619, inhibition of Rho-kinase by Y-27632 dramatically reduced both the onset and the duration of MLC-phosphorylation, the key event in the initiation of platelet shape change. Two novel kinases have been isolated that phosphorylate MLC and contain catalytic domains resembling that of Rho-kinase. These kinases bind to and are activated by the Rho-related protein CDC42, promoting cytoskeletal reorganization.24 CDC42 promotes filopod formation in fibroblasts, and translocates to the cytoskeleton in activated platelets.25 It might be possible that Y-27632 also inhibits these novel kinases, thereby contributing to the inhibition of shape change that is independent of an increase in cytosolic Ca2+.
During the Ca2+-dependent pathway of platelet shape change, induced by ADP or ionomycin, we observed a weak inhibition of shape change that was associated with a small reduction of phosphorylation of MLC in the presence of Y-27632. These results show that Rho-kinase plays only a minor role in phosphorylating MLC and shape change under these conditions. The weak activation of Rho-kinase might be caused by the increase of Ca2+ after ADP and ionomycin stimulation, or mediated by ADP receptor activation; it cannot be excluded that small amounts of ADP are released by ionomycin during shape change. BAPTA/EGTA treatment prevented MLC-phosphorylation and shape change induced by stimuli of the Ca2+-dependent pathway. It also delayed shape change, and reduced and delayed MLC phosphorylation induced by stimuli that did not increase cytosolic Ca2+. Because BAPTA/EGTA treatment also reduced the basal level of cytosolic Ca2+, this may indicate that the Rho/Rho-kinase pathway, albeit not regulated by cytosolic Ca2+, still requires the concentration of cytosolic Ca2+ in resting platelets for optimal activity.
Y-27632 did not completely inhibit MLC-phosphorylation induced by YFLLRNP or U46619 (Fig 4c and d), but almost completely abolished shape change. This may indicate that a certain level of MLC phosphorylation is required for shape change, and/or that Rho-kinase phosphorylates other proteins involved in platelet shape change. It is noteworthy that Rho-kinase phosphorylates vimentin, an intermediate filament protein, and ezrin/radixin/moesin proteins that are involved in the interaction of actin filaments with the plasma membrane.26 27Phosphorylation of these cytoskeletal proteins interferes with their function and is expected to affect the cytoskeletal rearrangements during shape change.
We also found that inactivation of Rho by C3-exoenzyme preferentially inhibited shape change by YFLLRNP as compared to ADP and ionomycin, corroborating our findings with the Rho-kinase inhibitor Y-27632. Shape change was not completely inhibited, possibly due to residual amounts of active Rho. After submission of our manuscript, a study using mouse platelets has been published reporting an inhibition of U46619-induced shape change in Gαq-deficient platelets by C3 exoenzyme and the Rho-kinase inhibitor Y-27632.28 These results support our conclusions in human platelets that the Rho/Rho-kinase pathway is pivotal in mediating the MLC phosphorylation and platelet shape change, when they are not regulated by an increase in cytosolic Ca2+. Recently, inhibition of RhoA has been found to inhibit the formation of focal adhesions in platelets that spread on fibrinogen.19 Spreading occurs subsequently to shape change, and depends on integrin αIIbβ3signaling. It is tempting to speculate that Rho-kinase activated during shape change is involved in the formation of focal adhesions through modulation of integrin αIIbβ3 activity or signaling.
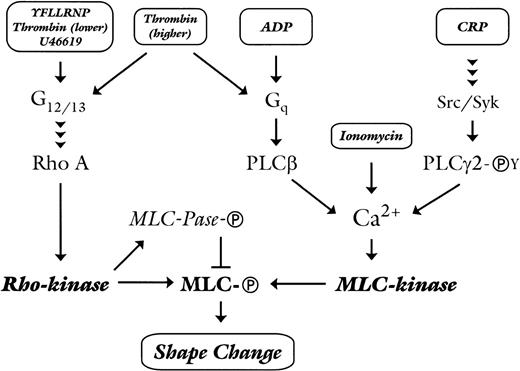
Our study has delineated two pathways of myosin phosphorylation and platelet shape change, one mediated by Rho-kinase and the other by the Ca2+-dependent activation of MLC kinase. Each pathway can be activated independently of each other, dependent on the type and concentration of the agonist. The tyrosine kinase–linked collagen receptor glycoprotein (GP) VI uses the Ca2+-dependent pathway to stimulate shape change. In contrast, stimuli that activate 7-transmembrane G protein–coupled receptors induce shape change via Rho-kinase, which is not regulated by an increase in cytosolic Ca2+ or via the Ca2+-dependent activation of MLC kinase. The ability of a particular 7-transmembrane receptor via either pathway is presumably related to couple to G12/13and Gq. As depicted in the model in Fig 6, thromboxane receptor activation by U46619 or thrombin receptor activation by YFLLRNP or low concentration of thrombin will activate the heterotrimeric G-proteins G12/1329 that are linked to the activation of Rho,30 and Rho-kinase leading to MLC phosphorylation and platelet shape change. This pathway is also present in mouse platelets.28 Activation of the purinergic P2Y1receptor by ADP will activate mainly the Gq/phospholipase Cβ/Ca2+ pathway31 that leads to cytosolic increase of Ca2+ and the Ca2+-dependent stimulation of MLC phosphorylation and shape change. This is in accordance with the result in Gαq knock-out mice that showed a loss of the Ca2+ and shape change response to ADP.23 In human platelets, ADP seems also to activate weakly Rho/Rho-kinase as indicated by the small inhibition of ADP-induced shape change and MLC phosphorylation by C3 exoenzyme and Y-27632. Activation of GPVI by CRP will sequentially activate the Src and Syk-tyrosine kinases with the subsequent tyrosine phosphorylation and activation of phospholipase Cγ2,32 thereby increasing cytosolic Ca2+ and stimulating Ca2+-dependent MLC-phosphorylation and shape change. Direct Ca2+-mobilization by ionomycin will also activate MLC kinase. Higher concentrations of thrombin will activate more strongly the Rho-kinase pathway and stimulate the Gq/phospholipase Cβ/Ca2+ pathway. Under these conditions, inhibition of either Rho-kinase or the Ca2+ pathway alone is insufficient to inhibit shape change, indicating that each pathway compensates for the loss of the other. Inhibition of both pathways is needed to completely inhibit shape change after the higher dose of thrombin (Fig1c), showing that a single agonist can induce shape change through the separate activation of two pathways (Fig 6).
Model depicting the dichotomous regulation of platelet shape change by Rho-kinase and Ca2+-dependent MLC-kinase. See text for details. Abbreviations: MLC-Ⓟ, phosphorylated myosin light-chain; MLC-kinase, Ca2+/calmodulin-dependent myosin light-chain kinase; MLC-Pase-Ⓟ, phosphorylated myosin light-chain phosphatase; PLCβ, phospholipase Cβ; PLCγ2-ⓅY, tyrosine-phosphorylated phospholipase Cγ2; Src/Syk, Src-family tyrosine kinases and tyrosine kinase Syk.
Model depicting the dichotomous regulation of platelet shape change by Rho-kinase and Ca2+-dependent MLC-kinase. See text for details. Abbreviations: MLC-Ⓟ, phosphorylated myosin light-chain; MLC-kinase, Ca2+/calmodulin-dependent myosin light-chain kinase; MLC-Pase-Ⓟ, phosphorylated myosin light-chain phosphatase; PLCβ, phospholipase Cβ; PLCγ2-ⓅY, tyrosine-phosphorylated phospholipase Cγ2; Src/Syk, Src-family tyrosine kinases and tyrosine kinase Syk.
Supported by Ernst und Berta Grimmke-Stiftung, Deutsche Forschungsgemeinschaft (Si 274/6-1, GRK 438, Ae11/5-1, and SFB 413), August-Lenz-Stiftung, Anglo-German Foundation (ARC-X-96), and the Wellcome Trust. S.P.W. is a British Heart Foundation Senior Research fellow.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Wolfgang Siess, MD, Institut für Prophylaxe und Epidemiologie der Kreislaufkrankheiten, Klinikum Innenstadt, Universität München, Pettenkoferstr. 9, D-80336 München, Germany; e-mail:wolfgang.siess@klp.med.uni-muenchen.de.