To the Editor:
McCloskey et al1 recently reported that spontaneous apoptosis of T cells from human immunodeficiency virus (HIV)-infected individuals occurred only in previously activated cells independent of CD95 receptor (R) expression or CD95 sensitivity. Using fluorescein isothiocyanate (FITC)-labeled anti-CD95 monoclonal antibody (MoAb), the authors found that T cells dying after 72 hours of in vitro culture were predominantly CD95-negative. However, in our own analysis of T cells from HIV-infected children, we could identify at least 3 different cell populations in the CD4+ T-cell subset using phycoerythrin (PE)-labeled anti-CD95 MoAb2(and unpublished results): (1) CD95-negative cells corresponding to resting/“naive” CD45RA+CD45ROneg T cells; (2) CD45RA+ cells that were recently activated and expressed low levels of both CD45RO and CD95; (3) CD95high CD45RO+ primed/“memory” T cells. We have previously shown that only resting/“naive” T cells from HIV-infected children were resistant to spontaneous and anti-CD95–induced apoptosis in vitro.2 Thus, the CD95neg CD45RO+ cells detected by McCloskey et al by an FITC-labeled anti-CD95 MoAb might in fact represent recently activated, CD95-low positive CD45RO-low positive T cells if analyzed with more sensitive staining methods using the appropriately labeled MoAb. The conclusion that only CD95-negative T cells of HIV-infected individuals are dying spontaneously during in vitro culture should, therefore, be controlled by using a PE-labeled anti-CD95 MoAb.
Based on their observation of an increased percentage of CD95-negative T cells undergoing spontaneous apoptosis in vitro, the authors speculate that mechanisms independent of the CD95 R/ligand (L) system may be involved in exaggerated lymphocyte apoptosis in HIV-1 infection. Indeed, as shown in Fig 1A, spontaneous apoptosis of freshly isolated peripheral blood T cells from HIV-infected individuals occurs already after short-term culture in vitro (16 to 20 hours, 37°C, 5% CO2); it can be partially blocked by addition of interleukin-2 (IL-2) to the culture medium and is not influenced by agents that block CD95 R/L interaction, eg, anti–APO-1 F(ab)′ fragments. On the other hand, anti-CD95 MoAb-induced apoptosis is completely inhibited by preincubation of T cells with anti–APO-1 F(ab)′ and is not influenced by addition of IL-2 (Fig 1B). The profound dysregulation of the CD95 R/L-system observed in pediatric HIV-infection3,4 is apparently not responsible for spontaneous cell death of isolated peripheral blood T cells from HIV-infected individuals. The CD95 system is, however, critically involved in activation-induced cell death (AICD) following crosslinking of the T-cell receptor (TCR)/CD3-complex.3,5 Interestingly, cocultivation of freshly isolated T cells from HIV-infected individuals with both anti-CD3 and anti-CD95 MoAb did always cause a higher rate of cell death than both of these agents alone (Fig 1C). This suggests that the CD95 pathway is not inhibited by simultaneous stimulation of the TCR/CD3-complex, and CD95-independent mechanisms contribute both to spontaneous cell death and to OKT3-induced AICD (eg, TRAIL/TRAIL-receptor mediated death6).
Inhibition of spontaneous T-cell death but not of anti-CD95–induced apoptosis by IL-2 and cellular activation. Isolated peripheral blood mononuclear cells (PBMC, 2 × 105cells/well) obtained from 10 HIV-1+ children and adolescents were cultured in the presence or absence of either anti-CD3 MoAb (OKT3; 10 μg mL−1) and/or anti-CD95 MoAb (anti–APO-1; 10 μg mL−1) and protein A (5 ng mL−1) and were collected after 21 ± 1 hours for determination of cell death in CD4+ and CD8+ cells as described.2-4 The percentage of specific anti-CD3– and anti-CD95–induced apoptosis was calculated according to the formula: 100 × [Experimentally Induced Cell Death (%) − Spontaneous Cell Death (%)]/[100 − Spontaneous Cell Death (%)]. In (A) and (B), data are given as arithmetic mean; error bars indicate the standard error of the mean. (A) Recombinant human IL-2 (30 U/mL; Biozol, Eching, Germany), but not preincubation with the antagonistic anti–APO-1 F(ab)′ fragment (5 μg mL−1),3 5 significantly reduced spontaneous T-cell death in vitro. (B) Signaling via the CD95 receptor, but not spontaneous cell death, was blocked by anti–APO-1 F(ab)′. (C) The effect of T-cell activation on anti–APO-1–induced apoptosis of freshly isolated CD4+ T cells was assessed in PBMC from 2 different patients stimulated with OKT3 and/or anti–APO-1 as described above. Numbers in the plots indicate the percentage of apoptotic cells that are identified by the typical change in light scatter characteristics (“shrinking death”). In patient no. 1 OKT3 induced less apoptosis than anti–APO-1, whereas in patient no. 2 more cell death was seen in OKT3-treated cells. However, in both experiments the combination of these MoAbs induced more apoptosis than each of them alone.
Inhibition of spontaneous T-cell death but not of anti-CD95–induced apoptosis by IL-2 and cellular activation. Isolated peripheral blood mononuclear cells (PBMC, 2 × 105cells/well) obtained from 10 HIV-1+ children and adolescents were cultured in the presence or absence of either anti-CD3 MoAb (OKT3; 10 μg mL−1) and/or anti-CD95 MoAb (anti–APO-1; 10 μg mL−1) and protein A (5 ng mL−1) and were collected after 21 ± 1 hours for determination of cell death in CD4+ and CD8+ cells as described.2-4 The percentage of specific anti-CD3– and anti-CD95–induced apoptosis was calculated according to the formula: 100 × [Experimentally Induced Cell Death (%) − Spontaneous Cell Death (%)]/[100 − Spontaneous Cell Death (%)]. In (A) and (B), data are given as arithmetic mean; error bars indicate the standard error of the mean. (A) Recombinant human IL-2 (30 U/mL; Biozol, Eching, Germany), but not preincubation with the antagonistic anti–APO-1 F(ab)′ fragment (5 μg mL−1),3 5 significantly reduced spontaneous T-cell death in vitro. (B) Signaling via the CD95 receptor, but not spontaneous cell death, was blocked by anti–APO-1 F(ab)′. (C) The effect of T-cell activation on anti–APO-1–induced apoptosis of freshly isolated CD4+ T cells was assessed in PBMC from 2 different patients stimulated with OKT3 and/or anti–APO-1 as described above. Numbers in the plots indicate the percentage of apoptotic cells that are identified by the typical change in light scatter characteristics (“shrinking death”). In patient no. 1 OKT3 induced less apoptosis than anti–APO-1, whereas in patient no. 2 more cell death was seen in OKT3-treated cells. However, in both experiments the combination of these MoAbs induced more apoptosis than each of them alone.
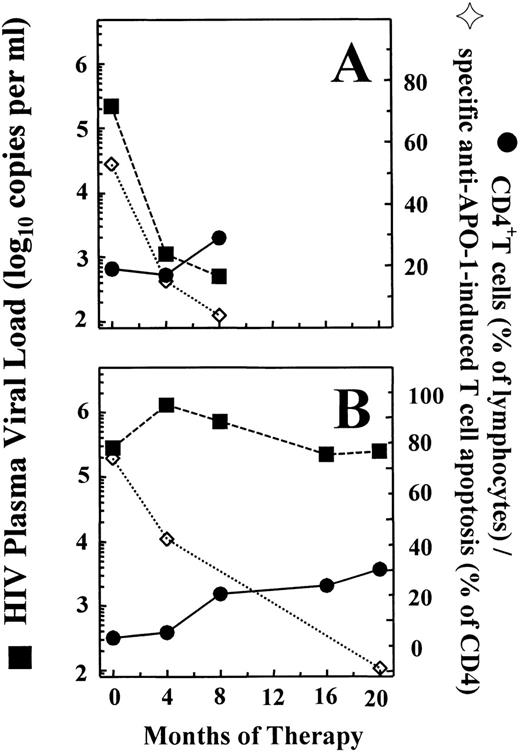
Thus, there are at least 3 different mechanisms responsible for programmed cell death of ex vivo–cultured T cells from HIV-infected individuals: (1) spontaneous cell death, which is CD95-independent but partially inhibitable by IL-2 and represents the classical “death-by-default” induced by growth-factor withdrawal7; (2) CD95-induced cell death, which is not inhibitable by IL-2 nor by stimulation via the CD3/TCR-complex; and (3) AICD via CD95-dependent and -independent pathways. It has not been clarified yet which of these mechanisms is of major importance for immunopathogenesis of HIV-1 infection in vivo. However, our previous observation that an increase in sensitivity of peripheral blood T cells toward CD95-induced apoptosis is seen already during early disease stages4 suggests that this may be a relevant mechanism in disturbed T-cell homeostasis, which is the central event in immunopathogenesis of HIV-1 infection and development of acquired immunodeficiency syndrome.8 The CD95 cell-surface receptor is expressed at high density on a variety of different cell types, including thymocytes and hematopoetic stem cells, and is also functionally active at least during certain stages of cellular activation and maturation.9,10 Immediately after starting highly active antiretroviral treatment (HAART), which caused an immediate increase in naive CD4+ and CD8+ T cells in HIV-1–infected infants and children, we observed a steep reduction in the sensitivity of peripheral blood T cells toward anti-CD95–induced apoptosis.11 Figure2 shows that normal CD95 sensitivity is maintained in clinically asymptomatic patients with stable CD4 counts during prolonged treatment with HAART, irrespective of the degree of concomitant suppression of viral replication as reflected by plasma viral load.
Development of HIV-1 plasma viral load (RNA-copies per milliliter; ▪), peripheral blood CD4 counts (percent of lymphocytes; •), and sensitivity of CD4+ T cells toward anti–APO-1–induced apoptosis (percent CD95-specific cell death; ◊) during highly active antiretroviral therapy (HAART) in 2 children infected perinatally with HIV-1. Patient A was a 10-year-old girl with no previous antiretroviral therapy. After starting HAART using lamivudine, stavudine, and nelfinavir, this patient showed an immediate decrease in sensitivity of CD4+ cells toward CD95-induced apoptosis, which was followed by a slow but gradual increase in CD4 counts and a decrease in plasma viral load to levels below the limit of detection (bDNA). Patient B was a 10-year-old boy who switched from combined treatment with zidovudine and lamivudine to HAART using lamivudine, stavudine, and nelfinavir. The reduction in sensitivity of CD4+ cells toward CD95-induced apoptosis was somewhat less pronounced, and a considerable increase in CD4 counts was seen only after 8 months of therapy. This patient never achieved complete repression of viral replication during HAART. However, despite the maintenance of high plasma viral load levels, he remained clinically well and showed a further increase in CD4 counts accompanied by the absence of CD95-induced T-cell death in vitro.
Development of HIV-1 plasma viral load (RNA-copies per milliliter; ▪), peripheral blood CD4 counts (percent of lymphocytes; •), and sensitivity of CD4+ T cells toward anti–APO-1–induced apoptosis (percent CD95-specific cell death; ◊) during highly active antiretroviral therapy (HAART) in 2 children infected perinatally with HIV-1. Patient A was a 10-year-old girl with no previous antiretroviral therapy. After starting HAART using lamivudine, stavudine, and nelfinavir, this patient showed an immediate decrease in sensitivity of CD4+ cells toward CD95-induced apoptosis, which was followed by a slow but gradual increase in CD4 counts and a decrease in plasma viral load to levels below the limit of detection (bDNA). Patient B was a 10-year-old boy who switched from combined treatment with zidovudine and lamivudine to HAART using lamivudine, stavudine, and nelfinavir. The reduction in sensitivity of CD4+ cells toward CD95-induced apoptosis was somewhat less pronounced, and a considerable increase in CD4 counts was seen only after 8 months of therapy. This patient never achieved complete repression of viral replication during HAART. However, despite the maintenance of high plasma viral load levels, he remained clinically well and showed a further increase in CD4 counts accompanied by the absence of CD95-induced T-cell death in vitro.
On the other hand, the beneficial clinical effect of IL-2 substitution12 in HIV-1–infected adults suggests that the death-by-default mechanism induced by IL-2 deficiency might be an important factor of disease progression in vivo. However, IL-2–treated patients also show a partial reconstitution of the naive T-cell compartment12 13; this argues against a decisive role of inhibition of spontaneous cell death of peripheral blood T cells in IL-2–induced T-cell reconstitution in HIV-infected individuals.
We previously described that both spontaneous T-cell death and CD95 sensitivity during pediatric HIV infection was confined to primed/memory cells and did not affect resting/“naive” T cells.2 Therefore, we recently proposed a model for the immunopathogenesis of HIV-1 infection based on the increased activation of the CD95 system (which is commonly observed in peripheral blood T cells from HIV-1+ individuals), which may also contribute to decreased T-cell regeneration in bone marrow and thymus.14 During the initial stages of the disease, a slight increase in loss of activated, memory-type T cells by direct, virus-induced and—more importantly—by indirect, activation-related mechanisms may not be sufficiently compensated for by an increase in T-cell regeneration due to the impaired function of thymus and bone marrow, thus leading to a constant decrease in naive T cells with disease progression.
Response
Despite extensive study, the role of the Fas/FasL system in HIV pathogenesis remains undetermined. While evidence exists both for1-1-1-7 and against1-8-1-12 CD95-mediated HIV-induced cell death, the most likely explanation is that both Fas-dependent and -independent pathways are at work, a concept supported by recent findings from our laboratory.1-13
Comments by Böhler and colleagues regarding our report indicate that they have misread the methods used and misconstrued the conclusions drawn. Their interpretation that “only CD95-negative T cells” were dying is incorrect; indeed, our data indicated the existence of both CD95-dependent and -independent mechanisms of apoptosis. By performing the TUNEL assay on cells labeled with anti-CD95 allophycocyanin MoAb, we observed that CD95-expressing cells were overrepresented in the live population. This finding strongly implicates Fas-independent mechanisms in HIV-induced cell death. Clearly, however, a CD95-positive population was present in the apoptotic population as well. The data presented by Böhler et al is, in fact, in agreement with our findings. We hypothesize that those TUNEL-positive cells which expressed CD95 may indeed be dying via a Fas/Fas ligand induced mechanism; our point was that not all cell death can be accounted for by this pathway.
The remark concerning the use of “FITC-labeled anti-CD95” for our 72-hour apoptosis data is also incorrect, leading them to claim that our labeling system was not sensitive enough. The data in question were collected with allophycocyanin-conjugated anti-CD95 MoAb, as stated. We purposely chose allophycocyanin because (1) its emission is far from cellular autofluorescence,1-14,1-15 (2) it has a large extinction coefficient and a high quantum yield,1-16,1-17 and (3) there is no spectral overlap from fluorescein (TUNEL) because the signals generated from the two laser beams are separated temporally. In fact, the relative “brightness” of allophycocyanin is comparable to that of phycoerythrin.1-18
Regulation of apoptosis is by necessity tightly controlled, with the expression of multiple pro- and anti-apoptotic molecules providing many checks and balances. The interaction of a chronic viral infection with the immune system may influence some of these regulatory pathways, thus lending further complexity. Mechanisms of HIV-induced cell death may differ during the disease course or following effective anti-retroviral therapy, and may depend on specific differences in individual patients, virus strains, treatment modalities, or other as yet unidentified factors. In agreement with our overall conclusions, Böhler et al state that both Fas-dependent and -independent mechanisms are responsible for HIV-mediated apoptosis; potential alternate mechanisms recently proposed include those mediated by TRAIL1-19 or by signaling through CD4 and CXCR4.1-20 As stated in our published report, our data indicate that multiple mechanisms of apoptosis induction are active during HIV infection, a notion that is supported by findings from other laboratories, including the data presented by Böhler and colleagues.

![Fig. 1. Inhibition of spontaneous T-cell death but not of anti-CD95–induced apoptosis by IL-2 and cellular activation. Isolated peripheral blood mononuclear cells (PBMC, 2 × 105cells/well) obtained from 10 HIV-1+ children and adolescents were cultured in the presence or absence of either anti-CD3 MoAb (OKT3; 10 μg mL−1) and/or anti-CD95 MoAb (anti–APO-1; 10 μg mL−1) and protein A (5 ng mL−1) and were collected after 21 ± 1 hours for determination of cell death in CD4+ and CD8+ cells as described.2-4 The percentage of specific anti-CD3– and anti-CD95–induced apoptosis was calculated according to the formula: 100 × [Experimentally Induced Cell Death (%) − Spontaneous Cell Death (%)]/[100 − Spontaneous Cell Death (%)]. In (A) and (B), data are given as arithmetic mean; error bars indicate the standard error of the mean. (A) Recombinant human IL-2 (30 U/mL; Biozol, Eching, Germany), but not preincubation with the antagonistic anti–APO-1 F(ab)′ fragment (5 μg mL−1),35 significantly reduced spontaneous T-cell death in vitro. (B) Signaling via the CD95 receptor, but not spontaneous cell death, was blocked by anti–APO-1 F(ab)′. (C) The effect of T-cell activation on anti–APO-1–induced apoptosis of freshly isolated CD4+ T cells was assessed in PBMC from 2 different patients stimulated with OKT3 and/or anti–APO-1 as described above. Numbers in the plots indicate the percentage of apoptotic cells that are identified by the typical change in light scatter characteristics (“shrinking death”). In patient no. 1 OKT3 induced less apoptosis than anti–APO-1, whereas in patient no. 2 more cell death was seen in OKT3-treated cells. However, in both experiments the combination of these MoAbs induced more apoptosis than each of them alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1829/5/m_blod41740002x.jpeg?Expires=1765368132&Signature=BzvXb0QFh-1EI5ViZcnoe4wuPj7wjj3SdcYeHnRk2jWGVB4Hbe1-rHQ3C2vpHpBbiz0oP-RdfhkAkgrCywX9WIxozJJp3yvT4MZUaori5bp9INUj7h3XUhcXeVvpWnosRlHYhFCqtKaJX6fOEz8TSkLi0ik~3odv3ySGbdK9H7It~VswcOaNUpoXzpBm2mY52e4NATefCyr0Od0~0Y7hd9IHDQdSzBVCetyySRTD8pIvhIm~IgbHuW1ZibxxmI42L4ObfLGSEN5o2J2Pd4sBYgm~GiB11H8GwQiboQ93QQQzX7MNK7avdSE8b1jeQ04p1loV~~SgMJJ4KyD1CzufJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)