Recently, culture conditions that stimulate the proliferation of primitive hematopoietic cells defined by various phenotypic and functional endpoints in vitro have been identified. However, evidence that they support a high probability of self-renewal leading to a large net expansion in vitro of transplantable cells with lympho-myeloid repopulating ability has been more difficult to obtain. The present study was designed to investigate whether the low overall expansion of human repopulating hematopoietic cells seen in vitro reflects a selective unresponsiveness of these rare cells to the growth factors currently used to stimulate them or, alternatively, whether they do proliferate in vitro but lose engrafting potential. For this, we used a high-resolution procedure for tracking and reisolating cells as a function of their proliferation history based on the loss of cellular fluorescence after staining with (5- and 6-) carboxyfluorescein diacetate succinimidyl ester. The results show that the vast majority of long-term culture-initiating cells and in vivo lympho-myeloid competitive repopulating units present in 5-day suspension cultures initiated with CD34+ human cord blood and fetal liver cells are the progeny of cells that have divided at least once in response to stimulation by interleukin-3, interleukin-6, granulocyte colony-stimulating factor, Steel factor, and Flt3-ligand. Thus, most human repopulating cells from these two sources are stimulated to undergo multiple divisions under currently used short-term suspension culture conditions and a proportion of these retain engraftment potential.
HEMATOPOIESIS originates in a small number of hematopoietic stem cells. These cells are defined by their ability to differentiate into all of the blood cell lineages as well as to generate progeny with the same unrestricted hematopoietic potential. Because specific markers of these unique functional properties of stem cells have not yet been identified, their detection and enumeration require the use of retrospective assays. These involve demonstrating an ability to repopulate all compartments of the hematopoietic system after intravenous injection of the cells under study into suitable myeloablated hosts.1 In the murine system, the use of histocompatible but genetically distinguishable donor and host combinations has been combined with limiting dilution analysis to allow many properties of stem cells to be elucidated.2-5 A similar approach to the quantitation of transplantable human stem cells has been possible using sublethally irradiated immunodeficient nonobese diabetic-scid/scid (NOD/SCID) mice as recipients.6 7
Self-renewal divisions are believed to be responsible for the expansion of the hematopoietic stem cell compartment that occurs both during fetal life and posttransplant, as well as for the stable maintenance of this population throughout normal adult life.8,9 However, the precise mechanisms that regulate the outcome of hematopoietic stem cell divisions are largely unknown. Recent progress in the development of methods for obtaining highly enriched populations of stem cells and the availability of an increasing number of recombinant growth factors to which they can respond has stimulated a plethora of studies to identify conditions that will support a net expansion of stem cells in vitro. These have shown that factors like Flt3-ligand (FL), Steel factor (SF), interleukin-6 (IL-6), IL-11, and thrombopoietin (TPO) are important synergizing growth factors active on these cells.7,10-12 However, to date, large (>10-fold) and continuing net expansions of cells with retention of stem cell activity have not been shown. One possible explanation for this may lie in the reversible loss of engraftment activity that might be related to the transit of stem cells through specific phases of the cell cycle.13 Thus, the activation of human stem cells from G0 into G1 might be expected to cause a similar rapid loss of their transplantability, as recently observed.14 If this reflects a transient change in the homing properties of stem cells,15 rather than an intrinsic alteration in their growth and differentiation potential, it should be possible to demonstrate the passage of some stem cells through multiple self-renewal divisions in vitro. To investigate this possibility, we have used a high-resolution procedure for tracking successive generations of hematopoietic cells in asynchronously dividing populations.16 In this procedure, cells are labeled at the beginning of the experiment with (5- and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE). The precise halving of fluorescence at each successive mitosis then allows multiple daughter generations to be reproducibly resolved. The application of this procedure to human CD34+ cell populations isolated from both cord blood and fetal liver and then cultured for 5 days in serum-free medium containing FL, SF, IL-3, IL-6, and granulocyte colony-stimulating activity (G-CSF) has shown that the majority of stem cells detectable after culture have already undergone multiple self-renewal divisions within the 5 days in vitro.
MATERIALS AND METHODS
Cells.
Samples of human fetal liver were obtained from elective abortuses of 10 to 16 weeks of gestation and dispersed either by pressing minced tissue fragments through a sieve or using dispase as described.17 Samples of heparinized cord blood were obtained from normal, full-term infants delivered by cesarean section. In both cases, institutional guidelines were adhered to for access and use of human material. Low-density cells (<1.077 g/mL) were isolated after centrifugation of the cells on Ficoll-hypaque (Pharmacia, Piscataway, NJ) and cryopreserved in 10% dimethyl sulfoxide plus 90% fetal calf serum (FCS; StemCell Technologies, Vancouver, BC, Canada) until required. Cells were then thawed, pooled (≥5 cord blood samples and ≥10 fetal liver samples per experiment), and cells expressing surface antigens characteristic of more mature hematopoietic cells (lin+ cells) were removed using StemSep columns (StemCell Technologies) according to the manufacturer’s instructions.
CFSE labeling and isolation of labeled cells.
Cells were washed, resuspended at 5 × 106 cells/mL in phosphate-buffered saline (PBS), and CFSE (Molecular Probes, Eugene, OR) was added at 20 μmol/L. After 10 minutes at 37°C, further uptake of the dye was stopped by addition of ice-cold Hanks’ balanced salt solution (HBSS) supplemented with 20% FCS. The cells were then washed twice, the second time in HBSS without FCS, and finally resuspended at 5 to 10 × 105 cells/mL in Iscove’s medium supplemented with BIT (BIT9500; StemCell Technologies), 10−4 mol/L 2-mercaptoethanol (Sigma Chemicals, St Louis, MO), 40 μg/mL low-density lipoproteins (Sigma), 50 ng/mL human TPO (Genentech, Palo Alto, CA), and 0.1 μg/mL colcemid (GIBCO-BRL, Burlington, Canada). Cells were then cultured overnight at 37°C to allow the efflux of all CFSE not stably bound to intracellular protein.16
After washing in HBSS with 2% FCS and 5% human serum (kindly provided by D. Hogge, Terry Fox Laboratory), CFSE-labeled cells were stained with Cy5-conjugated 8G12 (anti-CD34) antibody (kindly provided by P.M. Lansdorp, Terry Fox Laboratory) for 30 minutes at 4°C. The cells were then washed 2 times with HBSS containing 2% FCS with 1 μg/mL propidium iodide (PI; Sigma Chemicals) being added to the second wash. A narrow gate (32 channels wide using a 1,024-channel log amplifier) was then used to define a sort gate for isolating a subset of homogeneously CFSE+ PI− CD34+cells with low side-scattering characteristics, as described.16 Using a FACStar Plus cell sorter equipped with a 5-W argon laser and a 30-mW helium neon laser (Becton Dickinson, San Jose, CA), 2 adjacent populations of CFSE-stained CD34+cells were isolated and subsequently manipulated identically, but in parallel, to allow the majority of CD34+ cells to be used.
Short-term suspension cultures.
CFSE-stained CD34+ cells were cultured for 5 days at 5 to 10 × 104 cells/mL in 35-mm petri dishes (StemCell Technologies) in serum-free Iscove’s medium containing the same supplements described above, but with replacement of the TPO by 20 ng/mL IL-3 (Novartis, Basel, Switzerland), 20 ng/mL IL-6 (Cangene, Mississauga, Ontario, Canada), 20 ng/mL G-CSF (StemCell Technologies), 100 ng/mL SF (prepared and purified from cDNA transfected COS cells in our laboratory), and 100 ng/mL FL (Immunex Corp, Seattle, WA). After 72 hours an equal volume of fresh medium and growth factors was added to each culture. Another 2 days later, the cells were harvested, washed in HBSS with 2% FCS, restained with Cy5-conjugated 8G12 (anti-CD34) antibody, and the cells then resorted according to their CFSE fluorescence relative to a parallel aliquot of cells that had been cultured under the same conditions and for the same time but in the presence of 0.1 μg/mL colcemid (to inhibit cell division). This made it possible to fix the positions of the fluorescence peaks corresponding to the undivided cells and first-, second-, and third-generation cells present after 5 days in culture. Comparison of the colony-forming cell (CFC) content (see below) of the cultured cells before and after the second sort showed no significant selective progenitor loss or enrichment as a result of this procedure (recovery of cord blood CFC = 99% ± 9%, n = 4; recovery of fetal liver CFC = 86% ± 24%, n = 3).
In vitro progenitor assays.
CFC and 6-week long-term culture-initiating cell (LTC-IC) assays (on murine fibroblast feeders engineered to produce human SF, IL-3, and G-CSF) were performed as previously described.18
Competitive repopulation unit (CRU) assay.
Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were bred and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, BC, Canada) under sterile conditions in microisolator cages and were provided exclusively with autoclaved food and water. To assay human CRU, test cells plus 106irradiated (15 Gy) normal human bone marrow (carrier) cells were injected intravenously into 6- to 12-week-old mice that had just been given 350 cGy total body 137Cs irradiation. Mice were killed 6 to 8 weeks later and the contents of both tibiae and femurs suspended in HBSS plus 2% FCS. To minimize nonspecific binding of antibodies, human and murine Fc receptors were blocked first by incubating the cells in 5% human serum and 2.4G2 (an anti-mouse Fc receptor antibody19). Separate aliquots of cells were then stained for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)-conjugated human anti-CD34 (8G12) and phycoerythrin (PE)-conjugated human anti-CD19 and anti-CD20 antibodies (Becton Dickinson) for the detection of human (CD34−CD19/20+) B-lineage cells, or with FITC-conjugated human anti-CD15 (Becton Dickinson), anti-CD66b (Pharmacia), and PE-conjugated human anti-CD45 (Becton Dickinson) and anti-CD71 (OKT9) for the detection of human (CD45/71+ CD15/66b+) myeloid cells. Mice with detectable human lymphoid and myeloid engraftment (5 positive events each per 20,000 assessed) were counted as positive. All other mice were considered negative. Levels of specific staining were set based on parallel analyses of additional aliquots of the same test cells similarly incubated with irrelevant isotype-matched control antibodies labeled with the corresponding fluorochromes. Gates were then set to exclude levels of fluorescence which included greater than 99.99% of these negative control samples.
RESULTS
Expansion of committed and primitive human hematopoietic progenitors in short-term cultures of CFSE-stained CD34+ cord blood and fetal liver cells.
Table 1 shows the changes in total cell, CFC, and LTC-IC numbers seen when cord blood or fetal liver cells were first incubated overnight in the presence of TPO and colcemid, a CFSE+ CD34+ subpopulation then isolated, and the cells cultured for an additional 5 days in the presence of FL, SF, IL-3, IL-6, and G-CSF. During the 5 days of culture, the total cell expansion and the increase in CFC numbers in both types of culture were similar (≈35-fold). Recoveries of LTC-IC numbers were more variable but they also generally increased several fold. These results are similar to previously published results for cells cultured without prior CFSE staining.7 20
CD34+ cord blood and fetal liver cells proliferate rapidly but asynchronously in response to stimulation by FL, SF, IL-3, IL-6, and G-CSF.
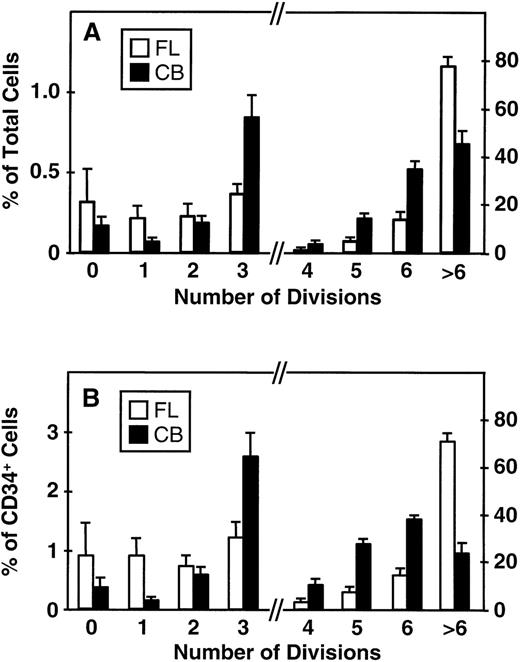
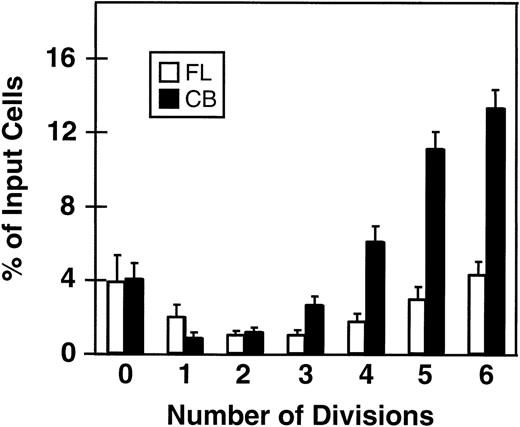
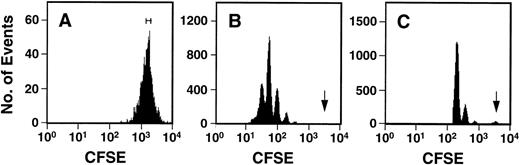
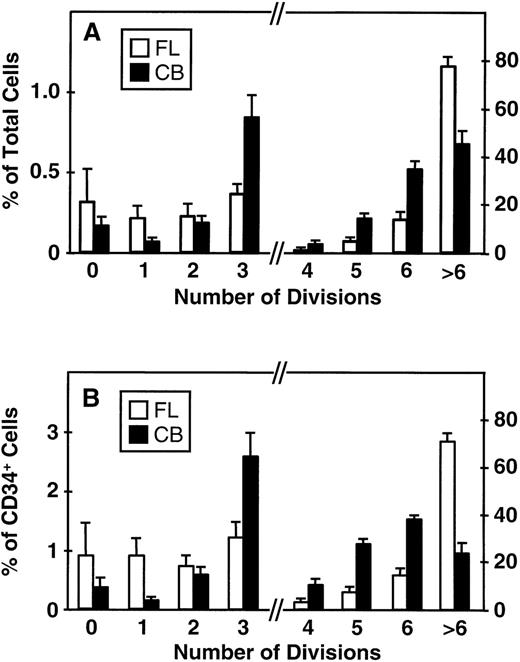
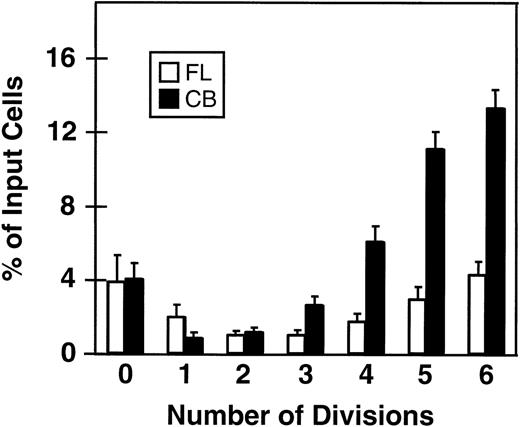
Fluorescence-activated cell sorting (FACS) analysis of cells harvested from 5-day cultures of CD34+ cord blood or fetal liver cells reproducibly resolved 6 generations of progeny, in addition to a small peak of residual undivided (very bright) cells (0.2% ± 0.1%, n = 6, and 0.3% ± 0.5%, n = 5, respectively, of the final cells in the cord blood and fetal liver cell cultures). These undivided cells represented, in both cases, ≈4% of the initial CD34+ cells originally seeded into the cultures. The results of a representative experiment are illustrated in Fig 1. The average distribution of cells among successive generations of progeny for all experiments (5 with fetal liver, 6 with cord blood) is shown in Fig 2A. Companion data for cells still expressing CD34 are shown in Fig 2B. As can be seen, the results for the cord blood and fetal liver cultures were similar, the majority of the cells in both types of culture being derived from cells that had divided at least 5 times, although a higher proportion of fetal liver cells divided more than 6 times. These data were used to calculate the proportion of the original cells that produced each of the different sized clones seen, assuming that all cell divisions were symmetric (with respect to subsequent proliferative activity) and that there was also no cell loss (Fig 3). The percentage of starting cells contributing to clones of different sizes was calculated by the equation ([a/2x]/b) where ais the number of cells derived from x divisions in vitro,x is the number of divisions, and b is the total number of initial cells. According to this calculation (and the underlying assumptions), the proportion of initial CD34+ cord blood or fetal liver cells that undergo only 1 to 3 divisions under the culture conditions used here is small and of similar magnitude for both tissues (3.5% to 4.0%). However, the proportion of initial CD34+cells able to complete >6 divisions was significantly higher for fetal liver cells than for cord blood cells (P < .05).
Representative FACS profiles of CFSE-stained cord blood cells before (A) and after (B and C) culture for 5 days in the presence of IL-3, IL-6, SCF, G-CSF, and FL. The initial sort gate used to isolate the cells placed in culture is indicated in (A). The arrows in (B) and (C) indicate the persisting undivided cells identified by comparison to a control aliquot of the cells cultured under the same conditions but in the presence of colcemid to suppress cell division. The profile shown in (C) was derived by gating on the highly fluorescent (CFSE+) cells to allow a better discrimination of the undivided population.
Representative FACS profiles of CFSE-stained cord blood cells before (A) and after (B and C) culture for 5 days in the presence of IL-3, IL-6, SCF, G-CSF, and FL. The initial sort gate used to isolate the cells placed in culture is indicated in (A). The arrows in (B) and (C) indicate the persisting undivided cells identified by comparison to a control aliquot of the cells cultured under the same conditions but in the presence of colcemid to suppress cell division. The profile shown in (C) was derived by gating on the highly fluorescent (CFSE+) cells to allow a better discrimination of the undivided population.
Distribution of total cells produced (A) and total CD34+ cells (B) produced by fetal liver (FL, □) and cord blood cells (CB, ▪) according to their proliferation history (0 to >6 divisions) during 5 days of suspension culture in the presence of IL-3, IL-6, SF, G-CSF, and FL. Values shown are the mean ± SEM from 5 (FL) and 6 (CB) experiments. Note change of scale on the ordinate axis: the lefthand side indicates the scale for cells that underwent ≤3 divisions, the righthand side indicates the scale for cells that underwent ≥4 divisions.
Distribution of total cells produced (A) and total CD34+ cells (B) produced by fetal liver (FL, □) and cord blood cells (CB, ▪) according to their proliferation history (0 to >6 divisions) during 5 days of suspension culture in the presence of IL-3, IL-6, SF, G-CSF, and FL. Values shown are the mean ± SEM from 5 (FL) and 6 (CB) experiments. Note change of scale on the ordinate axis: the lefthand side indicates the scale for cells that underwent ≤3 divisions, the righthand side indicates the scale for cells that underwent ≥4 divisions.
Minimal percentages of initial CD34+ fetal liver (FL, □) and cord blood cells (CB, ▪) with the ability to divide from 0 to 6 times in short-term cultures containing 5 growth factors (same experiments as shown in Fig 2). A similar calculation could not be made for cells that underwent more than 6 divisions; however, by subtraction this can be estimated as 78% ± 3% for fetal liver cells and 45% ± 5% for cord blood cells. Values shown are the mean ± SEM from 5 (FL) and 6 (CB) experiments.
Minimal percentages of initial CD34+ fetal liver (FL, □) and cord blood cells (CB, ▪) with the ability to divide from 0 to 6 times in short-term cultures containing 5 growth factors (same experiments as shown in Fig 2). A similar calculation could not be made for cells that underwent more than 6 divisions; however, by subtraction this can be estimated as 78% ± 3% for fetal liver cells and 45% ± 5% for cord blood cells. Values shown are the mean ± SEM from 5 (FL) and 6 (CB) experiments.
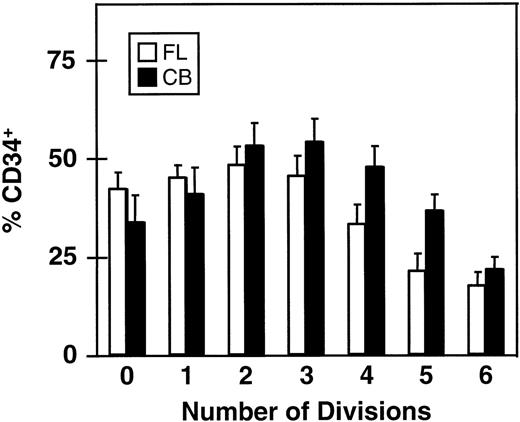
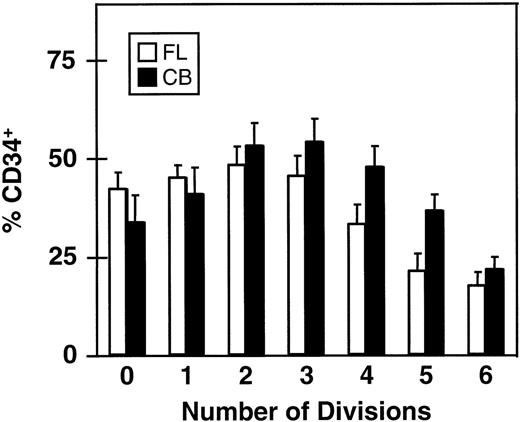
The rate of loss of CD34 expression as function of the number of divisions completed within 5 days is shown in Fig 4. Here it can be seen that greater than 15% of all cells in the clones (and single undivided cells) present at the end of the 5-day culture were still CD34+. This proportion was, as expected, lowest in the largest clones (>64 cells) and highest (≈50%) in the smaller clones (2 to 8 cells/clone), again assuming that asymmetric divisions or cell loss are not significant during the period studied.
Proportion of CD34+ cells in clones of different sizes derived from CD34+ fetal liver (FL, □) and cord blood cells (CB, ▪) after 5 days in culture with 5 growth factors (same experiments as shown in Figs 2 and 3). Values shown are the mean ± SEM.
Most LTC-IC in short-term cultures of CD34+ cord blood and fetal liver cells are confined to the smallest clones.
Because of previous reports that primitive human hematopoietic progenitors detected as LTC-IC are enriched in the PKH2bright (undivided) cell fraction of CD34+cells that have been stimulated for 7 days with IL-3, IL-6, and SF,21 22 we sorted the cultured cells in the above experiments according to the number of cell cycles they had completed (on the basis of their CFSE fluorescence) and then assayed each fraction for its LTC-IC (and CRU, see below) content. In a first set of experiments, the 2 fractions isolated consisted of 1 fraction of undivided cells and a second fraction containing all of the rest (with a gap ≈25 channels wide to minimize cross-contamination of the 2 fractions). As shown in Table 2, all or most (>88%) of the LTC-IC were consistently found in the fraction that had undergone at least 1 division in cultures initiated with either CD34+ cord blood or fetal liver cells. In the second series of experiments, the cells harvested from the 5-day cultures were separated into those that had undergone ≤2 divisions, and those that had undergone ≥3 divisions, again with a gap of ≈25 channels to minimize cross-contamination. In these latter experiments, most of the LTC-IC in the cord blood cultures were in the populations that had undergone ≤2 cell divisions, but a somewhat greater proportion of the fetal-liver–derived LTC-IC were found among later generations of progeny that had divided more rapidly (Table 3).
Distribution of human CRU among different generations of growth-factor–stimulated CD34+ cord blood and fetal liver cells.
In the above 2 series of experiments, only a small proportion (<20%) of all the cultured cells were used to perform cell counts, phenotyping studies, and CFC and LTC-IC assays. The remaining 80% to 93% were used to examine the CRU content by injecting the cells from each of the 2 fractions collected into sublethally irradiated NOD/SCID mice (the approximate proportion of the culture injected per mouse for each group can be derived directly from the data shown in Fig 3). As shown in Table 4, in 4 of 4 experiments the majority of human cells with any kind of NOD/SCID repopulating activity had completed at least 1 cell division within 5 days of culture. This included all of those with lympho-myeloid potential. Moreover, in 5 of 5 experiments a greater proportion of the CRU was found in the populations that had undergone at least 3 cell cycles (Table 5). Limiting dilution analysis of the data pooled from all like experiments was used to determine the frequencies of CRU in the subpopulations of cord blood cells isolated according to their pretransplant cycling history in culture. The average frequency of CRU in the population of cells that had divided less than 3 times was 1 per 14,000 cells (range defined by ± SEM, 1 per 9,600 cells to 1 per 20,700 cells). The corresponding value for cells that had divided 3 or more times was 1 CRU per 1,300,000 cells (range, 1 per 900,000 to 1 per 1,800,000 cells), with 63% the CRU detected in the cultured cells being found in this latter group. The calculated change in total CRU numbers in these experiments was an ≈2.4 fold increase. This compares favorably to our previous results with cord blood cells using similar culture conditions7and, again, argues against any likelihood of a toxic effect of the CFSE staining and sorting procedure.
DISCUSSION
In this study we show that the majority of transplantable hematopoietic stem cells present in 5-day cultures of human fetal liver or cord blood CD34+ cells stimulated with IL-3, IL-6, G-CSF, SF, and FL have executed at least 3 full cell cycles. These results demonstrate unequivocally that human hematopoietic stem cell activity assessed by their ability to regenerate lymphoid and myeloid progeny after 6 to 8 weeks in irradiated NOD/SCID mice7 23 is not lost inevitably or irreversibly when cells are activated mitogenically with soluble growth factors. In addition, our findings show that most, if not all, of the stem cells present in CD34+ populations of human cord blood and fetal liver can be stimulated to rapidly proliferate in vitro by this 5–growth factor combination.
At first glance, these findings appear to contradict those of others who have reported rapid losses of human14,22 and murine24 stem cell activity in the first hours after exposure of such cells to a similar combination of growth factors, and who have detected retained stem cell activity among those cells thought to have remained quiescent in 7-day cultures (because they did not show a major loss of PKH2 fluorescence). From such observations, it was concluded that retention of functions essential to the integrity of stem cell activity were lost rapidly upon cell activation and could be preserved by cells that were able to resist activation over prolonged periods of time in vitro. A similar model has recently been proposed based on measurements of CD34+CD38− cell outputs by sequentially replated single fetal liver cells.25 On the other hand, we previously showed that the magnitude of LTC-IC amplification within individual clones generated over a 10-day period in culture by adult human marrow CD34+CD38− cells is clone size–independent.26 Also, time-course studies of single-cell cultures of CD34+CD38− adult human marrow and cord blood cells have shown that greater than 95% of those able to respond to the combination of growth factors studied here will complete a first division within 5 to 8 days, respectively.27 Similarly, we and others have shown that these conditions can also support the retroviral infection of up to 30% of the human fetal liver or cord blood stem cells (CRU) present at the end of a 5- to 6-day infection protocol.28-30 Taken together, these findings argue strongly against the concept of a significant persisting quiescent population of transplantable stem cells in cultures of human cord blood and fetal liver cells stimulated by FL plus SF and IL-3, IL-6, and G-CSF. However, they do not rule out the possibility that such cells may exist at low frequency. One possible explanation for the apparent discrepancy between this conclusion and the results reported by Traycoff et al22 may lie in the different methodologies used to separate divided and undivided cells. It is possible that the lower resolution of PKH2 staining to discriminate cell division history allows some cells that divide up to 4 times to be misclassified as undivided, which could be sufficient to reconcile the present and previously published findings. In addition, recent evidence of cell-cycle–associated variations in engraftment potential has been presented.13 Such variations, if confirmed, would imply that the homing and differentiation/self-renewal properties of stem cells can be independently regulated and, hence, may be separately manipulated. Additional support for this hypothesis is provided by evidence of an involvement of α4β1 integrins31-33 and the chemokine receptor CXCR415 in stem cell homing, but not in the intrinsic ability of stem cells to proliferate and differentiate per se.33 34 The implication of such a mechanism for the findings reported in this study is that the CRU activity measured would have underestimated the true stem cell content of the various populations assessed because only those in G0 or G1 may have been detectable.
The present findings raise a number of other issues of interest to understanding the control of human stem cell self-renewal. Recently, much attention has been raised concerning the long-term sustainability of hematopoiesis by CD34+ cells.35-38 In the present studies, all cultures were initiated with highly purified CD34+ FACS-sorted cells. Hence the likelihood that the results obtained could be attributed to contaminating CD34− cells seems low. Moreover, the culture conditions used in this study were similar to conditions that have not been found to support the generation or maintenance of human hematopoietic repopulating activity by activated CD34− cells.38 Thus, it can be concluded that few, if any, of the stem cell progeny we have found to be the result of divisions in vitro originated from contaminating CD34− (lin−) cells. However, further studies will be required to investigate whether the in vivo self-regenerative activity39 of human stem cells amplified in vitro has also been retained, as has been shown in the murine system.11 It is also important to note that the yield of LTC-IC (and CRU) is lower than that predicted by the number of divisions they have undergone. Part of this decreased yield is likely due to loss of LTC-IC (and/or CRU) activity during asymmetric cell divisions in which one of the progeny differentiates. In addition, as noted above, CRU enumeration may be subject to cell-cycle changes leading to underestimates of stem cell numbers in proliferating populations.
Another interesting point is the discrepant rate of loss we observed in LTC-IC and in vivo lympho-myeloid repopulating activities. Given the evidence that some cells detectable as LTC-IC may represent “later” cell types than those identified as CRU,40the finding that repopulating activity persisted through more cell generations than LTC-IC activity (or was selectively associated with more rapidly cycling cells) was unexpected. However, other examples of a dissociation in LTC-IC and CRU function in murine cells have been reported,41,42 indicative of differences in the molecular mechanisms required for primitive cells to be detected in these 2 assays. It is also possible that the efficiencies of LTC-IC and CRU detection may be differentially affected by the culture conditions to which they were exposed here (eg, by changes in their cycling status, as discussed above). If cells with these differences were also to be differently distributed between progeny that had undergone different numbers of cell divisions, this would impact on the assumed numerical differences in CRU and LTC-IC ascribed to their cell-cycle history. Therefore, a next step will be to examine these issues by other experimental strategies.43
For clinical gene-therapy applications using retroviral vectors, a culture system that supports stem cell self-renewal divisions at practically useful frequencies is essential. At the same time, completion of a single division should be sufficient if access of the virus into the cell is not limiting. At least for human cord blood targets, our findings now show that prolonging the overall exposure of these cells to FL, SF, IL-3, IL-6, and G-CSF for more than 5 days is not advantageous. Improved yields of retrovirally transduced hematopoietic stem cells are, therefore, likely to benefit more from the pursuit of strategies for manipulating homing activity postinfection. Studies to investigate such possibilities are now underway.
ACKNOWLEDGMENT
The authors thank Maya Sinclaire and Jessica Maltman for assistance with the animal work, Gayle Thornbury, Giovanna Cameron, and Rick Zapf for assistance in cell sorting, the staff of Stem Cell Assay Service for initial hematopoietic cell processing, and Caroline Lonsdale and Tara Palmater for manuscript preparation. The authors also thank Cangene, Immunex, Novartis, StemCell, and Dr Peter Lansdorp (Terry Fox Laboratory) for generous gifts of reagents.
Supported by the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run, the National Institutes of Health (POI-HL55435), and Novartis (Canada), and a grant from the Dr. Mildred Scheel Stiftung für Krebsforschung, Bonn, Germany, to H.G. C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to C.J. Eaves, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, British Columbia, Canada V5Z 1L3; e-mail:connie@terryfox.ubc.ca.