CD40 antigen is a costimulatory molecule highly expressed on dendritic cells (DC) and activated B cells, which induces T-cell proliferation through the binding with CD40L receptor. In this study, we evaluated CD40 expression on normal CD34+blood cells and functionally characterized CD34+CD40+ and CD34+CD40− cell subsets. CD40, CD80, and CD86 antigens were constitutively expressed on 3.2% ± 4.5%, 0%, and 1.8% ± 1.2% CD34+ blood cells, respectively. However, after 24 hours in liquid culture with medium alone, or with tumor-necrosis-factor- (TNF-), or with allogeneic mononuclear cells 10.8% ± 3.8%, 75.3% ± 15.0% and 53.7% ± 17.0% CD34+ blood cells, respectively, became CD40+. After incubation for 24 hours with TNF- CD34+CD40+ blood cells expressed only myeloid markers and contained less than 5% CD86+ and CD80+ cells. Also, a 24-hour priming with TNF- or ligation of CD40 significantly increased the CD34+ blood cells alloantigen presenting function. Finally, purified CD34+CD40+ blood cells stimulated an alloreactive T-cell response in MLC, were enriched in granulocytic, monocytic, and dendritic precursors, and generated high numbers of DC in 11-14 d liquid cultures with GM-CSF, SCF, TNF- and FLT-3L. In contrast, CD34+CD40− cells were poorly immunogenic, contained committed granulocytic and erythroid precursors and early progenitors, and differentiated poorly toward the DC lineage. In conclusion, a short incubation with TNF- allows the selection of CD40+ blood progenitors, which may be a useful source of DC precursors for antitumor vaccine studies, and also a CD34+CD40− blood cell fraction that could be exploited in innovative strategies of allogeneic transplantation across HLA barriers.
CD40 ANTIGEN belongs to the tumor necrosis factor-receptor (TNFR) family1 and has been previously found on many cell types, including endothelial and epithelial cells and professional antigen presenting cells (APC).2 These latters, in fact, can induce immune responses by delivering to T lymphocytes both a first signal through the HLA:T-cell receptor binding and a second signal through costimulatory molecules such as B7-1 (CD80), B7-2 (CD86), and/or CD40, whereas the lack of this signal may induce T-cell unresponsiveness.3-6Recently, it has also been described that the CD40:CD40 ligand (CD40L) interaction mediates T-cell help for cytotoxic T lymphocytes.7,8 Furthermore, triggering of CD40 can induce maturation of B-cell precursors,9,10 as well as of CD34+ cord blood dendritic cell progenitors11and upregulates the expression of CD80 and CD86 in normal and neoplastic B cells, and in dendritic cells (DC), thus increasing their APC activity.12-16
In a previous report we showed the alloantigen-presenting capacity of a subset of normal human hematopoietic CD34+ marrow cells constitutively expressing CD18 and CD86,17 that were recently shown to be strictly committed to the dendritic lineage.18 Also, we showed the induction of CD80 and CD86 on a subset of normal granulocyte colony-stimulating factor (G-CSF)-mobilized CD34+ blood cells upon interaction with T cells.19 However, it is not known whether CD40 is expressed on a subset of human CD34+ blood cells, and if it may identify lineage-specific committed progenitors. In this study we show that although CD40 is constitutively expressed on less than 4% CD34+ blood cells, it can be rapidly induced on the majority of these cells by allogeneic mononuclear cells or by TNF-α. CD34+CD40+ blood cells stimulate allogeneic T cells potently and include both dendritic and other myeloid precursors. On the contrary, CD34+ blood cells that fail to upregulate CD40 after TNF-α stimulation are enriched in early progenitors and are not capable of inducing efficient allogeneic T-cell responses in vitro.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs).
Human MoAbs used in this study were the following: CD34 (HPCA-2) fluorescein isthyocianate (FITC), phycoerythrin (PE) or peridin chlorophyll protein (PerCP)-conjugated CD80 (B7-1), PE, CD3 PE, CD13 PE, CD33 PE, CD14 PE, and CD19 PE from Becton Dickinson (San José, CA); CD1a PE, CD40 FITC, and CD86 (B7-2) FITC or PE from PharMingen (San Diego, CA). Appropriate isotype controls were from Becton Dickinson and PharMingen.
Cell separation.
Blood and apheresis samples were obtained with informed consent from adult normal healthy donors. Peripheral blood stem cell donors were treated with 10 μg/kg/d subcutaneously of glycosilated G-CSF (Lenograstin; Rhone-Poulenc Rorer, Milan, Italy) for 5 to 6 days and stem cell collection began on day 5. All samples were separated by centrifugation over Fycoll/Hypaque (Nycomed Pharma, Oslo, Norway) gradients to obtain mononuclear cells (MNC). Light density cells were washed twice in phosphate buffer-saline with 1% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), and CD34+ cells were highly purified by MiniMACS high gradient magnetic separation column (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer instructions.20 To assess the purity of the CD34 separation, aliquots of the CD34+ cells were restained with IgG1 HPCA-2 FITC MoAb directed toward an epitope of the CD34 antigen different from the one targeted by the QBend 10 MoAb, used with the MiniMACS system. This procedure obtained a population of 98% CD34+ cells, with an overall yield greater than 90%. In selected experiments the CD34+ cells obtained by magnetic separation were incubated with 10 ng/mL TNF-α (Innogenetics, Zwijndrecht, Belgium) for 24 hours, and then stained with anti-CD34 PE and anti-CD40 FITC MoAbs for 30 minutes at 4°, washed twice, resuspended in 10% fetal calf serum (FCS)-enriched RPMI-1640 (Sera Lab, Crawley Down, Sussex, UK). Purified fractions of CD34+CD40+ and CD34+CD40− cells were obtained by fluorescence activated cell sorting on a FACS Vantage (Becton Dickinson). The sorting gates for CD34+CD40+and CD34+CD40− were set to obtain populations that expressed the 2 extreme levels of the CD40 molecule and did not overlap on reanalysis. Aliquots of CD34+CD40+and CD34+CD40− sorted fractions were reanalyzed on a FACS Calibur instrument (Becton Dickinson) to verify their purity.
Kinetic expression of CD40 on CD34+ blood cells.
The expression of CD40 on purified CD34+ blood cells was evaluated by flow cytometry. After isolation, 2.5 × 104CD34+ cells per well were plated in standard mixed leukocyte culture (MLC) with allogeneic MNC at 1:2 ratio, or TNF-α (10 ng/mL), or granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sandoz, Basel, Switzerland) (50 ng/mL), or with medium alone. Twenty-four hours after incubation the cells were collected in 5-mL tubes, washed, stained with anti-CD34 PerCP, CD40 FITC, and PE-conjugated MoAbs, or specific isotype controls, and then evaluated by cytofluorimetric analysis on a FACS Calibur instrument (Becton Dickinson).
Primary MLC.
Isolated CD34+ cells preincubated with TNF-α, GM-CSF, or medium for 24 hours, or in selected experiments, purified CD34+CD40+ and CD34+CD40− cells, were irradiated (3,000 cGy) and tested as stimulators in primary MLC. Autologous and third-party blood mononuclear control cells were also added where indicated. Cells were resuspended in medium containing RPMI-1640, 25 mmol/L HEPES, 1 U/mL penicillin, 1 g/mL streptomicin and 10% AB human serum that had been inactivated at 56° for 30 minutes. 5 × 104responder MNC, or nylon wool-purified T cells, were mixed with stimulators in round-bottomed 96-well plates for 6 days at 37°C in a 5% CO2-humified atmosphere. Cells were pulsed with 1 μCi/well 3H-thymidine for 18 hours before harvest on day 6. Stimulation index (SI) were calculated for each individual experiment as: SI = cpm (T-cell responders + stimulators)/cpm (T-cell responders). In selected experiments, a purified anti-TNFR2 (p80) MoAb (Genzyme, Cambridge, MA) (1 mg/mL), or an anti-CD40 MoAb (B-B20) (Oxford Biomarketing Ltd, Oxford, UK) (10 μL/well) that stimulates CD40 receptor mimicking CD40L molecule21 and is not mitogenic on T cells (data not shown), or specific isotype controls, were added in the MLC.
Colony-forming cells (CFU-C) and long-term culture-initiating cells (LTC-IC) assays.
Purified CD34+CD40+ and CD34+CD40− blood cell subsets were evaluated for CFU-C in semisolid medium.22 To measure the optimum clonogenic efficiency, 10% (vol/vol) of a selected lot of phytohemagglutinin-lymphocyte-conditioned medium was added and the final concentration of methylcellulose was 1.1%. Dendritic cell CFU (CFU-DC) were cultured as described,23 and TNF-α (10 ng/mL), GM-CSF (50 ng/mL), stem cell factor (SCF) (Amgen, Thousand Oaks, CA) (20 ng/mL), and FLT-3L (Immunex, Seattle, WA) (50 ng/mL) were used as stimulators. Granulocyte CFU (CFU-G), macrophage CFU (CFU-M), and erythroid progenitors (burst-forming unit-erythroid, BFU-E) were scored after 14 days of incubation at 37°C in a fully humidified 5% CO2atmosphere. CFU-DC were recorded as aggregates greater than 50 cells as previously described.23 Also, purified CD34+CD40+ and CD34+CD40− cell subsets were plated in long-term cultures onto irradiated murine stromal cells (M2-10B4) genetically engineered to produce G-CSF and interleukin-324with weekly half-medium change. After 5 weeks in culture, the cells were then evaluated for their secondary CFU-C activity, and the number of LTC-IC was calculated as earlier reported.22
Expansion of DC in liquid culture.
Liquid culture of purified CD34+CD40+ and CD34+CD40− blood cells was initiated with Iscove’s modified Dulbecco’s medium-20% FCS and antibiotics at an initial density of 4 × 104 cells/mL. All cultures were maintained for 14 days in the presence of TNF-α, GM-CSF, SCF, and FLT-3L. At weekly intervals, half of the medium was replaced by fresh medium and growth factors, and the generation of DC was assessed by phase-contrast microscopy and immunophenotyping on days 11 and 14.23
Statistical analysis.
For statistical analysis, the t-test was used.
RESULTS
Rapid induction of CD40 on CD34+ blood cells.
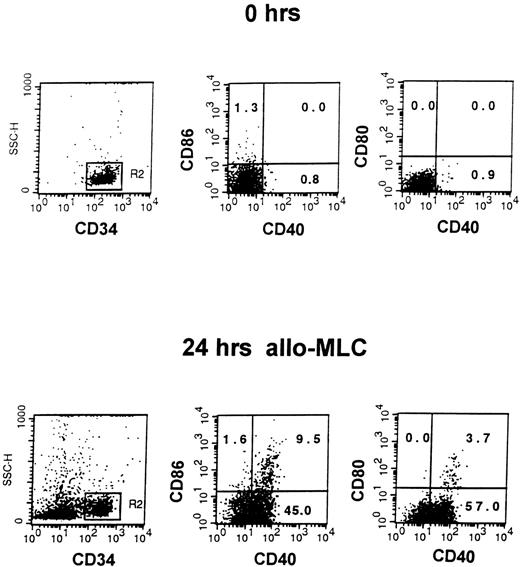
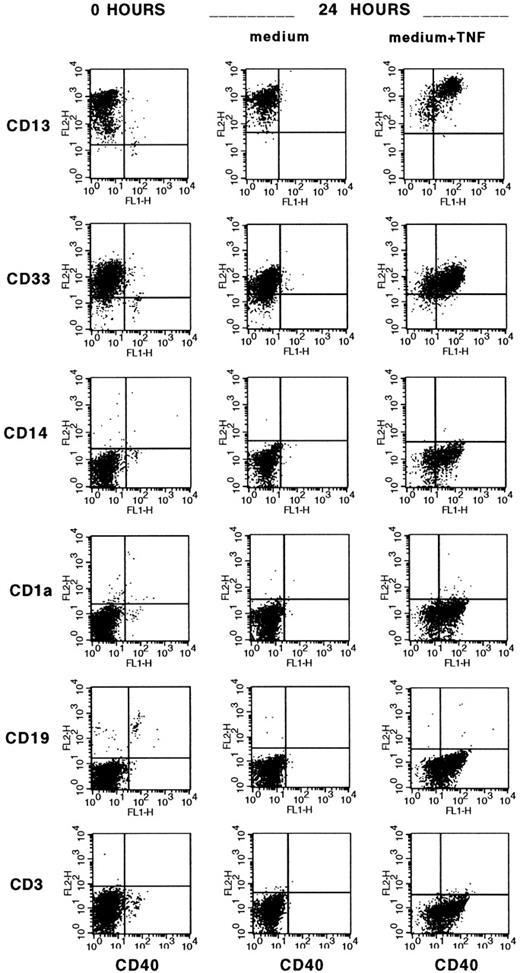
A small subset of CD34+ blood cells upregulate B7-1 (CD80) and B7-2 (CD86) costimulatory molecules 24 hours after CD4+or CD8+ T-cell contact and present alloantigen efficiently.19 In the first set of experiments, we evaluated the expression of CD40 costimulatory molecule on CD34+ blood cells before and after 24 hours of culture with medium alone, or with allogeneic MNC (alloMNC), TNF-α, or alloMNC + TNF-α. Immediately after isolation, on average 3.2% ± 4.5% CD34+ blood cells were positive for CD40 (n = 9 experiments). The proportion of CD34+CD40+blood cells increased to on average 10.8% ± 13.8% after 24 hours in medium alone (n = 5), 53.7% ± 17.0% after 24 hours in alloMLC (n = 5), and to 75.3% ± 15.0% after 24 hours in culture with TNF-α (n = 5), whereas the addition of TNF-α and responder cells did not further increase the number of CD40+ progenitors (71.3% ± 13.0%, n = 3). Moreover, upregulation of CD80 and CD86 on contact with alloMNC was detected in CD34+CD40+ blood cells, as shown in a representative example in Fig 1. After 24 hours of priming with TNF-α, CD34+CD40+ blood cells were shown to express CD13 and CD33 myeloid antigens by 3-color stainings, whereas lymphoid (CD3 and CD19), monocytic (CD14), and dendritic (CD1a) markers were negative, as shown in Fig2. CD80 and CD86 were expressed on 3.8% ± 2.3% and 4.3% ± 1.5% (n = 5), respectively, of the cells.
Induction of CD40, CD80, and CD86 on CD34+blood cells in alloMLC. Three-color staining with CD34PerCP, CD40FITC, and CD80PE or CD86PE MoAbs was performed on CD34+ blood cells immediately after separation (top row, 0 hrs) and after 1 day of culture with allogeneic mononuclear cells at 1:2 ratio (bottom row, 24 hrs alloMLC). Cells that fluoresced brightly for anti-CD34 MoAb were gated for analysis. The proportion of CD34+ cells expressing CD40 and/or CD80 or CD86 is shown in the appropriate quadrant of each figure.
Induction of CD40, CD80, and CD86 on CD34+blood cells in alloMLC. Three-color staining with CD34PerCP, CD40FITC, and CD80PE or CD86PE MoAbs was performed on CD34+ blood cells immediately after separation (top row, 0 hrs) and after 1 day of culture with allogeneic mononuclear cells at 1:2 ratio (bottom row, 24 hrs alloMLC). Cells that fluoresced brightly for anti-CD34 MoAb were gated for analysis. The proportion of CD34+ cells expressing CD40 and/or CD80 or CD86 is shown in the appropriate quadrant of each figure.
TNF- induction of CD40 on myeloid CD34+blood cells. Three-color staining with CD34PerCP, CD40FITC, and PE-conjugated MoAbs specific for myeloid (CD13, CD33), monocytic (CD14), dendritic (CD1a), and lymphoid (CD19, CD3) lineages was performed on freshly isolated CD34+ blood cells and after 24 hours of culture with medium alone or TNF- (10 ng/mL). Cells that fluoresced brightly for anti-CD34 MoAb were gated for analysis.
TNF- induction of CD40 on myeloid CD34+blood cells. Three-color staining with CD34PerCP, CD40FITC, and PE-conjugated MoAbs specific for myeloid (CD13, CD33), monocytic (CD14), dendritic (CD1a), and lymphoid (CD19, CD3) lineages was performed on freshly isolated CD34+ blood cells and after 24 hours of culture with medium alone or TNF- (10 ng/mL). Cells that fluoresced brightly for anti-CD34 MoAb were gated for analysis.
Enhanced CD34+ cell alloantigen-presenting function after priming with TNF-α.
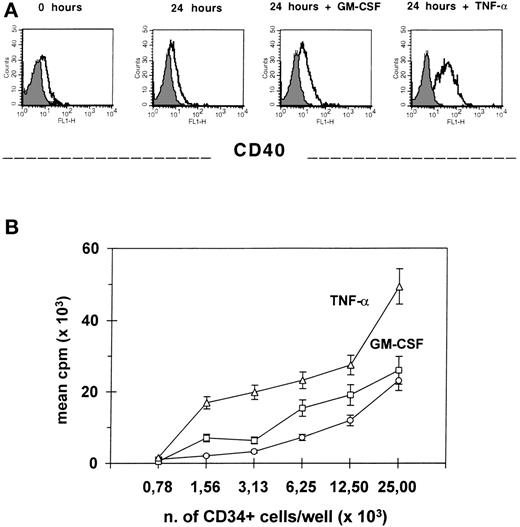
We previously showed the capacity of the CD34+ cell to stimulate alloT cells in primary MLC.17 19 Because TNF-α rapidly induces CD40 expression on the majority of CD34+blood cells and CD80 and CD86 on less than 5% of progenitors, we addressed the question whether it also modifies the APC function of these cells. In 3 separate experiments, purified CD34+ blood cells that were incubated for 24 hours with TNF-α, washed, irradiated (3,000 cGy), and mixed in primary MLC with alloMNC responders at 1:2 ratio induced a significantly higher T-cell proliferation (P = .01), as compared with CD34+ cells primed with either GM-CSF or medium alone. In fact, GM-CSF preincubation did not significantly modify CD34+ blood cells immunogenicity. In Fig3, one representative example is shown where different expression of CD40 on CD34+ blood cells correlates with different APC activity. Also, the presence of an anti–TNF-R2 MoAb in the MLC significantly reduced (P = .02; n = 3 experiments) the alloresponse to CD34+ blood cells as compared with MLC with a specific isotype control MoAb (data not shown).
Preincubation with TNF- increases CD34+blood cell alloantigen-presenting function. Freshly isolated CD34+ blood cells were either stained with CD40 MoAb or incubated for 24 hours with medium alone, TNF-, or GM-CSF, then washed and evaluated both for CD40 expression by flow cytometry (A) and, after irradiation, for their capacity of stimulating allogeneic MNC in primary MLC with 5 × 104 responders at different stimulator/responder ratios (B). Results are the mean cpm ± SEM of triplicate cultures. At 1:2 stimulator/responder ratio, the differences of the alloresponse to CD34+ blood cells preincubated with TNF- versus GM-CSF and medium, are both significant (P = .01).
Preincubation with TNF- increases CD34+blood cell alloantigen-presenting function. Freshly isolated CD34+ blood cells were either stained with CD40 MoAb or incubated for 24 hours with medium alone, TNF-, or GM-CSF, then washed and evaluated both for CD40 expression by flow cytometry (A) and, after irradiation, for their capacity of stimulating allogeneic MNC in primary MLC with 5 × 104 responders at different stimulator/responder ratios (B). Results are the mean cpm ± SEM of triplicate cultures. At 1:2 stimulator/responder ratio, the differences of the alloresponse to CD34+ blood cells preincubated with TNF- versus GM-CSF and medium, are both significant (P = .01).
Induction of CD86 on CD34+ cells via CD40.
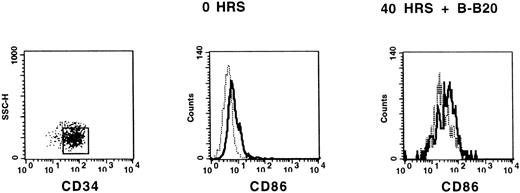
Ligation of CD40 receptor on professional APC results in potent activation with upregulation of accessory molecules and enhancement of their antigen-presenting function. To test whether a subset of CD34+ cells can express B7 costimulatory molecules on triggering with CD40, we evaluated the surface expression of CD86 and CD80 by flow cytometry on 3 × 104 purified CD34+ blood cells immediately after isolation and after incubation with B-B20 MoAb for 40 hours. CD86 was variably upregulated on CD34+ cells as shown in a representative example in Fig4, whereas CD80 was not induced by CD40 (data not shown). Importantly, the addition of the B-B20 MoAb to irradiated CD34+ blood cells 30 minutes before starting a primary MLC with nylon-wool-purified T cells at 1:2 ratio resulted in a significant increase (P = .02) of the alloantigen-presenting function of these cells (n = 4 experiments; Fig5). Thus, it is conceivable that ligation of CD40 receptor and release of TNF-α may both contribute to the activation of CD34+ APC by T cells.
Triggering of CD40 increases the expression of CD86 on CD34+ blood cells. Immunomagnetically separated CD34+ blood cells were stained with HPCA-2 (anti-CD34)–PE and anti–CD86-FITC MoAbs, or isotype control, immediately after isolation (0 hrs) and after 40 hours in liquid culture with B-B20 (anti-CD40) MoAb. CD34+ cells were gated for analysis and histograms represent staining with FITC-labeled isotype control (dashed line) and staining with CD86 FITC-labeled MoAb (solid line).
Triggering of CD40 increases the expression of CD86 on CD34+ blood cells. Immunomagnetically separated CD34+ blood cells were stained with HPCA-2 (anti-CD34)–PE and anti–CD86-FITC MoAbs, or isotype control, immediately after isolation (0 hrs) and after 40 hours in liquid culture with B-B20 (anti-CD40) MoAb. CD34+ cells were gated for analysis and histograms represent staining with FITC-labeled isotype control (dashed line) and staining with CD86 FITC-labeled MoAb (solid line).
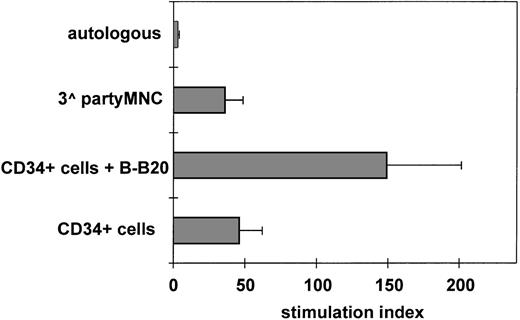
Triggering of CD40 enhances CD34+ blood cell alloantigen-presenting capacity. Purified CD34+blood cells were irradiated (3,000 cGy) and mixed with 5 × 104 allogeneic nylon-wool-purified T cells at 1:2 ratio with or without an anti-CD40 MoAb (B-B20) that is not mitogenic on T cells (data not shown), or an isotype-specific irrelevant control, and cultured for 6 days in MLC. No difference was observed in the response to CD34+ blood cells incubated without antibody or with isotype control. Autologous and third-party MNC were used as negative and positive controls. SI were calculated for each experiment. Results are represented as the mean SI ± SEM of 4 separate experiments. B-B20 MoAb significantly increased CD34+ blood cell alloantigen-presenting activity (P = .02).
Triggering of CD40 enhances CD34+ blood cell alloantigen-presenting capacity. Purified CD34+blood cells were irradiated (3,000 cGy) and mixed with 5 × 104 allogeneic nylon-wool-purified T cells at 1:2 ratio with or without an anti-CD40 MoAb (B-B20) that is not mitogenic on T cells (data not shown), or an isotype-specific irrelevant control, and cultured for 6 days in MLC. No difference was observed in the response to CD34+ blood cells incubated without antibody or with isotype control. Autologous and third-party MNC were used as negative and positive controls. SI were calculated for each experiment. Results are represented as the mean SI ± SEM of 4 separate experiments. B-B20 MoAb significantly increased CD34+ blood cell alloantigen-presenting activity (P = .02).
Immunogenic activity of G-CSF mobilized CD34+CD40+ and CD34+CD40− blood cells.
To address whether TNF-α priming may allow the identification of different hematopoietic progenitors, CD34+CD40+and CD34+CD40− cell populations were purified to greater than 98% degree by a 2-step procedure including a high gradient separation of CD34+ cells followed by FACS. Immunomagnetically isolated CD34+ cells were sorted into CD40+ and CD40− fractions using windows that allowed no overlap at reanalysis on a FACS Calibur. After irradiation (3,000 cGy), purified CD34+CD40+ and CD34+CD40− blood cells (2.5 × 104/well) and third-party MNC (5.0 × 104/well) were tested in primary MLC with alloMNC cells (5.0 × 104/well) from HLA-DR incompatible donors. In 3 separate experiments, allostimulating activity was significantly higher among CD34+CD40+ blood cells as opposed to CD34+CD40− cells (P = .01; Fig 6). These data prove that the subset of CD34+ cells capable of presenting alloantigen do upregulate CD40 rapidly after TNF-α priming, whereas CD34+ cells that fail to express CD40 may be either more immature or not committed to APC lineages.
CD34+CD40+ but not CD34+CD40+ blood cells induce T-cell alloresponses in primary MLC. 2 × 104 purified CD34+CD40+ and CD34+CD40− blood cells, or 5 × 104 autologous MNC were irradiated and tested in primary MLC with 5 × 104 allogeneic mononuclear responders. SI were calculated for each experiment. Results are represented as the mean SI ± SEM. CD34+CD40+ blood cells induced a significantly higher alloresponse than CD34+CD40− blood cells (P = .01) (n = 3 experiments).
CD34+CD40+ but not CD34+CD40+ blood cells induce T-cell alloresponses in primary MLC. 2 × 104 purified CD34+CD40+ and CD34+CD40− blood cells, or 5 × 104 autologous MNC were irradiated and tested in primary MLC with 5 × 104 allogeneic mononuclear responders. SI were calculated for each experiment. Results are represented as the mean SI ± SEM. CD34+CD40+ blood cells induced a significantly higher alloresponse than CD34+CD40− blood cells (P = .01) (n = 3 experiments).
CFU-C and LTC-IC in G-CSF mobilized CD34+CD40− and CD34+CD40+ blood cells.
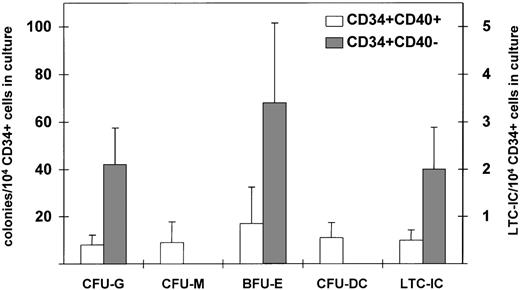
According to previous findings, CD34+ cells with alloantigen-presenting capacity are enriched in committed progenitors, and in particular, CD34+CD86+ marrow cells are specifically committed to the dendritic lineage.18 To address whether induction of CD40 by TNF-α may induce lineage commitment of CD34+ blood progenitors, purified CD34+CD40+ and CD34+CD40− blood cells were evaluated in short- and long-term culture assay. Incubation of CD34+blood cells with TNF-α for 24 hours did not affect the overall clonogenic activity of the progenitors (data not shown). In 3 separate experiments summarized in Fig 7, CD34+CD40+ blood cells contained progenitors committed to granulocytic, monocytic, dendritic, and erythroid lineages, whereas CD34+CD40− blood cells were highly enriched in granulocytic and erythroid precursors but failed to generate monocytic and dendritic colonies in vitro. Moreover, CD34+CD40− blood cells showed a 4-fold higher content of LTC-IC as opposed to CD34+CD40+blood cells. These results show that nonimmunogenic progenitor cells are devoid of APC precursors and contain both committed and early progenitors.
CFU-C and LTC-IC in CD34+CD40+ and CD34+CD40− blood cells. Purified CD34+CD40+ and CD34+CD40− blood cells were tested for their clonogenic activity in semisolid medium, and the results show the mean number ± SEM of colonies/104 cells plated according to the scale on the left side of the figure (n = 3 experiments) and for their content of LTC-IC. The results show the mean number ± SEM of LTC-IC/104 cells plated according to the scale on the right side of the figure (n = 3 experiments). Differences in the number of colony units between CD34+CD40+ and CD34+CD40− blood cells are not statistically significant.
CFU-C and LTC-IC in CD34+CD40+ and CD34+CD40− blood cells. Purified CD34+CD40+ and CD34+CD40− blood cells were tested for their clonogenic activity in semisolid medium, and the results show the mean number ± SEM of colonies/104 cells plated according to the scale on the left side of the figure (n = 3 experiments) and for their content of LTC-IC. The results show the mean number ± SEM of LTC-IC/104 cells plated according to the scale on the right side of the figure (n = 3 experiments). Differences in the number of colony units between CD34+CD40+ and CD34+CD40− blood cells are not statistically significant.
Expansion of DCs from CD34+CD40+blood cells.
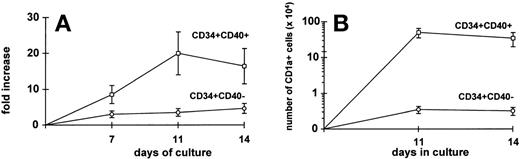
CD34+ blood progenitors represent an optimal source for generating potent DC in vitro.23 Because clonogenic assay suggested that committed dendritic precursors are included in the CD34+CD40+ cell fraction, we evaluated whether growth factors such as GM-CSF, TNF-α, SCF, and FLT-3L could allow in vitro expansion and differentiation into DC of purified CD34+CD40+ and CD34+CD40− blood cells separated after 24 hours of incubation with TNF-α. After 11 to 14 days of culture, CD34+CD40+ blood cells expanded more efficiently than CD34+CD40− blood cells (Fig8A). Also, it is likely that the peak of proliferation of CD34+CD40+ cells on day 11 rather than on day 14 may be because of the fact that these cells are enriched in committed precursors needing a shorter time to mature. Immunophenotypic characterization of DC was performed by staining the cells with CD1a, CD86, CD80, CD40, and HLA-DR MoAbs.23 On average, 20% of the cells generated from CD34+CD40+ and 5% of the cells generated from CD34+CD40− blood cells were CD1a+and HLA-DR++, as well as positive for costimulatory molecules (data not shown). In Fig 8B it is shown that the absolute mean number of CD1a+ DC per 10 × 104 stem cells plated was 2 log higher in cultures started with CD34+CD40+ blood cells than in cultures started with CD34+CD40− blood cells (n = 3 experiments). Also, CD34+CD40+ blood cells derived DC elicited potent allogeneic T-cell responses in MLC (data not shown). These data further show that hematopoietic progenitors committed to the DC lineage do upregulate CD40 after priming with TNF-α and proliferate in response to growth factors.
High expansion of DC from CD34+CD40+ blood cells. Purified CD34+CD40+ and CD34+CD40− blood cells were grown in liquid culture in the presence of GM-CSF, TNF-, SCF, and FLT-3L for 14 days (see Materials and Methods). The total expansion of each cell fraction at 7, 11, and 14 days is shown in the left quadrant (A), while the absolute number of CD1a+ cells derived from each cell subset at 11 and 14 days of culture is shown in the right quadrant (B). Results are represented as the mean ± SEM values of 3 separate experiments. After 11 or 14 days of culture, there is a 2-log difference in absolute number of CD1a+ cells among CD34+CD40+ versus CD34+CD40− blood cells (P = .07).
High expansion of DC from CD34+CD40+ blood cells. Purified CD34+CD40+ and CD34+CD40− blood cells were grown in liquid culture in the presence of GM-CSF, TNF-, SCF, and FLT-3L for 14 days (see Materials and Methods). The total expansion of each cell fraction at 7, 11, and 14 days is shown in the left quadrant (A), while the absolute number of CD1a+ cells derived from each cell subset at 11 and 14 days of culture is shown in the right quadrant (B). Results are represented as the mean ± SEM values of 3 separate experiments. After 11 or 14 days of culture, there is a 2-log difference in absolute number of CD1a+ cells among CD34+CD40+ versus CD34+CD40− blood cells (P = .07).
DISCUSSION
Hematopoietic CD34+CD86+ cells with antigen-presenting capacity have been previously identified in bone marrow and G-CSF–mobilized peripheral blood of healthy donors,17,19 where the constitutive expression of B7-2 (CD86) molecule on CD34+ marrow cells identifies committed DC precursors capable of stimulating T cells potently.18 In this study, we show that another costimulatory molecule, CD40, is rapidly upregulated on the majority of CD34+ blood cells on T-cell contact or TNF-α stimulation, can modulate CD86 expression on CD34+ cells, and identifies progenitors highly enriched in alloantigen-presenting cells. Moreover, CD34+CD40+ blood cells include precursors that are committed to the granulocytic, erythroid, and monocytic/dendritic lineage, whereas CD34+CD40− blood cells are enriched only in granulocytic and erythroid progenitors, contain the majority of LTC-IC, and are not immunogenic.
Recently, we observed the induction of CD80 and CD86 on a subset of CD34+ blood cells upon CD4+, CD8+ T cell, or MNC contact,19 raising the hypothesis that cellular signaling or soluble factors would mediate upregulation of B7 costimulatory molecules on CD34+ cells. In fact, mechanisms such as TNF-α stimulation and CD40 ligation are both involved in the activation pathway of B cells by T lymphocytes,25 and CD40 ligation of monocytes and DC results in high expression of accessory molecules, cytokine production, and a powerful capacity of these cells to present antigens to T cells.15,16 The expression of CD40 had been previously shown in human cord blood and bone marrow CD34+ cells isolated by immune panning and stained after overnight incubation at 37°C in medium supplemented with serum.26 Because we observed that, on average, less than 4% of freshly isolated CD34+ blood cells express CD40 and this proportion may spontaneously increase with high variability after 24 hours of incubation in medium, different data on cord blood and marrow cells might depend on either the timing of the staining after stem cell purification, because of a rapid modulation of CD40 receptor on the CD34+ cell surface, or on the effect of G-CSF treatment that might downmodulate CD40 expression on blood stem cells. However, our data also suggest that a 24-hour culture of CD34+ blood cells with alloMNC, or with TNF-α, always results in a high proportion of CD34+CD40+blood cells expressing myeloid markers and, in a small fraction, CD80 and CD86 molecules. Furthermore, because priming with TNF-α not only modifies the phenotype but also increases CD34+ blood cells capacity to stimulate allogeneic T cells in primary MLC, it is likely that secretion of TNF-α by T cells may play an important role in enhancing CD34+ APC activity. Similarly to what has been previously observed in mature B cells and professional APC,12,15,16 we could obtain the induction of CD86 on a small fraction of CD34+ blood cells and increased stem cell capacity of stimulating T cells by triggering CD40 receptor. Therefore, it is conceivable that both pathways through CD40:CD40L activation of a large fraction of progenitors and through CD86:CD28 binding on a smaller subset of progenitors may account for T-cell alloreactivity to CD34+ cells. In this case we might hypothesize that allogeneic transplantation of purified CD34+ cells may require profound immune suppression of the host to prevent stem cell rejection. Moreover, because a subset of donor CD34+ cells might rapidly upregulate CD40 and B7 costimulatory molecules and contribute to the activation of autologous T cells by presenting host antigens, after CD34+ cell selection, a further T-cell depletion of the graft may be necessary in case of major HLA disparity in the attempt of preventing acute graft-versus-host disease (GVHD), as recently reported.27
Our working hypothesis was that TNF-α induces CD40 mainly on CD34+ blood cells driven to differentiation into APC. To test this hypothesis we incubated CD34+ blood cells with TNF-α for 24 hours and isolated CD34+CD40+and CD34+CD40− blood cell subsets to test their immunogenic and clonogenic activity, as well as their capacity to generate mature DC in liquid culture. As previously suggested by experiments in cord blood,11CD34+CD40+ blood cells are enriched in progenitors committed to the dendritic lineage, but, interestingly, they include also granulocytic, monocytic, and erythroid precursors. These findings may suggest that among the mechanisms used by T cells to sustain normal human hematopoiesis the CD40 ligation on committed progenitors could play a direct role. Similar conclusions, in fact, were drawn also by Funakoshi et al,28 who used a syngeneic bone marrow transplantation mouse model to investigate on the role of a soluble recombinant CD40L, and showed that stimulation of CD40 by its ligand results both in a better immune reconstitution and in an accelerated recovery of neutrophils and platelets in mice that received a transplant.
Finally, CD34+ blood cells that lack CD40 expression even after 24 hours of incubation with TNF-α do not induce a proliferation of HLA mismatched T cells and are enriched in granulocytic and erythroid progenitors, and fail to generate DC in semisolid and liquid cultures. Also, they include the majority of early progenitors identified by in vitro long-term cultures. Because of these functional characteristics, we hypothesize that CD34+CD40− cells could be exploited in allogeneic transplantation where a major HLA disparity occurs. In fact, experimental models showed that the blockade of CD40L and/or CD28 signaling by MoAbs resulted in the abrogation of acute and chronic GVHD, or in the engraftment of incompatible solid organs.29-32 Therefore, because G-CSF mobilization allows the collection of high numbers of CD34+ blood cells from healthy donors, new strategies aimed at overcoming HLA barriers in allogeneic stem cell transplantation by preventing CD40:CD40L and B7:CD28 activation may include the infusion of purified CD34+CD40− blood cells. In this regard, however, preclinical studies in animal models are required to test whether CD34+CD40− contain true “stem cells.” Moreover, because human donor bone marrow cells enhance solid organ allograft,33 it might be hypothesized that infusion of donor purified CD40− stem cells may further facilitate a stable chimerism and T-cell unresponsiveness in the organ recipient, thus prolonging the allograft survival and eventually reducing the time of immunesuppression. In summary, our data suggest that a short incubation of CD34+ blood cells with TNF-α allows clear identification of a large fraction of CD40+committed hematopoietic myeloid progenitors, including DC precursors. Furthermore, it allows the selection of a smaller CD40−progenitor cell fraction, enriched in LTC-IC and in nonimmunogenic committed progenitors, that may be further investigated in new strategies for inducing tolerance after allogeneic transplantation.
Supported by Associazione Italiana per la Ricerca contro il Cancro (AIRC), Milan; and by Consiglio Nazionale Ricerche (CNR), Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked“advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Damiano Rondelli, MD, Institute of Hematology and Medical Oncology, “L. & A. Seràgnoli,” University of Bologna, via Massarenti, 9, 40138 Bologna, Italy; e-mail:drond@med.unibo.it.