Bruton’s tyrosine kinase (Btk) is a critical component in the B-cell antigen receptor (BCR)-coupled signaling pathway. Its deficiency in B cells leads to loss or marked reduction in the BCR-induced calcium signaling. It is known that this BCR-induced calcium signaling depends on the activation of phospholipase Cγ (PLCγ), which is mediated by Btk and another tyrosine kinase Syk and that the SH2 and pleckstrin homology (PH) domains of Btk play important roles in this activation process. Although the importance of the PH domain of Btk has been explained by its role in the membrane targeting of Btk, the functional significance of the SH2 domain in the calcium signaling has remained merely a matter of speculation. In this report, we identify that one of the major Btk-SH2 domain-binding proteins in B cells is BLNK (B-cell linker protein) and present evidences that the interaction of BLNK and the SH2 domain of Btk contributes to the complete tyrosine phosphorylation of PLCγ.
BRUTON’S TYROSINE KINASE (Btk) is a critical cytoplasmic tyrosine kinase in B-lymphocyte development. Mutations in the Btk gene are responsible for human X-linked agammaglobulinemia (XLA), which usually exhibits an almost complete block of B-cell maturation.1-3 Btk is also responsible for murine X-linked immunodeficiency (XID) in which a point mutation4,5 in the pleckstrin homology (PH) domain causes a less severe block of B-cell maturation. Although the molecular framework in which Btk participates in B-cell development is still not fully defined, accumulating data indicate that Btk is a critical component in B-cell antigen receptor (BCR)-coupled calcium signaling pathway. The first evidence came from an experiment with genetic dissection of the Btk gene in DT40 chicken B-lymphoma cells, which led to a complete loss of BCR-coupled inositol-1,4,5-trisphosphate (IP3) production and calcium flux.6 The reduced levels of BCR-coupled IP3 production and calcium flux seen in B cells from XLA patients7,8 and also from XID mice9 seem consistent with this observation and indicate the crucial role of Btk in BCR-coupled calcium signaling.
Btk has 4 distinct domains (PH, SH3, SH2, and catalytic [SH1]) from N to C termini).3 Although ectopic expression of wild-type Btk in Btk-deficient cells could restore the deficient BCR-coupled calcium signaling, Btk harboring mutations in its PH domain or SH2 domain could not restore it, suggesting that the functions of both the PH and SH2 domains as well as Btk kinase activity are required for BCR-coupled calcium signaling.6-8 The function of the PH domain of Btk in calcium signaling has been explained by its ability to bind phosphatidyl inositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3 ),10 a product of phosphatidyl inositol 3 (PI3) kinase. The binding of the PH domain of Btk to PtdIns-3,4,5-P3 seems to enhance the translocation of Btk to the cell membrane and promote the activation of Btk by membrane-anchoring protein kinases.11,12 The activated Btk, in turn, activates phospholipase Cγ (PLCγ), which is another critical component of calcium signaling, by means of tyrosine phosphorylation.6-8 This activation process of PLCγ has been suggested to be concerted with the activity of another tyrosine kinase Syk.6 The activation of PLCγ leads to the production of IP3 followed by the release of internal calcium storage through IP3 receptors, which finally triggers extracellular calcium entry through the calcium release activated channel by an as yet unknown mechanism.13,14 This scheme seems to explain quite well the molecular mechanism of BCR-coupled calcium signaling in which the function of the PH domain and the catalytic activity of Btk are indispensable. However, for the full understanding of the molecular framework involved in this process, several of the molecular connections remain to be investigated. First, although several reports have shown that the function of the Btk-SH2 domain is also indispensable for BCR-coupled calcium signaling,6-8 the significance of this domain has remained a matter of speculation and the binding partner of the SH2 domain of Btk has not been clarified in any reports. Second, recent studies have revealed that the interaction of PLCγ and a newly identified B-cell linker protein (BLNK)15 (alternatively termed SLP-6516) is also indispensable for the BCR-coupled calcium signaling. BLNK was reported to be tyrosinephosphorylated by activated Syk after BCR engagement and to bind to the SH2 domain of PLCγ,15 which leads to the colocalization of Syk and PLCγ, resulting in the activation of PLCγ by Syk. This observation was confirmed by an experiment using the genetic dissection of the BLNK gene in DT40 cells, in which BCR-coupled IP3 production and calcium mobilization were almost completely abolished.17Therefore, the present question is whether some molecular connection does exist between these two (Btk-PLCγ2 and Syk-BLNK-PLCγ2) signaling pathways essential for the BCR-coupled calcium signaling.
To clarify these matters, we attempted to identify ligands for the Btk-SH2 domain. In the present study, we report that one of the major Btk-SH2 domain binding proteins in B cells is BLNK and present evidence that the interaction of Btk and BLNK via the Btk-SH2 domain contributes to the complete tyrosinephosphylation of PLCγ by Btk.
MATERIALS AND METHODS
Cell lines and antibodies.
RAMOS cells, an EBV-negative Burkitt’s lymphoma cell line,18 were obtained from the Health Science Research Resources Bank (HSRRB, Osaka, Japan) and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 500 μmol/L 2-mercaptoethanol. Provided by Dr Takashi Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), 293 T cells19were maintained in Dulbecco’s modified Eagle’s medium with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. DT40 cells (chicken B-lymphoma cell line), which stably expressed T7-epitope tagged human Btk,20 were cultured in RPMI medium supplemented with 10% FCS, 1% chicken serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 500 μmol/L 2-mercaptoethanol, and 2 mmol/L glutamine. Anti-Btk monoclonal antibody (MoAb) 43-3B,21 anti-chicken BLNK polyclonal antibody,17 anti-chicken Syk polyclonal antibody,22 and anti-chicken PLCγ2 polyclonal antibody23 were described previously. Antiphosphotyrosine MoAb 4G10, anti-T7 tag MoAb, anti-Flag MoAb (M2), and F(ab′)2 fragment of goat anti-human IgM (μ-chain specific) were purchased from Upstate Biotechnology Inc (Lake Placid, NY), Novagen Inc (Madison, WI), Sigma Chemical Co (St Louis, MO), and Cappel ICN Pharmaceuticals Inc (Aurora, OH), respectively. Anti-chicken IgM MoAb M424 was kindly provided by Dr Max Cooper (University of Alabama, Birmingham, AL).
Expression constructs and mutagenesis.
Human wild-type and WW251LL [SH3-mutated Btk; Tryptophans 251 and 252 in the Btk-SH3 domain were replaced by Leucines] Btk cDNAs inserted into the pEF-BOS mammalian expression vectors were described previously.25 Human R307K [SH2-mutated Btk; Arginine 307 in the Btk-SH2 domain was replaced by Lysine] Btk cDNA was produced by means of a polymerase chain reaction-based site directed mutagenesis system (TaKaRa Co, Shiga, Japan) and inserted into the pEF-BOS vector. Porcine Syk cDNA26 and rat PLCγ227 cDNA in pApuro vector have been described elsewhere. The Flag-epitope tagged BLNK expression vector was generated by in-frame insertions of an oligonucleotide with a Flag sequence and chicken BLNK cDNA17 into pEF-BOS vector.
Purification and identification of GST-Btk SH2 domain binding protein.
The cDNA fragments (corresponding to amino acid residues 276 to 401) of human wild-type (Wild) and SH2-mutated (R307K) Btk were in-frame inserted into PGEX-2T vector (Pharmacia, Uppsala, Sweden). The GST expression vectors were transfected into NM522 cells with a pT-Trx (thioredoxin) expression vector.28 Purification of the GST proteins was performed as described previously.21Immobilization of GST-Btk-SH2 (Wild or R307K) on glutathione-Sepharose 4B beads (Pharmacia) was performed by incubating beads with 20 mmol/L dimethyl pimelimidate dihydrochloride (Nacalai Tesque, Kyoto, Japan) in 200 mmol/L sodium borate (pH 9) for 40 minutes at room temperature, followed by incubation in 200 mmol/L ethanolamine (pH 8) for 2 hours at room temperature. RAMOS cells (1 × 1010) were pelleted at 1,200 rpm for 6 minutes, resuspended in phosphate buffer saline (PBS) at a concentration of 1 × 108 cells/mL and stimulated with 50 μg/mL of F(ab′)2 fragment of goat anti-human IgM for 3 minutes at 37°C. After stimulation, the cells were pelleted and then lysed with the same volume of a lysis buffer [0.2 % NP40, 10 mmol/L HEPES (pH 7), 143 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mmol/L sodium orthovanadate]. The cell lysate was precleared with the GST protein immobilized on glutathione-sepharose 4B beads and then incubated with the GST-Btk-SH2 (Wild) protein immobilized on glutathione-Sepharose 4B beads for 2 hours at 4°C. Beads were washed with the lysis buffer 4 times and eluted with elution buffer 1 [1 % sodium dodecyl sulfate (SDS), 50 mmol/L Tris pH 7.5] for 30 minutes at 100°C. The eluate was then diluted 10-fold with the lysis buffer and incubated with antiphosphotyrosine MoAb 4G10 immobilized on protein A-Sepharose CL4B beads (Pharmacia) for 2 hours at 4°C. Beads were washed with the lysis buffer 4 times and eluted with elution buffer 2 [100 mmol/L phenyl phosphate (Nacalai Tesque), 150 mmol/L NaCl, 10 mmol/L Tris pH 7.5, 1 mmol/L sodium orthovanadate]. The eluate was concentrated by means of Centricon-10 (Millipore Co, Bedford, MA) and then boiled with 5 × SDS sample buffer for 5 minutes. The sample was fractionated by SDS-polyacrylamide gel electrophoresis, electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Problott; Applied Biosystems, Foster City, CA). The membrane was stained with Ponceau S (Nacalai Tesque) in 1% (vol/vol) acetic acid and the bands corresponding to 68-kD and 70-kD proteins (approximately 1 μg of each protein) were excised separately from the membrane, followed by in situ digestions with Achromobacter protease I (a lysylendopeptidase) as described.29 Molecular mass analysis was performed by Matrix-assisted Laser Desorption /Ionization time-of-flight (MALDI-TOF) mass spectrometry with a PerSeptive Biosystem Voyager-DE/RP (PE Biosystems, Foster City, CA).30 Peptide sequencing of the 4 main digested peptide fragments was performed with a Shimadzu PPSQ-2 protein sequencer (Shimadzu, Kyoto, Japan). The proteins were identified by searching a protein sequence database [National Center for Biotechnology Information (NCBI; nr 10.17.98), National Institutes of Health (NIH)].
Transfection.
The DNAs of Btk, Flag-BLNK, Syk, and PLCγ2 expression vectors (total 10 μg) were transfected into 293T cells with Lipofectamine (Life Technologies Inc, Rockville, MD) and the cells were harvested after 48 hours.
Immunoprecipitation.
For the coimmunoprecipitation assay 1 × 108 of DT40 cells (expressing the T7 epitope-tagged human Btk) were resuspended in 1 mL of PBS and preincubated for 15 minutes at 37°C. Cells were then stimulated with 4 μg/mL of the anti-chicken IgM MoAb M4 for 0, 2, 5, and 10 minutes at 37°C. Stimulation was terminated by cell lysis with the ice-cold lysis buffer described above. To detect the coprecipitation of BLNK with T7 epitope-tagged Btk, each cell lysate was incubated with 5 μg of the anti-T7 tag MoAb, followed by conjugation with protein A-Sepharose CL4B beads. The beads were washed with the lysis buffer 4 times and then boiled with 2% SDS sample buffer for 5 minutes. The samples were then electrophoresed and visualized by immunoblotting with the anti-chicken BLNK polyclonal antibody.
Immunoblotting analysis.
Immunoblotting analysis was performed as described previously.31 As primary antibodies, antiphosphotyrosine antibody 4G10 was used at a concentration of 1 μg/mL, anti-Btk MoAb 43-3B at 3 μg/mL. The anti-chicken BLNK polyclonal antibody was used at 1:4,000 dilution, the anti-chicken Syk polyclonal antibody at 1:1,000 dilution and the anti-chicken PLCγ2 polyclonal antibody at 1:2,000 dilution. Immunoreactive proteins were detected by means of the Enhanced Chemiluminescence System (Amersham, Buckinghamshire, UK). In some cases, the intensities of the bands were semi-quantitated by using Scanning Imager (Amersham).
RESULTS
Identification of the Btk-SH2 domain binding protein as BLNK.
Stimulation of BCR on RAMOS cells by crosslinking with anti-μ antibody rapidly induces tyrosine phosphorylation of multiple cellular proteins. To identify the phosphoproteins, which bind to the SH2 domain of Btk, cellular proteins were extracted from stimulated or unstimulated RAMOS cells and incubated with the GST-SH2 (Btk) fusion protein conjugated on glutathione S Sepharose beads. Immunoblotting analysis with the antiphosphotyrosine antibody revealed that doublet phosphoproteins (68 kD and 70 kD) in stimulated RAMOS cells prominently bound to the GST-SH2 (Btk) protein, whereas the binding or tyrosine phosphorylation of these proteins from unstimulated cells was weak (Fig 1A, left lanes). In contrast to the prominent binding of the 68-kD and 70-kD phosphoproteins to the GST-SH2 (Btk) (Wild) protein, no binding of these proteins was detected with a fusion protein of GST and a mutated SH2 [Arginine 307 in Btk was replaced by Lysin; GST-SH2 (R307K)] (Fig 1A, right lanes), indicating that the binding of these phosphoproteins was mediated via the conserved structure of the phosphopeptide-binding pocket in the Btk-SH2 domain. We then purified the 68-kD and 70-kD proteins by using a 2-step affinity purification procedure based on these proteins’ ability to bind the GST-SH2 (Btk) protein and the antiphosphotyrosine antibody (see Materials and Methods). The purified 68-kD and 70-kD proteins (Fig1B) were separately digested by Achromobacter protease I and the resulting peptides were subjected to MALDI mass spectrometry analysis (Fig 1C). A search of a comprehensive peptide mass database with the list of Achromobacter protease I-digested peptide fragments revealed that the peptide masses derived from the 68-kD and 70-kD proteins unambiguously matched with the theoretical peptide masses of the Achromobacter protease I-digested human BLNK-s (shortened form of BLNK15) and BLNK respectively, which are registered in the protein sequence database (NCBI) (Table 1). Furthermore, the amino acid sequences of 4 main peptides (Table 1), which were identified by protein microsequencing, were found to completely coincide with those of the reported BLNK-s and BLNK sequences. This result indicates that the Btk-SH2 domain binding proteins of 68 kD and 70 kD in B cells are human BLNK-s and BLNK, which was further confirmed by immunoblotting analysis by using an anti-BLNK antibody (data not shown).
Identification and purification of the Btk-SH2 domain-binding protein. (A) Lysates of RAMOS cells stimulated with anti-μ-antibody (+) or without stimulation (−) were incubated with the fusion protein of GST and the wild-type Btk-SH2 [SH2(Wild)] or mutated Btk-SH2 [SH2(R307K)] domain. Binding phosphoproteins were detected by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10. (B) Ponceau S staining of the purified proteins on PVDF membrane. (C) Peptide mass map obtained by MALDI mass analysis. The result obtained from the peptide mixture generated byAchromobacter protease I digestion of the 68-kD protein is representatively shown.
Identification and purification of the Btk-SH2 domain-binding protein. (A) Lysates of RAMOS cells stimulated with anti-μ-antibody (+) or without stimulation (−) were incubated with the fusion protein of GST and the wild-type Btk-SH2 [SH2(Wild)] or mutated Btk-SH2 [SH2(R307K)] domain. Binding phosphoproteins were detected by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10. (B) Ponceau S staining of the purified proteins on PVDF membrane. (C) Peptide mass map obtained by MALDI mass analysis. The result obtained from the peptide mixture generated byAchromobacter protease I digestion of the 68-kD protein is representatively shown.
Tyrosinephosphorylation of BLNK by Syk is responsible for the binding of BLNK to the Btk-SH2 domain.
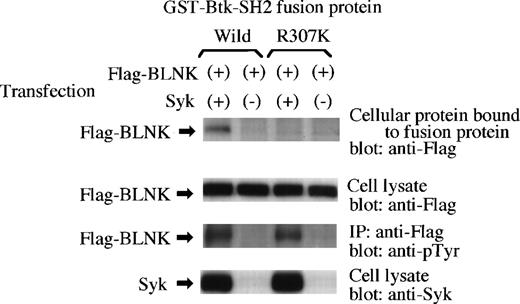
A recent study using tyrosine-kinase–deficient DT40 cells and a baculovirus expression system showed that the tyrosine kinase responsible for the phosphorylation of BLNK is Syk, not Btk nor Lyn.15 Therefore, we exmained whether the tyrosine phosphorylation of BLNK by Syk is sufficient for the binding of BLNK to the Btk-SH2 domain (Fig 2). A Flag-epitope tagged BLNK expression vector was transfected with or without Syk expression vector into 293T cells. As reported previously15and also shown in the third panel of Fig 2, BLNK was strongly tyrosinephosphorylated when it was coexpressed with Syk in this reconstitution system. Expressed proteins were extracted and the cell lysates were incubated with the GST-SH2 (Wild) or the GST-SH2 (R307K) proteins on glutathione S sepharose beads, followed by the detection of BLNK on the beads by means of immunoblotting with the anti-Flag antibody (Fig 2, top panel). Binding of BLNK on the GST-SH2 (Wild) protein, not on the GST-SH2 (R307K) protein, was detected only when BLNK was coexpressed with Syk. This result indicates that the tyrosinephosphorylation of BLNK by Syk is actually responsible for the binding of BLNK to the Btk-SH2 domain.
Tyrosinephosphorylation of BLNK by Syk is responsible for the binding of BLNK to the Btk-SH2 domain. Flag-BLNK was expressed with or without Syk in 293T cells and the cell extracts were incubated with the GST fusion protein of the Btk-SH2 domain. The binding of Flag-BLNK to GST proteins was detected by immunoblotting the cellular proteins bound to the fusion protein beads with the anti-Flag antibody (top panel). Expression of Flag-BLNK (second) or Syk (bottom) in the lysates was detected by immunoblotting with the anti-Flag antibody or the anti-Syk antibody. The tyrosinephosphorylation of Flag-BLNK by Syk was confirmed by immunoprecipitation (IP) with the anti-Flag antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10 (third).
Tyrosinephosphorylation of BLNK by Syk is responsible for the binding of BLNK to the Btk-SH2 domain. Flag-BLNK was expressed with or without Syk in 293T cells and the cell extracts were incubated with the GST fusion protein of the Btk-SH2 domain. The binding of Flag-BLNK to GST proteins was detected by immunoblotting the cellular proteins bound to the fusion protein beads with the anti-Flag antibody (top panel). Expression of Flag-BLNK (second) or Syk (bottom) in the lysates was detected by immunoblotting with the anti-Flag antibody or the anti-Syk antibody. The tyrosinephosphorylation of Flag-BLNK by Syk was confirmed by immunoprecipitation (IP) with the anti-Flag antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10 (third).
In vivo interaction of BLNK with Btk.
We then examined the in vivo association of Btk and BLNK in reconstituted cells (Fig 3A) and a B cell line (Fig 3B). Flag-BLNK and Btk were coexpressed with or without Syk in 293T cells. The association of Btk and BLNK was detected by immunoprecipitation with the anti-Flag antibody followed by immunoblotting with an anti-Btk antibody. As shown in lanes 1 and 2 of the top panel of Fig 3A, coexpression of Syk significantly potentiated the association of Btk and BLNK (approximately 3-fold increased binding as quantitated by Scanning Imager), indicating that the tyrosinephosphorylation of BLNK by Syk plays an important role in the association of BLNK and Btk also in vivo. However, it was noted that significant binding of Btk to BLNK was observed even when BLNK was not phosphorylated (Fig 3A, lane 2 of the top panel), suggesting that the association of Btk and BLNK in this reconstitution system was partly mediated in a phosphotyrosine-independent manner. Because of the presence of the proline-rich region in BLNK and the SH3 domain in Btk, it was presumed that this phosphotyrosine-independent association was mediated through the Btk-SH3 domain. This was confirmed by the fact that the SH3-mutated Btk (Tryptophans 251 and 252 in Btk were replaced by Leucines; WW251LL) hardly bound to BLNK when it was not tyrosinephosphorylated by Syk (Fig 3A, lane 4: the intensity of the binding is less than 10% compared with lane 3 of Fig 3A). The association of Btk and BLNK in these reconstituted cells was, thus, mediated through both the Btk-SH2 and SH3 domains (see Discussion).
(A) Association of Btk and BLNK in 293T cells. Flag-BLNK and Btk [wild-type or SH3-mutated (WW251LL)] were coexpressed with (+) or without (−) Syk. Coprecipitation of Btk with Flag-BLNK was detected by immunoprecipitation (IP) with the anti-Flag antibody followed by immunoblotting with the anti-Btk antibody 43-3B (top panel). (Without the expression of Flag-BLNK, Btk protein was not detected with the same procedure; data not shown.) The equality of the amounts of the Flag-BLNK protein in each immunoprecipitate was confirmed by reprobing the same filter with the anti-Flag antibody (second). Tyrosinephosphorylation of Flag-BLNK by Syk in precipitates was confirmed by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10 (third), and the equality of the amounts of the Btk protein in cell lysates was confirmed by immunoblotting with the anti-Btk antibody 43-3B (bottom). (B) Association of Btk and BLNK in DT40 cells. After DT40 cells, in which T7-Btk was stably expressed, were stimulated with the anti-chicken IgM antibody M4 for the indicated time periods, the cells were lysed and immunoprecipitated with the anti-T7 antibody. The coprecipitation of the endogenous BLNK was detected by immunoblotting with the anti-BLNK antibody (top panel). The equality of the amounts of T7-Btk protein in each of the immunoprecipitates was confirmed by reprobing the same filter with the anti-Btk antibody 43-3B (second). The tyrosinephosphorylation of BLNK (third) or that of T7-Btk (bottom) was detected by immunoprecipitating with the anti-BLNK antibody or the anti-T7 antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10.
(A) Association of Btk and BLNK in 293T cells. Flag-BLNK and Btk [wild-type or SH3-mutated (WW251LL)] were coexpressed with (+) or without (−) Syk. Coprecipitation of Btk with Flag-BLNK was detected by immunoprecipitation (IP) with the anti-Flag antibody followed by immunoblotting with the anti-Btk antibody 43-3B (top panel). (Without the expression of Flag-BLNK, Btk protein was not detected with the same procedure; data not shown.) The equality of the amounts of the Flag-BLNK protein in each immunoprecipitate was confirmed by reprobing the same filter with the anti-Flag antibody (second). Tyrosinephosphorylation of Flag-BLNK by Syk in precipitates was confirmed by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10 (third), and the equality of the amounts of the Btk protein in cell lysates was confirmed by immunoblotting with the anti-Btk antibody 43-3B (bottom). (B) Association of Btk and BLNK in DT40 cells. After DT40 cells, in which T7-Btk was stably expressed, were stimulated with the anti-chicken IgM antibody M4 for the indicated time periods, the cells were lysed and immunoprecipitated with the anti-T7 antibody. The coprecipitation of the endogenous BLNK was detected by immunoblotting with the anti-BLNK antibody (top panel). The equality of the amounts of T7-Btk protein in each of the immunoprecipitates was confirmed by reprobing the same filter with the anti-Btk antibody 43-3B (second). The tyrosinephosphorylation of BLNK (third) or that of T7-Btk (bottom) was detected by immunoprecipitating with the anti-BLNK antibody or the anti-T7 antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10.
To evaluate the contributions of the Btk-SH2 and SH3 domains to the binding with BLNK in B cells, the association of these molecules was investigated in DT40 cells, which stably expressed T7-epitope tagged Btk to evaluate the association precisely. After exposure to the anti-chicken IgM antibody M4, the cells were lysed and the cell lysates were immunoprecipitated with the anti-T7 antibody. The coprecipitation of the endogeneous BLNK was evaluated by means of immunoblotting with the anti-BLNK antibody (Fig 3B, top panel). Although the association of Btk and BLNK was observed to be weak in resting cells, BCR engagement rapidly strengthened the association. The time course of the association of Btk and BLNK was similar to that of the tyrosinephosphorylation of BLNK (Fig 3B, third panel) after BCR engagement. This finding meant that the association of Btk and BLNK in DT40 cells is phosphorylation dependent, thus indicating that the association is mainly mediated by the Btk-SH2 domain and phosphotyrosine(s) in BLNK.
Interaction of the Btk-SH2 domain and phosphorylated BLNK enhances the tyrosinephosphorylation of PLCγ2 by Btk.
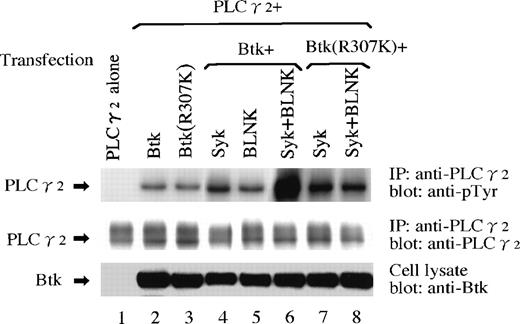
The BCR-induced PLCγ2 phosphorylation is known to be mediated by both Btk and Syk.6-8,22 Genetic dissections of Btk6or Syk22 in DT40 cells resulted in marked reductions of the PLCγ2 phosphorylation. This and other7,8 observations suggest that PLCγ2 serves as the in vivo substrate of Btk. However, it is not clear whether another B-cell cytoplasmic molecule mediates the efficient PLCγ2 phosphorylation by Btk. A recent study using BLNK-deficient DT40 cells showed that BCR-induced PLCγ2 phosphorylation is almost completely eliminated by the disruption of BLNK.17 When taken together with this report, our present observation that Btk associates with phosphorylated BLNK suggests a mechanism by which BLNK might mediate the efficient PLCγ2 phosphorylation by Btk. We tested this possibility by using a reconstituted cell system (Fig 4). An increase in PLCγ2 phosphorylation was observed when wild-type Btk was coexpressed with PLCγ2 in 293T cells (Fig 4, lane 2), which was further potentiated by the additional expression of Syk (Fig 4, lane 4). This PLCγ2 phosphorylation was not mediated by the Btk-SH2 domain because a comparison of the PLCγ2 phosphorylation by the wild-type Btk (Fig 4, lane 2) and by the SH2-mutated Btk (Btk [R307K], Fig 4, lane 3) did not show any significant changes (see Discussion). Although the additional expression of BLNK with Btk did not affect the PLCγ2 phosphorylation significantly (compare lanes 2 and 5, Fig 4), BLNK markedly enhanced the PLCγ2 phosphorylation when it was coexpressed with Btk and Syk (compare lanes 4 and 6, Fig 4), suggesting that the phosphorylated BLNK contributed to the efficient PLCγ2 phosphorylation by Btk. In contrast to this, the additional expression of BLNK with the SH2-mutated Btk and Syk did not enhance the PLCγ2 phosphorylation compared with when BLNK was not expressed (compare lanes 7 and 8, Fig 4). These observations suggest that the interaction of the Btk-SH2 domain and phosphotyrosine(s) in BLNK contributed to the complete tyrosinephosphorylation of PLCγ2 by Btk.
Tyrosinephosphorylation of PLCγ2 by Btk and Syk on BLNK. Indicated proteins were expressed in 293T cells. Tyrosinephosphorylation of PLCγ2 were detected by immunoprecipitating (IP) with the anti-PLCγ2 antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10 (top panel). The same filter was reprobed with the anti-PLCγ2 antibody to confirm the equality of the amounts of the PLCγ2 protein (middle). The expression of Btk in the cell lysates is also shown (bottom).
Tyrosinephosphorylation of PLCγ2 by Btk and Syk on BLNK. Indicated proteins were expressed in 293T cells. Tyrosinephosphorylation of PLCγ2 were detected by immunoprecipitating (IP) with the anti-PLCγ2 antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10 (top panel). The same filter was reprobed with the anti-PLCγ2 antibody to confirm the equality of the amounts of the PLCγ2 protein (middle). The expression of Btk in the cell lysates is also shown (bottom).
DISCUSSION
In the present study, we identified one of the major Btk-SH2 domain binding proteins in B cells as BLNK and showed that the interaction of the Btk-SH2 domain and phosphorylated BLNK contributes to the complete tyrosinephosphorylation of PLCγ2 by Btk. Our findings have identified a new molecular connection, which may contribute to the understanding of how each domain of Btk is involved in BCR-coupled calcium signaling.
BLNK was shown to bind the Btk-SH2 domain after its tyrosinephosphorylation by Syk. The experiment that used reconstituted cells showed that the tyrosinephosphorylation of BLNK by Syk plays an important role in its binding to Btk also in vivo. It should be noted, however, that, as seen in Fig 3A, a significant binding of Btk to BLNK was still observed even when BLNK was not phosphorylated, which suggested that both the SH2 and SH3 domains contributed to the binding of Btk to BLNK in the reconstitution system used in this study. The SH2 and SH3 domain-dependent binding observed here looked similar to that reported in the binding of Grb2 to BLNK,15,16 in which Grb2 constitutively bound to BLNK in resting cells but the binding was further enhanced by BCR stimulation15 or pervanadate stimulation.16 However, our coprecipitation experiment with results shown in Fig 3B indicated that the association of Btk and BLNK was weak in the resting B cells but rapidly became enhanced after BCR engagement, which suggested that the interaction between these molecules is phosphorylation dependent but not constitutive in B cells and underlined the primary importance of the Btk-SH2 domain in the interaction with BLNK. Moreover, in the reconstituted cells, the phosphorylated BLNK enhanced the PLCγ2 phosphorylation by Btk but not by the SH2-mutated Btk, indicating that the proper binding of Btk to BLNK via the Btk-SH2 domain is necessary for the complete tyrosinephosphorylation of PLCγ2. These results may well explain the previous observation that, although the restoration of BCR-induced calcium flux in Btk-deficient B cells required the Btk-SH2 domain, it was independent of the Btk-SH3 domain.7
The results obtained with the reconstitution experiment indicated that the interaction of the Btk-SH2 domain and the phosphorylated BLNK leads Btk to the proximity of PLCγ2, which also binds to the phosphorylated BLNK,15 resulting in efficient PLCγ2 phosphorylation. However, (and as also described in another report7) a certain level of PLCγ2 phosphorylation by Btk was observed even without the coexpression of BLNK in our reconstitution system. This observation does not directly mean the existence of a direct interaction between Btk and PLCγ2 in B cells and, in fact, it was observed that the BLNK-deficient DT40 cells eliminated BCR-induced PLCγ2 phosphorylation.17 The direct phosphorylation of PLCγ2 by Btk observed in these experiments probably reflects the difference of the intracellular distributions of these molecules in reconstituted cells from that in B cells or may be partly mediated by some ubiquitously expressed adaptor molecules. It is noted that, as apparently contradicting the finding of another report,7 no significant difference was observed in the PLCγ2 phosphorylation by wild-type Btk and the SH2-mutated Btk without the coexpression of BLNK in our reconstituted cells, suggesting that the direct interaction of Btk and PLCγ2 via the Btk-SH2 domain is unlikely.
The identification of the interaction between Btk and BLNK leads to the emergence of a new molecular scenario in BCR-coupled calcium signaling. BCR engagement activates Btk by means of membrane anchoring through the interaction with PtdIns-3,4,5-P3 (the Btk-PH domain is, thus, essential in this process)10,12 and after tyrosinephosphorylation by upstream kinases.11 Syk is also activated after BCR engagement and phosphorylates BLNK on its tyrosines,15 which allows the association of BLNK and Btk as shown in this study (the Btk-SH2 domain is, thus, essential in this process) and also the association of BLNK and PLCγ2.15 As Syk also colocalizes on the phosphorylated BLNK,15 BLNK might nucleate an activation complex, including Syk, Btk, and PLCγ2, for which PLCγ2 is fully tyrosinephosphorylated and thereby activated. The results of genetic dissection experiments of DT40 cells seem to support this molecular mechanism. Syk-deficient22or BLNK-deficient17 DT40 cells almost completely abrogated BCR-induced PLCγ2 phosphorylation and Btk-deficient6 DT40 cells exhibited a lower level (almost 3 times lower than that of wild-type cells) of the PLCγ2 phosphorylation. The residual phosphorylation of PLCγ2 in Btk-deficient cells may be caused by the activity of Syk, because in the above scenario Btk is not necessarily required for the Syk-dependent PLCγ2 phosphorylation, whereas Syk is required for the Btk-dependent PLCγ2 phosphorylation through the phosphorylation of BLNK. However, despite the presence of residual PLCγ2 phosphorylation, Btk-deficient DT40 cells showed complete loss of the BCR-induced calcium flux,6 which raises the possibility that Btk and Syk may mutually phosphorylate distinct tyrosines in PLCγ2 and that Syk-dependent phosphorylation alone may not be sufficient to activate PLCγ2. Precise characterization of the PLCγ2 phosphorylation sites by Btk and Syk should show the exact activation mechanism of PLCγ2.
Certain reported observations still remain difficult to be explained by the simple scenario described above. First, B cells from XID mice, which contain a mutation in the Btk-PH domain, were reported to exhibit only a limited (40% to 50%) reduction of BCR-induced IP3production and calcium flux,9 which is in contrast to the observation that the calcium signaling in Btk-deficient DT40 cells could barely be restored by PH-mutated Btk.6 Second, it was reported that B-cell lines established from 2 XLA patients exhibited a detectable but markedly blunted BCR-induced calcium flux, whereas the BCR-induced PLCγ2 phosphorylation in XLA B cells was not altered compared with that in normal B cells.7 As also suggested in previous reports,6,7 these discrepancies may reflect the species differences in the uses of PLCγ isoforms or Btk-related kinases. It should also be noted that a phosphorylation-independent activation mechanism of PLCγ was recently reported,32although its biologic significance in BCR-coupled calcium signaling has not yet been determined. These complicated observations may imply the presence of some redundancies in calcium signaling at least in some species or alternatively may indicate that multiple pathways must operate together to allow the IP3-gated internal calcium store to be released. Our study proposes that the molecular network including Btk and BLNK is one of the major pathways in BCR-coupled calcium signaling.
ACKNOWLEDGMENT
We thank Shigeyuki Arai and his colleagues (Fujisaki Institute, Hayashibara Biochemical Laboratories Inc, Okayama, Japan) for the collaboration to generate the anti-Btk MoAb 43-3B, Shunsuke Ishi (Tsukuba Life Science Center, RIKEN, Ibaraki, Japan) for providing pT-Trx expression vector, Hirohei Yamamura (Kobe University School of Medicine, Kobe, Japan) for providing porcine Syk cDNA, Toshio Miyawaki (Toyama Medical and Pharmaceutical University) for critical reading of the manuscript, and Noriko Kameoka for preparation of the manuscript.
Supported by Grants-in-Aid for Scientific Research (to S.T., M.I., and T. Kurosaki), Grants-in-Aid for Scientific Research on Priority Areas (to S.T. and T. Kurosaki) and Grants-in-Aid for COE Research (to T. Kishimoto) from the Ministry of Education, Science, Sports and Culture of Japan, the Ministry of Health and Welfare’s Research Grant for Specific Diseases, Japan (to S.T.), Grants from Mochida Science Foundation (to S.T.), and the Takeda Science Foundation (to T. Kurosaki).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Satoshi Tsukada, MD, PhD, Department of Molecular Medicine (formerly Medicine III), Osaka University Medical School, 2-2, Yamada-oka, Suita City, Osaka 565-0871, Japan; e-mail:tsukada@imed3.med.osaka-u.ac.jp.

![Fig. 1. Identification and purification of the Btk-SH2 domain-binding protein. (A) Lysates of RAMOS cells stimulated with anti-μ-antibody (+) or without stimulation (−) were incubated with the fusion protein of GST and the wild-type Btk-SH2 [SH2(Wild)] or mutated Btk-SH2 [SH2(R307K)] domain. Binding phosphoproteins were detected by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10. (B) Ponceau S staining of the purified proteins on PVDF membrane. (C) Peptide mass map obtained by MALDI mass analysis. The result obtained from the peptide mixture generated byAchromobacter protease I digestion of the 68-kD protein is representatively shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2357.419k40_2357_2364/6/m_blod41940001w.jpeg?Expires=1763904840&Signature=sVW0RIjKrRIEv8~SUV-N1rBayiUgZDlD3fYdQmSaFPXCu~PtTn-IIayzFIyzf9q~qquLESrambeF70f4eKzTU6QFcdLzN~xuxYsQ7CnrvNqlFIsl2O1ozAaaHreyIh0RoEQs5aOCyJClGtyXH9tYkn8ib4C0TdSZcDrruF3~etlAG~GaKDnv8tBAPK24BEwNNVLmtCnsccx~6m0Upc8WFYiV-zORbHBwHdzUqXO-MxlQrNnWV9v2G4~eQltU9pE33XcgvKajwEHX6N5Lw-LY5EAVaixSaNkvZwV0iy8pLLASeOKEdxpV3ADw4QmNGWv6FSGuRmwmi-GNrBPOahCRyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. (A) Association of Btk and BLNK in 293T cells. Flag-BLNK and Btk [wild-type or SH3-mutated (WW251LL)] were coexpressed with (+) or without (−) Syk. Coprecipitation of Btk with Flag-BLNK was detected by immunoprecipitation (IP) with the anti-Flag antibody followed by immunoblotting with the anti-Btk antibody 43-3B (top panel). (Without the expression of Flag-BLNK, Btk protein was not detected with the same procedure; data not shown.) The equality of the amounts of the Flag-BLNK protein in each immunoprecipitate was confirmed by reprobing the same filter with the anti-Flag antibody (second). Tyrosinephosphorylation of Flag-BLNK by Syk in precipitates was confirmed by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10 (third), and the equality of the amounts of the Btk protein in cell lysates was confirmed by immunoblotting with the anti-Btk antibody 43-3B (bottom). (B) Association of Btk and BLNK in DT40 cells. After DT40 cells, in which T7-Btk was stably expressed, were stimulated with the anti-chicken IgM antibody M4 for the indicated time periods, the cells were lysed and immunoprecipitated with the anti-T7 antibody. The coprecipitation of the endogenous BLNK was detected by immunoblotting with the anti-BLNK antibody (top panel). The equality of the amounts of T7-Btk protein in each of the immunoprecipitates was confirmed by reprobing the same filter with the anti-Btk antibody 43-3B (second). The tyrosinephosphorylation of BLNK (third) or that of T7-Btk (bottom) was detected by immunoprecipitating with the anti-BLNK antibody or the anti-T7 antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2357.419k40_2357_2364/6/m_blod41940003w.jpeg?Expires=1763904840&Signature=Ac1wBhCp2UMaKyNqLD6L9fBaIZVPo4xUp7NWp5EwcCUrh994QySyfvCrEYNUssAI6XW8KS1EAI6y-ODNjhug3W~6bR2wlaLC9MUjpYcvcUips3-YLiKuLKVtln93VV3TU6Xalel7pQVv2AToeFnhxCXA33Qb39UKmg1QuoPu4zvcNRlOfp70SxPV5DVaQLD6cNUAK3-5RvcGN5Y9chztzOP-XcHqbXtGYaeIzSf9bTCtTi3oDeXiQcSlG3vo4fJ3BznoXuV~CY~QHN7s~WUa-tmteHufDbon1-0neyPFI4IPeM6eMHVpgA56-Arzy~Sst1peTuIhmB6c7hz~Pnz~gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Identification and purification of the Btk-SH2 domain-binding protein. (A) Lysates of RAMOS cells stimulated with anti-μ-antibody (+) or without stimulation (−) were incubated with the fusion protein of GST and the wild-type Btk-SH2 [SH2(Wild)] or mutated Btk-SH2 [SH2(R307K)] domain. Binding phosphoproteins were detected by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10. (B) Ponceau S staining of the purified proteins on PVDF membrane. (C) Peptide mass map obtained by MALDI mass analysis. The result obtained from the peptide mixture generated byAchromobacter protease I digestion of the 68-kD protein is representatively shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2357.419k40_2357_2364/6/m_blod41940001w.jpeg?Expires=1763904841&Signature=LMCldUEPwcv-WGGPGATcIOsPoJt~jUJnnLoXhJG1onYUO8mBi9KIUsaTAh5bryf4mRRlMeJ0D2Dd0P1qG~2~NCPrEdFBn2cO3kETjAV4SP5nxP9aDgNpfZnf2xcvHaXVcNpsGXgCObwNdy3-H4Yhb6NSMi~fv3BOeLzPOaAKGNU5WL3I1mwY5nYHxxesegI0TM8AjOTI09WQnlfhFWSkL0nbPJAom5-drYBoYwOkZYuKlczuOZsf3lwi5o4UfcBIrkndyNxWfSn5yCJ9VtxoT0uVSxk8R31pD2K3n1iKx0UpQ2JZQhiCTqERYhaQxKSF~s0cx4nhWAO5rGFstWd4aA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. (A) Association of Btk and BLNK in 293T cells. Flag-BLNK and Btk [wild-type or SH3-mutated (WW251LL)] were coexpressed with (+) or without (−) Syk. Coprecipitation of Btk with Flag-BLNK was detected by immunoprecipitation (IP) with the anti-Flag antibody followed by immunoblotting with the anti-Btk antibody 43-3B (top panel). (Without the expression of Flag-BLNK, Btk protein was not detected with the same procedure; data not shown.) The equality of the amounts of the Flag-BLNK protein in each immunoprecipitate was confirmed by reprobing the same filter with the anti-Flag antibody (second). Tyrosinephosphorylation of Flag-BLNK by Syk in precipitates was confirmed by immunoblotting with the antiphosphotyrosine (anti-pTyr) antibody 4G10 (third), and the equality of the amounts of the Btk protein in cell lysates was confirmed by immunoblotting with the anti-Btk antibody 43-3B (bottom). (B) Association of Btk and BLNK in DT40 cells. After DT40 cells, in which T7-Btk was stably expressed, were stimulated with the anti-chicken IgM antibody M4 for the indicated time periods, the cells were lysed and immunoprecipitated with the anti-T7 antibody. The coprecipitation of the endogenous BLNK was detected by immunoblotting with the anti-BLNK antibody (top panel). The equality of the amounts of T7-Btk protein in each of the immunoprecipitates was confirmed by reprobing the same filter with the anti-Btk antibody 43-3B (second). The tyrosinephosphorylation of BLNK (third) or that of T7-Btk (bottom) was detected by immunoprecipitating with the anti-BLNK antibody or the anti-T7 antibody followed by immunoblotting with the antiphosphotyrosine antibody 4G10.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2357.419k40_2357_2364/6/m_blod41940003w.jpeg?Expires=1763904841&Signature=jv9Ma4GYDr38ZvTabDc~xh8fQwYYbHY0UbcxnRVxQeGFbqjtK0~r83F2ejY-xbjmiccjuopkrIObGRyPXKKQgXD0fcpP7NU5HxUMVO7qmeo3R9XbbpBeD~8EEorRwckHlM9joN4YhAgqpCcT3LWlN78NyZU9AGz7M6rw3QW3fWykx7aVRf9iM-r2STHEuN3iA4htbospSDG8FQo~IckA5Aruw~nMF9~HI-R4-6RdpT87AZ6gl8Lq1ANnKmIdc67eS~hFhx5qXrOTSpKtft1Dsgn8tE6KswcVJKpQ9XBmqSCUn1gFphUPRVmczuSsdkPQQiGlfVDNN8bl~spxKZPTWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
