Chronic granulomatous disease (CGD) is a group of inherited disorders in which phagocytes are unable to generate superoxide (O2−) due to genetic defects in any 1 of 4 essential NADPH oxidase components. Mutations in the X-linked gene for gp91phox, the large subunit of the flavocytochromeb558 heterodimer, account for the majority of CGD. An X-CGD patient in which a splice junction mutation results in an in-frame deletion of 30 nucleotides encoding amino acids 488 to 497 of gp91phox (▵488-497 gp91phox) has previously been reported. In this study, we generated myeloid PLB-985 cells expressing the mutant ▵488-497 gp91phox to further characterize its functional properties. These cells mimicked the phenotype of the patient’s neutrophils with normal expression of a nonfunctional ▵488-497 gp91phox flavocytochrome. Translocation of p47phox and p67phox to ▵488-497 gp91phox PLB-985 plasma membranes was not affected, as determined both in activated intact cells and in the cell-free system. Furthermore, a synthetic peptide corresponding to residues 488-497 of gp91phox was relatively ineffective in inhibiting O2− production in the cell-free oxidase assay (IC50, ∼500 μmol/L), suggesting that residues 488-497 of gp91phox are not directly involved in oxidase assembly. Mutant ▵488-497 gp91phox flavocytochrome failed to support iodonitrotetrazolium (INT) reduction, showing a disruption of electron transfer from NADPH to the FAD center of gp91phox. However, the FAD binding capacity of the mutant flavocytochrome was normal, as measured by equilibrium dialysis. Taken together, these results suggest that the ▵488-497 deletion in gp91phox disrupts electron transfer to FAD, either due to a defect in NADPH binding or to impaired delivery of electrons from NADPH.
PHAGOCYTES PLAY a critical role in host defense by producing reactive oxygen species against invading microorganisms. One of the most important enzymes in producing microbicidal oxidants is the superoxide (O2−)-generating NADPH oxidase.1 The NADPH oxidase is a multicomponent enzyme complex whose redox center is a membrane-associated flavocytochromeb558 heterodimer composed of gp91phox and p22phox. In addition, three cytosolic oxidase subunits, p47phox, p67phox, and a low molecular weight GTP binding protein Rac, are required for high level production of O2−. In resting phagocytes, the dormant oxidase is unassembled. However, upon phagocyte activation, the active oxidase complex is rapidly formed by translocation of the cytosolic oxidase components to the plasma membrane via interactions with the cytochrome.2 Subsequently, electrons are transferred from cytosolic NADPH to molecular oxygen (O2) at the external face of the membrane to generate O2−.3
Genetic deficiency of NADPH oxidase activity results in chronic granulomatous disease (CGD), a rare inherited disorder of host defense. Patients with CGD develop recurrent, often life-threatening bacterial and fungal infections due to impaired microbicidal oxidant generation by the patient’s phagocytes. CGD is caused by genetic defects in any 1 of the 4 oxidase components, p47phox, p67phox, gp91phox, and p22phox. Mutations in the X-linked gene for gp91phox account for approximately two thirds of CGD, with the remaining cases due to autosomal recessive mutations in the genes encoding p22phox, p47phox, or p67phox.1
The NADPH oxidase catalyzes the transfer of electrons from the substrate NADPH to O2, via intermediate flavin (FAD) and heme prosthetic groups, to produce O2−.3,4 The gp91phox polypeptide appears to be the oxidase subunit responsible for mediating electron transfer. We recently have shown that the 2 heme groups incorporated into the cytochrome heterodimer are located within gp91phox.5 The carboxyl terminus of gp91phox contains homologies to consensus FAD and NADPH binding domains of members of ferredoxin-NADP+reductase (FNR) family,6-8 although p67phox has also been reported recently to contain a functional NADPH-binding site and may also participate in the NADPH binding.9,10 Coexpression of both gp91phox and p22phox subunits are required to assemble a functional flavocytochrome capable of supporting O2− production.5In addition, expression of gp91phox in phagocytes is stabilized by association with its partner p22phox.11 12
The majority of missense mutations or in-frame deletions identified in X-CGD result in apparent instability of the gp91phox polypeptide, with either absent or markedly reduced expression of the mutant flavocytochromeb558. Rare mutations in which expression of flavocytochrome b558 is preserved have been informative in identifying important structural-function relationships of the cytochrome.13-15 An X-CGD patient in which a splice junction mutation results in an in-frame deletion of 30 nucleotides encoding amino acids 488 to 497 of gp91phox(Δ488-497 gp91phox) has previously been reported.16 A detailed functional analysis of the mutant cytochrome could not be performed due to the death of the patient. In this study, we stably transfected the Δ488-497 gp91phox cDNA into X-CGD PLB-985 cells, which lack endogenous gp91phox expression due to gene targeting,17 to create a cell line expressing the mutant Δ488-497 gp91phox flavocytochrome. This approach allowed us to perform functional studies on Δ488-497 gp91phox PLB-985 cells to further characterize the defect resulting in failure of O2−production. We found that deletion of gp91phoxresidues 488-497 did not affect translocation of the cytosolic subunits p47phox and p67phox to plasma membranes of activated Δ488-497 gp91phox PLB-985 cells. However, mutant Δ488-497 gp91phoxflavocytochrome failed to support iodonitrotetrazolium (INT) reduction, showing a defect of the proximal electron transfer pathway from NADPH to the FAD center of gp91phox. Partially purified Δ488-497 gp91phox had a normal capacity for FAD binding as determined by equilibrium dialysis against FAD. These results suggest that the Δ488-497 deletion disrupted electron transfer from NADPH to FAD, either due to a defect in NADPH binding or to impaired electron delivery from NADPH.
MATERIALS AND METHODS
In vitro mutagenesis and expression of recombinant gp91phox in promyelocytic PLB-985 cells.
The Δ488-497 gp91phox cDNA, which has an in-frame deletion of 30 nucleotides,16 was generated by oligonucleotide-directed mutagenesis by using the Sculptor in vitro mutagenesis kit (Amersham, Arlington Heights, IL). The mutant cDNA was verified by dideoxynucleotide sequencing and then subcloned into the Not I site of the mammalian expression vector, pEF-PGKpac. The pEF-PGKpac vector contains a mammalian EF-1α promoter to drive constitutive gp91phox expression and a linked expression cassette for puromycin-N acetyltransferase.18 In parallel, the vector containing the full-length human wild-type (WT) gp91phox cDNA was also constructed. The WT or the Δ488-497 gp91phox-containing vectors were transfected by electroporation into X-CGD PLB-985 cells.17 Clones were selected by limiting dilution in the presence of puromycin (1 μg/mL). To minimize any potential clone-to-clone variation in recombinant gp91phox expression or NADPH oxidase activity, 3 independent clones determined to express relatively higher levels of recombinant gp91phox were pooled and used for subsequent analysis.
Cell culture and granulocytic differentiation.
X-CGD PLB-985 cells (X-CGD), transfected PLB-985 cells expressing WT, or the deletion mutant (Δ488-497) gp91phox were maintained in RPMI 1640 medium containing 10% fetal calf serum and 2 mmol/L L-glutamine. To induce expression of endogenous NADPH oxidase subunits, cells were differentiated for 5 days by exposure to 0.5% dimethylformamide (DMF). Under these conditions, more than 80% of the cells had undergone granulocytic differentiation as determined by observation of morphological changes and nitroblue tetrazolium (NBT) test.12
Immunoblot and confocal microscopy analysis of recombinant gp91phox expression.
To evaluate cell surface expression of flavocytochromeb558, immunostaining with the 7D5 monoclonal antibody was performed as described.5,18 After staining, 3,000 to 5,000 cells were deposited on glass slides by centrifugation at 450 rpm for 5 minutes and were observed by confocal microscopy. Expression of recombinant gp91phox and p22phox was also determined by immunoblotting as described previously.12
Translocation analysis of cytosolic oxidase components.
Granulocyte-differentiated cells (1 × 108) were collected, washed, and then resuspended in 1 mL of relaxation buffer consisting of 10 mmol/L PIPES, pH 7.3, 100 mmol/L KCl, 3.5 mmol/L MgCl2, 3 mmol/L NaCl, and 1 mmol/L EGTA. To activate the NADPH oxidase assembly, cells were treated with either phorbol 12-myristate 13-acetate (PMA) or dimethyl sulfoxide (DMSO) vehicle control at a final concentration of 500 ng/mL for 10 minutes at 37°C. After the addition of 12 mL of cold phosphate-buffered saline (PBS) to stop the activation, cells were pelleted, resuspended in 1.5 mL of the relaxation buffer, and disrupted by sonication for 3 times at 6 seconds each at 20% power at 4°C. Subsequently, the sonicates were spun for 8 minutes at 500g, followed by 10 minutes at 2,000g. A total of 0.75 mL of the resulting supernatant was loaded on a discontinuous sucrose gradient (1.5 mL of 20% over 1.5 mL of 38%) and centrifuged at 41,000 rpm (204,275g) in an SW55 rotor (Beckman Instruments, Fullerton, CA) for 40 minutes at 4°C. After centrifugation, the 0.6 mL fraction of the top of the gradient was collected as cytosol, and a distinct band located in the interface of the 20% and 38% sucrose gradient was collected as the plasma membranes (∼0.6 mL). To remove sucrose, the membranes were mixed with 3.5 mL of cold PBS and centrifuged at 55,000 rpm (368,000g) for 30 minutes, and the resulting pellets were resuspended in 100 μL of relaxation buffer. Translocation of cytosolic oxidase components p47phox and p67phox to plasma membrane was detected by immunoblotting as described previously.12 Translocation assay was also performed in a cell-free system. Briefly, 100 μg of cellular membranes was mixed with 300 μg of cytosol isolated from granulocyte-differentiated cells indicated in the figure legends in 0.5 mL of relaxation buffer containing 10 μmol/L GTPγs. After the addition of 100 μmol/L sodium dodecyl sulfate (SDS), the mixture was incubated for 5 minutes at 37°C and then centrifuged for 30 minutes at 55,000 rpm (368,000g) at 4°C. The resulting pellets were collected and used for immunoblotting.
Measurement of NADPH oxidase activity.
O2− production by granulocyte-differentiated cells was measured both in whole cells and in the cell-free oxidase assay by monitoring the reduction of cytochrome c at 550 nm using a Thermomax microplate reader.12 In the assay using intact cells, PMA at a final concentration of 0.1 μg/mL was used to activate the NADPH oxidase of granulocyte-differentiated cells (a total of 2.5 × 105 cells in 200 μL of volume in a well). The cell-free assays using SDS as activator were performed as described previously,19,20 using flavocytochromeb558 partially purified from membranes of the PLB-985 cell lines and cytosol from neutrophil or granulocyte-differentiated PLB-985 cells. Membrane and cytosolic fractions were prepared by continuous centrifugation followed by cell disruption by sonication.21 Michaelis-Menton kinetics were analyzed using GraphPad Prism (San Diego, CA). For peptide inhibition assays, peptides were dissolved in assay buffer and added to the reaction mixture before the addition of SDS (100 μmol/L). Protein concentration was determined by BCA assay (Pierce, Rockford, IL). INT reductase activity was measured as described previously.20
Purification, relipidation, and reflavination of flavocytochromeb558.
Flavocytochrome b558 was partially purified from 3 × 109 cell equivalents of salt-washed PLB-985 cell membranes using the method described previously for flavocytochromeb558 purification from neutrophil membranes.22 This method uses mixed-bed (carboxylmethyl [CM], diethyl aminoethyl [DEAE] Sepharose CL-6B, amin-octyl agarose) and heparin chromatography. Relipidation and reflavination of the partially purified flavocytochromeb558 was also performed as described previously using phosphatidylcholine (type IIS; Sigma, St Louis, MO). For the determination of FAD binding constants, portions of the partially purified cytochrome were relipidated in the absence of FAD.
Spectroscopy.
Reduced minus oxidized difference spectra of detergent-solubilized membranes and partially purified flavocytochromeb558 were recorded as described previously using a Perkin-Elmer Lamda 18 spectrophotometer (Perkin-Elmer, Norwalk, CT).13
Determination of the affinity of flavocytochromeb558 for FAD.
The dissociation constant for FAD binding by the flavocytochromeb558 preparations was determined by equilibrium dialysis using Sialomed equilibrium dialyzers (AmiKa Corp, Columbus, MD). One-hundred–microliter aliquots (130 to 150 nmol/L in concentration) of partially purified flavocytochromeb558 samples that had been relipidated (but not reflavinated) were placed in one side of the dialysis chamber and dialyzed against 100 μL of the same buffer containing 100 nmol/L FAD. FAD standards were made by serial dilution of a freshly prepared stock solution of FAD. The concentration of the stock solution was determined from the absorbance at 450 nm using an extinction coefficient of 11.3 mmol/L−1. All FAD-containing solutions were protected from light. The concentration of flavocytochromeb558 was determined spectrophotometrically using an extinction coefficient of 21.6 mmol/L−1 at 559 nm for the reduced-minus oxidized heme (10.8 mmol/L−1 per mole flavocytochrome b558).22 Samples were dialyzed on ice for 4 hours, and the contents of each chamber were removed for FAD analysis. All samples and FAD standards were heated in a 100°C water bath for 3 minutes to release enzyme-bound FAD and centrifuged to remove denatured protein.
FAD was estimated using a modification of the method of Hinkkanen and Decker.23 The assay mixture consisted of 80 to 100 μL of sample (or FAD standard), 20 mmol/L 3, 5-dichlorobenzene sulfonic acid, 200 μmol/L 4-amino antipyrene, 4 U/mL horseradish peroxidase, 0.2 U/mL apo-D-amino acid oxidase, and 35 mmol/L D-proline in a total volume of 250 μL 100 mmol/L Tris, pH 8.6. The assay was performed at 37°C and the rate of formation of N-(4-antipyryl)-3-chloro-5-sulfonate-p-benzoquinone monoimine was observed for 60 minutes by measuring the increase in absorbance at 512 nm. The FAD concentration of the samples was calculated from a standard curve of 0 to 200 nmol/L FAD plotted against maximum rate of ΔA512. The Kd (FAD) for each sample was calculated from the final concentrations of FAD in the sample and buffer compartments, and the concentration of flavocytochromeb558 was added.
RESULTS AND DISCUSSION
Δ488-497 gp91phox PLB-985 cells mimic the phenotype of X-CGD Δ488-497 gp91phox neutrophils.
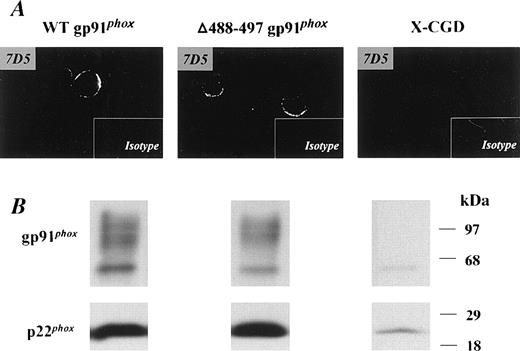
Stable expression of recombinant Δ488-497 gp91phox in X-CGD PLB-985 cells mimicked the phenotype originally reported for neutrophils isolated from an X-CGD patient with the same Δ488-497 deletion in gp91phox.16 Expression of Δ488-497 gp91phox in transfected PLB-985 cells was examined by immunoblotting (Fig 1B). The level of recombinant Δ488-497 gp91phox was similar to that of recombinant WT gp91phox expressed in PLB-985 cells. A marked increase in expression of p22phoxwas seen in both transgenic Δ488-497 gp91phox and WT gp91phox PLB-985 cells (Fig 1B). This is consistent with previous observations indicating that coexpression of both gp91phox and p22phox and subsequent heterodimer formation is important for stable expression of each flavocytochrome b558subunit.11,12,24 We have previously shown that the transgenically expressed recombinant WT gp91phox is processed and targeted normally into the plasma membrane in promyelocytic PLB-985 cells.18 To determine whether Δ488-497 gp91phox was expressed in the plasma membranes, transfected cells were stained with 7D5, a monoclonal antibody that interacts with an extracellular epitope of gp91phox, and examined by confocal microscopy. As shown in Fig 1A, membrane surface staining was present in both Δ488-497 gp91phox as well as WT gp91phox PLB-985 cells. No positive signal above the background was obtained in X-CGD PLB-985 cells, consistent with the absence of gp91phox in the cells (Fig 1B).
Expression of recombinant WT gp91phoxand ▵488-497 gp91phox flavocytochromeb558 in transgenic PLB-985 cells. X-CGD PLB cells were transfected with either WT or ▵488-497 gp91phox cDNAs, and the expression of recombinant gp91phox/p22phox heterodimer was examined by immunoblotting (B). Five micrograms of cellular membranes was loaded. (A) Confocal microscopy observation of membrane surface expression of recombinant gp91phox. The indicated cells were stained with the gp91phoxmonoclonal antibody, 7D5, as described previously,18 and mouse IgG1 was used as an isotype control. Imaging amplifications: ×360 for 7D5 staining and ×148 for IgG1 staining.
Expression of recombinant WT gp91phoxand ▵488-497 gp91phox flavocytochromeb558 in transgenic PLB-985 cells. X-CGD PLB cells were transfected with either WT or ▵488-497 gp91phox cDNAs, and the expression of recombinant gp91phox/p22phox heterodimer was examined by immunoblotting (B). Five micrograms of cellular membranes was loaded. (A) Confocal microscopy observation of membrane surface expression of recombinant gp91phox. The indicated cells were stained with the gp91phoxmonoclonal antibody, 7D5, as described previously,18 and mouse IgG1 was used as an isotype control. Imaging amplifications: ×360 for 7D5 staining and ×148 for IgG1 staining.
We next performed reduced minus oxidized difference spectroscopy on transgenically expressed flavocytochrome b558partially purified from membranes isolated from Δ488-497 gp91phox, WT gp91phox, and X-CGD PLB-985 cells. Virtually identical spectra characteristic of flavocytochrome b558 were seen for both the Δ488-497 gp91phox and WT gp91phox flavocytochrome preparations, demonstrating the normal incorporation of heme groups in the gp91phox deletion mutant (Fig 2). As expected, X-CGD samples lacked specific absorption at 558 nm (Fig 2).
Reduced minus oxidized difference spectrum of flavocytochrome b558 samples partially purified from membranes of X-CGD, WT gp91phox, and ▵488-497 gp91phox PLB-985 cells. Flavocytochromeb558 samples were partially purified from cellular membranes of the indicated cells and dithionite-reduced minus oxidized difference spectrum of the samples were analyzed as described in the Materials and Methods. Results shown are from one representative of triplicate analyses.
Reduced minus oxidized difference spectrum of flavocytochrome b558 samples partially purified from membranes of X-CGD, WT gp91phox, and ▵488-497 gp91phox PLB-985 cells. Flavocytochromeb558 samples were partially purified from cellular membranes of the indicated cells and dithionite-reduced minus oxidized difference spectrum of the samples were analyzed as described in the Materials and Methods. Results shown are from one representative of triplicate analyses.
NADPH oxidase activity in Δ488-497 gp91phoxPLB-985 cells was determined in both intact cells and in the cell-free oxidase assay. After granulocytic differentiation for 5 days to induce the expression of the endogenous p47phox and p67phox oxidase subunits, WT gp91phox PLB-985 cells produced O2− after stimulation with PMA, as expected (Table 1). In contrast, Δ488-497 gp91phox PLB-985 cells were unable to generate O2− (Table 1). To confirm that the cellular defect in the NADPH oxidase in Δ488-497 gp91phox PLB-985 cells was related to the mutation in gp91phox, cell-free oxidase assays were performed using combinations of cytosol and membranes prepared from WT gp91phox and Δ488-497 gp91phox PLB-985 cells. As shown in Table 2, membranes isolated from Δ488-497 gp91phox PLB-985 cells failed to support O2− generation in combination with cytosol from either WT gp91phox or Δ488-497 gp91phox PLB-985 cells, demonstrating that absence of NADPH oxidase activity resulted from a defect in the cellular membranes containing the mutant flavocytochromeb558.
Assembly of the NADPH oxidase by translocation of p47phox and p67phox to the plasma membrane is not affected by the Δ488-497 deletion in gp91phox.
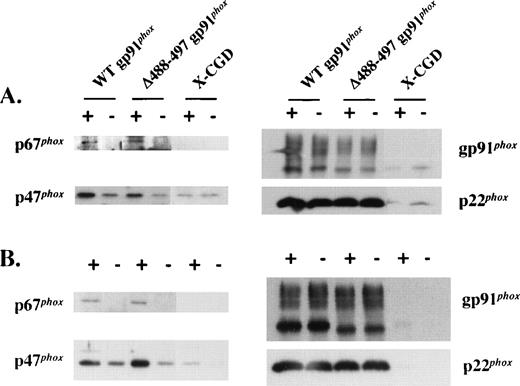
Upon phagocyte activation, cytosolic oxidase components translocate to the plasma membrane to assemble the functional NADPH oxidase. Multiple contact points between p47phox and the gp91phox and p22phox subunits of flavocytochrome b558 have been described previously.14,25-29 Among these, a missense mutation predicting an Asp-Gly substitution at residue 500 of gp91phox has been reported to lead to defective translocation of p47phox and p67phox.14 The proximity of the Δ488-497 deletion in gp91phox to Asp500 prompted us to test whether translocation of p47phox and p67phox to the plasma membrane during oxidase assembly was affected by this deletion. As shown in Fig 3A, PMA-stimulated translocation of p47phox and p67phox to the plasma membrane in intact Δ488-497 gp91phoxPLB-985 cells was similar to that seen for WT gp91phox PLB-985 cells. A similar result was obtained in a cell-free oxidase reconstitution system using SDS for activation (Fig 3B). The band seen below the 68 kD marker protein is the high mannose 65-kD precursor of gp91phox.24 We have previously shown that this species is localized in the ER, as determined by cell fractionation using a 10% to 60% continuous sucrose gradient.24 The membranes isolated by discontinuous sucrose gradient (20% and 38%) in the current experiment may be contaminated with intracellular membranes including ER, which may account for the presence of the precursor in the preparation (Figs 1B and 3B). A small amount of p47phox but not p67phox was seen in WT and Δ488-497 gp91phox-transfected PLB-985 granulocytes even in the absence of PMA, as well as in X-CGD PLB-985 cells (Fig 3A and B). This likely reflects nonspecific binding of p47phoxto membranes, which has also been observed by others.30Alternatively, an unexpected priming of PLB-985 granulocytes during culture at 37°C may cause the translocation of p47phox to the membrane, which is flavocytochrome-independent.
Translocation of p47phox and p67phox to plasma membrane in intact cells activated with PMA and in the cell-free oxidase reconstitution assay stimulated with SDS. (A) The indicated cells undergone granulocytic differentiation for 5 days were stimulated with (+) or without (−) PMA (500 ng/mL) for 10 minutes at 37°C, and the plasma membranes were prepared on discontinuous sucrose gradients and analyzed for translocation of p47phox and p67phox by immunoblot analysis using p47phox and p67phox antibodies (left panel). After stripping, the blots were reprobed with gp91phox and p22phox monoclonal antibodies to show an equal loading (right panel). Each lane was loaded with 5 μg of proteins. (B) Membranes separated from the indicated cells were mixed with 3-fold of neutrophil cytosol in the cell-free oxidase reconstitution assay. After 10 minutes of incubation at 25°C in the presence (+) or absence (−) of 100 μmol/L SDS, the membranes were reisolated by ultracentrifugation and detected for the translocation of p47phox and p67phox (left panel) by immunoblot analysis as described in (A). Each lane was loaded with 5 μg of proteins.
Translocation of p47phox and p67phox to plasma membrane in intact cells activated with PMA and in the cell-free oxidase reconstitution assay stimulated with SDS. (A) The indicated cells undergone granulocytic differentiation for 5 days were stimulated with (+) or without (−) PMA (500 ng/mL) for 10 minutes at 37°C, and the plasma membranes were prepared on discontinuous sucrose gradients and analyzed for translocation of p47phox and p67phox by immunoblot analysis using p47phox and p67phox antibodies (left panel). After stripping, the blots were reprobed with gp91phox and p22phox monoclonal antibodies to show an equal loading (right panel). Each lane was loaded with 5 μg of proteins. (B) Membranes separated from the indicated cells were mixed with 3-fold of neutrophil cytosol in the cell-free oxidase reconstitution assay. After 10 minutes of incubation at 25°C in the presence (+) or absence (−) of 100 μmol/L SDS, the membranes were reisolated by ultracentrifugation and detected for the translocation of p47phox and p67phox (left panel) by immunoblot analysis as described in (A). Each lane was loaded with 5 μg of proteins.
To provide additional evidence that gp91phoxresidues 488-497 are not essential for oxidase assembly, we synthesized a peptide corresponding to residues 488-497 and tested its ability to inhibit O2− production in the cell-free oxidase assay using membrane and cytosol isolated from normal neutrophils. As shown in Fig 4, peptide 488-497 inhibited oxidase activity only at high concentrations (IC50, ∼500 μmol/L), whereas a peptide derived from residues 86-102 of gp91phox (containing a probable p47phox binding motif28,29) had an IC50 of 2 μmol/L (Fig 4). The 488-497 peptide was also much less potent at inhibiting O2− production compared with other peptides derived from gp91phox domains proposed as binding sites for cytosolic oxidase components, including peptide 491-504 containing Asp500 (IC50, 10 μmol/L),14peptide 559-570 (IC50, 28 μmol/L),31 and peptide 550-569 (IC50, 4 μmol/L).32 Consistent with our results, Kanegasaki’s group has reported that IC50 of peptide 484-502 was greater than 300 μmol/L.32
Effect of peptide 488-497 on NADPH oxidase activity in the cell-free oxidase assay. Plasma membrane (8 μg) and cytosolic fractions (20 μg) separated from normal neutrophils were used in the cell-free assay. Peptide 488-497 of gp91phox (•) as well as a control peptide corresponding to residues 86-102 of gp91phox containing a putative p47phox binding site 86-93 (▪) were added to the assay before the addition of SDS, and O2−generation was measured. The activity of superoxide production in the absence of peptides was 216 ± 22 nmol/min/mg of membrane proteins. The data represent the mean ± SD of 3 separate experiments.
Effect of peptide 488-497 on NADPH oxidase activity in the cell-free oxidase assay. Plasma membrane (8 μg) and cytosolic fractions (20 μg) separated from normal neutrophils were used in the cell-free assay. Peptide 488-497 of gp91phox (•) as well as a control peptide corresponding to residues 86-102 of gp91phox containing a putative p47phox binding site 86-93 (▪) were added to the assay before the addition of SDS, and O2−generation was measured. The activity of superoxide production in the absence of peptides was 216 ± 22 nmol/min/mg of membrane proteins. The data represent the mean ± SD of 3 separate experiments.
Electron transport from NADPH to FAD is disrupted in Δ488-497 gp91phox.
We have previously shown that electrons are transferred to INT primarily from the flavin center of gp91phox.4 We have used this method to show that the proximal electron transfer pathway (from NADPH to flavin) is normal in a mutant form of gp91phox in which there is an amino acid substitution affecting one of the heme redox potentials.13 It was therefore of interest to see if the proximal electron transport pathway of the Δ488-497 mutant was functional. Partially purified flavocytochrome b558from membranes of WT gp91phox PLB-985 cells was able to support INT reductase activity as efficiently as flavocytochrome b558 purified from neutrophils (not shown). In contrast, flavocytochrome b558 from Δ488-497 gp91phox PLB-985 cells was incapable of INT reductase activity, suggesting that either flavin or NADPH binding is affected in this mutant. As expected, the equivalent purification fraction from X-CGD PLB-985 cells also had no activity (Fig 5).
INT reductase activity in flavocytochromeb558 purified from membranes of X-CGD, ▵488-497 gp91phox, and WT gp91phoxPLB-985 cells. INT reductase activity was determined in the 96-well microtiter plate assay as described in the Materials and Methods. Each well contained the equivalent of 0.5 pmol flavocytochromeb558 and 2.5 × 106 cell equivalents of neutrophil cytosol. The results are expressed as the mean ± SEM.
INT reductase activity in flavocytochromeb558 purified from membranes of X-CGD, ▵488-497 gp91phox, and WT gp91phoxPLB-985 cells. INT reductase activity was determined in the 96-well microtiter plate assay as described in the Materials and Methods. Each well contained the equivalent of 0.5 pmol flavocytochromeb558 and 2.5 × 106 cell equivalents of neutrophil cytosol. The results are expressed as the mean ± SEM.
The Δ488-497 mutant flavocytochromeb558 has a normal affinity for FAD.
To evaluate the capacity of the Δ488-497 gp91phoxmutant to bind FAD, we measured the affinity of the partially purified flavocytochrome b558 preparations for FAD by equilibrium dialysis as described in the Materials and Methods. Both WT gp91phox and Δ488-497 gp91phox flavocytochrome preparations had virtually identical affinities for FAD of approximately 66 nmol/L (Table 3). These values are consistent with the literature values of 20 to 85 nmol/L.33-35 An equivalent volume of the X-CGD PLB fraction eluted from the heparin column that corresponded to the peak fractions of the Δ488-497 gp91phox and WT gp91phoxflavocytochrome b558 showed no ability to bind FAD. In all cases, the recovery of FAD from each equilibrium dialysis experiment (sample + buffer) was 100% ± 4%.
Analysis of NADPH binding in the Δ488-497 gp91phox flavocytochrome b558.
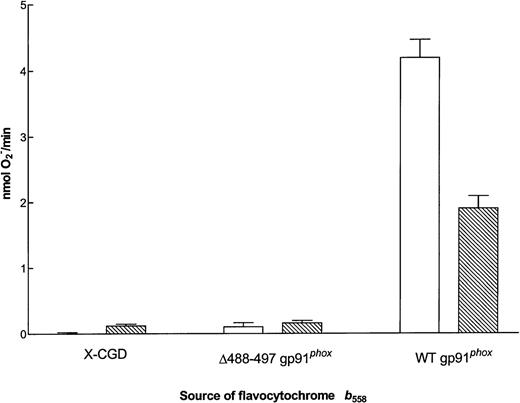
Residues 488-497 have been postulated to lie near the NADPH binding domain of gp91phox.36 To address whether the Δ488-497 mutation alters the affinity of flavocytochromeb558 for NADPH, we measured the Km for NADPH on mutant flavocytochrome partially purified from membranes of Δ488-497 gp91phox PLB-985 cells. The NADPH oxidase has the ability to use NADPH or NADH as substrate, although the Km for NADPH is approximately 10-fold lower (∼40 μmol/L).37-39 The Km for NADPH of the enzyme in the cell-free system using neutrophil membranes was found to be 57.1 ± 1.8 μmol/L (n = 22), and the Km of purified neutrophil flavocytochrome b558 was 27.5 ± 1.3 μmol/L (n = 17; A. Cross, unpublished data). The Km of the flavocytochrome purified from membranes of WT gp91phox PLB-985 cells was found to be 30.4 ± 1.5 μmol/L (not shown). No activity was evident in the cytochrome purified from the Δ488-497 gp91phox PLB-985 cells. Increasing the substrate concentration to as high as 4.9 mmol/L NADPH did not induce any O2− formation from the Δ488-497 mutant and neither did the addition of 4.9 mmol/L NADH (Fig 6). This suggests that either the Δ488-497 gp91phox cannot bind the substrate NADPH or it cannot support electron transfer from NADPH to FAD.
O2− generating activity of flavocytochrome b558 purified from membranes of X-CGD, ▵488-497 gp91phox, and WT gp91phox PLB-985 cells. Cell-free O2− assays were performed as described in the Materials and Methods using the equivalent of 0.5 pmol flavocytochromeb558 per well and 2.5 × 106 cell equivalents of neutrophil cytosol. (□) The maximum rate of O2− production with 4.9 mmol/L NADPH as substrate (mean ± SEM); (▧) the maximum rate of O2− production with 4.8 mmol/L NADH as substrate.
O2− generating activity of flavocytochrome b558 purified from membranes of X-CGD, ▵488-497 gp91phox, and WT gp91phox PLB-985 cells. Cell-free O2− assays were performed as described in the Materials and Methods using the equivalent of 0.5 pmol flavocytochromeb558 per well and 2.5 × 106 cell equivalents of neutrophil cytosol. (□) The maximum rate of O2− production with 4.9 mmol/L NADPH as substrate (mean ± SEM); (▧) the maximum rate of O2− production with 4.8 mmol/L NADH as substrate.
Over the past 10 years, several groups have tried to identify the NADPH binding component of the oxidase complex by labeling with32P- or 3H-labeled NADPH analogues. However, different results have been reported by different groups, including a 66-kD cytosolic protein,40 an approximately 32-kD cytosolic protein,41 a 52-kD membrane-associated protein,42 and p67phox,9therefore leaving the issue still uncertain. By comparison with known NADPH binding regions of members of Ferredoxin-NADP+Reductase family, Segal’s group,6 Rotrosen et al,8 and Sumimoto et al7 have proposed that the apparent NADPH binding pocket resides in the carboxyl terminal portion of gp91phox. This postulation has subsequently been strongly supported by the experiments showing that reflavinated and relipidated membrane fractions isolated from normal neutrophils are capable of supporting O2− generation in the absence of any cytosolic proteins.43 44
We attempted to analyze the ability of the Δ488-497 mutant flavocytochrome b558 to bind NADPH by affinity labeling using the photoaffinity label [4-N-(4-azido-2-nitrophenyl) aminobutyryl] NAD[32P].45 Despite using a number of different experimental conditions, it was not possible to convincingly, or reproducibly, label WT gp91phox from either neutrophils or WT gp91phox PLB-985 cell membranes. Therefore, we were not able to unambiguously determine if the Δ488-497 mutant can bind NADPH.
Taylor et al36 have predicted a 3-dimensional structure of gp91phox using Ferredoxin-NADP+Reductase as a template and proposed that an α-helical loop composed of residues 484-503 lies over the NADPH binding cleft. During oxidase activation, access of NADPH into the binding site could potentially be regulated by interactions of this loop with cytosolic oxidase components.36 Although the impaired translocation of cytosolic oxidase subunits reported for a gp91phoxmutant with Asp500Gly substitution indirectly supports this hypothesis,14 we found no evidence that gp91phox residues 488-497 are required for interactions with cytosolic oxidase components during oxidase assembly. Deletion of these residues did not affect translocation of p47phox and p67phox, and the peptide corresponding to residues 488-497 was a weak inhibitor of superoxide production in the cell-free oxidase assay. It therefore is likely that the 488-497 deletion in gp91phox either leads to a failure of NADPH to bind or a defect in the subsequent transfer of electrons to FAD. Either would be consistent with the observed absence of INT reductase activity for the Δ488-497 gp91phox flavocytochrome b558. The data presented here further imply that simply removing the loop is insufficient to expose a functional NADPH binding site.
The majority of patients with CGD present in early childhood with severe, recurrent bacterial and fungal infections. However, the CGD patient with the Δ488-497 gp91phox mutation was in good health until 69 years of age, when he developed an infection due to Burkholderia cepacia.16 Patient neutrophils were reported to have trace amounts of O2− production, which may have accounted for this milder phenotype, although we could not confirm the presence of residual O2−-generating activity in Δ488-497 gp91phox PLB-985 cells. Also, note that the grandson of this patient died at 5 years of age due toBurkholderia cepacia pneumonia,16 suggesting that other factors influencing host defense may have accounted for the late age of presentation in the index patient.
ACKNOWLEDGMENT
The authors thank Drs Dirk Roos and Arthur J. Verhoeven (Central Laboratory of The Netherlands Blood Transfusion Service, Amsterdam, The Netherlands) for kindly providing anti-gp91phox and anti-p22phoxmonoclonal antibodies 48 and 449, respectively. In addition, Dr David Lambeth (Emory University, Atlanta, GA) provided polyclonal anti-p47phox and Dr Paul Heyworth (The Scripps Research Institute, La Jolla, CA) provided polyclonal anti-p67phox antibodies. The anti-flavocytochrome b558 monoclonal antibody 7D5 was a generous gift from Dr Michio Nakamura (Nagasaki University, Nagasaki, Japan).
Supported in part by Grants No. RO1 HL45635 and PO1 HL 353586 to M.C.D. and AI-24838 to A.R.C.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mary C. Dinauer, MD, PhD, Wells Center for Pediatric Research, Cancer Research Bldg, Room 466, 1044 W Walnut St, Indianapolis, IN 46202; e-mail: mdinauer@iupui.edu.