We have developed a time-lapse camera system to follow the replication history and the fate of hematopoietic stem cells (HSC) at a single-cell level. Combined with single-cell culture, we correlated the early replication behavior with colony development after 14 days. The membrane dye PKH26 was used to monitor cell division. In addition to multiple, synchronous, and symmetric divisions, single-sorted CD34+/CD38− cells derived from fetal liver (FLV) also gave rise to a daughter cell that remained quiescent for up to 8 days, whereas the other daughter cell proliferated exponentially. Upon separation and replating as single cells onto medium containing a cytokine cocktail, 60.6% ± 9.8% of the initially quiescent cells (PKH26 bright) gave rise again to colonies and 15.8% ± 7.8% to blast colonies that could be replated. We have then determined the effects of various regulatory molecules on symmetry of initial cell divisions. After single-cell sorting, the CD34+/CD38− cells derived from FLV were exposed to flt3-ligand, thrombopoietin, stem cell factor (SCF), or medium containing a cytokine cocktail (with SCF, interleukin-3, interleukin-6, granulocyte-macrophage colony-stimulating factor, and erythropoietin). Whereas mitotic rate, colony efficiency, and asymmetric divisions could be altered using various regulatory molecules, the asymmetric division index, defined as the number of asymmetric divisions versus the number of dividing cells, was not altered significantly. This observation suggests that, although lineage commitment and cell proliferation can be skewed by extrinsic signaling, symmetry of early divisions is probably under the control of intrinsic factors.
HEMATOPOIETIC STEM CELLS (HSC) are characterized by the dual abilities to self-renew and to differentiate into progenitors of all the mature blood cell lineages. These 2 features are evident after bone marrow transplantation and require that the HSC undergo rounds of asymmetric divisions to generate mature cells of the distinct blood lineages as well as cells to sustain long-term hematopoiesis.1 The 2 daughter cells from a HSC may be initially equivalent, but subsequent cell divisions must result in different fates of the progeny cells.2 It has been suggested that the hallmark of a stem cell might be its ability to divide asymmetrically to produce a daughter cell identical to the mother and another cell committed to differentiation.3 4Alternatively, a balance between symmetrical cell divisions that result in self-renewal versus that which result in differentiation might be able to maintain the stem cell pool and provide a source of multipotent progenitors. Even if the latter were true, asymmetric division must have occurred during ontogenesis of these 2 populations, and further asymmetric divisions must occur during their multilineage differentiation.
A central question in developmental biology is how a single cell can divide to produce 2 daughter cells that adopt distinct fates.4 Theoretically, daughter cells with different fates can arise by means of the following mechanisms. First, they may be different from each other at the time of cell division, ie, due to intrinsic factors. A parental factor, such as a transcription factor, may be distributed unevenly to the daughter cells. Second, the daughter cells may be similar at the time of cell division, but become different upon subsequent exposure to environmental signals, such as a cytokine, ie, due to extrinsic factors.2 4
Thus far, remarkably little is known if and how hematopoietic stem cells divide in a self-renewing, asymmetric fashion. Recently, studies of asymmetric division of neural stem cells in Drosophila and mammals have provided exciting and new insights into the mechanism of stem cell division and might serve as a model for hematopoietic reconstitution.3,5-7 These studies demonstrated that at least 3 types of asymmetric divisions can be found in neural progenitors. (1) In Drosophila, neuroblasts (NB) undergo a series of oriented asymmetric divisions to renew themselves and produce smaller ganglion mother cells. (2) In the peripheral nervous system, a sensory organ precursor (SOP) responsible for forming external sensory organs (ie, sensory bristles) generates an organ by dividing asymmetrically to form precursor cells to produce 2 outer support cells (a hair and a socket cell), a sensory neuron, and a sheath cell.6 (3) In the central nervous system, MP2 precursors are a pair of embryonic neural precursors that divides only once to produce 2 different postmitotic neurons.
By analyzing the colony-forming potentials of individual daughter cells in the hematopoietic system, Leary et al8,9 have demonstrated that approximately 10% of the cell divisions of multipotent progenitors are asymmetric. Mayani et al10 also described asymmetry of cell division of hematopoietic progenitors. Recently, Brummendorf et al,11 from the same group, reported a linkage between cell division rate and asymmetric cell divisions. They showed that the proliferative potential and cell cycle properties were unevenly distributed among daughter cells derived from single-sorted HSC from fetal liver (FLV) and that expansion potential is associated with asymmetric division. According to this evidence asymmetric cell divisions seemed to occur in 3% to 20% of the cultured cells and lineage commitment did not seem to be influenced by cytokines. Based on all these observations, it has been suggested that stem cell differentiation is a stochastic event.
The discoveries in neural stem cells have been made possible by imaging studies of neuroblast divisions and development.4Visualization of cell divisions with time-lapse camera systems thus represents a powerful tool to study cell replication and symmetry of division. In this study, we have monitored the replication history of human candidate HSC with a time-lapse camera system and demonstrated that asynchronous or asymmetric divisions of CD34+ cells indeed occur. This asymmetry is shown by different replication behavior of the 2 daughter cells. One daughter cell remained quiescent, whereas the other multiplied to yield hundreds to thousands of cells after 10 days. Mitotic activity as well as asymmetric divisions decrease with ontogenic age, ie, are more frequent among CD34+/CD38− cells derived from FLV than those from adult bone marrow, whereas the fraction of asymmetric divisions stays unchanged. Monitoring of asymmetrical divisions represents a measurable and reproducible measure of candidate HSC populations.
MATERIALS AND METHODS
Human hematopoietic progenitor cell preparations.
FLV samples were obtained from legal abortions at 17 to 24 weeks of gestational age and were supplied by Advanced Bioscience Resources, Inc (Alameda, CA). FLV cells were prepared by homogenizing the tissue through a Cell Strainer (Becton Dickinson Labware, Lincoln Park, NJ) and were washed once in RPMI 1640 containing 5% fetal calf serum (FCS; Germini, Calabasas, CA). Umbilical cord blood (UCB) specimens were collected at the University of California, San Diego delivery room as well as supplied by Advanced Bioscience Resources Inc, using heparin (Marsam, Cherry Hill, NJ) as anticoagulant. Healthy subjects were recruited for adult bone marrow samples (ABM). Approximately 40 to 50 mL of bone marrow was drawn from multiple sites (10 to 15 mL each time) from the posterior iliac crest. Subjects gave informed consent to donate marrow for research. Low density mononuclear cells (MNC) from FLV, UCB, and ABM were obtained by Ficoll-Hypaque (Histopaque 1077; Sigma Chemical Co, St Louis, MO) separation and washing. All projects involving human subjects and use of human tissues have been reviewed and approved by the Human Subjects Committee of the University of California, San Diego.
Labeling with PKH-26.
The procedure for staining cells using PKH-26 (in a kit from Sigma) was provided by the manufacturer. Briefly, CD34+ cells (from different hematopoietic tissues) were washed using PRMI 1640 medium without serum at room temperature and resuspended in 1 mL of Diluent C as supplied in the kit. Then, 1 mL of Diluent C containing 8 × 106 molar PKH-26 dye was mixed with the cells and incubated at room temperature for 5 minutes. The staining reaction was halted by adding 2 mL of phosphate-buffered saline (PBS) containing 1% FCS and incubating for 1 additional minute. The cells were washed with 4 mL of 10% FCS/RPMI 1640 medium. It has been demonstrated that the PKH dye binds tightly to the lipid layer of cell membrane and is distributed equally between the daughter cells after each division.12 13 Light and phase contrast microscopy in preliminary experiments did not show differences in the morphology of the cells after staining.
Flow cytometry and index sorting.
The hematopoietic progenitor cell preparations marked with PKH-26 were stained with CD34 (HPCA-2 fluorescein isothiocyanate [FITC]; Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA) and CD38 (Cy-chrome; Pharmingen, San Diego, CA). Cells were stained with monoclonal antibodies for 30 minutes on ice and washed 2× with 2% FCS/RPMI 1640 medium. Flow cytometric sorting was performed on a FACStarPlus equipped with an Argon ion laser tuned at 488 nm. Single cells of CD34+/CD38− subsets were deposited singly onto a 72-well Terasaki plate (Robinson Scientific, Sunnyvale, CA) with the use of an Automated Cell Deposition Unit (ACDU) and Index Sorting Device according to preset sort-gates. Data acquisition was performed using Lysys II (BDIS) software.13 The pulse processor module index sorting device permitted the linkage of list-mode data of each cell to the location of the well in the microtiter dish. Three-color, 5-parameter multidimensional analysis was performed on a FACScan using PAINT-A-GATEPLUS software (BDIS). This program allows the log-transformation of side scatter that permits easier delineation of different hematopoietic cell populations.
Single-cell suspension culture technique.
In the single-cell suspension culture system,13 14 each well contained a mixture of myeloid long-term culture medium (Stem Cell Technology, Vancouver, British Columbia, Canada) containing 12.5% horse serum, 12.5% FCS, 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 0.2 mmol/L I-inositol, 20 mol/L folic acid, and antibiotics and supplemented with 2.5 U/mL recombinant human erythropoietin (Epo; Amgen, Thousand Oaks, CA), 10 ng/mL recombinant human interleukin-3 (IL-3), 500 U/mL recombinant human (rh) IL-6, 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), 2.5 ng/mL recombinant human basic fibroblast growth factor (bFGF), 10 ng/mL recombinant human insulin-like growth factor-1 (IGF-1; Collaborative Research, Bedford, MA), and 50 ng/mL recombinant human stem cell factor (SCF; Genzyme, Boston, MA). This combination of cytokines is referred to as “cocktail” in subsequent experiments. For 96-well microtiter plates, the cells were cultured in 200 μL, and for the 72-well Terasaki plates, the culture volume is 20 μL. All cultures were incubated in 5% CO2 in air at 37°C in a fully humidified incubator. Cell growth was scored for the presence of dispersed cells, cell clusters, or a mixture of both on days 10 to 14. Cells were scored as dispersed cells when an expansion of a minimum of 40 cells was generated that appeared as dispersed round translucent cells after 10 to 14 days of culture. Others grew into clusters of typically erythroid colonies or myeloid colonies and were scored according to the typical morphology. Other single cells grew into a mixture of clusters among dispersed blast cells.
With our single-cell suspension culture technique, we were able to define precisely the colony efficiency (CE), the growth pattern, and the replating potential of each phenotype of CD34+cells.15 CE is defined as the percentage of wells each initially containing 1 single cell that developed into colonies after 10 to 14 days. Blast colony efficiency (BE) is defined as the percentage of colonies that showed dispersed (blast colonies) and mixed growth pattern (blasts with clusters in between) that have the potential to give rise to further colonies when replated in our suspension culture system.14 15
Experiments were also performed to test the influence of serum-free medium (QB60; kindly provided by Dr Ronald Brown, Quality Biologics, Gaithersburg, MD) on kinetics and symmetry of cell division, as well as on colony formation, as compared with the above-described myeloid long-term culture medium. In subsequent experiments, the impact of various regulatory molecules on symmetry of cell division was also determined by the addition of specific cytokines instead of the above-described cocktail. The concentrations of regulatory molecules used were as follows: 50 ng/mL SCF, 100 ng/mL thrombopoietin (TPO; kindly provided by Amgen, Thousand Oaks, CA), and 100 ng/mL Flt3-ligand (FL3-L; kindly provided by Dr Douglas Williams, Immunex Corp, Seattle, WA).
Time-lapse camera system.
Time-lapse measurements of cells in multiple microscope fields over long periods of time (hours to days) require instrumentation that can operate in an automatic manner. Briefly, images were acquired using an inverted fluorescent microscope (Nikon Diaphot 300; Nikon Inc, Melville, NY) with a 4× objective such that an entire well of a Terasaki plate can be observed in a single image (1,938 × 1,523 μm) field of view. Illumination was provided by a 100-W Mercury arc lamp that passed through a 41003 filter set (Chroma Technologies, Brattleboro, VT). Digitized images were acquired and stored on a SGI O2 workstation (Silicon Graphics, Mountain View, CA). A motorized X, Y, Z stage (Ludl, Hawthorn, NY) moved the stage between wells so that multiple images could be rapidly collected. All acquisition and processing functions were controlled by the Isee software (Inovision Corp, Durham, NC), which allowed for the analysis of multiples from the list to create a composite image that showed changes in cell shape or position over time.16 Cells in a Terasaki plate can be simultaneously tracked in a single experiment and revisited at prescribed time intervals.
After the cells were deposited as single cells, the replication history of HSC was monitored initially every 3 to 12 hours for 7 to 10 days. For each experiment, the number of wells analyzed were 72 to 216. The replication history of the HSC was measured using the PKH membrane dyes (see above), which were available in both green (PKH2) and red (PKH26) forms. These dyes consist of a fluorophore attached to an aliphatic carbon backbone that binds irreversibly to the lipid bilayer. With each cell division, the fluorescent intensity of the PKH dye is reduced by one half. Thus, one can determine, using the time-lapse camera system, the replication history of the daughter cells. We determined the kinetics of cell division (by measuring the doubling times), whether both daughters divided symmetrically, and under which conditions the cells underwent asymmetric division. Initial- ly, we performed these experiments with CD34+cells derived from FLV. The same plates were kept in culture for 10 to 14 days whenever possible. The colony efficiency and growth patterns were determined at the end of this period. The relationship between short-term kinetics and symmetry of division and the outcome of long-term culture was studied.
Mitosis index is defined as the number of single-sorted cells that have shown cell division after 8 days versus the total number of cells of the same phenotype deposited. Asymmetric division is defined as the number of cells that demonstrated at least 1 asymmetric division during the course of 8 days versus the total number of cells deposited. The asymmetric division index (ADI) is defined as the number of cells that demonstrated at least 1 asymmetric division during the course of 8 days divided by the number of dividing cells.
Statistical analysis.
For statistical analysis, a personal computer program, Testimate (supplied by IDV Daten analyse, Gauting-Munich, Germany), was used. Data reported were given as the mean ± standard deviations or as the median and range, wherever applicable. The Student’st-test was applied to verify the differences in mitotic rate, colony efficiency, percentage of asymmetric divisions, and ADI of CD34+/CD38− cells between 2 subgroups. The Kruskal-Wallis Analysis was applied to validate the differences in CD34+/CD38− cells from the MNC samples from various sources, eg, FLV, or UCB compared with ABM. Wherever possible, the paired t-test was applied to verify the difference between matched observations, eg, control and treated preparations handled in parallel, or cells with symmetric divisions and those with asymmetric divisions from the same sample.
RESULTS
In the first series of experiments, we have monitored the replication history of hematopoietic progenitors that were CD34+. CD34+ cells, without further fractionation derived from FLV, UCB, or ABM, were used for the initial experiments. The cells were stained in bulk with PKH26 for visualization in fluorescence light, followed by resorting as single cells in medium containing the above-described cytokine cocktail, and were deposited onto a Terasaki plate. At least 72 wells and up to 216 wells were analyzed for each experiment. Preliminary experiments using light and phase-contrast microscopy demonstrated that there was no significant difference in morphology between cells before and after PKH26 staining or between cells before and after the first divisions. Image analysis included simultaneous assessment of cell number and fluorescence intensity in each of 72 wells at defined intervals, eg, every 3 to 12 hours for 10 days. This enabled us to define precisely the replication history of each single CD34+ cell. We found that the first division typically occurred at 36 to 38 hours after being seeded. After the first division, the subsequent doubling times were 12 hours, irrespective of the cell source, ie, from different ontogenic ages. The majority (∼65% to 75%) of the CD34+ cells showed multiple synchronous symmetric divisions within a single well. However, approximately 30% of the single-sorted CD34+ cells derived from FLV gave rise to a daughter cell that remained quiescent for up to 8 days, whereas the other daughter cell multiplied exponentially. Such asynchronous divisions represented asymmetric divisions with respect to the replication behavior of the 2 daughter cells.
We have then focused our studies on CD34+/CD38− cells derived from FLV, because our previous experiments indicated that this subset contained significantly higher frequencies of candidate stem cells with self-renewal capacity.14 15 Preliminary experiments also demonstrated that 39.7% ± 10.3% (mean ± SD) of CD34+/CD38− cells derived from FLV underwent asymmetric divisions and were consistently and significantly higher than that of CD34+/CD38+ cells (30.7% ± 6.9%, n = 5, P = .0325, paired t-test).
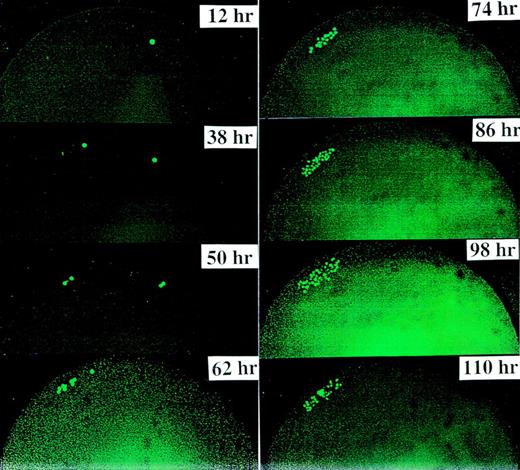
Figure 1demonstrates a typical symmetric division of 1 CD34+/CD38− cell derived from FLV. We confirmed that the first division of this cell type also occurred consistently at 36 to 38 hours after culturing; thereafter, the cell doubling time was every 12 hours. The daughter cells continued to divide every 12 hours, giving rise to 4 cells at 48 to 50 hours, 8 cells at 60 to 62 hours, 16 cells at 72 to 74 hours, 32 cells with dim fluorescence at 84 to 86 hours, and so forth.
This figure demonstrates a typical symmetric division of one CD34+/CD38− cell derived from FLV. We found that the first cell division occurred consistently at 36 to 38 hours after culturing. Thereafter, the cell doubling time was 12 hours. Previous and subsequent experiments with shorter observation intervals (eg, every 3 hours) have demonstrated that cells might migrate, partly due to movements of the culture plates and partly due to the migratory property of the progenitor cells themselves.
This figure demonstrates a typical symmetric division of one CD34+/CD38− cell derived from FLV. We found that the first cell division occurred consistently at 36 to 38 hours after culturing. Thereafter, the cell doubling time was 12 hours. Previous and subsequent experiments with shorter observation intervals (eg, every 3 hours) have demonstrated that cells might migrate, partly due to movements of the culture plates and partly due to the migratory property of the progenitor cells themselves.
Divisional history of 1 single CD34+/CD38− cell stained with PKH26, derived from an FLV sample. At 0 hour, a cell showing bright fluorescence is depicted. At 38 hours, the cell has divided, yielding 2 daughter cells with bright PHK26 fluorescence. At 98 hours, 1 cell has maintained bright fluorescence among 32 cells with dim fluorescence. On day 8 (at 194 hours), 1 cell with bright fluorescence has remained among several hundreds of other cells. The upper 4 pictures were taken with fluorescence microscopy. The bottom picture shows the same cell culture on day 8 in both phase contrast and fluorescence microscopy.
Divisional history of 1 single CD34+/CD38− cell stained with PKH26, derived from an FLV sample. At 0 hour, a cell showing bright fluorescence is depicted. At 38 hours, the cell has divided, yielding 2 daughter cells with bright PHK26 fluorescence. At 98 hours, 1 cell has maintained bright fluorescence among 32 cells with dim fluorescence. On day 8 (at 194 hours), 1 cell with bright fluorescence has remained among several hundreds of other cells. The upper 4 pictures were taken with fluorescence microscopy. The bottom picture shows the same cell culture on day 8 in both phase contrast and fluorescence microscopy.
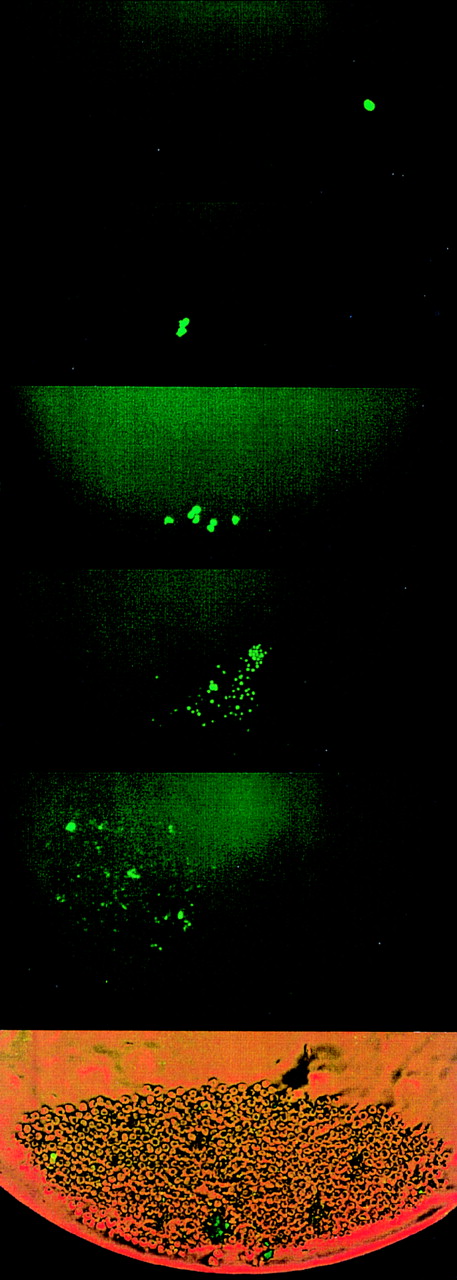
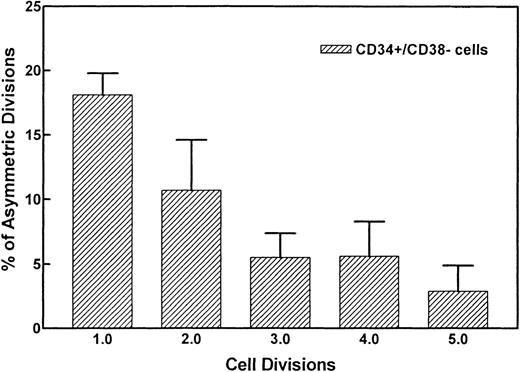
Figure 2 demonstrates the divisional history of 1 single CD34+/CD38− cell that has divided asymmetrically, as monitored by time-lapse camera system over a period of 8 days. In the first 36 to 38 hours, the image confirmed that 1 single cell with very bright fluorescence was deposited in the well. After 36 to 38 hours, 2 cells with bright PKH26 fluorescence were observed. Seventy-two to 74 hours after culturing, 1 bright cell was observed among 8 other cells with dim fluorescence. Whereas the 1 PKH26 bright cell maintained its fluorescence intensity and remained quiescent, the other cells continued to divide symmetrically to give rise to 16, 32, 64 cells, etc, every 12 hours, such that on day 8, the same bright cell was observed among hundreds of fluorescence-negative cells, thus providing evidence that asymmetric divisions occurred among CD34+/CD38− cells derived from FLV. Other CD34+/CD38− cells initially gave rise to 2 daughter cells that appeared equivalent after the first mitosis, but then divided asymmetrically after the second division. Figure 3 shows a typical example, ie, 1 parental cell gave rise to 2 daughter cells, which in turn gave rise to 4 cells, with 1 of 4 cells then remaining quiescent, whereas the other 3 multiplied symmetrically, giving rise to altogether 7 (6 + 1) cells at 60 hours after culturing. We have analyzed the percentages of asymmetric divisions found after the first, second, third, and up to the fifth cell division. The results of 4 experiments are summarized in Fig 4. Forty-two percent and 25% of all the asymmetric divisions occurred during the first and second mitosis, respectively. Asymmetric divisions are rarely found in the third (13%), fourth (13%), and fifth (7%) waves of cellular divisions.
Other CD34+/CD38−cells initially gave rise to 2 daughter cells that appeared equivalent, but then divided asymmetrically after the second or third division. This figure depicts a typical example, ie, 1 parent cell gave rise to 2 daughter cells (at 38 hours), which in turn gave rise to 4 cells, with 1 of them then remaining quiescent and the other 3 multiplying symmetrically in subsequent divisions, yielding 7 cells at 60 hours, ie, on day 3. Thereafter, 1 cell maintained bright PKH26 fluorescence, whereas the other 6 multiplied to yield hundreds of cells after 9 days, which all showed very dim to nondetectable fluorescence.
Other CD34+/CD38−cells initially gave rise to 2 daughter cells that appeared equivalent, but then divided asymmetrically after the second or third division. This figure depicts a typical example, ie, 1 parent cell gave rise to 2 daughter cells (at 38 hours), which in turn gave rise to 4 cells, with 1 of them then remaining quiescent and the other 3 multiplying symmetrically in subsequent divisions, yielding 7 cells at 60 hours, ie, on day 3. Thereafter, 1 cell maintained bright PKH26 fluorescence, whereas the other 6 multiplied to yield hundreds of cells after 9 days, which all showed very dim to nondetectable fluorescence.
Percentages of asymmetric divisions found after the first, second, third, and the fifth cell division. After the fifth cycle, it became difficult to discern symmetry of divisions. Forty-two percent and 25% of all asymmetric divisions occurred during the first and second mitosis, respectively.
Percentages of asymmetric divisions found after the first, second, third, and the fifth cell division. After the fifth cycle, it became difficult to discern symmetry of divisions. Forty-two percent and 25% of all asymmetric divisions occurred during the first and second mitosis, respectively.
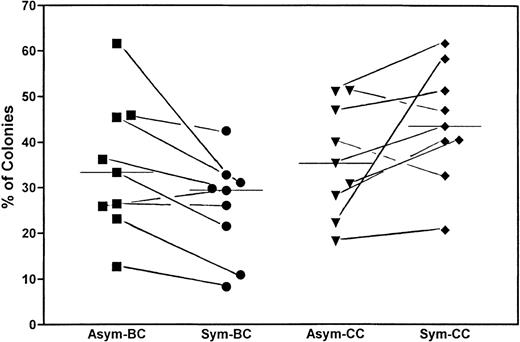
To correlate the replication behavior of the CD34+/CD38− cells in the first 8 days with the growth pattern of the corresponding single cell after culture, we have continued to incubate the plates for 10 to 14 days, as previously described.15 Our hypothesis is that cells showing asymmetric divisions would give rise to more blast colonies with dispersed and mixed growth patterns, whereas cells showing symmetric divisions gave rise to more clusters. Figure 5 depicts the results from 9 experiments. The median percentage of blast colonies (BC; which included wells with dispersed and mixed growth pattern) was 33.3% for cells showing asymmetric divisions and 29.4% for cells showing symmetric divisions. The median percentage of cluster colonies (CC) was 35.3% for cells showing asymmetric divisions and 43.5% for those with symmetric divisions. Although the colony data varied widely among individual samples, they remained fairly consistent for cells within the same specimen. In 8 of the 9 experiments, the percentage of BC was higher in cells showing asymmetric divisions (Asym) as compared with those showing symmetric divisions (Sym). Paired t-test also confirmed a decrease, albeit of marginal significance, in BC in the cells with symmetric division, with P = .0348.
Correlation between replication behavior of the CD34+/CD38− cells in the first 4 days with the growth pattern of the corresponding single cell after 14 days of culture. Wells containing cells showing asymmetric divisions (Asym) were compared with those showing only symmetric divisions (Sym) in their abilities to form BC, reflected in dispersed and mixed growth patterns, and their abilities to form CC after a total of 14 days of culture. The figure summarizes the results of 9 experiments. The percentages of the cells that gave rise to colonies versus those cells that have initially divided are shown. Although the colony data varied widely among samples, the paired t-test showed a significant decrease of BC among cells showing symmetric divisions versus those showing asymmetric divisions. The bars represent the corresponding medians.
Correlation between replication behavior of the CD34+/CD38− cells in the first 4 days with the growth pattern of the corresponding single cell after 14 days of culture. Wells containing cells showing asymmetric divisions (Asym) were compared with those showing only symmetric divisions (Sym) in their abilities to form BC, reflected in dispersed and mixed growth patterns, and their abilities to form CC after a total of 14 days of culture. The figure summarizes the results of 9 experiments. The percentages of the cells that gave rise to colonies versus those cells that have initially divided are shown. Although the colony data varied widely among samples, the paired t-test showed a significant decrease of BC among cells showing symmetric divisions versus those showing asymmetric divisions. The bars represent the corresponding medians.
Because our conventional culture system made use of FCS, which might contain minute quantities of various regulatory molecules that could induce differentiation or apoptosis and hence affect symmetry of divisions, we have compared the use of serum-free medium (QB-60) versus serum-containing medium. The results of 5 experiments are summarized in Table 1. Whereas there was no significant difference in mitotic rate, colony efficiency, asymmetric division, and ADI between cells in sera-containing media and those without, cells cultured in serum-free medium showed minimum interference by background fluorescence and were therefore used for subsequent studies.
To test the viability of the PKH26 bright cells, we have, on the one hand, applied propidium iodine to the wells with quiescent cells at 32+1 to 512+1 cell stages (days 4 to 8). All of the cells were examined in light and fluorescence microscopy and 70.3% ± 4.7% of the PKH26 bright cells were shown to be viable. To examine the functional integrity of the quiescent cells with bright PKH26 fluorescence derived from asymmetrically divided HSC, we separated the fluorescence bright cells from PKH26 dim cells at 32+1 to 64+1 cells stage (after 96 to 108 hours). They were then replated as single cells in 96-well plates with medium containing the cytokine cocktail. Culture of such cells for an additional 10 to 14 days showed that 60.6% ± 9.8% of the PKH26 bright cells (n = 137 cells from 3 different samples) gave rise to colonies, whereas only 15.9% ± 11.1% of the PKH26 dim cells (n = 121 cells from 3 different samples) did so. A total of 15.8% ± 7.8% of the PKH26 bright cells gave rise to colonies with dispersed growth pattern, which upon replating gave rise to a third generation of colonies.13-15 Colonies with dispersed growth pattern were observed in 2.5% ± 2.5% of the PKH26 dim cells, none of which showed replating potential.
After establishing that asymmetric divisions occurred among CD34+/CD38− cells, we have determined the percentages of asymmetric divisions among samples derived from different ontogenic ages. CD34+/CD38−cells derived from FLV, UCB, or ABM were sorted, stained with PKH26, and deposited as single-sorted cells. Divisions were monitored every 12 to 24 hours for up to 8 days. Mitotic rate, symmetry of the initial divisions, colony efficiencies, and ADI were documented. The data from at least 5 experiments (number of cells analyzed was 72 to 216 per measurement point) are summarized in Table2. Whereas the mitotic rate, colony efficiency, and percent of asymmetric divisions all decreased with ontogenic age, ie, from FLV, UCB, to ABM, the fraction of cells undergoing asymmetric division among dividing cells, ie, ADI, was consistently at 45%, irrespective of ontogenic age.
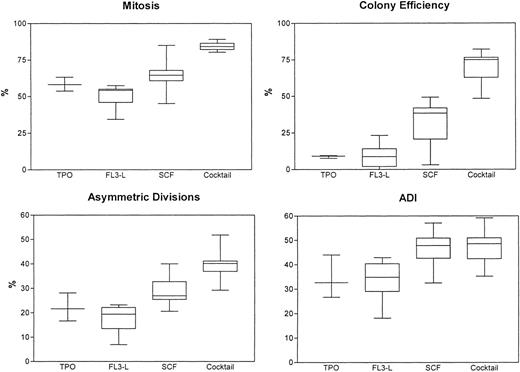
We have then compared the use of medium alone without addition of any regulatory molecules versus our conventional cytokine cocktail. In these series of 5 experiments, we found that, with medium alone, the cells died after approximately 3 to 4 days and, hence, the mitotic rate, ADI, and colony efficiency were low to nonmeasurable, with cell debris and background fluorescence. We then determined the effects of various regulatory molecules on symmetry of initial cell divisions. After single-cell sorting and deposition of CD34+/CD38− cells derived from FLV, the cells were exposed to regulatory molecules such as FL3-L, TPO, rhSCF, or a combination of the 3 or to medium containing the cocktail. Cell divisions were monitored every 6 to 12 hours for up to 8 days. The results (mean ± SD) from 6 experiments are summarized in Table 3 and Fig6. The mitotic rate, colony efficiency, and cells undergoing asymmetric divisions decreased significantly upon exposure to FL3-L, TPO, or SCF as compared with the cocktail. With the exception of FL3-L, which induced a marginal decrease, ADI did not change significantly upon exposure to different regulatory molecules or combinations thereof and has remained at approximately 40%. This invariance was also confirmed in 2 experiments using a combination of FL3-L+TPO+SCF. The ADI was 43.9% and 45.7%, respectively. On monitoring the replication history, we also observed that, with FL3-L, TPO, or SCF, the time interval between exposure to cytokines and first mitosis was 48 to 50 hours instead of 36 to 38 hours. Thus, although mitotic rate, cloning efficiency, divisional kinetics, and asymmetric divisions could be altered when using various regulatory molecules, the ADI was not altered significantly.
Mitotic rates, asymmetric divisions, colony efficiencies, and ADI of CD34+/CD38− candidate hematopoietic stem cells upon exposure to regulatory molecules: recombinant human TPO, FL3-L, and SCF. Cytokine cocktail (containing Epo, IL-3, IL-6, GM-CSF, SCF, bFGF, and IGF-1) was used for comparison.
Mitotic rates, asymmetric divisions, colony efficiencies, and ADI of CD34+/CD38− candidate hematopoietic stem cells upon exposure to regulatory molecules: recombinant human TPO, FL3-L, and SCF. Cytokine cocktail (containing Epo, IL-3, IL-6, GM-CSF, SCF, bFGF, and IGF-1) was used for comparison.
DISCUSSION
The fate of HSC during the first hours and days after transplantation in vivo or after seeding onto culture plates in vitro has been largely unknown. Conventional stem cell assays have attempted to estimate their proliferative and differentiating potential by making use of their ability to form colonies after incubation for 14 days.17,18Various modifications of long-term cultures (eg, long-term culture initiating cells [LTC-IC]) based on the use of stromal feeder layer derived from bone marrow have been used to estimate repopulating potential of stem cells, but such assays were not able to determine another dimension of stem cell activity, which is self-renewal capacity.19-22 Recent advances in the understanding of neural stem cell biology might serve as a model for HSC development.3 4 Based on these studies of neural stem cells, a fundamental property of stem cell development seems to be asymmetric division, during which the generation of cell diversity requires daughter cells to adopt different pathways. A central question in HSC biology is if and how a single HSC can divide to produce 2 progeny cells that adopt distinct fates.
To follow the precise replication history and the fate of HSC at a single-cell level, we have applied a time-lapse camera system to directly monitor early cell divisions. Combined with index sorting and single-cell culture to measure the colony formation of various CD34+ subsets, we were able to correlate the early replication behavior with colony development. The following conclusions can be drawn from the use of this technology. First, we have confirmed definitively that approximately 30% of the single-sorted CD34+ cells derived from FLV gave rise to a daughter cell that remained quiescent for up to 8 days, whereas the other daughter cell proliferated exponentially. Such asynchronous divisions probably represented asymmetric divisions and were found more frequently among CD34+/CD38− cells than in CD34+/CD38+ cells. These divisions could be observed during the first and subsequent rounds of mitosis among CD34+/CD38− cells. Second, the percentage of such asymmetric divisions decreased with ontogenic age, ie, higher in CD34+/CD38− cells derived from FLV than in those from UCB or ABM. However, despite the fact that asymmetric divisions, along with mitotic rate and colony efficiency, decreased significantly with ontogenic age, the ADI, ie, the ratio of cells undergoing asymmetric divisions versus dividing cells, remained constant at approximately 40%. Third, we have demonstrated that cells showing asynchronous or asymmetric divisions gave rise to more blast colonies than those showing symmetric divisions, a phenomenon consistent with the observations by Young et al.23 Upon replating as single cells onto fresh medium, 15.8% ± 7.8% of the PHK bright cells gave rise to colonies with dispersed growth pattern and demonstrated replating potential, whereas none of the PKH dim cells had replating potential. Fourth, whereas significant changes in mitotic rate, colony formation, and asymmetric divisions were dependent on exposure to regulatory molecules, the ADI remained unchanged at approximately 40%. This interesting finding supports the notion that growth factors are not essential for determining the symmetry of divisions and hence the fate of the daughter cells. Thus, commitment decision to self-renewal versus to differentiation is probably controlled by intrinsic programming and not by regulatory molecules.24,25 In a series of experiments, Ogawa et al have reported disparate differentiation in paired hematopoietic progenitors.8,9,26,27 Initially in a murine stem cell model,26,27 later confirmed in the human HSC,8,9 they demonstrated that approximately 20% of the progenitors divided asymmetrically, giving rise to different differentiation pathways from paired daughter cells from a single progenitor. When mouse-derived primitive progenitors were cultured individually, asymmetric divisions took place in almost 20% of the cases and always involved multipotent progenitors.26,27Symmetric divisions involved both multipotent and monopotent progenitors and occurred in the rest. The same group obtained similar results in studies of human HSC.8,9 Given this evidence, they suggested that stem cell differentiation is a stochastic process. Mayani et al10 described asymmetry of cell division of hematopoietic progenitors. In their studies, individually sorted human cord blood-derived primitive hematopoietic cells were allowed to undergo 1 division, after which the 2 daughter cells were physically separated and cultured in either the same or different cytokine combinations. These investigators used cytokine combinations favoring erythropoiesis (mast cell growth factor [MGF]+IL-6+IL-3+Epo) or myelopoiesis (MGF+IL-6+fusion protein of IL-3 and GM-CSF+macrophage colony-stimulating factor [M-CSF]+granulocyte-colony-stimulating factor [G-CSF]) in the culture media. Asymmetric division was defined as a division that yields 2 daughter cells with distinct functional properties, ie, 1 of the daughter cells gave rise to erythroid and the other to myeloid or mixed colonies, corresponding to asymmetric division of peripheral sensory organ progenitors described in neural stem cells.3 According to these investigators, asymmetric divisions occurred in 3% to 17% of the cultured cells and lineage commitment did not seem to be influenced by cytokines. The fundamental question of whether asymmetric divisions of HSC with 1 cell remaining quiescent and maintaining self-renewal capacity occur was not addressed by these studies. With our present technology, we were able to visualize the behavior of dividing CD34+/CD38− cells during the first rounds of cell divisions and to correlate this behavior with their corresponding fate in further cell culture. The focus of our study was on an earlier level in the hierarchy of HSC development, corresponding to the asymmetric cell divisions of primitive neuroblasts.3
Denkers et al28 recently described a similar time-lapse recording system of human hematopoietic progenitors in culture. With this present technology, we were able to define retrospectively cells that gave rise to asymmetric divisions, with 1 daughter cell that remained PKH26 bright and hence quiescent after mitosis and another that gave rise to multilineage progenitors, as shown in the formation of typical erythroid and myeloid clusters. More blast colonies could be derived from single-sorted cells with a quiescent daughter cell, whereas more clusters could be derived from those with symmetric divisions. Using a similar technique to identify quiescent HSC, Young et al23 also reported that cell production capacity was largely attributed to cells exhibiting quiescent behavior, which is consistent with our observations. When the PKH bright cells were replated onto fresh medium containing cytokine cocktail, each single-picked cell gave rise to colonies with dispersed and cluster growth patterns. Under the present conditions, we have not yet been able to establish that these cells would undergo further rounds of asymmetric divisions and, hence, represent precise replication of the mother cells.
Two mechanisms may be responsible for the adoption of different fates by the daughter cells. (1) The intracellular or intrinsic mechanism involves an inherited determinant that is asymmetrically segregated into 1 daughter cell at the time of division.4,6,7 (2) The extracellular or extrinsic mechanism may result from communication of the daughter cells with each other or with surrounding cells.4 Current research indicates a stereotypic mechanism for the asymmetric division of stem cells. Evidence from neural stem cell research supports the idea that asymmetric divisions are defined mostly by cell-autonomous information, whereas extrinsic signal might also be involved initially in instructing the asymmetric fates of daughter cells. Our observation of a fairly consistent ADI, irrespective of ontogenic age or of exposure to regulatory molecules, supports this hypothesis. The results indicate that, although the pattern of commitment can be skewed by extrinsic signaling, the proportion of asymmetric divisions is probably under the control of intrinsic factors.
Using a different approach, Brummendorf et al11 also drew similar conclusions from studies of single-sorted candidate HSC from FLV. They reported that the results from culturing and replating of hematopoietic progeny cells from single-sorted HSC were indicative of asymmetric divisions in primitive hematopoietic cells. The proliferative potential and cell cycle properties were shown to be unevenly distributed among daughter cells derived from single-sorted HSC from FLV. Judging from the continuous generation of functional heterogeneity among clonal progeny of HSC, they suggested that intrinsic control of stem cell fate is more likely than extrinsic.
Interestingly, Reddy et al29 also demonstrated that HSC from mouse bone marrow took 36 to 40 hours to complete the first division and then only 12 hours to complete each of 5 subsequent divisions. Our present study in human HSC derived from FLV, UCB, or ABM confirmed this inertia of the first mitosis and that each of the subsequent divisions took only 12 hours. The inertia for the first division might represent just an artifact caused by the trauma to the cells due to the preparation from the primary tissue, subsequent sorting, and staining procedures. However, subsequent divisions then took 12 hours in our culture conditions with the cytokine cocktail. Divisions resulting in 1 quiescent daughter cell as well as symmetric divisions resulting in equivalent daughter cells also occurred in intervals of 12 hours. This was a surprising finding, because the sorted population probably represented a relative heterogenous mixture of cells. We have as yet no satisfactory explanation for this phenomenon. Our preliminary experiments with “early” regulatory molecules showed a slight prolongation of the cell doubling times. However, this observation requires further confirmation and analysis.
Our technology will permit precise definition of cytokine and cellular determinants of replication behavior of primitive progenitors. In continuation of the present project, we will define if cellular determinants such as the number and type of stroma cells will have an impact on symmetry of HSC divisions. Furthermore, intracellular determinants have been shown to define the symmetry of division of neural stem cells. Recent genetic analysis has identified several proteins that differentially segregate during division and may be involved in determining the asymmetry of the division. These important cell fate determinants range from transcription factors (such as PROS)30 to modulations of cell-cell interactions (such as Numb and Notch)6 and are asymmetrically localized during division of neuroblasts.6,7,31-33 Simultaneously, mounting evidence indicates that transcription factors may play a key role in the differentiation of hematopoietic progenitors.34 Our method may enable us to correlate the expression of such factors and symmetry of cell division and to define the differential expression of such factors in the daughter cells of a single CD34+/CD38− cell that adopt different fates.
Supported by National Institutes of Health Grants No. R01 DK49619-01 and U19 AI36612-01 and by the Pete Lopiccola Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Anthony D. Ho, MD, Department of Medicine V, Hospitalstr. 3, 69115 Heidelberg, Germany.