Abstract
It has recently become clear that distinct subsets of CD8 T cells, analogous to their CD4 counterparts, exist in rodents and humans. To examine functional differences between human CD8 T-cell subsets, we generated Tc1, Tc2, and Tc0 T-cell clones from the peripheral blood of healthy individuals. The majority of CD8 T-cell clones generated displayed a classic Tc1 phenotype, but 10% to 20% secreted interleukin (IL)-4 in addition to interferon-γ (Tc0 phenotype). Generation of Tc2 clones was dependent on the use of anti-CD3 and anti-CD28 as the primary stimulus. The cytokine profiles of established clones remained susceptible to modification by the addition of IL-12 and IL-4. In addition, IL-12 enhanced and IL-4 inhibited the growth of Tc1 but not Tc2/0 CD8 T-cell clones. Significant functional differences were observed between the subsets. Tc2/0 clones expressed CD30 and CD40 ligand at a much higher level than Tc1 clones. Both Tc1 and Tc2/0 clones showed comparable cytotoxicity and produced similar levels of perforin and Fas L. However, Tc2 clones were much more resistant to activation-induced cell death and less susceptible to apoptosis by direct Fas ligation. Moreover, Tc1 and Tc2 clones had opposing effects on the development of CD4 effectors, promoting type 1 and type 2 responses, respectively. These data provide evidence for profound differences between human CD8 T-cell subsets that may be important in their functions as cytotoxic or immunoregulatory cells. (Blood. 2000;95:231-240)
The importance of distinct subsets of CD4 T lymphocytes in the etiology of a variety of immune-mediated diseases has become clear in the last 10 years. Two distinct populations of mouse T-helper (Th) clones were first identified by Mosmann et al.1,2 Th1 cells secrete interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-β and are primarily involved in cell-mediated immune responses, whereas Th2 cells secrete IL-4, IL-5, IL-6, and IL-10 and fulfill an important role in humoral and allergic immune responses.3 Human CD4 T-cell clones have similar, but not identically restricted, cytokine profiles.4 Human Th1 and Th2 subsets are usually defined according to IFN-γ/IL-4 production because the synthesis of IL-2, IL-6, and IL-10 is not stringently restricted to a single subset. Although the expression of type 1 and type 2 cytokines was initially considered to be mutually exclusive, T cells expressing both type 1 and type 2 cytokines have been identified during differentiation5,6 and among terminally differentiated cells.7
Historically, CD8 T cells have been regarded as a homogeneous population of cytotoxic cells producing only a limited number of cytokines. More recently, it has become clear that CD8 T cells have the potential to produce a much wider array of cytokines, and the existence of distinct subsets of CD8 T cells that are similar to their CD4 counterparts has been established in the mouse,8-10rat,11,12 and human.13 14 Analogous to the Th1/Th2 terminology, these subsets were termed Tc1 and Tc2.
The presence of different cytokines in the T-cell microenvironment appears to be the major factor that determines the differentiation of precursor T cells. IL-12, transforming growth factor-β, and IFN-γ induce differentiation of naive CD4 into Th1 but not Th2 cells, whereas IL-4 is essential for the differentiation of naive CD4 cells into Th2 cells and inhibits the development of Th1 cells.15-19 IL-6 also has been implicated in Th2 differentiation.20 IFN-γ and IL-4 also induce the differentiation of naive CD8 cells into type 1 and type 2, respectively, whereas IL-12 promotes the development of Tc1 cells.8,18 21
After differentiation, effector T cells show a stable cytokine pattern and rarely, if ever, switch to the opposite phenotype or revert to their precursor state. However, some modifications have been described: Th0 cells can shift toward Th1 or Th2 in response to cytokines,22 and human Th2 clones can transiently express IFN-γ after IL-12 treatment.23-25 Th1 cells are reported to be irreversible, although mouse Th1 cells can produce IL-4 when stimulated in the presence of IL-4.24 IL-4 also inhibits the ability of differentiated Tc1 cells to synthesize IL-226 and down-regulates other functions, including cytokine synthesis, proliferation, and long-term cytotoxicity.26 27
In addition to their classic role in the killing of infected cells, CD8 T cells play a role in the regulation of activation and differentiation of CD4 cells. This regulation could be mediated through secreted products (cytokines, chemokines) or by cell–cell interaction. CD8 T cells can alter the balance of Th1/Th2 responses in vivo28,29 by influencing the development of IL-4– or IFN-γ–secreting CD4 cells.30,31 In addition, CD8 T cells appear to play a role in the development of CD4 perforin-mediated cytotoxicity30 and also have been reported to suppress CD4 proliferative responses32,33 through the inhibition of costimulatory interactions. CD8 cells are also capable of influencing other components of the immune response, such as recruitment of eosinophils into the lungs during respiratory syncytial virus infection or allergic asthma,31,34,35 activation of macrophages,36 and regulation of antibody production by B cells.10,12 37-39
We have generated human CD8 T-cell clones which, according to cytokine production, can be classified into 3 subsets: Tc1 clones producing IFN-γ and a variety of other cytokines but no IL-4, Tc0 clones producing IL-4 in addition to IFN-γ and other cytokines, and Tc2 clones producing IL-4 but no IFN-γ. We investigated the effects of IL-4 and IL-12 on the proliferation and cytokine production of these subsets. In addition, the 3 subsets were compared for the expression of surface markers, cytotoxic capacity, resistance to activation-induced cell death (AICD), and their ability to direct the development of CD4 effectors. These data represent the first systematic analysis of the functional heterogeneity of circulating human CD8 T cells.
Materials and methods
Reagents
Lymphoprep was purchased from Nycomed (Birmingham, UK), CD8 and CD4 magnetic beads and Detach-a-bead from Dynal (Wirral, UK), and Hanks balanced salt solution (HBSS), fetal calf serum (FCS), RPMI 1640, Iscove's Modified Dulbecco's Medium (IMDM),L-glutamine, and 2-mercaptoethanol from Gibco BRL (Paisley, UK). Recombinant human IL-2 was purchased from Chiron (Harefield, UK), IL-4 from Serotec (Oxford, UK), and IL-12 from R&D systems (Abingdon, UK). Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled antibodies to various cell surface markers were purchased from Becton Dickinson (Oxford, UK), except for antibodies to perforin and Fas ligand, which were from Alexis (Nottingham, UK). All antibody pairs and recombinant cytokines for enzyme-linked immunosorbent assay (ELISA) were from Cambridge Biosciences (Cambridge, UK). Anti-CD3 and anti-Fas L (NOK1,2) monoclonal antibodies (MoAbs) were from Becton Dickinson (Oxford, UK). Anti-CD28 antibody was from CLB (Amsterdam, The Netherlands), and anti-Fas (CH11) was from Upstate Biotechnology (Lake Placid, NY). Antilymphocyte function-associated antigen-1 (LFA-1) MoAb was from Alexis (Nottingham, UK). Cytotoxicity detection kit lactate dehydrogenase (LDH) was supplied by Boehringer Mannheim (Lewes, UK), and OKT3 hybridoma was purchased from European Collection of Animal Cell Cultures (ECACC) (Salisbury, UK). Staphylococcal enterotoxin B (SEB) was obtained from Toxin Technologies (Sarasota, FL). All other reagents were from Sigma-Aldrich Ltd. (Poole, UK).
Purification of CD8 T cells
Peripheral blood (20 to 60 mL) was collected from healthy volunteers by venipuncture into sodium citrate and diluted 1:1 with HBSS, layered onto Lymphoprep, and centrifuged at 800g for 20 minutes at 18°C. The interface containing peripheral blood mononuclear cells (PBMCs) was then collected and washed with HBSS. PBMCs were resuspended either at 1 × 106/mL in complete media to be used as feeders or in RPMI 1640/2% FCS at 1 to 5 × 107/mL for the purification of CD8 cells.
CD8 cells were isolated by positive selection using anti-CD8 magnetic beads, which were added at a 3:1 bead to target cell ratio and mixed by rotation for 60 minutes at 4°C. Bound CD8 cells were released by addition of the Detach-a-bead antibody with rotation for 60 minutes at room temperature. Purified CD8 T cells were then incubated with anti-CD4 magnetic beads in the same way as described earlier to remove contaminating CD4 or CD4/CD8 double-positive cells. The purity of the isolated CD8 T cells was determined by FACScan (Becton Dickinson, Oxford, UK) and was consistently found to be > 99% pure, as assessed by staining with PE-labeled anti-CD8 and FITC-labeled anti-CD4.
Generation of CD8 T-cell clones
Aliquots of purified CD8 cells were prepared at concentrations from 1 × 104/mL to 1 × 101/mL in complete medium (RPMI 1640, 10% normal human AB serum [HS], 1% sodium pyruvate, 1% nonessential amino acids, 50 mmol/L 2-mercaptoethanol). Purified CD8 cells were seeded in Terasaki plates at concentrations of 0.3, 1, 3, 10, and 30 cells/well with 104 irradiated (4000 rad) autologous feeders in a total volume of 20 μL of complete medium supplemented with phytohemagglutinin (PHA) at 5 μg/mL and recombinant human IL-2 at 50 U/mL. On day 5, the cultures were fed with 10 μL of complete medium and on day 10, the plates were scored for cell growth and the cloning frequency determined as described by Taswell.40 Wells deemed to be positive were transferred to 96-well microtiter plates and were restimulated in 200 μL of complete medium with PHA (5 μg/mL final concentration), IL-2 (50 U/mL final concentration), and 1 × 105/well irradiated (4000 rad) autologous feeders as before. Every 3 to 4 days, half the medium was replaced with fresh complete medium supplemented with IL-2 (20 U/mL). Every 14 days, clones were restimulated with PHA and irradiated feeder cells as before. In some experiments, to determine the effect of different cytokines on the cloning frequencies and cytokine profiles, we generated CD8 clones and cultured them in complete medium supplemented with IL-4 (100 U/mL), IL-2 (50 U/mL) + IL-4 (100 U/mL), or IL-2 (50 U/mL) + IL-12 (100 U/mL), as described earlier.
Alternatively, purified CD8 cells were first stimulated in bulk cultures with either plate-bound anti-CD3 and anti-CD28 or anti-CD28 MoAb alone in complete media supplemented with IL-2 (25 U/mL) and IL-4 (50 U/mL). Twenty-four–well plates were coated with phosphate-buffered saline (PBS) containing 5 μg/mL CD3 and 2 μg/mL CD28 (200 μL/well) overnight at 4°C. After 6 days, CD8 cells were washed, counted, and plated out at limiting dilution and stimulated with PHA, IL-2, and autologous feeders, as before.
Flow cytometric analysis
Clones were analyzed for cell surface marker expression using FITC- and PE-conjugated antibodies to CD3, CD4, CD8, CD28, CD30, and CD40 ligand. Cells were used 14 days after PHA restimulation with and without phorbol myristate acetate (PMA) and ionomycin treatment (18 hours). Perforin was detected by intracellular staining. Briefly, PMA- and ionomycin-stimulated cells were fixed with 4% formaldehyde and then permeabilized using Becton Dickinson permeabilization solution before incubation with the labeled antibody. Fas ligand expression was determined on the surface 6 hours after stimulation, and also in the presence of Brefeldin A (protein secretion inhibitor) by intracellular staining, or in the presence of metalloprotease inhibitor (KB8301) 5 hours after anti-CD3 stimulation. Data were acquired and analyzed on Becton Dickinson FACSCalibur using Cellquest software (Becton Dickinson, Oxford, UK).
Cytokine analysis by ELISA
On day 14 of the cycle, clones (1 × 106/well) were stimulated in 2 mL of complete medium with ionomycin (400 ng/mL) and PMA (10 ng/mL) for 24 hours. Cell-free supernatants were collected after 24 hours and stored at −20°C. The quantitative measurement of a range of cytokines was performed using commercially available ELISA antibody pairs according to the manufacturer's instructions. Cytokine levels were calculated by reference to standard curves constructed using recombinant cytokines calibrated against Quantikine ELISA kit standards (R&D Systems, Abingdon, UK).
Intracellular cytokine staining
Cells were stimulated either with PMA and ionomycin or with plate-bound anti-CD3 and anti-CD28 MoAbs in the presence of Brefeldin A at 5 μg/mL. A portion of cells was left unstimulated as a control. After 6 or 18 hours, the cells were washed in PBS/0.2% bovine serum albumin and fixed in 500 μL of 4% formaldehyde for 20 minutes. After washing, cells were permeabilized using 250 μL of permeabilization solution (Becton Dickinson) for 10 minutes, incubated with the appropriate antibodies for 30 minutes at 4°C, washed, and fixed with 1% paraformaldehyde.
Cytotoxic assay
Day-14 clones were stimulated using plate-bound anti-CD3 (OKT3 supernatant) overnight. Cells were washed, counted, and adjusted to a range of concentrations in fresh RPMI with 2% HS. Target cells (OKT3 hybridoma cell line, 2 × 104/well) were cocultured with effector cells at a range of effector to target ratios (0.3:1 to 50:1) in round-bottom tissue culture plates in 200 μL of RPMI/2% HS. Supernatants were collected after 4 hours of culture. We used 1% Triton X to determine maximum lysis, and target cells in medium alone to determine spontaneous lysis. Spontaneous LDH release by effector cells also was determined. The levels of LDH were determined using a Boehringer Mannheim cytotoxicity detection kit according to the manufacturer's instructions. Percentage lysis was calculated according to a modified standard formula: (OD [optical density] experimental − OD spontaneous targets − OD spontaneous effectors)/(OD maximum − OD spontaneous targets) × 100.
Apoptosis induction
Cells (clones at day 14 of the cycle) were purified over Lymphoprep to remove debris and dead cells. Viable cells were diluted to 1 to 5 × 105 cells/well and stimulated with immobilized anti-CD3 for 4 to 6 hours. Control cells were left unstimulated. In some experiments, anti-Fas ligand antibodies (NOK1 and NOK2 at 10 μg/mL) were added to the cultures to determine the involvement of Fas–Fas ligand interaction in the AICD. In other experiments, anti–LFA-1 antibody was added (at 1 or 10 μg/mL) to investigate the importance of adhesion for the induction of AICD in Tc1 clones. After treatment, the cells were washed in PBS, resuspended in 200 μL of 1× binding buffer (10 mmol/L HEPES/NaOH, pH 7.4, 140 mmol/L NaCl, 2 mmol/L CaCl2), and incubated for 15 minutes in the dark at room temperature with 2.5 μL annexin V–FITC and 10 μL propidium iodide (50 μg/mL). Then 300 μL of binding buffer was added to each tube, and samples were acquired immediately (within 1 hour). Annexin V–positive, propidium iodide–negative cells were considered apoptotic. To measure direct anti-Fas induction of apoptosis, we incubated clones with anti-Fas antibody (CH11 at 1 μg/mL) for 4 hours at 37°C in 5% CO2 and stained them as described. Control tubes contained irrelevant IgM antibody.
The effect of IL-4 and IL-12 on proliferation and cytokine production of Tc1 and Tc0/2 CD8 T-cell clones
We also investigated the effect of different cytokines on proliferation and cytokine profiles of established CD8 T-cell clones. On day 14 of the cycle, cloned cells were washed and stimulated (1 × 105/well) in 200 μL of complete medium with PMA (10 ng/mL) and ionomycin (400 ng/mL) in the presence of IL-12 or IL-4 (0-10 000 U/mL). On day 4, the cells were washed and restimulated with PMA and ionomycin. After 24 hours, supernatants were collected for cytokine analysis, and the cells were incubated for another 6 hours in the presence of 3H-thymidine (0.5 μCi/well) and then harvested. Growth was determined by tritium incorporation, which was measured using a Canberra Packard Matrix 96 β counter (Pangbourne, Berkshire, UK) and expressed as counts per minute (cpm).
Effects of CD8 clones on CD4 differentiation
CD8 clones were cocultured with autologous CD4 T cells in the presence of antigen-presenting cells (APC). To ensure interaction between CD4, CD8, and APC's superantigen, SEB was used as a stimulus. First, CD8 clones (Tc1 and Tc2) reactive to SEB were identified. These clones were cocultured with autologous PBMCs depleted of CD8 cells, at varying ratios in the presence of SEB at 2.5 μg/mL. Two different approaches were used. Short-term cocultures (6 days) were followed by overnight PMA/ionomycin stimulation in the presence of Brefeldin A and staining with CD3 APC, CD8 peridinin chloropyll protein (PerCP), IL-4 PE, and IFN-γ FITC. By gating on CD3+CD8−cells, cytokine production of CD4 effector cells could be examined. In the second approach, CD8 clones and PBMCs depleted of CD8 were cocultured for 14 days at a ratio of 1:1. On day 14, CD4 cells were positively selected using magnetic beads, as described previously, and the resulting population was > 99% pure as assessed by CD4/CD8 staining. CD4 cells were plated at limiting dilution at 0.3 cells/well. The cloning procedure was followed as described for CD8 clones, and after 2 stimulation cycles, newly generated CD4 clones were screened for cytokine production after 18 hours of PMA/ionomycin stimulation.
Data analysis
Cloning frequencies were calculated using weighted mean statistics as described by Taswell.40 All data are expressed as mean ± SEM. Student's t test and Wilcoxon signed-rank test were used to assess statistical significance.
Results
Generation of distinct subsets of CD8 T-cell clones
Human CD8 clones were generated from highly purified (> 99%) peripheral blood CD8 T cells using PHA, autologous feeders, and IL-2. The cloning frequency varied from 0.23 to 0.61 among different experiments and donors. The cytokine profiles were determined by the concentration of cytokines in the clone culture supernatants 24 hours after PMA and ionomycin stimulation. Based on IL-4 and IFN-γ production, it is possible to group them into 2 subsets: 1 producing IFN-γ and no detectable IL-4 (Tc1) and the other producing both IFN-γ and IL-4 (Tc0). Of more than 500 clones produced from 5 donors using this protocol, none failed to secrete IFN-γ. Furthermore, no significant difference in the amount of IFN-γ produced by the 2 subsets was observed17 (17.1 ± 9.4 ng/mL for Tc0 as compared with 20.5 ± 10 ng/mL for Tc1). The majority (80% to 90%) of the clones produced were Tc1, with approximately 10% to 20% of clones showing a Tc0 cytokine profile. IL-5 and IL-13 were predominantly produced by Tc0 clones. Although some Tc1 clones produced IL-5 (57%) and IL-13 (52%), this was usually at a low level (mean ± SEM IL-5 production for Tc1 clones: 1302 ± 417 pg/mL compared with 6318.9 ± 1817 pg/mL for Tc0,P < .02; and 1462 ± 557 pg/mL compared with 4298 ± 769.95 pg/mL for IL-13, P < .005).
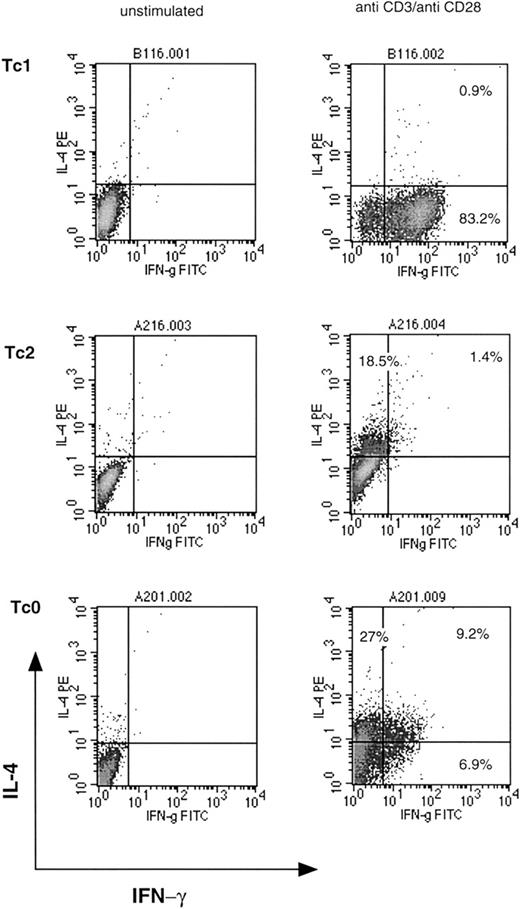
Production of other cytokines such as IL-2, granulocyte macrophage colony-stimulating factor, IL-6, IL-10, and TNF-α was not restricted to a particular subset and was common to most clones. Similar cytokine profiles were observed in 3 separate cloning experiments from different donors. Using a modified protocol, a small number of Tc2 clones were generated from the same donor. Stimulation with anti-CD3 plus anti-CD28 or anti-CD28 alone for 6 days in the presence of IL-2 and IL-4, followed by a standard limiting dilution procedure, resulted in a small number of clones (10% to 13%) that were classified as type 2. These clones either did not produce any detectable IFN-γ, or IFN-γ production was < 5 ng/mL. Cytokine profiles of representative Tc1, Tc2, and Tc0 clones are shown in Figure 1. Tc0 clones appear to include cells that produce either IL-4 or IFN-γ, or both. This has been described previously in Th0 clones41and reflects the independent nature of cytokine regulation. Phenotypic studies were subsequently performed using clones from a single donor. In our hands, Tc2 clones were very similar to Tc0 clones (in terms of characteristics and function) and were, in some experiments, grouped together for further analysis.
Cytokine profiles of representative Tc1, Tc2, and Tc0 clones.
Clones were stimulated with plate-bound anti-CD3 and anti-CD28 MoAbs for 18 hours in the presence of Brefeldin A. Cells were washed, fixed, permeabilized, and incubated with anti–IFN-γ FITC and anti–IL-4 PE antibodies. Quadrants were set with reference to an unstimulated control. Stimulated cells stained with isotype control antibodies gave similar profiles. Percentages of cells positive for the particular cytokine are indicated.
Cytokine profiles of representative Tc1, Tc2, and Tc0 clones.
Clones were stimulated with plate-bound anti-CD3 and anti-CD28 MoAbs for 18 hours in the presence of Brefeldin A. Cells were washed, fixed, permeabilized, and incubated with anti–IFN-γ FITC and anti–IL-4 PE antibodies. Quadrants were set with reference to an unstimulated control. Stimulated cells stained with isotype control antibodies gave similar profiles. Percentages of cells positive for the particular cytokine are indicated.
IL-12 and IL-4 influence the cytokine profile of CD8 clones generated
To assess the effects of IL-4 and IL-12 on the cytokine profile of the clones generated, we conducted parallel cloning experiments with different cytokine combinations (Table 1). In the presence of IL-4, both with and without IL-2, the number of IL-4–secreting clones was increased from 15% to 74% and 70%, respectively. The proportion of IL-5–producing clones was not significantly changed (66% in IL-2, 77% in IL-4, 53% in IL-2 + IL-4). IL-4 did not inhibit the generation of IFN-γ–producing clones and, using this method, none were generated that did not produce IFN-γ (Tc2). Mean IFN-γ, IL-4, and IL-5 production was largely unaffected. Levels of IL-10 appeared reduced, but this did not reach statistical significance (P < .09).
The presence of IL-12 resulted in a decrease in the proportion of IL-4–secreting clones from 25% to 12% and, more dramatically, in IL-5–producing clones from 80% to 22% (Table 1). The percentage of IL-10–producing clones increased from 19% to 55%, and mean IFN-γ production was also significantly increased in IL-12–generated clones (P < .0001) as compared with clones generated in the presence of IL-2 alone. The mean IL-4 production was decreased 5-fold (P < .05), and IL-5 production more than 20-fold (P < .0002).
Tc1 and Tc2 clones differ in their surface marker expression but show similar levels of cytotoxicity
Cell surface CD8, CD28, CD30, and CD40L expression and intracellular perforin were determined by flow cytometry 18 hours after stimulation of clones with PMA and ionomycin (Figure2). Perforin levels increased from 6 to 24 hours after stimulation, at which point all cells appeared positive. No difference was observed in the levels of perforin between Tc1 and Tc2/Tc0 clones at either time. Significant differences were observed in the levels of CD30 expression: 8 of 11 Tc0 and Tc2 clones showed a high proportion of CD30-positive cells (40% to 60%), as compared with < 10% CD30 expression by all the Tc1 clones. Striking differences also were observed in the levels of CD40 ligand expression. The majority of Tc2/0 clones tested (8/11) showed significant expression of CD40 ligand (20% to 70% positive cells). Only a low level of expression was observed (< 10%) on the Tc1 clones. There were no consistent differences in the levels of CD28 expression.
Tc1 and Tc0 clones differ in their surface marker expression.
Day-14 clones were stimulated with PMA and ionomycin for 24 hours and stained using FITC- and PE-labeled antibodies. (A) The surface phenotype of a representative Tc1 clone (left panel) and Tc2 clone (right panel). The percentage of cells positive for a particular marker is indicated. Tc0 clones were found to be very similar to Tc2 clones in terms of surface markers (not shown). Fas L expression was measured 6 hours after stimulation (A) and 18 hours after stimulation in the presence of protein secretion inhibitor (B). Perforin was measured by intracellular staining. Solid lines indicate marker expression; broken lines indicate isotype control staining.
Tc1 and Tc0 clones differ in their surface marker expression.
Day-14 clones were stimulated with PMA and ionomycin for 24 hours and stained using FITC- and PE-labeled antibodies. (A) The surface phenotype of a representative Tc1 clone (left panel) and Tc2 clone (right panel). The percentage of cells positive for a particular marker is indicated. Tc0 clones were found to be very similar to Tc2 clones in terms of surface markers (not shown). Fas L expression was measured 6 hours after stimulation (A) and 18 hours after stimulation in the presence of protein secretion inhibitor (B). Perforin was measured by intracellular staining. Solid lines indicate marker expression; broken lines indicate isotype control staining.
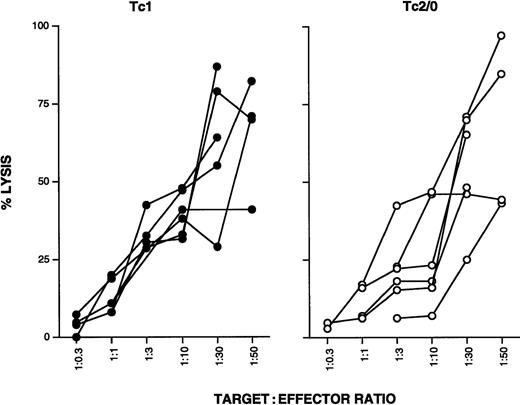
Six Tc1 and 6 Tc2/0 clones were further compared for their cytolytic function. Nonspecific CD3-mediated lysis was measured using the OKT3 hybridoma as target cells at a range of effector to target ratios. All clones tested showed similar levels of cytotoxicity, and no differences were observed between Tc1 and Tc2/0 clones (Figure3).
Tc1 and Tc0 clones show comparable levels of cytotoxicity.
Six Tc1 and Tc0/2 clones were stimulated with plate-bound anti-CD3 for 18 hours. Cells were washed, counted, and cocultured with OKT3 target cells at different effector to target cell ratios. After 4 hours, culture supernatants were collected and LDH levels were measured using a cytotoxicity detection kit; 1% Triton X was used to determine maximum lysis, and target cells in medium alone were used to determine spontaneous lysis. Spontaneous LDH release by effector cells was also determined. Color development was measured at 490 nm. Percentage lysis was calculated according to a modified standard formula: (OD experimental − OD spontaneous targets − OD spontaneous effectors)/(OD maximum − OD spontaneous targets) × 100.
Tc1 and Tc0 clones show comparable levels of cytotoxicity.
Six Tc1 and Tc0/2 clones were stimulated with plate-bound anti-CD3 for 18 hours. Cells were washed, counted, and cocultured with OKT3 target cells at different effector to target cell ratios. After 4 hours, culture supernatants were collected and LDH levels were measured using a cytotoxicity detection kit; 1% Triton X was used to determine maximum lysis, and target cells in medium alone were used to determine spontaneous lysis. Spontaneous LDH release by effector cells was also determined. Color development was measured at 490 nm. Percentage lysis was calculated according to a modified standard formula: (OD experimental − OD spontaneous targets − OD spontaneous effectors)/(OD maximum − OD spontaneous targets) × 100.
Tc1 and Tc2 clones are differentially susceptible to apoptosis
All Tc1 clones tested (> 6) were highly susceptible to AICD after 5 hours of stimulation with anti-CD3 (Figure4). The percentage of apoptotic cells induced in this way varied from 14% to 50%, with a mean of 30% ± 3.5%. In contrast, Tc2 clones were much more resistant to apoptosis. Most Tc2 clones showed complete resistance, whereas others showed low levels of apoptosis, with a mean of 12% ± 4.3% (2 representative clones; Tc1 and Tc2 are shown in Figure4). Anti-CD3–induced AICD was at least partially Fas–Fas L dependent because a proportion of cells could be rescued by the addition of NOK2 antibody (anti–Fas L MoAb). Interestingly, both groups were susceptible to induction of apoptosis by direct ligation with anti-Fas MoAb (CH11), although type 2 clones were less so (mean apoptotic cells 33% ± 3.8% compared with 50% ± 3.6% for Tc1;P < .02). This indicates that there may be differences in Fas L expression or function between the subsets. Fas L expression was examined by standard surface staining after 6 hours of PMA/ionomycin stimulation, under which conditions all clones (Tc1 and Tc2/0) appeared negative. We assumed that the Fas L was being cleaved and lost from the surface, so we investigated the expression of Fas L after stimulation in the presence of a protein secretion inhibitor (Brefeldin A). Under these conditions, Fas L was readily detectable, but no differences were observed between the subsets (Figure 2B). Furthermore, levels of Fas L expression were the same after stimulation in the presence of a metalloprotease inhibitor, KB8301, known to prevent the cleavage of Fas L from the surface (data not shown). This suggests that differences in Fas L function rather than levels of expression cause differential susceptibility to AICD.
Tc1 and Tc2 clones show differential susceptibility to apoptosis.
AICD was induced in a number of Tc1 and Tc2 clones by 5 hours of stimulation with immobilized anti-CD3. After treatment, the cells were stained with annexin V–FITC and propidium iodide and analyzed as described in Materials and Methods. The percentage of apoptotic cells is shown in the lower right quadrants. Induction of apoptosis was partially blocked by anti–Fas L antibody (NOK2). Apoptosis was also induced by direct Fas ligation (CH11, anti-Fas MoAb). Two representative clones are shown.
Tc1 and Tc2 clones show differential susceptibility to apoptosis.
AICD was induced in a number of Tc1 and Tc2 clones by 5 hours of stimulation with immobilized anti-CD3. After treatment, the cells were stained with annexin V–FITC and propidium iodide and analyzed as described in Materials and Methods. The percentage of apoptotic cells is shown in the lower right quadrants. Induction of apoptosis was partially blocked by anti–Fas L antibody (NOK2). Apoptosis was also induced by direct Fas ligation (CH11, anti-Fas MoAb). Two representative clones are shown.
The importance of the interaction between LFA-1 and intercellular adhesion molecule (ICAM)-1 in Th1-mediated B-cell apoptosis has been demonstrated.42 We investigated whether blocking the homotypic interaction between LFA-1 and ICAM-1 by the addition of anti–LFA-1 antibody could partially or completely rescue Tc1 clones by interfering with the induction of AICD. However, we found that blocking the LFA-1–ICAM-1 interaction had no effect (mean apoptotic cells 40 ± 15 vs 46.3 ± 11 without or with anti–LFA-1, respectively). The anti–LFA-1 antibody was shown to be effective in blocking a 2-way mixed lymphocyte reaction, indicating that it effectively blocked cell adhesion at the concentrations used (not shown).
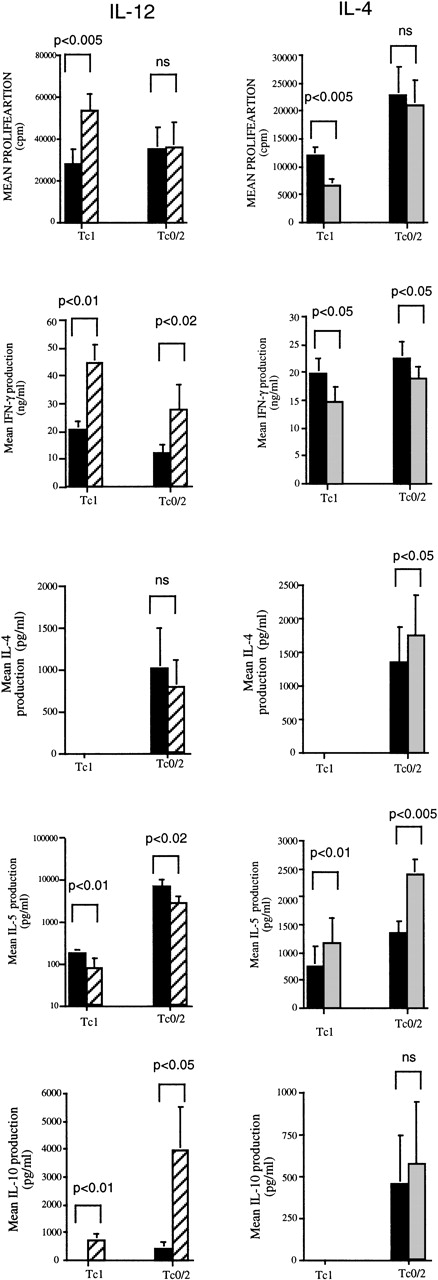
IL-12 increases and IL-4 inhibits proliferation of Tc1 but not Tc0/2 CD8 T-cell clones
The effects of IL-4 and IL-12 on the growth of established Tc1 and Tc0 CD8 T-cell clones were investigated. Tc1 (n = 8) and Tc0/2 (n = 8) CD8 T-cell clones were stimulated with PMA and ionomycin in medium containing 0 to 10 000 U/mL of IL-12 or IL-4, as described in Materials and Methods. IL-12 increased the proliferation of all Tc1 clones (mean increase 117.5% ± 30%) at the highest concentration of IL-12 (Figure 5). Conversely, Tc0/2 clones were relatively unaffected by IL-12 (mean 4.0% ± 4.7%). IL-4 had an opposite, although much weaker, effect. All 8 Tc1 clones tested showed a decrease in proliferation (mean 38% ± 4.2% at the highest concentration of IL-4 tested), whereas Tc0/2 clones were unaffected, with a mean change in proliferation of +0.1% ± 5.8% (Figure 5).
The effects of IL-12 and IL-4 on proliferation and cytokine production of Tc1 and Tc0/2 CD8 T-cell clones.
Cloned cells at 1 × 105/well were incubated with PMA (10 ng/mL) and ionomycin (400 ng/mL) in the presence of IL-12 or IL-4 (0 to 10 000 U/mL). All experiments were performed in triplicate. On day 4, the cells were washed and restimulated with PMA and ionomycin in the absence of cytokines. At 24 hours, supernatants were collected and the cells were incubated in the presence of3H-thymidine (0.5 μCi/well) for 6 hours and then harvested. Mean cpm values for 8 Tc1 and 8 Tc0/2 clones are shown. Levels of IFN-γ, IL-4, IL-5, and IL-10 in the supernatants were measured by ELISA (as described in Materials and Methods). All results are expressed as mean ± SEM for 8 clones under control conditions (filled bars) and at 10 000 U/mL IL-4 or IL-12 (shaded bars).
The effects of IL-12 and IL-4 on proliferation and cytokine production of Tc1 and Tc0/2 CD8 T-cell clones.
Cloned cells at 1 × 105/well were incubated with PMA (10 ng/mL) and ionomycin (400 ng/mL) in the presence of IL-12 or IL-4 (0 to 10 000 U/mL). All experiments were performed in triplicate. On day 4, the cells were washed and restimulated with PMA and ionomycin in the absence of cytokines. At 24 hours, supernatants were collected and the cells were incubated in the presence of3H-thymidine (0.5 μCi/well) for 6 hours and then harvested. Mean cpm values for 8 Tc1 and 8 Tc0/2 clones are shown. Levels of IFN-γ, IL-4, IL-5, and IL-10 in the supernatants were measured by ELISA (as described in Materials and Methods). All results are expressed as mean ± SEM for 8 clones under control conditions (filled bars) and at 10 000 U/mL IL-4 or IL-12 (shaded bars).
IL-12 and IL-4 modulate the cytokine production of Tc1 and Tc0/2 CD8 T-cell clones
Cytokine production by 8 Tc1 and Tc0/2 clones stimulated with PMA and ionomycin in medium containing 0 to 10 000 U/mL of IL-12 or IL-4 was examined. IL-12 had a strong modulatory effect on the cytokine profiles of both Tc1 and Tc2/0 clones. In all of the clones tested, IL-12 significantly increased IFN-γ production, in some cases by more than 4-fold. Interestingly, the most dramatic increases were observed in some of the Tc0/2 clones, whereas in Tc1 clones, the increase ranged from 35% to 188% (Figure 5), which could largely be accounted for by the increase in proliferation of these cells. IL-12 also had a dramatic effect on IL-10 production. The majority of Tc1 clones (7/8) and half of the Tc0/2 clones (4/8) produced undetectable amounts of IL-10 in the absence of IL-12. However, in the presence of IL-12, all but 4 of these clones showed significant production of IL-10 ranging from 100 to 4000 pg/mL. IL-4 suppressed IFN-γ production in Tc1 and to a lesser extent in Tc2/0 clones, although for Tc1 clones, the levels of suppression are in keeping with the decrease in proliferation that was observed in these conditions. IL-4 had no effect on IL-10 production by either Tc1 or Tc0/2 clones (Figure 5).
IL-12 significantly decreased IL-5 production by both Tc1 and Tc2/0 clones, whereas IL-4 production was decreased in some, but remained constant in other, Tc2/0 clones. IL-4 increased IL-5 production by the majority of Tc1 clones (Figure 5) in the range of 20% to 30% at the highest IL-4 concentration; however, a few clones showed a more substantial increase of 2- to 5-fold. Because IL-4 suppresses proliferation of these clones, these increases may represent much greater effects on a per-cell basis. The effect on Tc2/0 clones was variable, with only some clones showing a significant increase in IL-5 production. The addition of IL-4 enhanced subsequent IL-4 production in most Tc0/2 clones, although only 3 of 8 showed a significant increase (2- to 4-fold). None of the Tc1 clones could be switched to produce IL-4.
Tc1 and Tc2 clones exert opposite effects on CD4 differentiation
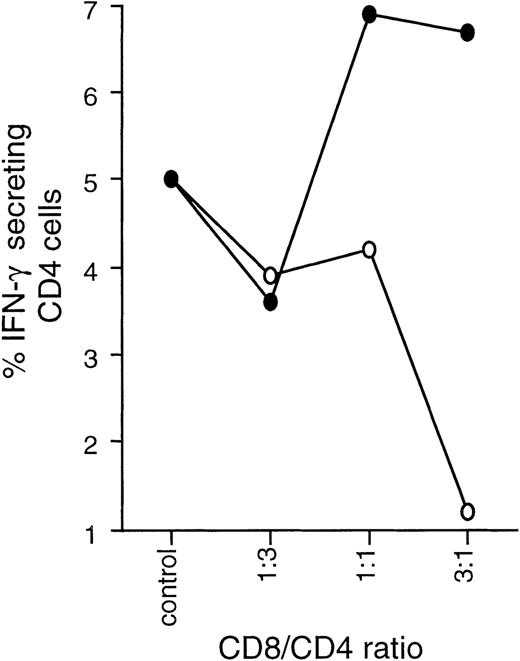
The effect of Tc1 and Tc2 clones on the differentiation of CD4 effectors was studied in a series of coculture experiments involving autologous CD8-depleted PBMCs and Tc1 or Tc2 clones stimulated with SEB. In short-term (6 day) coculture experiments, Tc1 clones strongly promoted IFN-γ synthesis and resulted in predominantly Th1 effectors. Tc2 clones had the opposite effect, enhancing IL-4 production and down-regulating IFN-γ, resulting in predominantly type 2 effectors (Figure 6).
Tc1 and Tc2 clones differentially regulate Th1/Th2 development.
Tc1 (■) and Tc2 (○) clones were cocultured with autologous PBMCs previously depleted of CD8 cells (CD4 + APC) at varying ratios and in the presence of SEB at 2.5 μg/mL. After 6 days, cultures were stimulated overnight with PMA/ionomycin in the presence of Brefeldin A and stained with CD3 APC, CD8 PerCP, IL-4 PE, and IFN-γ FITC. By gating on CD3+CD8−cells, cytokine production of CD4 effector cells could be examined. Results are expressed as percentage of CD4 cells positive for IL-4 or IFN-γ.
Tc1 and Tc2 clones differentially regulate Th1/Th2 development.
Tc1 (■) and Tc2 (○) clones were cocultured with autologous PBMCs previously depleted of CD8 cells (CD4 + APC) at varying ratios and in the presence of SEB at 2.5 μg/mL. After 6 days, cultures were stimulated overnight with PMA/ionomycin in the presence of Brefeldin A and stained with CD3 APC, CD8 PerCP, IL-4 PE, and IFN-γ FITC. By gating on CD3+CD8−cells, cytokine production of CD4 effector cells could be examined. Results are expressed as percentage of CD4 cells positive for IL-4 or IFN-γ.
We also cocultured Tc2 and Tc1 clones with CD8-depleted PBMCs in the presence of SEB for 14 days, positively selected CD4 cells, and generated CD4 clones using the PHA/IL-2 protocol. After 2 rounds of mitogen stimulation, clones were screened for cytokine production. Significant differences were observed in the levels of secreted cytokines and in the subset distribution of the generated clones. Coculture with Tc2 clones resulted in a greater proportion of Th2 clones (from 17% to 43%) and prevented the generation of Th1 clones. Conversely, coculture with Tc1 cells resulted in an increased proportion of Th1 (from 13% to 50%) and decreased the proportion of Th2 clones (from 17% to 6%) (Table 2). Mean cytokine production of CD4 clones was similarly affected. Clones generated in the presence of Tc1 cells produced almost 5 times more IFN-γ and half the IL-4, whereas clones from Tc2 cocultures produced approximately half the IFN-γ and more IL-4 and IL-5 than CD4 clones generated from control cultures. Interestingly, coculture with either type 1 or type 2 CD8 clones decreased the subsequent cloning efficiency of the CD4 clones (Table 2). No differences were observed in levels of proliferation or the numbers of CD4 cells recovered in mixed cultures compared with controls.
Discussion
The existence of distinct cytokine-producing subsets within the CD8 T-cell population is becoming increasingly accepted. However, little is known about the immunobiology of these subsets. We have generated a large number of CD8 clones that can be defined as Tc1 (IFN-γ, no IL-4), Tc0 (IFN-γ, IL-4), or Tc2 (no IFN-γ, IL-4). The majority of human CD8 T-cell clones described to date can be classified as Tc1, although CD8 clones secreting IL-4 have been generated from patients with lepromatous leprosy,13 cutaneous leishmaniasis,41 HIV,14 and periodontitis,42 and from the peritoneum of healthy individuals.43 CD8 T cells that secrete IL-4 and IL-5 are also present in the lung,44,45 especially in asthmatic patients.35 The method for generating mitogen-driven CD8 clones from the peripheral blood of healthy donors used in the study reported here resulted in a majority of clones with a Tc1 cytokine profile and a minority (10% to 20%) with a Tc0 profile. A small number of Tc2 clones have been established after a modification of stimulation conditions, suggesting that the predominance of the type 1 and lack of type 2 phenotype may be, at least in part, an artifact of the in vitro culture.
The importance of the microenvironment in the development of different subsets is well documented. It is possible that Tc2 CD8 cells exist in significant numbers only in certain disease states or are confined to particular immune compartments. However, our data show that CD8 cells with the potential to become Tc2 exist, albeit at a low frequency, in the peripheral blood of normal individuals. As for CD4 cells, CD8 cells are susceptible to the polarizing effects of IL-4 and IL-12, which promote type 2/0 and type 1, respectively. However, the majority of CD8 T-cell clones generated from peripheral blood develop from memory/effector cells, and although polarizing cytokines do influence the proportion of Tc0/Tc2 clones, they are unable to completely redirect their development. Medical history and atopic status are likely to influence the proportion of Tc0/Tc2 clones from each individual.
The effects of cytokines such as IL-4, IFN-γ, and IL-12 on the proliferation and cytokine production of established CD8 T-cell populations or clones have not been extensively studied previously. We observed that IL-12 strongly promoted the proliferation of Tc1 clones, whereas IL-4 suppressed growth of the clones. The Tc0 and Tc2 clones were unaffected by either cytokine. We chose PMA and ionomycin stimulation 14 days after the addition of feeders to ensure that the response was accessory cell–independent and that only direct effects of cytokines on CD8 T cells were assessed. Our data clearly demonstrate that cytokines regulate CD8 responses not only at the level of differentiation, but also at the level of expansion (proliferation) and cytokine production of established effector cells. These effects could serve to maintain and fine-tune polarized CD8 effector responses during prolonged immune activation.
The data currently available on the specific cell surface phenotypes associated with different CD8 T-cell subsets are somewhat limited. CD30 has been implicated as a marker for Tc0 and Tc2 CD8 T cells in both healthy and diseased subjects.46,47 Our findings are in agreement with these reports because significant expression of CD30 as well as CD40L was restricted to the Tc2/0 subset. The functional significance of these costimulatory molecules is, as yet, unclear. Recent reports have suggested that CD30 may be involved in the induction of cell death.48-50 The proposed CD30-CD30L pathway appears independent of the Fas–Fas L and TNF pathways and could play an important role in the deletion of T cells after the resolution of an immune response. Differential expression of CD30 on distinct subsets may serve to preferentially delete or maintain a specific population, thus influencing the memory T-cell repertoire. Expression of CD40L coupled with IL-4 production could identify a subset of cells capable of interacting with and providing help for B cells,10,51 although this type of interaction may be compromised by their capacity to kill APC. It is possible that CD40L-expressing Tc2 clones could interact with APC, inducing IL-12 secretion by macrophages and dendritic cells,52,53 with subsequent IFN-γ production.54 This could serve as a control mechanism for dampening type 2–mediated immunopathology.
For Th1 and Th2 CD4 T-cell subsets, different cytokine profiles are closely associated with specific functions; however, such a correlation has yet to be defined for CD8 T cells. Although mouse Tc1 and Tc2 clones are both reportedly cytotoxic,8,9,55 it appears that the mechanisms they use for killing, and hence their effectiveness in eliminating particular targets, may differ. The inability of Tc2 cells to kill through a Fas-dependent pathway has been reported using perforin knockout mice.56 Further evidence of reduced cytolytic activity by Tc214,57 and even noncytolytic CD8 T cells has been described.58 All subsets of our CD8 clones (Tc1, Tc0, and Tc2) showed efficient anti-CD3–mediated killing of OKT3 targets and expressed comparable levels of perforin. A clear advantage for Tc2 cytotoxic cells could be their ability to kill in a type 2 environment where proliferation of Tc1 cells would be suppressed. This is in agreement with a report by Mosmann27showing that IL-4 treatment affects proliferation, cytokine secretion, and long-term cytotoxicity of Tc1 cells. In addition, cytotoxic cells secreting a wide array of cytokines (e.g., Tc2, Tc0) would be capable of effective killing of infected cells while at the same time providing additional signals to other cells to help amplify and direct the immune response. Both Tc1 and Tc2 cells are able to mediate inflammation,59 which again may be an advantage in responses to different pathogens as inflammation could be coupled to different effector functions.
Recent reports indicate that Th1 cells are more susceptible to activation-induced apoptosis than Th2 cells.60-62 Whereas some studies found that this was related to differences in the levels of Fas L expression,60,63 others found Fas L levels to be comparable.61-64 We observed that Tc2 clones are much more resistant to anti-CD3–mediated AICD. This process appears to be mediated primarily through the Fas–Fas L interaction and can be partly abrogated by anti–Fas L antibodies. In our hands, the subsets expressed comparable levels of Fas ligand, suggesting that differences in the susceptibility to AICD did not result from the differences in Fas L expression. This raises the possibility that Fas L on Tc2 cells is not functional or is only partially functional. Although we have not investigated Fas-dependent cytotoxicity, this hypothesis is in agreement with reports56 that Tc2 cells show only marginal levels of Fas-mediated killing. Alternatively, other proteins that regulate the Fas-mediated death pathway, such as Fas-associated protein (FAP), Fas-associated death domain (FADD), and Fas-associated death domainlike IL-1β converting enzyme (FLICE),61,62may play a role in protecting Tc2 cells. However, because anti–Fas L antibody provides only partial rescue in Tc1 cells, other mechanisms in addition to Fas signaling are likely to be involved in AICD. Tc2 resistance to AICD was probably not due to a lack of cell–cell adhesion because blockade of the LFA-1–ICAM-1 interaction failed to inhibit AICD in Tc1 cells. In certain situations, it may be desirable to suppress (or terminate) a type 1 inflammatory response or to promote a humoral immune response. Preferential depletion of Tc1 and Th1 cells through AICD would be one possible mechanism. It is interesting to note that the cloning procedure results in a majority of Tc1 clones despite their increased propensity toward apoptosis. AICD results in death of a proportion of cells while the surviving cells go on to proliferate.65 This would explain the relatively low cloning frequency (usually < 50%), indicating that a proportion of cells die during cloning.
The microenvironment plays a crucial role in directing the T-cell response toward type 1 or type 2 cytokine secretion. This may be due to the influence of secreted products (cytokines, chemokines) of resident cells or APCs or to the nature of direct interactions, including TcR-ligand affinity, ligand density, and costimulation. We wanted to investigate the capability of CD8 Tc1 and Tc2 clones to influence the development of CD4 effectors. Tc1 clones favored the development of CD4 effectors that were Th1-biased, whereas Tc2 clones had the opposite effect. CD8-dependent suppression of Th2 responses and induction of IFN-γ production have been reported in other systems and have mostly been attributed to the high levels of IFN-γ.28,29,31 36However, we have also demonstrated that Tc2 clones not only can promote Th2 effectors but also can efficiently suppress the development of Th1 cells. The observed effects are consistent with cytokine-mediated regulation, as Tc2 clones produced IL-4 and IL-10, and Tc1 clones produced high levels of IFN-γ, which could act directly or indirectly on developing CD4 cells. The importance of cell contact, either direct or through the APC, could not be ruled out and is a matter for further investigation. Interestingly, both Tc1 and Tc2 clones appeared to have a suppressive effect on the subsequent generation of CD4 clones, evident from significantly reduced cloning frequencies. Because coculture with CD8 clones did not appear to affect the initial proliferative response or the number of CD4 cells recovered, we speculate that the CD8 clones, either through secretion of a cytokine (possibly IL-10) or through inhibition of costimulation, impair the long-term proliferative capacity of SEB-reactive CD4 cells.
The data described in this article clearly indicate that human CD8 T cells represent a broad spectrum of cells with a wide array of potential functions. It seems likely that Tc1 and Tc2 cells will develop and function under the influence of a particular microenvironment, probably alongside Th1 and Th2 responses, respectively. In addition to their role in protecting against infection through the efficient killing of target cells, CD8 T cells themselves have the potential to play an important role in the regulation of the immune response. They appear capable of influencing the activation or suppression of other cell types including macrophages,36eosinophils,31,34,35 and B cells,10,12,37-39 51and, as we have demonstrated, they can direct the development of CD4 T cells into Th1 and Th2 effectors.
This work was supported by grants from the Medical Research Council, Bayer-Yakuhin, and Glaxo-Wellcome.
Reprints:David M. Kemeny, Department of Immunology, GKT School of Medicine, Rayne Institute, 123 Coldharbour Lane, London, SE5 9NU, United Kingdom; e-mail: david.kemeny@kcl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.