Abstract
In human immunodeficiency virus (HIV)-1 infection, highly increased T-cell turnover was proposed to cause exhaustion of lymphocyte production and consequently development of AIDS. Here, we investigated cell proliferation, as measured by expression of the Ki-67 nuclear antigen, in peripheral blood CD4+ and CD8+ lymphocyte subpopulations before and during highly active antiretroviral therapy (HAART). In untreated HIV-1 infection, both the percentage and number of Ki-67+CD4+ and CD8+ lymphocytes were significantly increased, compared with values obtained from healthy individuals. A more than 10-fold increase in the percentage of dividing naive CD4+ T cells in the blood was found when the number of these cells were below 100 per μL.. HAART induced an immediate decline in Ki-67 antigen expression, despite often very low CD4+ T-cell numbers, arguing against increased proliferation being a homeostatic response. After approximately 24 weeks of HAART treatment, a transient increase in the number of proliferating cells was seen, but only in the CD4+CD27+ memory pool. In the CD8+ T-cell compartment, the number of dividing cells was elevated 20- to 25-fold. This increase was most notable in the CD27+ CD 45RO+ and CD27−CD45RO+ memory CD8+ T-cell pool, corresponding with the degree of expansion of these subsets. Reduction of plasma HIV-RNA load by HAART was accompanied by a decrease in numbers and percentages of dividing cells in all CD8+T-cell subsets. Taken together, our results indicate that peripheral T-cell proliferation is a consequence of generalized immune activation. (Blood. 2000;95:249-255)
Several hypotheses have been presented to explain the loss of CD4+ T lymphocytes, the major hallmark of human immunodeficiency virus (HIV)-1 infection that leads to severe immune depletion and ultimately AIDS and death. The model of high lymphocyte turnover has received considerable attention over the past few years.1,2 On the basis of the observation that during the first few weeks of highly active antiretroviral therapy (HAART) the number of CD4+ T cells increases, it was postulated that HIV-1 infection leads to a rapid turnover of lymphocytes, reflecting a new balance between production and death of lymphocytes. The increased production rate would eventually lead to exhaustion of the CD4+ T-cell renewal capacity and result in CD4+ lymphocyte depletion. Indeed, in simian immunodeficiency virus (SIV)-infected macaques increased T-cell production was observed3; however, there was a parallel rise in the turnover of B cells and NK cells.4 Moreover, the highest increase was observed in CD8+ T cells, the compartment that is initially expanded and does not get depleted until very late in the course of HIV-1 infection.5-9
HIV-induced lymphocyte depletion and subsequent recovery after HAART appeared to be variable when studied at the level of T-cell subsets. Infection with HIV-1 induces an early decline in the number of naive CD4+ and CD8+ and memory CD4+ T lymphocytes. Conversely, the memory and activated CD8+T-cell compartments expand initially. Only shortly preceding progression to AIDS, the numbers of these latter cell types fall as well.10-12 HAART induces an early rise of memory CD4+ and CD8+ T-cell numbers, accompanied by a slow increase in naive CD4+ and CD8+T-cell numbers.13,14 From this, it was concluded that different mechanisms are involved in the dynamics of depletion and recovery of the various T-lymphocyte subsets. These mechanisms include interference of HIV with de novo T-cell production capacity15 (D. R. Clark et al, submitted for publication) and enhanced sequestration of cells in lymphoid tissues.14,16-18 Recently several groups have reported on cross-sectional studies of T-cell production levels. Most of these reports studied total CD4+ and CD8+ T-cell populations, and did not discriminate between naive and memory subsets.3,7,8,19 20
To answer the question whether increased T-cell proliferation could be involved in CD4+ lymphocyte depletion, we measured expression of the Ki-67 antigen in naive and memory CD4+T-cell subsets in the blood. Furthermore, we investigated the role of T-cell proliferation in the expansion and depletion of naive, memory, and effector CD8+ T lymphocyte subsets. Our approach was longitudinal: cell division rates were studied from the stage of chronic untreated HIV-1 infection up to a year of treatment with HAART.
Materials and methods
Patients
To study T-cell turnover before and during antiretroviral treatment, sequential cryopreserved peripheral blood samples from HIV-1 infected participants of the CHEESE study were used. Cryopreservation was performed using a computerized freezing device that results in optimal quality of viably frozen cells for functional studies.21Frozen blood samples were stored in liquid nitrogen. No differences in Ki-67 expression were found between freshly isolated or frozen cells in a pilot experiment (data not shown). In the multicenter CHEESE study, patients received either zidovudine (Retrovir; 200 mg, 3 times daily), lamivudine (Epivir; 150 mg, twice daily), and saquinavir soft gel capsules (Fortovase; 1200 mg, 3 times daily), or zidovudine, lamivudine, and indinavir (Crixivan; 800 mg, 3 times daily). The inclusion criteria for this trial were no previous treatment with antiretroviral therapy, except for AZT < 12 months, CD4+T-cell count < 500 per μL, or HIV RNA > 10 000 copies per mL, or CDC stage B or C.22 Of 60 participants, 16 were selected on the basis of baseline CD4+ T-cell numbers and the availability of frozen peripheral blood mononuclear cell (PBMC) samples. Patients were equally distributed among both therapy arms. Expression of the Ki-67 antigen was analyzed at 5 timepoints: during untreated HIV infection (t = 0) and after 4 (t = 4), 12 (t = 12), 24 (t = 24), and 48 (t = 48) weeks of therapy. As controls, cryopreserved PBMC from 5 HIV-negative blood bank donors were used.
Monoclonal antibodies
CD4-PerCP, CD8-PerCP, CD45RO-PE mAb, and Streptavidin-APC were obtained from Becton Dickinson (San Jose, CA). Biotinylated CD27 mAb was purchased from the CLB (Amsterdam, The Netherlands), and FITC-labeled Ki-67 mAb was purchased from Immunotech (Marseille, France).
Flow cytometry
CD4+ and CD8+ T cells were subdivided into naive (CD45RO−/ CD27+), CD27+memory (CD45RO+/CD27+), CD27−memory (CD45RO+/CD27−), and CD27− effector (CD45RO−/CD27−) cells, as previously described.23,24 In contrast to these earlier studies, CD45RA mAb was replaced by CD45RO mAb and the definition of the T-cell subsets was adjusted to the used mAb combination. Cell proliferation was studied by measuring expression of the Ki-67 antigen, which is expressed by cells in late G1, S, G2, and M phase of the cell division circle.25-28
Cryopreserved peripheral blood mononuclear cells were thawed and incubated with CD4− or CD8−PerCP mAb, CD45RO−PE, and biotinylated CD27 mAb. After washing with phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA), cells were incubated with Streptavidin-APC. Red blood cells were lysed and lymphocytes fixated with fluorescence-activated cell sorter (FACS) Lysing Solution (Becton Dickinson). Subsequent permeabilization was performed by incubating cells with FACS permeabilization buffer (Becton Dickinson), after which cells were stained intracellularly with Ki-67-FITC mAb. Cells were fixed, using Cellfix (Becton Dickinson), and analyzed on a FACS Calibur (Becton Dickinson) with Cellquest software. All incubation steps were performed at 4°C for 20 minutes; for fixation and permeabilization, samples were kept at room temperature for 10 minutes.
Viral load
Plasma viral load was determined with RT-PCR detecting HIV-1 RNA (Roche Amplicor Monitor Standard Assay, Roche Diagnostics, Branchburg, NJ).
Statistical analysis
Patient characteristics at baseline and after various timepoints during treatment with HAART were compared using Wilcoxon Signed Ranks test and Mann-Whitney U test for comparison with healthy control values. Correlations were calculated using Spearman's correlation coefficients.
Results
Ki-67 antigen expression in chronic HIV-1 infection
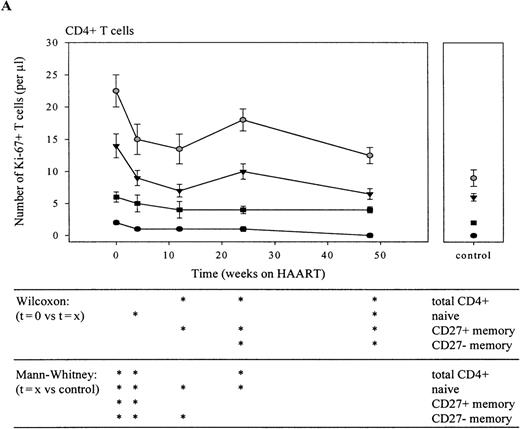
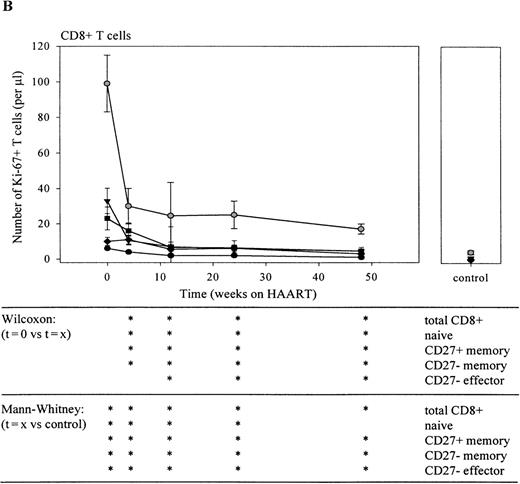
During chronic untreated HIV-1 infection, both the percentage and the absolute number of Ki-67+ CD4+ and CD8+ T cells was significantly increased (Figure1, Tables1 and2; P = .001), compared with healthy controls. The percentage of Ki-67 expression was significantly elevated in all subsets (P < .005), except for the CD8+ CD27− effector T-cell population (Figure 1). Naive T cells showed a 10- (CD4) to 20- (CD8) fold elevation in Ki-67+ T-cell percentage, and in the memory and effector T-lymphocyte compartment, Ki-67 expression was increased up to 7-fold (Figure 1). When absolute numbers instead of percentages of dividing cells were analyzed, cell proliferation in the CD4+ T-cell subsets was elevated < 3-fold, whereas in the CD8+ compartment, a 20- to more than 50-fold rise was observed (Tables 1 and 2). This increase was most notable in the CD27+ memory and CD27− memory lymphocyte population (see t = 0, Figure 4B).
Percentage of Ki-67+ T lymphocytes within the CD4+ (A) and CD8+ (B) peripheral blood T-cell populations of healthy individuals (gray bars) and untreated HIV-infected patients (black bars).
An asterisk represents statistical difference compared with control value (P < .005; Mann-Whitney U test).
Percentage of Ki-67+ T lymphocytes within the CD4+ (A) and CD8+ (B) peripheral blood T-cell populations of healthy individuals (gray bars) and untreated HIV-infected patients (black bars).
An asterisk represents statistical difference compared with control value (P < .005; Mann-Whitney U test).
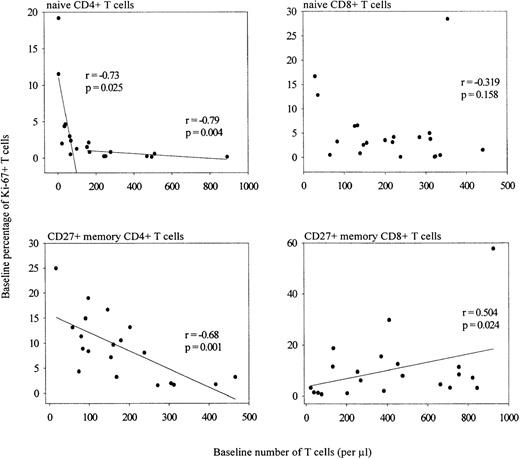
The percentage of naive Ki-67+ CD4+ T lymphocytes was inversely correlated with the number of naive cells (Figure 2), but did not correlate with plasma viral load (data not shown). Strikingly, only when the number of naive CD4+ T cells dropped below 100 cells per μL, the proportion of Ki-67 expressing cells in this subset exceeded 2% (naive CD4 count > 100/μL: r = −0.79, P = .004; naive CD4 count < 100/μL: r = −0.73, P = .025; stratification arbitrary). The same overall trend, but not a significant correlation, was observed for the naive CD8 T-cell compartment (Figure 2). The size of the CD27+ memory CD4+ T-cell pool was negatively correlated with the percentage of proliferating cells in this compartment (Figure 2; r = −0.68, P = .001). In the CD8+ T-cell compartment, the opposite was observed. Here, an increase in the percentage of Ki-67 expressing cells correlated positively with the size of the CD27+ memory T-lymphocyte subset (Figure 2; r = 0.50, P = .024) and the size of the CD27− memory T-cell subset (data not shown; r = 0.52, P = .016). No correlation was found between the same parameters in the CD4+ CD27− memory or the CD8+ CD27− effector T-cell population, or between Ki-67 antigen expression and viral load (data not shown).
Correlation between the number of CD4+ and CD8+ naive or CD27+ memory T cells and the percentage of Ki-67+ naive or CD27+ memory cells of untreated HIV-infected and noninfected individuals (Spearman's correlation coefficients).
Correlation between the number of CD4+ and CD8+ naive or CD27+ memory T cells and the percentage of Ki-67+ naive or CD27+ memory cells of untreated HIV-infected and noninfected individuals (Spearman's correlation coefficients).
Longitudinal analysis of immune recovery during HAART
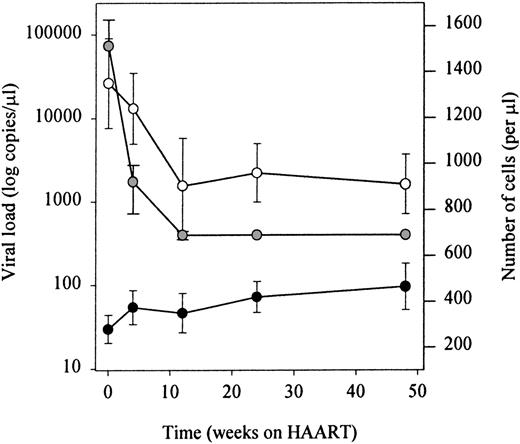
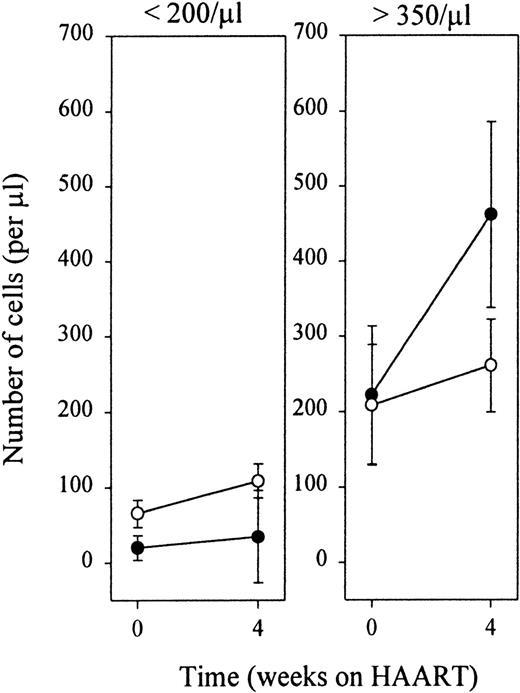
Infection with HIV-1 resulted in CD4+ T-cell depletion and expansion of the CD8+ T-cell pool as has been described previously (t = 0 in Figure 4A and B). Treatment with antiretroviral therapy resulted in a significant decrease of plasma HIV-RNA to levels below the limits of detection (lower limit of detection 400 RNA copies/mL). In parallel, total numbers of CD4+ T cells increased, and a fall in total numbers of CD8+ T lymphocytes was observed (Figure 3). Figure4A shows that the therapy-induced rise in CD4+ T cells observed in the first 4 weeks of HAART was accounted for by an increase of both CD27+ memory and naive T lymphocytes. Stratification of patients into 2 groups, based on their baseline naive CD4+ T-cell counts, revealed that the HAART-induced restoration of the naive subset was better in patients with higher baseline naive CD4+ T-cell values (Figure 5). After 1 year of treatment, numbers of naive and CD27+ memory CD4+ T cells had risen significantly compared with pretreatment levels, but were still lower compared with control levels (Figure 4A,P < .05). In the same period, as depicted in Figure 4B, the number of naive CD8+ T cells increased. The CD27+ and CD27− memory and CD27− effector CD8+ T-lymphocyte subsets remained significantly expanded, compared with control values (P < .05), despite an initial decrease in cell number that was significant for CD27+ and CD27−memory T cells after 1 and 3 months, respectively (P < .05).
Longitudinal analysis of lymphocyte recovery and plasma HIV-RNA load decline.
White dots represent HIV-RNA load, black dots CD4+ T cells and gray dots CD8+ T cells.
Longitudinal analysis of lymphocyte recovery and plasma HIV-RNA load decline.
White dots represent HIV-RNA load, black dots CD4+ T cells and gray dots CD8+ T cells.
CD4+ and CD8+ T-cell subset recovery.
Naive (circles), CD27+ memory (triangles), CD27− memory (squares), and CD27−effector (diamonds) lymphocytes within the CD4+ (A) and CD8+ (B) T-cell compartments are shown. Sequential patient values were compared using the Wilcoxon Signed Ranks test, and the Mann-Whitney U test was used for comparison with healthy individuals (*P < .05).
CD4+ and CD8+ T-cell subset recovery.
Naive (circles), CD27+ memory (triangles), CD27− memory (squares), and CD27−effector (diamonds) lymphocytes within the CD4+ (A) and CD8+ (B) T-cell compartments are shown. Sequential patient values were compared using the Wilcoxon Signed Ranks test, and the Mann-Whitney U test was used for comparison with healthy individuals (*P < .05).
Early recovery of CD4+ naive and CD27+ memory T lymphocytes.
Patients were stratified according to baseline naive T-cell values. Black and white dots represent naive and CD27+ memory cells, respectively.
Early recovery of CD4+ naive and CD27+ memory T lymphocytes.
Patients were stratified according to baseline naive T-cell values. Black and white dots represent naive and CD27+ memory cells, respectively.
Ki-67 antigen expression during HAART-induced immune recovery
Immediately on reduction of plasma HIV-RNA load by HAART, the total number (Figure 6A and B) and the percentage (data not shown) of CD4+ and CD8+Ki-67+ T cells and of Ki-67+ T lymphocytes in all subsets declined. In the CD4+ T-cell compartment, this decline was relatively slow when compared with the rapid decline observed in the CD8+ T-cell population. Furthermore, after 6 months of HAART, a temporary increase in the number of Ki-67+ lymphocytes was observed in the total CD4+ T-cell population. After 1 year of treatment, numbers of dividing CD4+ T cells had returned to normal. Interestingly, the percentage of proliferating CD4+ naive and CD27+ memory T lymphocytes was still significantly elevated at that time point (P < .05; data not shown). In the CD8+ T-cell compartment, the steepest decline in the number of Ki-67+ T cells was observed in the first 4 weeks of treatment (figure 6B; P < .05 for t = 0 vs t = 4 in total CD8+ T cells and in naive, CD27+ and CD27-memory T-cell subsets). After 1 year, the percentage (data not shown), but not the absolute number of Ki-67+CD27+ and CD27− memory and Ki-67+ CD27− effector T lymphocytes had returned to normal values (P < .008).
Longitudinal analysis of Ki-67 antigen expression in CD4+ (A) and CD8+ (B) T lymphocyte subsets and total populations.
Decline of the number of total (gray circles), naive (black circles), CD27+ memory (black triangles), CD27−memory (black squares), and CD27− effector (black diamonds) Ki-67+ T cells is shown. Sequential patient values were compared using the Wilcoxon Signed Ranks test, and the Mann-Whitney U test was used for comparison with healthy individuals (*P < .05).
Longitudinal analysis of Ki-67 antigen expression in CD4+ (A) and CD8+ (B) T lymphocyte subsets and total populations.
Decline of the number of total (gray circles), naive (black circles), CD27+ memory (black triangles), CD27−memory (black squares), and CD27− effector (black diamonds) Ki-67+ T cells is shown. Sequential patient values were compared using the Wilcoxon Signed Ranks test, and the Mann-Whitney U test was used for comparison with healthy individuals (*P < .05).
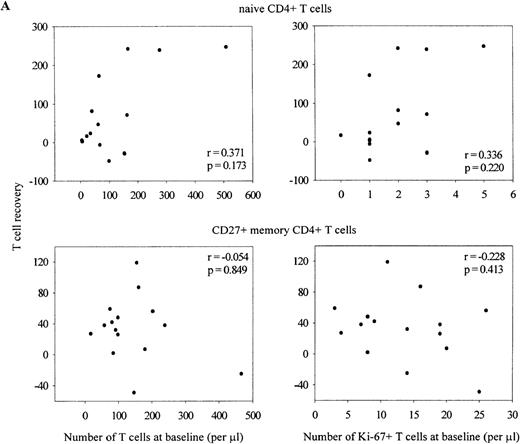
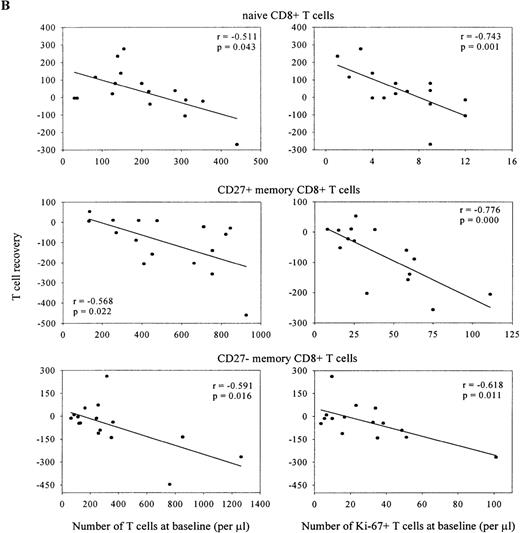
Despite the severe depletion of the naive T-cell pool, the number of Ki-67+ naive T lymphocytes fell immediately on introduction of HAART (Figure 6A and B). Figure 7depicts data of the individual Ki-67 decline in naive CD4+T lymphocytes. Reduction of Ki-67 expression was relatively slow in 2 patients who had less than 100 naive CD4+ T cells per μL and the highest percentage of dividing naive CD4+ T cells at baseline. However, as is shown in Figure8A, no correlation was found between early naive, CD27+ memory or CD27− memory (data not shown) CD4+ T-cell recovery with the baseline size of these subsets or with the number of dividing lymphocytes. In the CD8+ T-cell compartment (Figure 8B), recovery of the naive subset correlated negatively with its baseline size and with the number of dividing cells within this pool (r = −0.511,P = .043 and r = −0.743, P = .001 respectively). Finally, patients with the most pronounced reduction in size of the CD27+ or CD27− memory T-cell subset had highest numbers of CD27+ or CD27− memory CD8+ T lymphocytes (r = −0.568, P = .022 and r = −0.591,P = .016), and highest numbers of dividing cells (r = −0.776, P = 0.000 and r = −0.618,P = .011) at baseline. A similar trend, albeit not significant, was observed for the CD27− effector T-cell subset (data not shown).
HAART-induced decline in the percentage of proliferating Ki-67+ naive CD4+ T cells.
The courses of individual patients are shown. Black dots represent patients with baseline naive CD4+ T-cell numbers < 300/μL (n = 9), gray dots represent individuals with baseline naive CD4+ T-cell counts > 300/μL but < 400/μL (n = 5) and white dots represent patients with baseline naive CD4+T-cell count > 600/μL (n = 2).
HAART-induced decline in the percentage of proliferating Ki-67+ naive CD4+ T cells.
The courses of individual patients are shown. Black dots represent patients with baseline naive CD4+ T-cell numbers < 300/μL (n = 9), gray dots represent individuals with baseline naive CD4+ T-cell counts > 300/μL but < 400/μL (n = 5) and white dots represent patients with baseline naive CD4+T-cell count > 600/μL (n = 2).
Early recovery of the naive and CD27+memory CD4+ (A) and naive, CD27+ memory and CD27− memory CD8+ (B) T-cell pool.
Correlations are shown between T-cell subset recovery in the first 4 weeks of HAART (delta t = 0 vs t = 4) and baseline size of subset or number of Ki-67+ cells within each subset (Spearman's correlation coefficients).
Early recovery of the naive and CD27+memory CD4+ (A) and naive, CD27+ memory and CD27− memory CD8+ (B) T-cell pool.
Correlations are shown between T-cell subset recovery in the first 4 weeks of HAART (delta t = 0 vs t = 4) and baseline size of subset or number of Ki-67+ cells within each subset (Spearman's correlation coefficients).
Discussion
In this longitudinal study, we measured expression of the Ki-67 antigen, which is taken as a measurement for proliferating cells, in peripheral blood lymphocyte subsets of HIV-1 infected patients. In untreated HIV-1 infection, both the percentage and number of Ki-67+ CD4+ and CD8+ lymphocytes were significantly increased, compared with values obtained from healthy individuals. These data confirm previous (mainly cross-sectional) studies,5,7,8 29 showing that T-cell turnover is increased maximally 2- to 3- fold in the CD4+, and 6- to 7- fold in the CD8+ T-cell population. However, when investigating the role of T-cell proliferation in the pathogenesis of HIV-1 infection, lymphocyte subsets rather than total CD4+ or CD8+ T-cell pools should be studied, as naive, memory, and effector T lymphocytes follow different courses of depletion or expansion. We therefore analyzed changes in expression of the Ki-67 antigen within naive, CD27+ and CD27− memory and CD27− effector CD4+ and CD8+ lymphocyte populations before and during the course of HAART.
The finding that in untreated HIV-1 infection, the number of proliferating cells was elevated 2 to 3 times in all CD4+T-cell subsets is in good agreement with earlier results demonstrating that the number of apoptotic cells within the CD4 population was equally distributed over all subsets.30 Together, these data show that, in general, HIV-1 infection affects both naive and memory peripheral blood CD4+ T cells to a similar degree. The early decrease in the number of naive CD4+ T cells could therefore reflect interference of the virus with T-cell renewal capacity rather than with peripheral production of these cells.
The median percentage of dividing naive CD4+ T cells was elevated 10-fold. Higher increases in percentages of Ki-67+CD4+ naive T cells were observed only when the number of naive cells fell below 100 cells per μL. When depletion of lymphocytes would be the driving force for the increase in peripheral naive CD4+ T-cell production, one would expect the high rate of cell division to be maintained during treatment with HAART until the number of cells would be normalized. We observed, however, an immediate decline in the number and percentage of Ki-67+naive CD4+ lymphocytes, although CD4+ T cells were still severely depleted. From this we conclude that increased Ki-67 expression in the naive CD4+ T-cell subset was caused by generalized immune activation rather then being a T-cell homeostatic response to compensate for T-cell depletion.
Furthermore, our data show that the early therapy-induced increase in CD4+ T cells is accounted for by a rise in the number of both naive and CD27+ memory T cells. Our finding that, in both subsets, the percentage and number of dividing lymphocytes decreased during antiretroviral treatment points toward other mechanisms than peripheral expansion involved in recovery of the CD4+ T-cell pool. It confirms previous reports indicating that in the first 4 weeks of HAART, redistribution of trapped CD27+ cells significantly adds to therapy-induced increase in peripheral blood CD4+ T-cell numbers.14,16 17
Whereas early restoration of the CD27+ memory CD4+ T-lymphocyte compartment was fairly constant between patients, recovery of the naive CD4+ T-cell pool was variable. In patients with less severe depletion of naive CD4+ T cells, a relatively steep increase in the number of naive lymphocytes was observed during the first 4 weeks of HAART, confirming recently published data by Hengel et al.31 After this first steep rise, replenishment occurred at a slower rate. Several factors may contribute to restoration of the naive lymphocyte compartment, such as reappearance of preexisting naive T cells in the peripheral blood, as well as de novo T-cell production from a thymic source. The relative importance of each mechanism in recovery of the immune system needs to be established. Recently, it was reported that thymic production of T cells increased substantially during HAART. Its dependence on disease progression remains to be studied.15
In contrast to the changes occurring in the CD4+ T-cell compartment, in the naive CD8+ T-cell pool, percentages of dividing cells were elevated regardless of the size of this subset. This points toward a distinct regulation of peripheral T-cell production rates in CD4+ and CD8+ cells. The high proliferation rate in the latter population most likely reflects antigen-induced expansion. The degree of expansion of the CD8+ T-cell subset correlated with the degree of cell proliferation during chronic HIV-1 infection observed in that particular subset. Ongoing antigenic pressure during untreated HIV-1 infection could lead to continuous recruitment of naive CD8+ T cells, thereby maintaining increased percentages of Ki-67 antigen expression in this subset. During HAART, both the percentage and the total numbers of dividing cells declined, suggesting that activation-related cell division was the driving force for expansion of the primed subset during chronic HIV-1 infection. The early therapy-induced drop in the number of primed CD8+ T cells reached a plateau after 6 months, at which the CD27+or CD27−CD45RO+ memory and CD27−CD45RO− effector CD8+ lymphocyte pool remained significantly expanded. Although the absolute number of dividing primed CD8+ T cells remained elevated, the percentage of Ki-67+ T cells within this subset returned to normal values. Using tetrameric HLA-peptide complexes, it has been shown that HAART reduced the frequency of HIV-1 specific cytotoxic T lymphocytes (CTLs) several fold.32 The early decline in primed CD8+ T cells that we observed could therefore reflect apoptosis of HIV-specific CTLs.
The numbers of dividing cells in healthy individuals found in this study correspond well to the range of earlier results obtained with Ki-67 staining7,8 and with deuterated glucose labeling of dividing cells.19 However, when studying HIV-1 infected patients, in these reports, the average total peripheral blood production of proliferating CD4+ T cells was 5.2 × 107 (range 2.7-8.3 × 107) and 10.8 × 107 (range 7.1-12.9 × 107) of CD8+ T cells. These values are lower than those reported in our study (11.4 × 107 [range 2.4-21.0 × 107] and 49.2 × 107[range 7.0-648.5 × 107], respectively), most likely because of the more advanced disease stage of our patients (see Table 3).
After the initial HAART-induced decline, we observed a transient rise in the number of proliferating CD4+ T cells after 6 months of therapy, after which these numbers declined further. Because this rise was only due to increased numbers of proliferating CD27+ memory cells, and coincided with dissolvement of the depletion of this subset, it could reflect improvement of the functional capacity of these cells. Indeed, T-cell function, as measured by the in vitro proliferative response to CD3 and CD28 mAb, improved and reached a plateau phase after 6 months of HAART (data not shown). Whereas in our study, the rise in T-cell proliferation did not exceed pretreatment values, in a study reported by Fleury et al,7 a clear increase in cell production was seen over baseline after 6 months of HAART. In that report, the baseline CD4+ T-cell counts were relatively high, on average over 600 cells per μL. We found the transient rise in numbers of dividing cells to be more pronounced in patients with higher baseline CD4+ T-cell counts (data not shown), pointing toward variances in the stage of HIV-1 infection as the cause of the observed differences.
Taken together, our data are compatible with the idea that most of the elevated cell division and cell death in HIV-1 infection is related to strong persistent immune activation30,33 rather than a response to or the cause of lymphocyte depletion. Therefore, alternative factors leading to T-cell decline must be involved in HIV-1 pathogenesis. As has been proposed previously, CD4+ T-cell depletion may be due to a diminished capacity for T-cell renewal as a direct or indirect result of HIV-1 infection or could be related to an intrinsically low capacity for renewal in adults, which cannot even compensate for a slightly enhanced T-cell death due to HIV-1 infection and generalized immune activation.34-37 Furthermore, we observed a clear distinction between CD4+ and CD8+ T cells, with respect to proliferation rates. A similar dichotomy was found before, when much more cell activation induced death and shortened telomeres were found in the CD8+ T-cell fraction.5 38 This may fit well with differential requirements for clonal expansion to exercise CD4+ helper and CD8+ effector cell functions. Because cell division diminished rapidly with HAART even when T-cell numbers were still very low, our results suggest that the increased peripheral lymphocyte proliferation is not a homeostatic response to T-cell depletion.
Acknowledgments
We thank patients and physicians who participated in the CHEESE study, and Dr R. A. W. van Lier for critical reading of the manuscript.
Reprints:Mette D. Hazenberg, Department of Clinical Viro-Immunology, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.