Abstract
To determine the relative in vivo importance of IL-1 release after allergen challenge to the subsequent endothelial adhesion and recruitment of eosinophils, the authors used ovalbumin sensitization and inhalation challenge to induce airway eosinophilia in IL-1 receptor type 1-deficient and control wild-type mice. Bronchoalveolar lavage (BAL) eosinophil recruitment in IL-1 receptor type 1-deficient mice challenged with ovalbumin (24.3% ± 6.3% BAL eosinophils) was significantly reduced compared with wild-type mice (63.7% ± 2.5% BAL eosinophils). To determine whether the inhibition of eosinophil adhesion to vascular endothelium contributed to the inhibition of eosinophil recruitment in IL-1 receptor type 1-deficient mice, the authors used intravital microscopy to visualize the rolling and firm adhesion of fluorescence-labeled mouse eosinophils in the microvasculature of the allergen-challenged mouse mesentery. Eosinophil rolling, eosinophil firm adhesion to endothelium, and transmigration across endothelium (peritoneal eosinophils) were significantly inhibited in allergen-challenged IL-1 receptor type 1-deficient mice compared with wild-type mice. Overall, these studies demonstrate that cytokines such as IL-1, released after allergen challenge, are important in the induction of endothelial cell adhesiveness, a prerequisite for the recruitment of circulating eosinophils. (Blood. 2000;95:263-269)
The recruitment of eosinophils from the bone marrow, where they are produced across inflamed postcapillary venules to tissue sites, is a multistep process.1-3 Bone marrow–derived eosinophils circulate intravascularly until they are exposed to inflamed endothelium, as noted at sites of allergic inflammation.4 Several cytokines (IL-1, tumor necrosis factor (TNF), IL-4)5-8 and mediators (histamine)9 released at sites of allergic inflammation are important upregulators of adhesion molecule expression by the endothelium. In vitro cytokines such as IL-1 and TNF induce human umbilical vein endothelial cells to express several adhesion molecules, including VCAM-1, ICAM-1, E-selectin, and P-selectin.5-7,10In contrast, cytokines such as IL-4 (induces VCAM-1 but not ICAM-1 or E-selectin)7,8 and mediators such as histamine (induces P-selectin)9 induce the expression of a more restricted profile of endothelial cell adhesion molecules. In vivo specific endothelium-expressed adhesion molecules, such as P-selectin11,12 and VCAM-1,11,13 are important to the initial eosinophil rolling on inflamed endothelium, whereas endothelium-expressed adhesion molecules, such as ICAM-111,14,15 and VCAM-1,13 15 are important to the subsequent eosinophil firm adhesion to endothelium.
We have, therefore, sought to determine the relative importance of an individual cytokine, IL-1, to eosinophil adhesion to endothelium and to the subsequent recruitment of eosinophils in a mouse model using IL-1 receptor type 1-deficient mice challenged with allergen. The potential importance of IL-1 to eosinophilic inflammation and asthma has been suggested by previous studies16 demonstrating the increased expression of IL-1β mRNA and protein in the airway of patients with symptomatic asthma compared with those with asymptomatic asthma. In addition, increased nocturnal levels of IL-1β at 4 amcompared to 4 pm have also been noted in the nocturnal BAL fluid of patients with asthma who have associated nocturnal airflow obstruction.17 During asthma episodes resident alveolar macrophages18 and airway epithelium19 are potential sources of IL-1β, whereas recruited eosinophils also express IL-1.20 In vitro study results have demonstrated that neutralizing antibodies to IL-1β partially block the upregulation of VCAM-1 and ICAM-1 expression by human bronchial tissue after allergen challenge.21 IL-1β release has been detected at sites of human cutaneous allergic reactions,22and human cutaneous allergic late-phase reactions can be inhibited by soluble IL-1 receptors.23
Two forms of IL-1, IL-1α and IL-1β, exert their biologic effect by binding to IL-1 receptors.24 Two types of IL-1 receptor have been cloned and classified as IL-1 receptor type 1 (IL-R type 1) and IL-1 receptor type 2 (IL-1 R type 2).25-28 Both IL-1 α and IL-1 β bind to the IL-1 R type 1 with high affinity and elicit biologic responses through IL-1 R type 1-mediated signal transduction pathways.29,30 In contrast, IL-1 R type 2 (which has a short 29 amino acid intracellular domain) does not transduce an intracellular signaling response when either IL-1α or IL-1β bind to this receptor.31 We have used IL-1 R type 1-deficient mice to study the role of IL-1 (compared with other proadhesive cytokines and mediators released at sites of allergic inflammation) in upregulating endothelial cell adhesiveness for circulating eosinophils at sites of allergic inflammation. We hypothesized that IL-1 (α or β) released at sites of allergen challenge16,17 binds to the IL-1 R type 1 expressed by endothelial cells and would upregulate endothelial cell adhesion molecule expression in wild-type mice but not in IL-1 R type 1-deficient mice. Endothelial cells express only IL-1 R type 1 and not IL-1 R type 2.31-33 In this study we demonstrated by intravital microscopy that eosinophils exhibit reduced rolling and firm adhesion to endothelial cells in postcapillary venules of IL-1 R type 1-deficient mice compared with wild-type mice challenged with allergen. We also demonstrated that this reduced endothelial cell adhesiveness for eosinophils in IL-1 R type 1-deficient mice results in the reduced transmigration of eosinophils to allergen-challenged tissues (lung and peritoneal cavity).
Materials and methods
IL-1 R type 1-deficient mice
IL-1 R type 1-deficient mice were kindly provided by Dr. J. Peschon (Immunex, Seattle, WA).34 C57Bl/6 × 129 J hybrid background wild-type female mice aged 8 to 10 weeks were used as controls. Previous studies34 with these IL-1 R type 1-deficient mice demonstrated that they generate IgG1 and IgE responses to antigen similar to those noted with C57BL/6 and 129 background wild-type mice. Additional studies35 demonstrate the loss of IL-1 β-mediated functional responses in IL-1 R type 1-deficient mice (ie, injection of murine IL-1 β in vivo in wild-type mice induced fever and weight loss, whereas injection of IL-1 β does not elicit this response).
Mouse model of eosinophilic pulmonary inflammation
Pulmonary eosinophilia in mice was induced as previously described in this laboratory.36 In brief, wild-type or IL-1 R type 1-deficient mice were sensitized by intraperitoneal injection with 50 μg ovalbumin/1 mg alhydrogel (Aldrich Chemical, Milwaukee, WI) in 0.9% sterile saline on days 0 and 12. Nonsensitized mice received 1 mg alhydrogel in 0.9% saline. On day 24, the appropriate groups of mice (n = 4 mice/group) were exposed 3 times (at 1-hour intervals) to an aerosol of ovalbumin (10 mg/mL) in 0.9% saline (nonsensitized mice received saline only) for 30 minutes. The aerosolized ovalbumin protocol was repeated every second day thereafter for 8 days. The aerosol was generated at 6 L/min by a nebulizer (Ultra-Neb 99; Devilbis, Somerset, PA) that produces a mean particle diameter of 3.9 mm into a closed chamber of 800 cm3. Mice were killed by CO2 asphyxiation 3 hours after the last aeroallergen challenge.
Bronchoalveolar lavage cells
Bronchoalveolar lavage (BAL) cells from wild-type and IL-1 R type 1-deficient mice were recovered by lavage with 1 mL phosphate-buffered saline (PBS) through a tracheal catheter. The resultant BAL cells were immediately separated from BAL fluid by centrifugation (700gfor 5 minutes). An appropriate PBS dilution of the recovered BAL cells was added to trypan blue, and the viability and total number of BAL white blood cells were counted with a hemocytometer. Differential leukocyte counts were performed after brief acetone fixation and staining of the BAL cells with May–Grünwald–Giemsa stains. Eosinophil, neutrophil, and mononuclear cell percentages on each slide were assessed by counting at least 300 cells in random high-power fields using a light microscope (magnification × 40) to display the slide image on a TV monitor (Videometric 150 image analysis program; American Innovision, San Diego, CA).
Quantitation of lung eosinophils
The number of eosinophils in lung tissue was determined using a sensitive method dependent on the presence of a cyanide-resistant eosinophil peroxidase.36,37 Excised lungs from ovalbumin or PBS-challenged wild-type or IL-1 R type 1-deficient mice were placed in Tissue Tek OCT compound (Sakura Finetek USA, Torrance, CA), snap-frozen in liquid nitrogen, and stored at −70°C. Cryosections (5-μm thick) of lung tissue were cut onto microscope slides and fixed for 5 minutes in acetone. Slides were rehydrated in PBS for 5 minutes. The lung sections were incubated at room temperature for 1 minute in the presence of cyanide buffer (10 mmol/L potassium cyanide, pH 6). Slides were then rinsed in PBS and incubated for 10 minutes with the peroxidase substrate DAB (3, 31-diaminobenzine) (Vector Laboratories, Burlingame, CA). Tissue sections were subsequently washed in PBS before counterstaining with hematoxylin. Cells that stained dark brown, which is characteristic of the eosinophil cyanide-resistant peroxidase,36 37 were counted in 4 random high-power fields (magnification × 40) in each lung section. Results were expressed as the number of eosinophils per high-power field (hpf) of lung tissue.
Immediate hypersensitivity skin test
Wild-type and IL-1R type 1-deficient mice were sensitized to ovalbumin as described above. On day 32, 50 μL ovalbumin antigen or diluent control was injected into the shaved backs of the different groups of mice. Immediately after antigen administration, 200 μL 1% Evans blue dye was injected into the tail veins of the mice.38 Bluing size of the skin (measured as the largest transverse diameter in millimeters) at the challenged site was assessed 15 minutes later.
Mouse model of peritoneal eosinophilic inflammation: ragweed allergen immunization and peritoneal allergen challenge
Techniques used for ragweed immunization and challenge were similar to those previously described in this and other laboratories.11,39 40 Mice were immunized by a series of 5 injections of a 1:1000 dilution of a ragweed pollen extract (Miles, Spokane, WA). On days 0 and 1, 0.1 mL was injected subcutaneously, and on days 6, 8, and 14, 0.2 mL was injected subcutaneously. A control group of ragweed-immunized mice (challenged with PBS diluent) and nonimmunized mice (prepared by subcutaneous injections of isotonic saline instead of the ragweed pollen extract) was kept on the same immunization schedule. Each group of mice studied consisted of 3 to 5 mice. Mice were challenged on day 20 by the intraperitoneal injection of 0.2 mL ragweed allergen (or control PBS diluent).
Assessment of eosinophils in the peritoneal cavity
At time points before (day 0) and after immunization, and before and 48 hours after intraperitoneal allergen challenge (day 22), the mice were killed by cervical dislocation. Two mL PBS containing 6 U/mL heparin was injected intraperitoneally, the abdomen was massaged, and the peritoneal infusion was collected after the peritoneum was opened. An appropriate PBS dilution of the recovered peritoneal fluid was added to trypan blue, and the viability and total number of white blood cells were assessed and counted with a hemocytometer. Differential leukocyte counts were taken after brief acetone fixation and staining of the peritoneal cells with May–Grünwald–Giemsa stains. Eosinophil percentage on each slide was assessed by counting at least 300 cells in random high-power fields using a light microscope (magnification × 40) to display the slide image on a TV monitor (Videometric 150 image analysis program; American Innovision, San Diego, CA).
Isolation of murine eosinophils from IL-5 transgenic mice for fluorescent labeling
Mouse eosinophils of 85% to 95% purity and >98% viability were purified from IL-5 transgenic mice (kindly provided by Dr Colin Sanderson)41 using a Percoll gradient as previously described.11 IL-5 transgenic mice (aged 10 weeks) had peripheral blood leukocyte differential cell counts exhibiting about 40% to 60% eosinophils. The contaminating white blood cells comprised about 30% to 40% T lymphocytes, about 2% mononuclear cells, and about 10% neutrophils. Eosinophils with at least 98% viability and >90% purity were selected and labeled with carboxy fluorescein diacetate (CFDA; Molecular Probes, Eugene, OR) as previously described in our laboratory for labeling of murine and human eosinophils.1 11 CFDA-labeled eosinophils were resuspended at a concentration of 0.5 × 107 cells/200 μL PBS containing 0.01% glucose and were kept at room temperature in the dark until used.
FACS analysis of mouse eosinophil expression of L-selectin and VLA-4
Expression of L-selectin and VLA-4 α integrins on purified populations of murine eosinophils derived from IL-5 transgenic mice was determined on a FACSstar flow cytometer (Becton Dickinson, Mountain View, CA).42 Eosinophils were incubated with the primary monoclonal antibodies (mAb) MEL-14 (a rat antimouse L-selectin IgG2a mAb), PS/2 (a rat antimouse α 4 integrin IgG2b mAb), or species- and isotype-matched mAb on ice for 30 minutes, washed, and incubated with goat-antirat–fluorescein isothiocyanate IgG (Sigma, St. Louis, MO) for an additional 30 minutes. Surface expression was determined after gating for eosinophils by characteristic forward and side light scatter.
Preparation of mice for detection of eosinophil rolling in the peritoneal microcirculation
Ragweed-sensitized IL-1R type 1-deficient or control wild-type mice (25–35 g body weight) were prepared for intravital microscopy 24 hours after the final peritoneal ragweed or PBS challenge. We previously reported11 that this 24-hour time point after allergen challenge is optimal for visualizing allergen-induced intravascular eosinophil rolling and adhesion in this mouse model. It was chosen because it slightly precedes peak eosinophil tissue recruitment, which peaks 24 to 48 hours after allergen challenge.11 38-40 Mice were anesthetized with subcutaneous injections of saline solution containing a cocktail of ketamine hydrochloride and xylazine (7.5 mg and 2.5 mg, respectively, per 100 mg body weight). Mice were then placed on a heating pad maintained at 37°C. A midline incision was made, and the mesentery was gently exteriorized and spread on a heated glass window (37.5°C) of the stage of a Leitz intravital microscope. The exteriorized portion of mouse mesentery was kept continuously moist with endotoxin-free isotonic saline solution (pH 7.4). Other parts of the intestine that were exposed but not microscopically observed were kept moist with isotonic saline-soaked cotton pads, and the mesentery was covered with plastic wrap. To minimize endotoxin contamination, the plastic wrap was presoaked with 1% E-Toxa-Clean (Sigma) overnight and then rinsed in 70% ethanol and endotoxin-free distilled water and given a final wash with sterile isotonic saline solution.
Visualization of eosinophils in the mouse mesentery
Fluorescent CFDA-labeled eosinophils were injected into the tail veins of mice previously sensitized with ragweed allergen and challenged with either ragweed or saline 24 hours before intravital fluorescence microscopy. All studies were conducted 0 to 2 hours after exteriorization of the mouse mesentery. Rolling of mouse eosinophils in mesenteric venules was made visible by stroboscopic epi-illumination using a video-triggered xenon lamp and a Leitz Ploemopak epi-illuminator (Leitz, Wetzlar, Germany) using an I2 filter block. All images were recorded through a silicon-intensified tube camera (SIT68; Dage MTI, Michigan City, IN) using a × 10 or a × 20 water immersion objective (Nikon, Melville, NY) as described previously.11 The rolling fraction (Rf) of CFDA-labeled mouse eosinophils in ragweed-challenged mice (wild-type control and IL-1 R type 1-deficient mice) was determined by frame-by-frame analysis as previously described.11 Rolling eosinophils were quantitated by counting the number of eosinophils interacting with the vessel wall in 1 minute in a plane perpendicular to a vessel axis, whereas cells that were stationary for at least 1 minute were considered adherent eosinophils.
Statistics
The numbers of eosinophils in lung, BAL fluid, and peritoneal cavity were compared by Student's t-test using a statistical software package (InStat, San Diego, CA). Rolling fractions of injected eosinophils were compared by multiple comparisons of paired data by Student's t-test using a statistical software package (SigmaStat; Jandel Scientific, San Rafael, CA).P < .05 was considered statistically significant. All results are given as mean ± SEM.
Results
Mouse model of eosinophilic lung inflammation
Broncho-alveolar lavage.
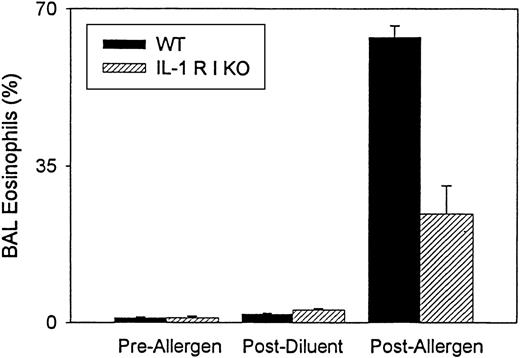
As previously described,36 sensitization and ovalbumin allergen challenge of wild-type mice (n = 3 experiments) induce significant BAL eosinophilia (63.7% ± 2.5% BAL eosinophils) compared with mice that were not sensitized or challenged with ovalbumin (1.1% ± 0.2% BAL eosinophils) (P = .004) or compared with mice immunized with ovalbumin and challenged with PBS diluent (1.9% ± 0.2% BAL eosinophils) (P = .0001). Neutrophils comprised <2% of BAL cells before or after allergen challenge and after diluent challenge. Mononuclear cells comprised the remainder of the BAL cells.
In contrast to wild-type mice, IL-1 R type 1-deficient mice (n = 3 experiments), immunized and challenged with ovalbumin, developed significantly less BAL eosinophilia (IL-1 R type 1-deficient mice 24.3% ± 6.3% BAL eosinophils vs. wild-type mice 63.7% ± 2.5% BAL eosinophils) (P = .001) (Figure1). Neither IL-1 R type 1-deficient mice not sensitized and challenged with ovalbumin (1.1% ± 0.3% BAL eosinophils) nor IL-1 R type 1-deficient mice immunized with ovalbumin and challenged with PBS diluent (2.9% ± 0.7% BAL eosinophils) developed significant BAL eosinophilia.
Quantitative analysis of bronchoalveolar lavage eosinophils in IL-1 R type 1-deficient mice.
The number of eosinophils in bronchoalveolar lavage (BAL) fluid was enumerated before and after allergen challenge and after diluent challenge in wild-type (WT) and IL-1 R type 1-deficient (IL-1 R I KO) mice. Results are expressed as the percentage of eosinophils in BAL fluid (n = 3 experiments). There was a significant inhibition of BAL eosinophilia in IL-1 R type 1-deficient mice after allergen challenge compared with wild-type mice after allergen challenge (P = .001).
Quantitative analysis of bronchoalveolar lavage eosinophils in IL-1 R type 1-deficient mice.
The number of eosinophils in bronchoalveolar lavage (BAL) fluid was enumerated before and after allergen challenge and after diluent challenge in wild-type (WT) and IL-1 R type 1-deficient (IL-1 R I KO) mice. Results are expressed as the percentage of eosinophils in BAL fluid (n = 3 experiments). There was a significant inhibition of BAL eosinophilia in IL-1 R type 1-deficient mice after allergen challenge compared with wild-type mice after allergen challenge (P = .001).
There was a significant increase in BAL total leukocytes in wild-type mice after allergen challenge (wild-type mice, 0.58 ± 0.08 × 105 BAL leukocytes before allergen challenge vs. 15.61 ± 2.08 × 105 BAL leukocytes after allergen challenge) (P = .0002) (n = 3). The number of BAL leukocytes before allergen challenge was not significantly different in IL-1 R type 1-deficient mice (0.56 ± 0.11 × 105 BAL leukocytes) than in wild-type mice (0.58 ± 0.08 × 105 BAL leukocytes) (P = NS) (n = 3). The number of BAL leukocytes recovered after allergen challenge was significantly less in IL-1 R type 1-deficient mice (6.10 ± 0.61 × 105 BAL leukocytes) than in wildtype mice (15.61 ± 2.08 × 105 BAL leukocytes) (P = .001) (n = 3). There was no significant difference in the number of BAL lymphocytes in wild-type mice compared with IL-1 R type 1-deficient mice.
Lung.
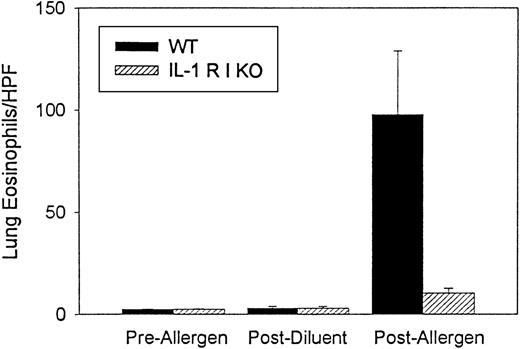
Ovalbumin sensitization and challenge also induced significant lung tissue eosinophilia (97.8 ± 31.3 lung eosinophils/hpf) (n = 3 experiments) compared with mice that were not sensitized or challenged with ovalbumin (2.5 ± 0.3 lung eosinophils/hpf) (P = .05) or compared with mice immunized with ovalbumin and challenged with PBS diluent (3.0 ± 0.9 lung eosinophils/hpf) (P = .002) (Figure 2). Analysis of lung sections of IL-1 R type 1-deficient mice immunized and challenged with ovalbumin also demonstrated a significant reduction in lung eosinophils (IL-1 R type 1-deficient mice 10.5 ± 2.3 lung eosinophils/hpf vs. wild-type mice 97.8 ± 31.3 lung eosinophils/hpf) (n = 3 experiments) (P = .01) (Figure 2). There was no significant difference in the number of lung eosinophils in wild-type mice compared with IL-1 R type 1-deficient mice not immunized or challenged with ovalbumin (2.5 ± 0.3 lung eosinophils/hpf in wild-type vs. 3.1 ± 0.8 lung eosinophils/hpf in IL-1 R type 1-deficient mice).
Quantitative analysis of lung eosinophils in IL-1 R type 1-deficient mice.
The number of eosinophils in cryosections of lung tissue was enumerated before and after allergen challenge and after diluent challenge in wild-type and IL-1 R type 1-deficient mice using a method to detect the presence of eosinophil peroxidase. Results are expressed as the number of lung eosinophils (expressed as eosinophils/hpf lung tissue) (n = 3 experiments). There was a significant inhibition of lung eosinophilia in IL-1 R type 1-deficient mice after allergen challenge compared with wild-type mice after allergen challenge (P = .01).
Quantitative analysis of lung eosinophils in IL-1 R type 1-deficient mice.
The number of eosinophils in cryosections of lung tissue was enumerated before and after allergen challenge and after diluent challenge in wild-type and IL-1 R type 1-deficient mice using a method to detect the presence of eosinophil peroxidase. Results are expressed as the number of lung eosinophils (expressed as eosinophils/hpf lung tissue) (n = 3 experiments). There was a significant inhibition of lung eosinophilia in IL-1 R type 1-deficient mice after allergen challenge compared with wild-type mice after allergen challenge (P = .01).
We also performed quantitative analysis of peribronchial and alveolar–interstitial eosinophil cell counts. In wild-type mice approximately 27.3% ± 3.7% of the total lung eosinophils were peribronchial, whereas 72.7% ± 3.7% of the total lung eosinophils were alveolar–interstitial. IL-1 R type 1-deficient mice had significantly reduced numbers of both peribronchial eosinophils (82.5% ± 3.6% inhibition vs. wild-type mice) (P = .01) and alveolar–interstitial eosinophils (91.8% ± 6.1% inhibition vs wild-type mice) (P = .01).
To determine whether differences in sensitization to antigen could account for the differences in eosinophil recruitment between wild-type and IL-1 R type 1-deficient mice, we performed immediate hypersensitivity skin testing in the different groups of mice. Wild-type and IL-1 R type 1-deficient mice sensitized with ovalbumin and challenged intradermally with ovalbumin developed equivalently sized immediate hypersensitivity skin bluing reactions when Evans blue dye was injected into their tail veins (wild-type mice, 11 ± 2 mm; IL-1R type 1-deficient mice 10 ± 2 mm).
Mouse model of eosinophilic peritonitis in IL-1R type 1–deficient mice
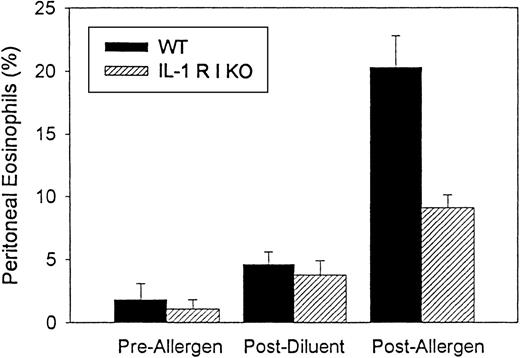
Wild-type mice (n = 3 separate experiments), when immunized and challenged with ragweed allergen, developed significant peritoneal cavity eosinophilia (20.3% ± 2.5% eosinophils) compared with wild-type mice not challenged with ragweed (1.8% ± 1.3% eosinophils) (P = .003) or compared with wild-type mice immunized with ragweed and challenged with PBS diluent (4.6% ± 1.0% eosinophils) (P = .004). In contrast to wild-type mice, IL-1-R type 1-deficient mice immunized with ragweed developed less peritoneal eosinophilia when challenged with intraperitoneal injection of ragweed allergen (IL-1-R type 1-deficient mice 9.1% ± 1.7% peritoneal eosinophils; about 55% inhibition compared with ragweed-challenged wild-type mice) (P = .02) (Figure 3). There was no significant difference in the number of peritoneal eosinophils in wild-type than in IL-1 R type 1-deficient mice not immunized or challenged with ragweed (1.8% ± 1.3% peritoneal eosinophils in wild-type vs. 1.1% ± 0.7% peritoneal eosinophils in IL-1-R type 1-deficient mice).
Comparison of eosinophil peritoneal recruitment in IL-1 R type 1 deficient and wild-type mice.
Ragweed-sensitized mice (IL-1 R type 1-deficient or control wild-type mice) (n = 3 experiments) were challenged with an intraperitoneal injection of ragweed allergen. Forty-eight hours later the percentage of transmigrated peritoneal eosinophils was assessed by light microscopy. IL-1 R type 1-deficient mice developed significantly less peritoneal eosinophilia than control wild-type mice after allergen challenge (P = .02).
Comparison of eosinophil peritoneal recruitment in IL-1 R type 1 deficient and wild-type mice.
Ragweed-sensitized mice (IL-1 R type 1-deficient or control wild-type mice) (n = 3 experiments) were challenged with an intraperitoneal injection of ragweed allergen. Forty-eight hours later the percentage of transmigrated peritoneal eosinophils was assessed by light microscopy. IL-1 R type 1-deficient mice developed significantly less peritoneal eosinophilia than control wild-type mice after allergen challenge (P = .02).
Percentage increases in peritoneal eosinophils in wild-type mice after allergen challenge were associated with a significant increase in the number of total peritoneal leukocytes (35.7 ± 6.9 × 105 peritoneal leukocytes before allergen challenge vs. 72.5 ± 8.9 × 105 peritoneal leukocytes after allergen challenge) (P = .02). There was no significant increase in the total number of peritoneal leukocytes in IL-1 receptor type 1-deficient mice (37.3 ± 10.0 × 105peritoneal eosinophils before allergen challenge vs. 45.2 ± 9.8 peritoneal eosinophils after allergen challenge) (P = .38).
Expression of L-selectin and VLA-4 by mouse eosinophils
Mouse eosinophils were purified from IL-5 transgenic mice for infusion to the peritoneal microcirculation of allergen-challenged wild-type mice. These purified mouse eosinophils expressed the eosinophil rolling receptors L-selectin and α 4 integrin, as assessed by FACS analysis.
Intravital microscopy and eosinophil rolling and adhesion in IL-1 R type 1–deficient mice
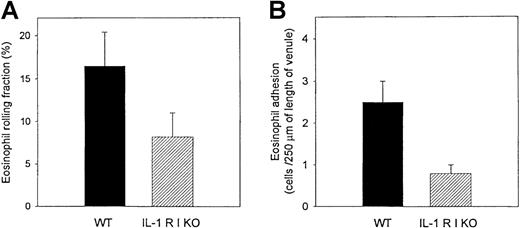
The passage of the fluorescence-labeled eosinophils in the allergen-challenged mesenteric circulation was made visible by stroboscopic epi-illumination (Figure 4). We previously demonstrated36 that peritoneal ragweed challenge induces a significant increase in eosinophil rolling in the mesenteric venules of wild-type mice challenged with ragweed compared with wild-type mice challenged with PBS diluent. Eosinophil rolling in venules of ragweed-challenged IL-1 R type 1-deficient mice (eosinophil rolling fraction 8.2% ± 2.8%) was significantly reduced than it was in ragweed-challenged wild-type mice (eosinophil rolling fraction 16.5% ± 3.9%) (P = .05) (Figure5A).
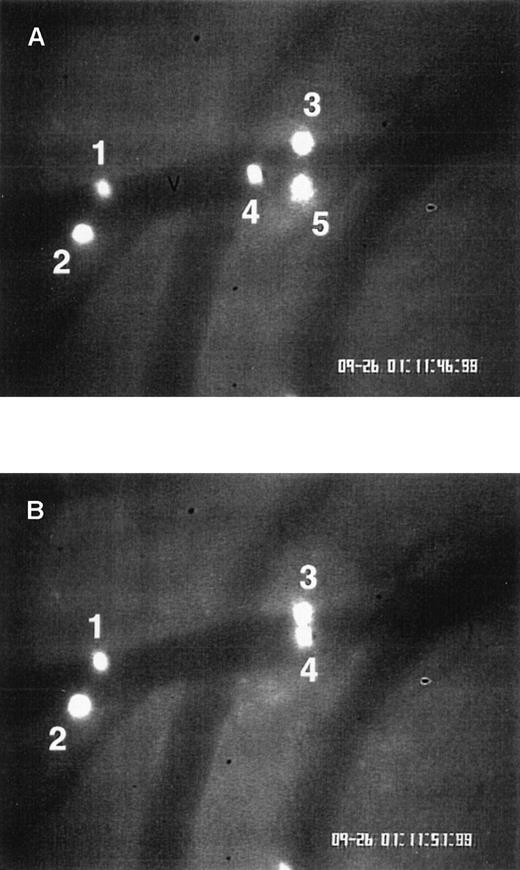
Eosinophil rolling and firm adhesion visualized by intravital videomicroscopy.
Ragweed-sensitized wild-type mice were challenged with an intraperitoneal injection of ragweed. Twenty-four hours after intraperitoneal ragweed allergen challenge, fluorescence-labeled eosinophils were injected through the tail vein and visualized in vivo in the peritoneal microcirculation using intravital videomicroscopy. (A) A videotape image of eosinophils flowing through a postcapillary venule (V) is depicted. Blood flow in the venule is from left to right. (B) An image of the same venule is depicted, taken 5 seconds later than the image in panel A. Eosinophils 1, 2, and 3 remain stationary (same positions in panels A and B), representing eosinophils adherent to the venular endothelium. Eosinophil 4 transiently rolled on endothelium and is visible in panels A and B. Eosinophil 5 (panel A) became detached from the endothelium and is no longer visible in panel B.
Eosinophil rolling and firm adhesion visualized by intravital videomicroscopy.
Ragweed-sensitized wild-type mice were challenged with an intraperitoneal injection of ragweed. Twenty-four hours after intraperitoneal ragweed allergen challenge, fluorescence-labeled eosinophils were injected through the tail vein and visualized in vivo in the peritoneal microcirculation using intravital videomicroscopy. (A) A videotape image of eosinophils flowing through a postcapillary venule (V) is depicted. Blood flow in the venule is from left to right. (B) An image of the same venule is depicted, taken 5 seconds later than the image in panel A. Eosinophils 1, 2, and 3 remain stationary (same positions in panels A and B), representing eosinophils adherent to the venular endothelium. Eosinophil 4 transiently rolled on endothelium and is visible in panels A and B. Eosinophil 5 (panel A) became detached from the endothelium and is no longer visible in panel B.
Comparisons of eosinophil rolling and firm adhesion in IL-1 R type 1-deficient and control wild-type mice using intravital microscopy.
Ragweed-sensitized mice (IL-1 R type 1-deficient or control wild-type mice) were challenged with intraperitoneal injections of ragweed. Twenty-four hours later fluorescence-labeled mouse eosinophils were infused intravenously into mouse tail veins. Mouse mesentery was visualized by intravital microscopy, and intravascular eosinophil rolling and adhesion to endothelium were recorded on videotape. The number of eosinophils rolling on endothelium in ragweed-challenged IL-1 R type 1-deficient mice was significantly less than that observed in ragweed-challenged control wild-type mice (P = .05) (panel A). Similarly, the number of eosinophils adherent to endothelium in ragweed-challenged IL-1 R type 1-deficient mice was significantly less than that observed in ragweed-challenged control wild-type mice (P = .002) (panel B). Six mice were studied in each experiment (n = 2 experiments), and 3 to 6 blood vessels were analyzed per mouse.
Comparisons of eosinophil rolling and firm adhesion in IL-1 R type 1-deficient and control wild-type mice using intravital microscopy.
Ragweed-sensitized mice (IL-1 R type 1-deficient or control wild-type mice) were challenged with intraperitoneal injections of ragweed. Twenty-four hours later fluorescence-labeled mouse eosinophils were infused intravenously into mouse tail veins. Mouse mesentery was visualized by intravital microscopy, and intravascular eosinophil rolling and adhesion to endothelium were recorded on videotape. The number of eosinophils rolling on endothelium in ragweed-challenged IL-1 R type 1-deficient mice was significantly less than that observed in ragweed-challenged control wild-type mice (P = .05) (panel A). Similarly, the number of eosinophils adherent to endothelium in ragweed-challenged IL-1 R type 1-deficient mice was significantly less than that observed in ragweed-challenged control wild-type mice (P = .002) (panel B). Six mice were studied in each experiment (n = 2 experiments), and 3 to 6 blood vessels were analyzed per mouse.
As observed with eosinophil rolling, reduced levels of eosinophil adhesion were observed in IL-1 R type 1-deficient mice compared with wild-type mice challenged with ragweed (0.5 ± 0.2 adherent eosinophils/250 μm blood vessel length in ragweed-challenged IL-1 R type 1-deficient mice vs. 2.5 ± 0.8 adherent eosinophils/250 μm blood vessel length in ragweed-challenged wild-type mice) (P = .002) (Figure 5B). The reduced number of firmly adherent eosinophils in IL-1 R type 1-deficient mice resulted from an effect on eosinophil rolling and an effect on integrin-mediated firm adhesion, as evidenced by the fact that eosinophil firm adhesion was inhibited to a greater degree (about 80%) than eosinophil rolling (about 51%).
Discussion
In this study we demonstrated that IL-1 R type 1-deficient mice exhibited reduced BAL and lung eosinophil recruitment compared with wild-type mice when challenged by inhaled allergen. This suggested that IL-1 released at sites of allergic inflammation in the lung might have contributed to the recruitment of eosinophils during episodes of allergen-induced asthma. The mechanism by which IL-1 induced eosinophil recruitment was most likely not a direct effect on eosinophil chemotaxis but was more likely an indirect effect on eosinophils mediated by IL-1 upregulation of endothelial cell adhesion molecules that bind circulating eosinophils. This hypothesis is supported by our intravital videomicroscopy studies demonstrating that eosinophils exhibited reduced rolling and firm adhesion to allergen-challenged endothelium in IL-1 R type 1-deficient mice in vivo. IL-1, by binding to the IL-1 R type 1 on endothelial cells, induces endothelial cells to express adhesion receptors capable of supporting eosinophil rolling (P-selectin, VCAM-1)10,11,13 and of supporting eosinophil firm adhesion (ICAM-1, VCAM-1)11 14 in vivo. The absence of IL-1 R type 1 on endothelial cells in IL-1 R type 1-deficient mice could therefore have resulted in reduced expression of these adhesion molecules and the reduced eosinophil rolling and adhesion we noted. An additional consequence of the reduced eosinophil rolling and endothelial cell adhesion could be the reduced eosinophil recruitment we noted in the lung and in the peritoneal cavity of IL-1 R type 1-deficient mice after allergen challenge.
Additional endothelial adhesion molecules induced by IL-1 include E-selectin5 and P-selectin.10 Although IL-1 can induce E-selectin expression, E-selectin preferentially supports neutrophil and not eosinophil rolling in vivo.43 Therefore, the inability of IL-1 to induce E-selectin expression by endothelial cells in IL-1 R type 1-deficient mice is unlikely to have accounted for the reduced eosinophil rolling noted in IL-1 R type 1-deficient mice. The importance of P-selectin to eosinophil recruitment has been demonstrated using P-selectin–deficient mice11,36,44 and intravital videomicroscopy to demonstrate that eosinophil rolling and firm adhesion are inhibited in P-selectin–deficient mice challenged with allergen.11 Thus the inability of IL-1 to induce P-selectin expression by endothelial cells in IL-1 R type 1-deficient mice could have contributed to the reduced eosinophil rolling and recruitment we noted. Attempts to quantitate differences in endothelial adhesion molecule levels (ICAM-1, VCAM-1, P-selectin) using immunohistochemistry in wild-type versus IL-1 R type 1-deficient mice proved technically difficult because the method is not quantitative and because grading differences in the color intensity of positive cells (as opposed to the presence or absence of color in positive and negative cells) are subjective. We have therefore indirectly inferred (based on our intravital microscopy study results demonstrating reduced eosinophil rolling and adhesion on endothelium) that IL-1 R type 1-deficient mice have reduced levels of endothelial adhesion molecule expression compared with wild-type mice.
Allergen challenge induces the release of several cytokines (IL-1, IL-4, TNF)22,45,46 and chemokines (exotoxin, RANTES, MCP-4, MIP1-α)47,48 with overlapping functions that can influence levels of eosinophil recruitment in vivo. The availability of cytokine-deficient mice has allowed the investigation of the relative importance of individual cytokines to eosinophil recruitment after allergen challenge. Previous studies using cytokine-deficient mice other than IL-1 R type 1-deficient (ie, IL-4 deficient mice challenged with aerosolized allergen) also demonstrate reduced eosinophil recruitment to the lung.49 Studies using IL-4 deficient mice did not directly investigate whether the mechanism of reduced eosinophil recruitment in IL-4 deficient mice was a consequence of reduced eosinophil adhesion to endothelium, as we have now demonstrated in IL-1 R type 1-deficient mice, or of alternative mechanisms (IL-4 influences IgE responses and VCAM -1 adhesion molecule expression by endothelium).7 Previous studies demonstrate that IL-1 R type 1-deficient mice generate IgE responses to antigen similar to those noted in background wild-type mice.34 Our immediate hypersensitivity skin test results in IL-1 receptor type 1-deficient and wild-type mice support this conclusion and suggest that differences in sensitization to allergen cannot explain our results.
We have noted that the number of eosinophils rolling in the ragweed-challenged mouse peritoneal microcirculation is less than that noted by studies of neutrophils in other mouse models of peritoneal inflammation50 51 for several potential reasons. One is that intravital microscopy studies of neutrophils in the peritoneal microcirculation have predominantly focused on endogenous neutrophils in the microcirculation and not on adoptively transferred neutrophils, as in our eosinophil studies. Thus the number of neutrophils in the peritoneal microcirculation available to interact with endothelial cells is far greater in those studies than in our studies, in which the adoptively transferred eosinophils circulate to the allergen-challenged peritoneum and to vascular beds other than the peritoneal microcirculation. In addition, the degree and type of peritoneal inflammation induced varies according to the stimulus used in different models of inflammation (ie, ragweed allergen in our eosinophil studies vs. TNF, lipopolysaccharides, bacteria in neutrophil studies). The ragweed stimulus we used induced a lower total peritoneal inflammatory cell response than that reported in studies of neutrophils. Nevertheless, sufficient numbers of eosinophils can be observed in the allergen-challenged peritoneal microcirculation to make this a useful method to study mechanisms of eosinophil recruitment in vivo.
Our study suggests that IL-1 released at sites of allergic inflammation signaled through endothelium-expressed IL-1 R type 1 to promote eosinophil rolling and adhesion. Because rolling, adhesion, and transmigration of eosinophils across endothelium are sequential steps, interruption of the adhesion cascade at an early step in IL-1 R type 1-deficient mice reduced eosinophilic tissue inflammation. Although the IL-1 receptor provides a potential therapeutic target to inhibit eosinophilic inflammation, an IL-1 receptor antagonist would not only inhibit the recruitment of eosinophils, it could inhibit the recruitment of other circulating leukocytes (and eosinophils) using the same profile of endothelial adhesion receptors induced by IL-1. However, this study underscored the importance of IL-1 as a key mediator of the eosinophilic inflammatory response associated with allergic inflammation.
Acknowledgments
The authors thank Dr J. Peschon (Immunex, Seattle, WA) for providing IL-1 R type 1-deficient mice and Dr Colin Sanderson (Perth, Australia) for providing IL-5 transgenic mice, Greg Hughes and Mark Santoz for technical assistance, and Lanesha Hill for expert secretarial support during the preparation of the manuscript.
Supported by National Institutes of Health grants AI 33977 and AI 38425 (DHB) and AI 35796 and California Tobacco-Related Disease Research program grant 7RT0197 (PS).
Reprints:David H. Broide, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0635; e-mail:dbroide@ucsd.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.