Abstract
In a double-blind, placebo-controlled, randomized study, 10 healthy men received either a single dose of 480 μg granulocyte colony-stimulating factor (G-CSF) or saline. Blood taken from the volunteers was stimulated with 10 μg/mL endotoxin and released cytokines were measured by enzyme-linked immunosorbent assay. Expression of G-CSF receptors on leukocytes was examined by flow cytometry and reverse transcriptase-polymerase chain reaction. Functional activity of these receptors was tested by challenging isolated leukocyte populations to release cytokines with endotoxin in the presence of G-CSF. The G-CSF treatment attenuated the release of the proinflammatory cytokines tumor necrosis factor (TNF)-, interleukin (IL)-12, IL-1β, and interferon (IFN)-γ in ex vivo lipopolysaccharide (LPS)-stimulated whole blood. In blood from untreated volunteers the presence of G-CSF in vitro also attenuated the LPS-stimulated release of these cytokines. G-CSF in vitro also attenuated TNF- release from elutriation-purified monocytes. In the presence of 10 ng/mL recombinant TNF-, the attenuation of LPS-inducible IFN-γ release by G-CSF was blunted in whole blood. However, G-CSF had no such effect on IFN-γ release from isolated lymphocytes stimulated with anti-CD3 or a combination of TNF- and IL-12. G-CSF receptor expression was detected in human neutrophils and monocytes but not in lymphocytes by means of RT-PCR as well as flow cytometry. These results indicate that G-CSF receptors expressed on monocytes are functional in modulating monokine release. We conclude that the attenuation of IFN-γ release from lymphocytes is not a direct effect of G-CSF on these cells but is rather due to the inhibition of monocytic IL-12 and TNF- release by G-CSF. (Blood. 2000;95:270-276)
Granulocyte colony-stimulating factor (G-CSF) is a cytokine that regulates the proliferation of hematopoietic cells in the bone marrow. This endogenous signaling protein not only stimulates the production of neutrophils, but also shortens their maturation time in the bone marrow.1-3 G-CSF also modulates neutrophil functions, for example, by increasing phagocytosis and antibody-dependent cell-mediated cytotoxicity, as well as by priming neutrophils for oxidative burst.1,2 G-CSF exerts its biologic effects by binding to its specific cell surface receptor. G-CSF receptors are expressed mainly on neutrophils and their precursors, but also have been reported on other cell types such as platelets, endothelial cells, placenta, small-cell lung cancer cell lines, and monocytes.3-10
Recently, new properties of G-CSF as a modulator of the inflammatory response were discovered. Mice, pretreated with G-CSF, were protected against an otherwise lethal dose of lipopolysaccharide (LPS); this protection was accompanied by a suppression of LPS-induced serum tumor necrosis factor (TNF)-α levels.11 G-CSF also modulates the inflammatory response in humans. Blood from healthy volunteers treated with 480 μg G-CSF showed an attenuated release capacity for the proinflammatory cytokines TNF-α, GM-CSF, and interferon (IFN)-γ when stimulated with LPS ex vivo.12,13 In extension of these findings, volunteers treated with 5 μg/kg G-CSF and then exposed to a low dose of endotoxin 24 hours later exhibited attenuated serum levels of TNF-α and interleukin (IL)-6 compared to levels under placebo treatment in a crossover design study.14 These results demonstrate that G-CSF not only acts on neutrophils and their precursors but also influences the release of cytokines derived from monocytes and lymphocytes. We therefore concluded that G-CSF also exerts anti-inflammatory effects in addition to recruiting and activating neutrophils.
In this study, we focused on characterizing these anti-inflammatory effects of G-CSF in humans. Because the mechanisms by which G-CSF influences monocytic and lymphocytic cytokine release are not known, we were particularly interested in whether G-CSF affects monocytes and lymphocytes directly or whether other mediators participate in transmitting the signal.
We considered whole blood and isolated leukocytes to be a suitable system to investigate this question because G-CSF also shows its anti-inflammatory effects in vitro. To identify possible target cells of G-CSF, we studied the expression of G-CSF receptors on leukocyte populations by means of flow cytometry and reverse transcriptase-polymerase chain reaction (RT-PCR).
Materials and methods
G-CSF treatment of healthy volunteers
The double-blind and placebo-controlled study was conducted in accordance with the precepts of the Helsinki declaration and approved by the Institutional Review Board of the University of Konstanz. All volunteers gave written informed consent before entry into the study. To exclude major disease and acute infections, all subjects underwent a thorough physical examination. Blood and urine samples were examined by routine clinical chemistry. Ten healthy men were admitted to the study. Treatment assignments were made through use of a randomization approach that is stratified by weight (≤ 80 kg versus > 80 kg) and randomized into 2 groups. The placebo group consisted of 4 volunteers (age 28.5±2.2 years, weight 75.8±3.2 kg) and the treatment group of 6 volunteers (age 27.3±0.7 years, weight 78.5±2.4 kg). The treatment group received a single subcutaneous injection of 1.6 mL with 480 μg filgrastim (Neupogen 48, Hoffmann LaRoche/Amgen, Basel, Switzerland) at 9 am. At the same time, the placebo group received a saline injection of 1.6 mL. Directly before injection, as well as 8 hours and 24 hours after injection of filgrastim, a 10-mL blood sample was withdrawn from the volunteers and used for the experiments.
White blood cell counts
Differential white blood cell (WBC) counts were carried out in EDTA-anticoagulated blood using a Coulter STKS (Coulter, Krefeld, Germany). Blood smears were prepared in parallel to verify the results of this automated differential WBC. The leukocyte differential counts were also confirmed by flow cytometric analysis (FACS Calibur, Becton Dickinson, Heidelberg, Germany) using fluorescence-conjugated anti-CD45 and anti-CD14 antibodies (Becton Dickinson).
Whole blood incubations
To study the LPS-induced cytokine release of whole blood, 800 μL RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 2.5 IU/mL heparin (Liquemin, Hoffmann La Roche, Grenzach-Whylen, Germany) and 100 IU/mL penicillin/streptomycin (Biochrom) was pipetted into a polypropylene reaction tube (Eppendorf, Hamburg, Germany) and 10 μg endotoxin from Salmonella abortus equi (Sigma, Deisenhofen, Germany) was added. Finally, 200 μL heparinized whole blood was added and the tubes were incubated for 24 hours at 37°C and 5% CO2. After incubation, the tubes were shaken and blood cells were sedimented by centrifugation (16 000g, 2 minutes). The cell-free supernatants were stored at −80°C until cytokine measurement.
In vitro incubation with G-CSF
For these experiments, whole blood incubations were performed with blood from untreated healthy volunteers and incubated in vitro with G-CSF (Neupogen 48) at various concentrations from 1 ng/mL to 300 ng/mL. G-CSF was tested alone as to induction of cytokine release, or blood was additionally stimulated with 10 μg endotoxin from Salmonella abortus equi. In some experiments 10 ng/mL IL-12 (R&D-Systems, Wiesbaden, Germany) and 10 ng/mL TNF-α (gift from Bender, Vienna, Austria) were also added.
Isolation of leukocyte populations
Peripheral blood mononuclear cells (PBMC) were prepared in cell preparation tubes (Vacutainer CPT, sodium citrate, Becton Dickinson) according to the manufacturer's instructions. After centrifugation (20 minutes, 1650g), the white layer above the gel containing the PBMC was removed and the cells were washed 3 times with RPMI 1640. Cell numbers were adjusted to 5 × 106 cells/mL in RPMI 1640 supplemented as described above.
Neutrophils were isolated from heparinized blood by density gradient centrifugation: 7 mL blood was layered on top of 6 mL Mono-Poly Resolving Medium, Ficoll Hypaque (ICN Biomedicals, Eschwege, Germany) in a polypropylene test tube and centrifuged at 300g for 30 minutes. The second white layer from the top containing the neutrophils was removed and the cells were washed twice with ice-cold phosphate-buffered saline (PBS) containing 1 mM EDTA, pH 7.4, and centrifuged at 450g for 10 minutes at 4°C. For lysis of erythrocytes, the cell pellet was resuspended in 30 mL ice-cold sterile water for 30 seconds, then 10 mL 0.6 mol/L KCl solution was added. After centrifugation (10 minutes, 350g, 4°C) the cell pellet was resuspended in RPMI 1640 supplemented as described above, and the cell number was adjusted to 107 cells/mL. The cells were kept on ice until addition to the PBMC incubation.
Monocytes were isolated by counter-current centrifugal elutriation (J2-MC centrifuge equipped with a JE-6B rotor, Beckman, Munich, Germany) as described previously.15 Monocytes were free of attached platelets because neither soluble phosphodiesterase (PDE) 3 nor PDE 5 activities, both highly expressed in platelets, were detectable in monocyte lysates (data not shown, see reference 16). Monocyte purity was > 90% as checked by anti-CD14 staining in flow cytometric experiments. Monocytes were adjusted to 105cells/mL in RPMI 1640 supplemented as described above.
For the isolation of lymphocytes, a protocol from Julius et al17 was adapted. Nylon wool (synthetic filter wool for aquarium filters) was incubated overnight at 37°C and 5% CO2 in endotoxin-free water, then filled into 10-mL syringes (Becton Dickinson), rinsed with RPMI 1640, and incubated with 10 mL RPMI 1640 for 1 hour at 37°C and 5% CO2. The medium was then replaced by 10 mL PBMC suspension in RPMI 1640 and incubated for 45 minutes at 37°C and 5% CO2. After the incubation time, the nonadherent lymphocytes were gained by rinsing the syringes with 40 mL RPMI 1640. Lymphocytes were pelleted by centrifugation (10 minutes, 400g) and adjusted to 106 cells/mL in RPMI 1640 plus 10% fetal-calf serum (Biochrom). Lymphocyte purity was > 90% as checked in flow cytometric experiments using anti-CD14-FITC (Becton Dickinson) and anti-CD45-PerCP (Becton Dickinson).
Stimulation of isolated leukocyte populations
Isolated leukocyte populations were stimulated to release cytokines, and the effect of G-CSF on cytokine release was studied in the different cell systems. PBMC (5 × 105) with or without neutrophils (106) or elutriation-purified monocytes (105) in 1 mL RPMI 1640 with 2.5 IU/mL heparin and 100 IU/mL penicillin/streptomycin were stimulated with 10 μg/mL LPS ± 100 ng/mL G-CSF and incubated for 24 hours at 37°C and 5% CO2.
Lymphocytes (106/mL) were stimulated with a combination of 100 ng/mL IL-12 and 100 ng/mL TNF-α or 1 mL cell suspension was plated on 24-well cell culture plates (Greiner, Frickenhausen, Germany) coated overnight with 500 μL PBS containing 10 μg/mL anti-CD3-antibody (Orthoclone OKT-3, Cilag GmbH, Sulzbach, Germany) and incubated for 24 hours at 37°C and 5% CO2.
Cytokine measurement
After incubation of the stimulated leukocytes for 24 hours, the released cytokines in the cell-free supernatants were quantified by sandwich enzyme-linked immunosorbent assay (ELISA). Antibody pairs for TNF-α, IL-1β, and IFN-γ were purchased from Endogen (Munich, Germany). Recombinant IFN-γ (Thomae, Biberach, Germany), recombinant IL-1β (Endogen), and recombinant TNF-α (Bender, Vienna, Austria) were used as standards. ELISA plates (Greiner) were coated overnight at 4°C with 50 μL/well coat antibody in 0.1 mol/L NaHCO3, pH 8.2. After blocking with 200 μL/well PBS supplemented with 3% bovine serum albumin (BSA) (Serva, Heidelberg, Germany), pH 7.0, for 2 hours at room temperature, the plates were washed twice with PBS/0.05% Tween-20. Sample (50 μL/well) and tracer-antibody (50 μL/well) in PBS/BSA 3% were added and incubated for 2 hours. After 6 wash cycles, plates were incubated for 30 minutes with streptavidin-peroxidase (Dianova, Hamburg, Germany; 1 μg/mL in PBS/BSA 3%, 100 μL/well). After 8 washes, 100 μL/well tetramethyl benzidine liquid substrate solution (Sigma) was added and incubated at room temperature for 5 to 30 minutes. After addition of 50 μL/well stop solution (1 mol/L H2SO4), absorption was measured at 450 nm using a reference wavelength of 690 nm. IL-12 was measured using the IL-12 Quantikine kit (R&D Systems) according to the manufacturer's instructions.
Flow cytometry
For leukocyte subtyping 50 μL EDTA blood was stained with 10 μL anti-CD14-FITC (Becton Dickinson) and 10 μL anti-CD45-PerCP (Becton Dickinson). For detection of G-CSF receptors, blood was additionally stained with 10 μL fluorescent G-CSF phycoerythrin (G-CSF-PE, Fluorokine kit, R&D Systems) or with 10 μL anti-G-CSF-receptor-PE (anti-CD114-PE, Serotec, Oxford, UK). Streptavidin-PE (10 μL streptavidin-PE/50 μL blood) provided in the Fluorokine kit was used to control the specificity of the G-CSF-PE staining. For the staining with anti-CD114-PE, control experiments were performed using an isotype antibody (PE mouse IgG1κ, Pharmingen, 10 μL antibody solution/50 μL blood). After incubation for 30 minutes at room temperature, 1 mL BD Lysing Solution (Becton Dickinson) was added to lyse erythrocytes and fixate cells. After 10 minutes, cells were washed twice with 1 mL Cell Wash buffer (Becton Dickinson) and measured in a FACS Calibur flow cytometer (Becton Dickinson) using Cell Quest software (Becton Dickinson). For each blood sample 50 000 cells were counted.
For quantitative comparison of fluorescent signals, a fluorescence standard curve was recorded using Quantibrite beads (Becton Dickinson), labeled with defined amounts of PE.
RNA preparation
RNA from purified neutrophils, monocytes, and lymphocytes was prepared with QIAamp RNA Blood mini kit plus additional DNAse digestion (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Reverse transcription
One μg RNA was reverse transcribed in a 20-μL reaction mixture, containing 2.5 μM oligo dT16 (GibcoBRL, Karlsruhe, Germany), 5 mM MgCl2, dNTP (1 mM each), 1 U/μL RNase inhibitor, 2.5 U/μL murine leukemia virus reverse transcriptase in 1 × PCR buffer (all reagents by Perkin-Elmer Applied Biosystems, Weiterstadt, Germany). Probes were incubated at 21°C for 10 minutes, at 42°C for 15 minutes, at 94°C for 5 minutes, and at 5°C for 5 minutes in a GeneAmp PCR System 2400 (Perkin-Elmer Applied Biosystems).
Polymerase chain reaction
For the PCR, 100 ng cDNA (2 μL RT probe) was added to PCR master mix, containing 4 mM MgCl2, 400 nM sense as well as antisense primer specific for human G-CSF receptor (Biognostic, Göttingen, Germany) or 18S rRNA (Ambion, Austin, TX) in 1 × Lightcycler DNA Master SYBR Green1 (Roche Diagnostics, Mannheim, Germany). PCR was performed in a LightCycler Instrument (Roche Diagnostics), using the following thermal settings: denaturation at 95°C for 30 seconds and cycling at 95°C for 0 seconds, 60°C for 5 seconds, and 72°C for 11 seconds. Amplification was followed online and the PCR reaction was stopped in the logarithmic phase (cycle 19 for G-CSF receptor, cycle 18 for 18S rRNA). Additionally, a melting curve analysis was performed after PCR to check specificity of the reaction. The PCR product was visualized by gel electrophoresis (1.5% agarose gel) and staining with ethidium bromide (1 μg/mL). Quantification of PCR products was performed with Image Master VDS and analysis software (Amersham Pharmacia Biotech, Freiburg, Germany) using mass calibration by GeneRuler 100 bp marker (MBI Fermentas, St. Leon-Rot, Germany). For normalization, the amount of G-CSF receptor amplicon was divided by the amount of 18 S rRNA amplicon of the respective sample.
Statistical analysis
All data are given as means ± SEM. Cytokine release was calculated per milliliter blood, that is, corrected for the dilution factor of 5 as 20% blood was used, or calculated per cell number. Statistical analyses were performed using GraphPad Instat (GraphPad Software, San Diego, CA). For comparison of 2 groups, a paired 2-tailed Student's t test was performed. Statistical analysis of 3 or more groups was performed with a paired Tukey-Kramer test. Significance testing for the G-CSF treatment study was done for IL-12, IL-1β, and TNF-α by comparing the cytokine release per monocyte of each volunteer 8 hours and 24 hours after treatment with the individual pretreatment value in a paired Tukey-Kramer test. This testing accounts for interindividual variances of cytokine formation. For IFN-γ release, the data could not be tested against individual pretreatment values because circadian differences were observed for IFN-γ release. In the afternoon, the release was about twice as high as in the morning. We therefore compared the G-CSF group with the placebo group at each time point using an unpaired 2-tailed Student's ttest.
Results
Effect of G-CSF treatment on WBC
Leukocyte counts in the G-CSF group increased from 7100±1200 cells/μL to 34 900±3600 cells/μL (P < .001) 24 hours after treatment, whereas the placebo group showed no changes in leukocyte numbers (6100±500 before treatment, 5500±300 24 hours after treatment). The differential WBC count revealed that the increase of leukocyte numbers in the G-CSF group was due to an increase of neutrophil as well as monocyte numbers. In the G-CSF group, neutrophils increased about 6-fold over control values from 3700±800 cells/μL to 21 300±1900 cells/μL (P < .001) and monocyte counts increased 3.3 times from 440±50 cells/μL to 1470±400 cells/μL (P < .05). Lymphocyte and eosinophil numbers were not significantly influenced by the G-CSF treatment (data not shown).
Ex vivo cytokine release modulation after G-CSF treatment
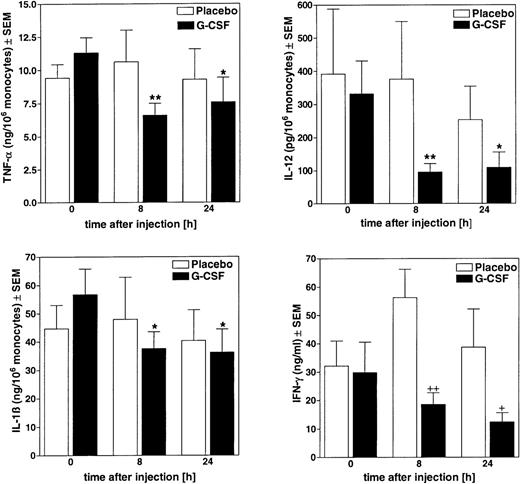
In blood taken from the volunteers 8 hours and 24 hours after G-CSF treatment, no significant changes were found in the total LPS-inducible release of TNF-α, IL-12, and IL-1ß. However, when cytokine release was calculated per monocyte count, which had increased in the G-CSF treated group, the release capacity of blood from G-CSF-treated volunteers was reduced 8 hours after treatment by 42%±5% for TNF-α, by 67%±4% for IL-12, and by 31%±7% for IL-1β (Figure 1); the placebo group showed no significant changes. Similar results were also obtained in blood taken 24 hours after treatment.
Ex vivo attenuation of LPS-stimulated cellular cytokine release in blood from G-CSF-treated donors.
Blood from volunteers was drawn at different times after injection of G-CSF (480 μg) or placebo and stimulated ex vivo with 10 μg/mL LPS. After 24 hours of incubation at 37°C, released cytokines were measured in cell-free supernatants by ELISA. Amounts of TNF-α, IL-12, and IL-1β were calculated per monocyte counts; IFN-γ release was expressed per milliliter whole blood. Data represent means ± SEM of the G-CSF treatment group (n = 6) and of the placebo group (n = 4). *P < .05, **P < .01 versus pretreatment values (t = 0 hours). +P < .05,++P < .01 versus placebo group.
Ex vivo attenuation of LPS-stimulated cellular cytokine release in blood from G-CSF-treated donors.
Blood from volunteers was drawn at different times after injection of G-CSF (480 μg) or placebo and stimulated ex vivo with 10 μg/mL LPS. After 24 hours of incubation at 37°C, released cytokines were measured in cell-free supernatants by ELISA. Amounts of TNF-α, IL-12, and IL-1β were calculated per monocyte counts; IFN-γ release was expressed per milliliter whole blood. Data represent means ± SEM of the G-CSF treatment group (n = 6) and of the placebo group (n = 4). *P < .05, **P < .01 versus pretreatment values (t = 0 hours). +P < .05,++P < .01 versus placebo group.
In blood from G-CSF-treated volunteers an attenuation of LPS-inducible IFN-γ release was found, that is, 8 hours after treatment IFN-γ release was reduced by 67%±7% and at 24 hours by 68%±8% of placebo control values (P < .01).
In vitro cytokine release modulation in the presence of G-CSF
Granulocyte colony-stimulating factor was added in vitro to whole blood from untreated individuals and incubated for 24 hours. In the concentration range from 1 to 300 ng/mL G-CSF alone did not induce any cytokine release. If 10 μg/mL LPS was additionally present in these blood incubations, concentrations > 10 ng/mL G-CSF attenuated the LPS-induced release of TNF-α, IL-12, IL-1β, and IFN-γ in a concentration-dependent manner (see Table). Three hundred ng/mL G-CSF reduced TNF-α release by 50%±4% in comparison with LPS control (P < .001), IL-12 by 42%±7% (P < .001), IL-1β by 23%±3% (P < .001), and IFN-γ by 40%±3% (P < .001).
Granulocyte colony-stimulating factor also attenuated the release of TNF-α and IL-1ß when cytokine release was stimulated with lower endotoxin concentrations in the range of 100 pg/mL to 100 ng/mL (data not shown).
Table. In vitro attenuation of cytokine release in LPS-stimulated human whole blood by G-CSF
| . | LPS . | + G-CSF (ng/ml) . | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 3 . | 10 . | 100 . | 300 . | ||||
| TNF-α (ng/ml) | 9.4 ± 0.8 | 8.6 ± 1.1 | 6.8 ± 0.71-165 | 7.5 ± 0.71-165 | 6.1 ± 0.81-165 | 5.2 ± 0.71-165 | ||
| IL-12 (pg/ml) | 245 ± 54 | 237 ± 53 | 217 ± 52 | 201 ± 501-165 | 195 ± 471-165 | 174 ± 461-165 | ||
| IL-1β (ng/ml) | 39.1 ± 2.0 | 43.2 ± 3.1 | 37.0 ± 2.2 | 31.9 ± 1.91-165 | 34.3 ± 2.31-160 | 30.7 ± 2.21-165 | ||
| IFN-γ (ng/ml) | 26.8 ± 4.6 | 28.7 ± 5.8 | 20.0 ± 4.3* | 19.8 ± 3.6* | 18.1 ± 3.31-165 | 17.1 ± 3.41-165 | ||
| . | LPS . | + G-CSF (ng/ml) . | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 3 . | 10 . | 100 . | 300 . | ||||
| TNF-α (ng/ml) | 9.4 ± 0.8 | 8.6 ± 1.1 | 6.8 ± 0.71-165 | 7.5 ± 0.71-165 | 6.1 ± 0.81-165 | 5.2 ± 0.71-165 | ||
| IL-12 (pg/ml) | 245 ± 54 | 237 ± 53 | 217 ± 52 | 201 ± 501-165 | 195 ± 471-165 | 174 ± 461-165 | ||
| IL-1β (ng/ml) | 39.1 ± 2.0 | 43.2 ± 3.1 | 37.0 ± 2.2 | 31.9 ± 1.91-165 | 34.3 ± 2.31-160 | 30.7 ± 2.21-165 | ||
| IFN-γ (ng/ml) | 26.8 ± 4.6 | 28.7 ± 5.8 | 20.0 ± 4.3* | 19.8 ± 3.6* | 18.1 ± 3.31-165 | 17.1 ± 3.41-165 | ||
20% whole blood in RPMI 1640 was stimulated with 10 μg/ml LPS in the presence of different concentrations of G-CSF. After 24 h incubation at 37°C released cytokines were measured in the cell-free supernatants by ELISA. Data represent means ± SEM of 17 to 20 different donors.
p < .05,
p < .01,
p < .001 versus LPS control.
Expression of G-CSF receptors on human leukocytes
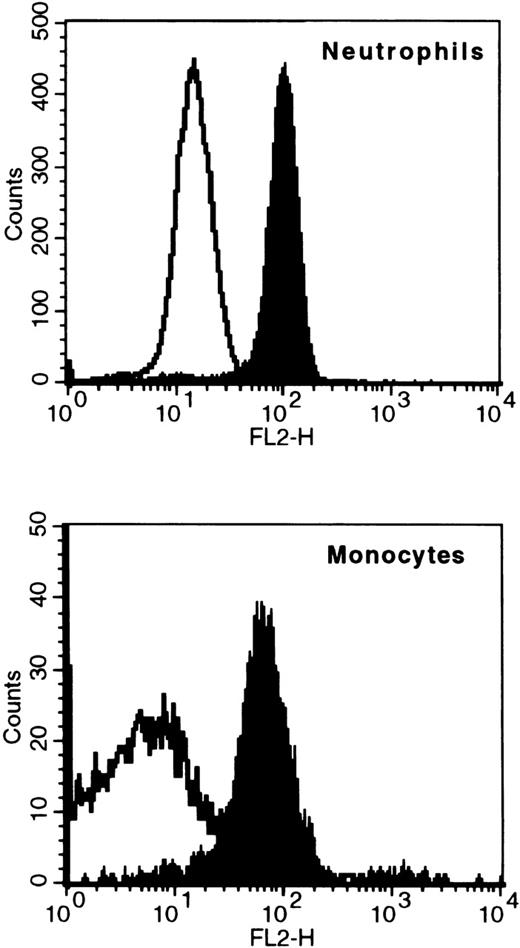
To study the distribution of G-CSF receptors on human leukocytes, whole blood was stained with PE-labeled G-CSF (G-CSF-PE) or an antibody against the G-CSF receptor (anti-CD114-PE). For an accurate definition of leukocyte populations, blood was additionally stained with anti-CD45-PerCP and anti-CD14-FITC. The specificity of G-CSF-PE staining was checked by control staining with streptavidin-PE, which would have indicated unspecific binding of PE to the cells. Positive staining with G-CSF-PE was found on neutrophils and monocytes, but not on eosinophils and lymphocytes. G-CSF-PE staining of 6 different blood samples showed a mean fluorescence signal of 66±4 for neutrophils and 31±6 for monocytes; streptavidin-PE staining resulted in a mean fluorescence signal of 7±1 for neutrophils and 10±2 for monocytes. The data of a representative sample are shown in Figure2.
Binding of fluorescent G-CSF to neutrophils and monocytes.
Whole blood was stained with fluorescent G-CSF (G-CSF-PE, indicated by black shading) or an unspecific staining control (Streptavidin-PE, no black shading). The populations of neutrophils or monocytes were gated by counterstaining with anti-CD14-FITC and anti-CD45-PerCP.
Binding of fluorescent G-CSF to neutrophils and monocytes.
Whole blood was stained with fluorescent G-CSF (G-CSF-PE, indicated by black shading) or an unspecific staining control (Streptavidin-PE, no black shading). The populations of neutrophils or monocytes were gated by counterstaining with anti-CD14-FITC and anti-CD45-PerCP.
As a second detection system for G-CSF receptors, staining with anti-G-CSF-receptor antibody was performed. Specificity of this staining was controlled with a PE-labeled isotype antibody. Positive staining was found again only on neutrophils and monocytes, but not on lymphocytes or eosinophils, confirming the results of the G-CSF-PE staining. The data of a stained blood sample are depicted in Figure3.
Binding of G-CSF receptor antibodies to neutrophils and monocytes.
Whole blood was stained with an anti-G-CSF receptor antibody (CD114-PE, indicated by black shading) or an isotype control antibody (isotype-PE, no black shading). The populations of neutrophils or monocytes were gated by counterstaining with anti-CD14-FITC and anti-CD45-PerCP.
Binding of G-CSF receptor antibodies to neutrophils and monocytes.
Whole blood was stained with an anti-G-CSF receptor antibody (CD114-PE, indicated by black shading) or an isotype control antibody (isotype-PE, no black shading). The populations of neutrophils or monocytes were gated by counterstaining with anti-CD14-FITC and anti-CD45-PerCP.
A quantitative analysis of fluorescence signals was performed using calibration beads with defined amounts of bound PE. With the additional information from the manufacturer that 1 fluorochrome is bound per antibody, it was possible to calculate the number of anti-CD114-PE bound per cell. The staining of 8 different blood samples with anti-CD114 resulted in 2330±100 molecules bound per neutrophil (390±40 isotype control antibodies bound per neutrophil) and 1570±130 bound per monocyte (68±15 isotype control antibodies bound per monocyte). Elutriation-purified, platelet-free monocytes showed the same binding characteristics of G-CSF-PE and anti-CD114-PE as seen in whole blood (data not shown).
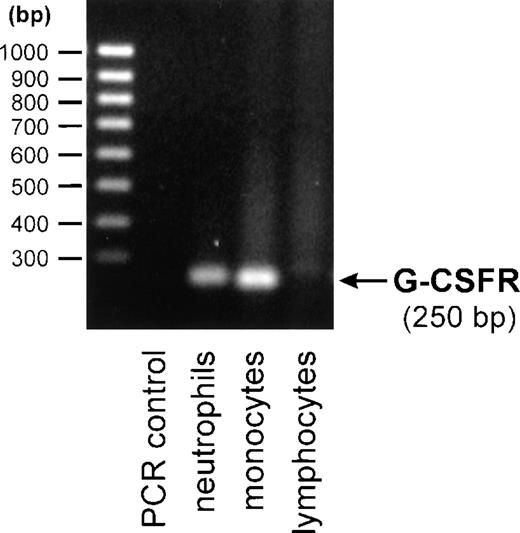
To further support this finding, we also performed RT-PCR analysis to detect G-CSF receptor specific mRNA. The G-CSF receptor amplicon was detected in RNA preparations of purified neutrophils and monocytes, but not in purified lymphocytes (Figure4). Normalization to the housekeeping gene 18S rRNA showed comparable amounts of G-CSF receptor amplicon in monocytes and neutrophils.
Detection of human G-CSF receptor mRNA by RT-PCR.
Total mRNA from purified neutrophils, monocytes, or lymphocytes was reverse transcribed and amplified by 19 cycles of PCR using G-CSF receptor-specific primers. PCR products were analyzed on a 1.5% agarose gel and visualized by ethidium bromide staining.
Detection of human G-CSF receptor mRNA by RT-PCR.
Total mRNA from purified neutrophils, monocytes, or lymphocytes was reverse transcribed and amplified by 19 cycles of PCR using G-CSF receptor-specific primers. PCR products were analyzed on a 1.5% agarose gel and visualized by ethidium bromide staining.
Effect of G-CSF on TNF- release in purified leukocyte populations
Our results from flow cytometry and RT-PCR suggest that G-CSF receptors are expressed also on monocytes. We therefore investigated whether these receptors on monocytes are functional in mediating the G-CSF effect on monokine release. To differentiate direct effects of G-CSF on monocytes from those due to possible cellular cross-talk, its influence on isolated leukocyte populations was studied. In whole blood, 100 ng/mL G-CSF reduced the LPS-induced TNF-α release by 37%±4% (P < .001) compared with controls (Figure5). Purified PBMC combined with purified neutrophils exposed to G-CSF reduced their TNF-α release by 24%±7% compared with LPS-stimulated control cells (P < .05). PBMC, in the absence of neutrophils, showed a reduction by 25%±3% (P < .01). In elutriation-purified, platelet-free monocytes G-CSF inhibited the TNF-α release by 56%±7% of the LPS control (P < .05). These results indicate that G-CSF directly influences monocytic TNF-α release and that the presence of neutrophils or platelets is not required for the decrease of monokine release under these conditions.
In vitro attenuation of cytokine release by G-CSF in whole blood or isolated leukocyte populations.
Whole blood (20%) in RPMI 1640, isolated PBMC (5 × 105/mL) with or without isolated neutrophils (106/mL), or elutriation-purified monocytes (105/mL) were stimulated with 10 μg/mL LPS ± 100 ng/mL G-CSF. White bars indicate the absence of G-CSF; black bars, its presence. After 24 hours of incubation at 37°C, released cytokines were measured in the cell-free supernatants by ELISA. Data represent means ± SEM of 19 different donors for whole blood, 7 donors for PBMC, and 5 donors for monocytes. *P < .05, **P < .01, ***P < .001 versus values of respective LPS samples.
In vitro attenuation of cytokine release by G-CSF in whole blood or isolated leukocyte populations.
Whole blood (20%) in RPMI 1640, isolated PBMC (5 × 105/mL) with or without isolated neutrophils (106/mL), or elutriation-purified monocytes (105/mL) were stimulated with 10 μg/mL LPS ± 100 ng/mL G-CSF. White bars indicate the absence of G-CSF; black bars, its presence. After 24 hours of incubation at 37°C, released cytokines were measured in the cell-free supernatants by ELISA. Data represent means ± SEM of 19 different donors for whole blood, 7 donors for PBMC, and 5 donors for monocytes. *P < .05, **P < .01, ***P < .001 versus values of respective LPS samples.
G-CSF and lymphocytic IFN-γ release
Although flow cytometry and RT-PCR experiments indicated that lymphocytes do not express G-CSF receptors, G-CSF treatment in vivo (see Figure 1) or the presence of G-CSF in vitro (see Table 1) attenuated the lymphocytic IFN-γ release in whole blood stimulated with LPS. To examine the possibility of direct effects of G-CSF on lymphocytes, isolated lymphocytes were stimulated to release IFN-γ with anti-CD3 or a combination of IL-12 and TNF-α in the absence or presence of G-CSF. G-CSF, 100 ng/mL and 300 ng/mL, which significantly reduced IFN-γ release in whole blood, showed no significant influence on IFN-γ release from isolated lymphocytes (data not shown).
The fact that G-CSF failed to affect isolated lymphocytes directly, but suppressed IFN-γ release in whole blood, suggests that another cell type mediates the G-CSF signal to lymphocytes. Because LPS fails to induce IFN-γ release in isolated lymphocytes (data not shown), induction of lymphokine release by LPS appears to be due to secondary mediators, most likely stimulatory monokines. This raises the possibility that G-CSF exerts its effect on lymphocytes not via suppressive signals but via attenuation of lymphocyte stimulatory signals. We expected such a mediator to be able to regulate lymphocytic IFN-γ release, to be formed by a G-CSF-sensitive cell type and that the release of this mediator is influenced by G-CSF. Prime candidates were the cytokines IL-12 and TNF-α because they are both formed by G-CSF-sensitive monocytes and because their secretion is reduced by G-CSF. Therefore, we tested if TNF-α and IL-12 are able to regulate IFN-γ release in whole blood. Actually, a combination of IL-12 and TNF-α (10 ng/mL, respectively) induced a significant IFN-γ release (P < .01) in whole blood (Figure 6).
Influence of IL-12 or TNF- on LPS-stimulated IFN-γ production in the presence or absence of G-CSF.
IL-12 (10 ng/mL) or TNF-α (10 ng/mL) or a combination of both was added to 20% whole blood in RPMI 1640. A set of samples was additionally stimulated with 10 μg/mL LPS ± 100 ng/mL G-CSF. After 24 hours of incubation at 37°C, released cytokines were measured in the cell-free supernatants by ELISA. Data represent means ± SEM of 8 different donors. ++P < .01 versus control, *P < .05, **P < .01 versus respective LPS samples.
Influence of IL-12 or TNF- on LPS-stimulated IFN-γ production in the presence or absence of G-CSF.
IL-12 (10 ng/mL) or TNF-α (10 ng/mL) or a combination of both was added to 20% whole blood in RPMI 1640. A set of samples was additionally stimulated with 10 μg/mL LPS ± 100 ng/mL G-CSF. After 24 hours of incubation at 37°C, released cytokines were measured in the cell-free supernatants by ELISA. Data represent means ± SEM of 8 different donors. ++P < .01 versus control, *P < .05, **P < .01 versus respective LPS samples.
If the hypothesis that G-CSF affects IFN-γ release by limiting the amounts of released IL-12 and TNF-α holds true, then a supplementation of TNF-α and IL-12 should cancel out the attenuation of IFN-γ release by G-CSF. To test this, we added IL-12 and TNF-α to LPS-stimulated whole blood in the absence or presence of 100 ng/mL G-CSF. TNF-α or IL-12 alone or a combination of both increased LPS-stimulated IFN-γ release in whole blood in the presence or absence of G-CSF (see Figure 6). In LPS-stimulated whole blood, G-CSF reduced the release of IFN-γ by about 40% (see Figure 6). In the presence of 10 ng/mL IL-12, more IFN-γ was formed than in the respective LPS-controls, but still G-CSF significantly inhibited IFN-γ release (see Figure 6). When, however, 10 ng/mL TNF-α or a combination of 10 ng/mL TNF-α and 10 ng/mL IL-12 was present in the medium, G-CSF could not attenuate IFN-γ release. These findings suggest that the curtailment of the release of these 2 monocyte-derived factors represents the cause for the inhibition of the release of IFN-γ by G-CSF as observed in whole blood.
Discussion
Granulocyte colony-stimulating factor is considered a lineage-specific growth and maturation factor for neutrophils. Only few publications report effects on other cell populations.5-10In this study, we showed that G-CSF not only increases the number of circulating neutrophils, but also leads to monocytosis. The treatment of healthy volunteers with G-CSF also resulted in an altered ex vivo LPS-inducible cytokine release. The release of TNF-α per monocyte and of total IFN-γ was significantly reduced as described in previous studies with G-CSF-treated healthy volunteers.12,13Additionally, we noticed an attenuated release of the proinflammatory monokines IL-1β and IL-12 when calculated per monocyte. Because the release of IFN-γ from lymphocytes was also reduced, we conclude that G-CSF is able to reduce the release of the major mediators of the inflammatory response. Recently, similar observations were made in G-CSF-treated volunteers challenged with LPS in vivo.14
In flow cytometry experiments, we studied the expression of G-CSF receptors on blood cells. Because it is known that G-CSF receptors are expressed on neutrophils,9 18-20 we used the staining of neutrophils as a positive control. Fluorescent G-CSF and an antibody against the G-CSF receptor stained neutrophils and monocytes, indicating G-CSF receptors also on human monocytes. The monocyte population was stained uniformly with G-CSF-PE or anti-G-CSF receptor antibody indicating a homogeneous monocytic expression of G-CSF receptors not confined to a subset of monocytes. The staining of monocytes was comparable to the staining of neutrophils referring to comparable receptor densities. RT-PCR revealed comparable amounts of G-CSF receptor mRNA in both cell populations.
The finding that the antibody against the G-CSF receptor bound to neutrophils and monocytes suggests that the receptors on both cell types have structural similarity, at least at the antibody binding site. Whether these receptors are identical or whether one of the known splicing variants21of the G-CSF receptor is expressed on monocytes must be investigated further.
Not only G-CSF treatment of volunteers, but also the presence of G-CSF in vitro attenuated the LPS-inducible release of these cytokines. Because G-CSF apparently also affected cytokine release in simple cellular systems, we were able to study the mechanisms of G-CSF action on monocytic and lymphocytic cytokine release. An earlier report described that G-CSF only reduced TNF-α release from cultivated human monocytes in the presence of neutrophils.22 It was suggested that G-CSF does not act on monocytes directly, but indirectly via neutrophil participation. We therefore investigated the effect of G-CSF on monokine release in the presence or absence of neutrophils. We observed that G-CSF attenuated the release of TNF-α in PBMC either in the presence or in the absence of neutrophils. Therefore, we also conclude from these functional data that G-CSF acts directly on monocytes. This deduction is also supported by the observation that G-CSF reduced the release of TNF-α in a mixed lymphocyte reaction with purified PBMC.23
Because G-CSF receptors were also described on platelets,5we also examined the effect of G-CSF on elutriation-purified monocytes, which were free of attached platelets. Because G-CSF also reduced the release of TNF-α from platelet-free monocytes, we conclude that G-CSF directly influences monocytes and is unlikely to affect monocytes via attached platelets.
Isolated lymphocytes, however, did not show the reduction of IFN-γ release in response to G-CSF observed in whole blood. In addition, G-CSF receptors were not detectable on lymphocytes in flow cytometric studies and specific mRNA was not detectable in RT-PCR analysis. These results indicate that lymphocytes are not direct target cells of G-CSF. However, we could show a reduction of IFN-γ release in LPS-stimulated whole blood of G-CSF-treated volunteers and also an attenuation of LPS-induced IFN-γ release in the presence of G-CSF in vitro. These seemingly contradictory results could be explained as follows. The monokines IL-12 and TNF-α both stimulate lymphocytes to release IFN-γ. However, G-CSF attenuates the release of these monokines and therefore lymphocytes are stimulated less, resulting in less IFN-γ secretion. This hypothesis is supported by the findings that IFN-γ release was induced by a combination of IL-12 and TNF-α, and that G-CSF did not inhibit LPS-induced IFN-γ release when TNF-α was supplemented.
In general, we propose from our findings that the G-CSF receptor on monocytes attenuates the inflammatory response, protecting against a fatal overactivation of the immune system. This view is supported by our previous finding that G-CSF treatment protected mice and rats against otherwise lethal endotoxin challenge, a model of septic organ failure.11 Furthermore, the reduced release of IL-12 and TNF-α mediated by G-CSF could result in reduced induction of IFN-γ release in lymphocytes.
Acknowledgments
The excellent technical assistance of Anke Biedermann, Gregor Pinski, Ina Seuffert, Daniela Fischer, and Gina Gensheimer is greatly appreciated. We are indebted to Volker Gimple for the assistance in analyzing the data from the G-CSF treatment study.
Supported by the Deutsche Forschungsgemeinschaft Grant We 686/18-1 and Land Baden-Württemberg Grant FP 699/97.
Reprints:Thomas Hartung, Biochemical Pharmacology, University of Konstanz, D-78457 Konstanz, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal