Abstract
Adult T-cell leukemia (ATL) is characterized by infiltration of various tissues by circulating ATL cells, a finding often associated with a poor prognosis. Leukocyte migration from the circulation into tissues depends on integrin-mediated adhesion to the endothelium, and integrins are tightly regulated by several factors, such as chemokines. In this study, we focused on the interaction between chemokines and chemokine receptors on ATL cells to understand factors involved in ATL cell infiltration of lymphoid organs. We compared freshly isolated ATL cells from patients with and without lymphoid organ involvement for the expression of the chemokine receptor CCR7/EBI1, the functional receptor for secondary lymphoid-tissue chemokine (SLC), which is expressed at high levels by high endothelial venules of lymph nodes and Peyer's patches. Reverse transcriptase-polymerase chain reaction and flow cytometric analysis, using anti-CCR7 monoclonal antibody (CCR7.6B3), revealed that ATL cells from patients with lymphoid organ involvement expressed significantly more CCR7/EBI1 than control CD4+CD45RO+ T cells and ATL cells from patients without lymphoid organ involvement. Consequently, significantly more ATL cells from patients with lymphoid organ involvement than control CD4+CD45RO+ T cells and ATL cells from patients without lymphoid organ involvement adhered to surfaces coated with ICAM-1 and SLC or EBI1-ligand chemokine (ELC), another ligand for CCR7/EBI1, under static and flow conditions and migrated toward SLC or ELC at a low concentration (30 ng/ml). These findings suggest that increased CCR7/EBI1 expression plays a role in lymphoid organ infiltration of ATL cells. (Blood. 2000; 30-38)
Adult T-cell leukemia (ATL) is a peripheral CD4+ T-cell malignancy caused by human T-cell leukemia virus type 1 (HTLV-1). A frequent manifestation of ATL is infiltration of various organs, such as the lymph nodes, liver, spleen, lungs, skin, and intestinal tract, by leukemic cells. ATL cell infiltration often poses serious clinical problems for patients, affecting the disease profile and prognosis. Tissue infiltration by ATL cells probably reflects the cells' biological properties, such as the expression and function of relevant adhesion molecules and the adhesive interactions with endothelial cells.
Lymphocytes migrate into lymph nodes, Peyer's patches, and other secondary lymphoid organs by interacting with organ-specific adhesion molecules expressed by high endothelial venules (HEVs).1,2A multistep model involving cell adhesion and activation of leukocyte integrins has been proposed for selective leukocyte trafficking, including the process of lymphocyte homing. According to this model, the sequential involvement of several receptor-ligand pairs selected from many potential combinations results in lymphocyte subset-specific homing. The process involves an initial tethering and rolling step mediated by L-selectin on the surfaces of lymphocytes and a specific complex of glycoproteins, known as peripheral lymph node addressin, expressed on the endothelium.3,4 The rolling interaction is followed by firm arrest of cells,5,6 which is mediated by β2 integrin, αLβ2 (LFA-1) binding to ICAM-1 or ICAM-2 on the endothelium. Then, lymphocytes transmigrate across the endothelium into the tissues. Treatment of lymphocytes with pertussis toxin (PTX) inhibits the αLβ2 (LFA-1)-mediated adhesion to HEVs, indicating that a G-protein–linked signal-transduction mechanism is a component of these processes. Chemokines that bind to G-protein–coupled receptors have emerged as candidates for these integrin activation signals. However, although in vitro static adhesion assays have shown that some chemokines increase integrin-mediated lymphocyte adhesion,7-11 this effect does not appear to be rapid or robust enough to account for the intravascular firm arrest of lymphocytes in HEVs. Furthermore, these chemokines are not normally produced by HEV cells of lymph nodes or Peyer's patches.
Recently, a lymphocyte-specific chemokine termed secondary lymphoid-tissue chemokine (SLC), also known as 6Ckine, Exodus-2, and thymus-derived chemotactic agent 4, was reported to be expressed at high levels in HEV cells and areas of T-cell accumulation in lymph nodes, Peyer's patches, and spleen.12-19 SLC is a highly efficacious chemoattractant for lymphocytes, including naive T cells, but does not attract monocytes or neutrophils. Furthermore, SLC induced firm adhesion of lymphocytes via β2 integrin binding to ICAM-1 under static and flow conditions, suggesting that SLC plays a role in lymphocyte homing.16,20,21 SLC is a high-affinity functional ligand for CCR7/EBI1, which was originally identified as a receptor induced by Epstein-Barr virus infection in B cells.22 CCR7/EBI1 is expressed on T, B, and mature dendritic cells and is up-regulated in CD4+ T cells in response to infection with human herpesvirus (HHV)-6 and HHV-7.23-29 A recent study demonstrated that each of the 3 chemokines expressed in lymph nodes, SLC, EBI1-ligand chemokine (ELC), and stromal cell-derived factor (SDF)-1α, could promote integrin-mediated arrest of human lymphocytes under flow conditions.20 ELC is another high-affinity functional ligand for CCR7/EBI1.30 SDF-1α is the only ligand for CXCR4 to be identified so far.31-33 In this study, we compared ATL cells from patients with and without lymphoid organ infiltration for CCR7/EBI1 and CXCR4 expression levels and have demonstrated that increased CCR7/EBI1 expression correlates with infiltration of lymphoid organs by ATL cells.
Materials and methods
Cells
The human T-cell line HUT78 was obtained from the American Type Culture Collection (Rockville, MD) and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (Life Technologies, Gaithersburg, MD). Human umbilical vein-derived endothelial cells (HUVECs) were purchased from Takara Shuzo (Kyoto, Japan).
ATL patients
Mononuclear cells were isolated by Ficoll-Conray density gradient centrifugation from lymph nodes and peripheral blood samples from 16 patients with involvement of lymphoid organs, such as the lymph nodes or spleen, and from 12 patients without lymphoid organ involvement. Control mononuclear cells prepared from peripheral blood (PBMCs) were obtained from 8 healthy volunteers. The control lymph node samples were prepared from the reactive lymph nodes of HTLV-1-seronegative individuals who had undergone abdominal surgery. ATL was diagnosed according to the following clinical criteria: serum antibodies against HTLV-1–associated antigens were present; mononuclear cells showed the morphologic characteristic of highly convoluted nuclei; the results of phenotypic analysis of ATL cells with anti-CD2, anti-CD4, and anti-CD25 monoclonal antibodies (mAbs); and the HTLV-1 proviral genome were integrated monoclonally in the cells. Using Shimoyama's criteria,34 the 16 ATL patients with lymphoid organ involvement were diagnosed as having acute ATL. Of these, 6 had involvement of the skin, gut, or bone marrow. The infiltration of the lymph organs, skin, gut, or bone marrow by ATL cells was confirmed radiographically and histochemically by examining biopsy samples or specimens obtained during autopsy. Of the 12 patients with noninfiltrating ATL, 8 and 4 had acute and chronic ATL, respectively. The relevant clinical data, profiles, and extent of organ involvement of each of the subjects are summarized in the Table.
Highly purified CD4+ T cells were enriched by negative immunoselection from mononuclear cells, using a multiple mAb mixture and immunomagnetic beads, as described previously.35Briefly, PBMCs of ATL patients and control samples were incubated for 30 minutes at 4°C with a cocktail of mAbs against CD8, CD11b, CD14, CD16, and CD20. After washing, Dynabeads (Japan Dynal, Tokyo, Japan) were added, incubated for 1 hour at 4°C, and then removed with a Dynal magnetic particle concentrator. The proportion of ATL cells (CD4+ and CD25+ cells) in the negatively selected cells of each ATL patient was greater than 90%, and the ATL cells in all the samples expressed CD45RO. CD4+CD45RO+ T cells from control samples were purified by the same negative immunoselection technique, using additional monoclonal antibodies (mAbs) against HLA-DR and CD45RA, and the proportion of CD4+CD45RO+ T cells in each control sample was approximately 90%.
Antibodies and reagents
Mouse mAbs to CD8 (OKT8), CD11b (OKM1), and HLA-DR (OKIa1) were obtained from the American Type Culture Collection (Rockville, MD). Mouse mAbs, anti-CD14 (322A-1), anti-CD18 (7E4), anti-CD20 (B1), anti-CD45RA (2H4), fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (OKT4), phycoerythrin (PE)-conjugated anti-CD25 (B1.49.9), and PE-anti-CD45RO (UCHL1) were purchased from Coulter Co (Hialeah, FL). Mouse mAbs to CD16 (3G8) were purchased from Chemicon International Inc (Temecula, CA). Anti-human CXCR4 mAb (12G5), recombinant human ELC/macrophage inflammatory protein (MIP)-3β, SLC/6Ckine, and ICAM-1 were purchased from R&D Systems Inc (Minneapolis, MN).
The anti-CCR7 mAb, designated CCR7.6B3 (IgG1, κ), was produced by fusing SP2/0-Ag14 cells with spleen cells from BALB/c mice immunized with CCR7-peptide, YIGDNTTVDYTLFESLC (H.H. et al, unpublished data). CCR7.6B3 antibody was found to be specific for CCR7 by flow cytometric analysis, using other chemokine receptor-transfected cells. This antibody is useful for flow cytometric analysis but not for immunohistochemistry or immunoblotting, because it cross-reacts with intracellular proteins.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
The total cellular RNA was extracted from control CD4+CD45RO+ T cells and isolated ATL cells by the acid phenol-guanidinium isothiocyanate extraction method, as described previously.36 The expression of CCR7/EBI1 in ATL cells and CD4+CD45RO+ T cells from control samples was analyzed by RT-PCR, using an RNA PCR kit (Takara Shuzo) and the GeneAmp PCR system 2400 (Perkin Elmer, Foster, CA), as described previously.37 First-strand cDNA was synthesized from 1 μg total cellular RNA and subjected to 28 PCR cycles (94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute) with specific primers for CCR7/EBI1 (forward, 5′-TCCTTCTCATCAGCAAGCTGTC-3′ and reverse, 5′-GAGGCAGCCCAGGTCCTTGAAG-3′), as described by Yanagihara et al.28 As a control, specific primers for β actin purchased from Clontech (Palo Alto, CA) were used. After staining the primers with ethidium bromide, the density of each band was analyzed with FluorImager SI (Molecular Dynamics, Sunnyvale, CA).
In vitro static adhesion assay
Adhesion of ATL cells and control CD4+CD45RO+ T cells to HUVECs and ICAM-1 was assayed as described by Tanaka et al.11 Briefly, HUVECs were placed on 96-well culture plates, cultured to confluence in EBM-2 medium (Takara Shuzo), washed with phosphate-buffered saline (PBS), and then stimulated with 1 ng/ml interleukin-1β (IL-1β) (Otsuka, Tokyo, Japan) for 4 hours at 37°C. ICAM-1 protein (25 ng/well) in PBS was applied to 96-well culture plates, which were incubated at 4°C overnight. The binding sites were then blocked with PBS/1% bovine serum albumin (BSA) for 2 hours at 37°C to reduce nonspecific attachment. Cells, which had been labeled with 51Cr (Dupont NEN, Wilmington, DE) in RPMI 1640 medium supplemented with 1% BSA, were untreated or preincubated with anti-CD18 mAb (7E4, 20 μg/ml) for 20 minutes at room temperature or with 100 ng/ml PTX (Sigma, St. Louis, MO) at 37°C for 1.5 hours. Untreated or treated cells (2 × 105) were incubated with SLC or ELC at a concentration of 30 ng/ml at 37°C for 30 minutes, and then were added to each well. The plates were incubated at 37°C for 30 minutes, then gently washed twice with RPMI 1640 medium at room temperature to remove all the nonadherent cells. The adherent cells in each well were lysed with 250 μl of 1% Triton X-100 (Sigma), and the radioactivity of the contents of each well was measured using a γ-counter. The data are expressed as the mean percentage of the binding of indicated cells from triplicate experiments.
Flow adhesion assay
Adhesion substrates were prepared as described by Tangemann et al.21 Briefly, adhesion substrates were generated by coating 10 μg/ml ICAM-1 in Tris-buffered saline (TBS), pH 9.0, onto petri dishes (Corning, San Mateo, CA) for 2 hours at room temperature. After washing the substrates with PBS, chemokine was coated at a concentration that induced maximal activation effects (10 μg/ml) in TBS, pH 9.0, for 1 hour. The substrates were washed and blocked with 3% BSA for 2 hours at room temperature.
A rheoscope combining a transparent 0.8° cone and substrate-coated dishes with an inverted microscope (Olympus Optics Co, IMT-2, Tokyo, Japan) was used as described previously.38 Images were captured using a video camera (Sony Corp, DXC-101, Tokyo, Japan), a videotape recorder (Sony, SLO-420), and an image processor (Nippon Avionics Co, TVIP-2000, Tokyo, Japan). Cells (106/ml in Hank's balanced salt solution supplemented with 0.2% BSA) were perfused onto each substrate-coated dish at 1 dyne/cm2, and a single field of view (4 × objective) was recorded for the first 4 minutes. Arrested cells were defined as those that remained stationary during the 15-second interval by overlaying the succession of captured images in the computer analysis. The number of arrested cells is shown by the total number accumulated as arrested cells for the first 4 minutes. Experiments were performed in triplicate.
Chemotactic assay
Chemotactic assays for control CD4+CD45RO+ T cells and ATL cells were performed in polycarbonate membrane, 6.5-mm diameter, 5-μm pore size transwell cell culture chambers (Costar Corp, Cambridge, MA). Aliquots (100 μl) of cells (5 × 106/ml) suspended in RPMI 1640/0.5% BSA were added to the upper chambers, and either SLC or ELC, to produce a final concentration of 30 ng/ml, was added to the lower wells. The cells were allowed to migrate for 2 hours at 37°C in a 5% CO2incubator, after which the filters were fixed with 1% glutaraldehyde in PBS for 30 minutes and stained with 0.5% toluidine blue overnight. Cell migration was quantified by counting cells in each lower chamber and cells adhering to the bottom part of the polycarbonate filter. Each assay was performed in triplicate.
Flow cytometric analysis
Flow cytometric analysis of freshly isolated ATL cells and control CD4+CD45RO+ T cells was conducted as described previously.35 The cells (2 × 105) were washed once with 3% FCS-PBS and incubated with anti-CCR7, anti-CXCR4, IgG1, or IgG2a mouse mAbs (negative control), at a saturating concentration (10 μg/ml), on ice for 30 minutes. After washing with 3% FCS-PBS, the cells were stained with FITC-conjugated goat anti-mouse IgG (Cappel, Westchester, PA) on ice for 30 minutes, washed, and resuspended in 3% FCS-PBS. The fluorescence intensity was analyzed using a profile flow cytometer (Epics; Coulter). The mean fluorescence intensity (MFI) for CCR7 or CXCR4 on positive cells was corrected by subtracting MFI obtained with the nonbinding control IgG1 or IgG2a mouse mAb, respectively.
Results
ATL cells from patients with lymphoid organ involvement expressed more CCR7/EBI1 than those without involvement of lymphoid organs
One of the major features of ATL cells is their marked infiltration into tissues. Leukocyte migration from the circulation into tissues is mediated by adhesion of leukocyte integrins to their ligands, including ICAM-1, on endothelial cells. Leukocyte integrins are normally inactive, and chemokines are capable of inducing integrin activation. SLC is a unique chemokine: It is the first chemokine found to be expressed at strikingly high levels in HEV cells of lymph nodes and Peyer's patches and to have the characteristics required to mediate homing of lymphocytes. Therefore, to investigate ATL infiltration of lymphoid organs, we focused on the expression of CCR7/EBI1, the functional receptor for SLC and ELC, on ATL cells. We used RT-PCR to analyze CCR7/EBI1 expression. The optimal conditions for detection and quantitation of CCR7/EBI1 expression were established by determining the relative yields of the PCR products of the CD4+CD45RO+ T cells and HUT 78 cells after various numbers of PCR cycles. After 35 cycles, the yield of the CCR7/EBI1-specific product of each of these cells reached a plateau, whereas after 25 to 33 cycles, it was in the exponential range, enabling the initial amounts of mRNA template in the samples to be compared (data not shown). The β actin-specific product was amplified exponentially by 20 to 30 PCR cycles (data not shown). Therefore, the CCR7/EBI1 and β actin-specific products of CD4+CD45RO+ T cells and ATL cells were placed in the same tube and subjected to 28 PCR amplification cycles.
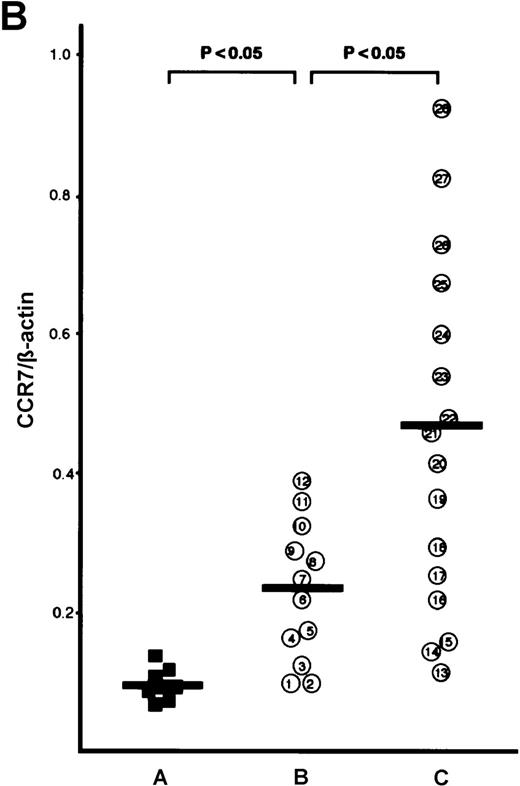
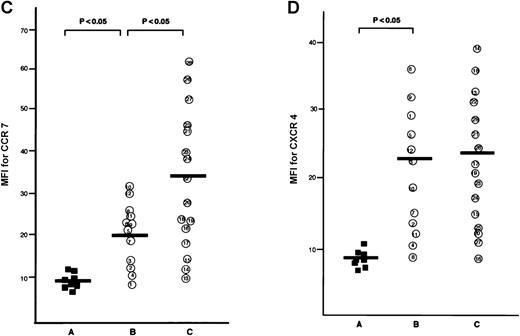
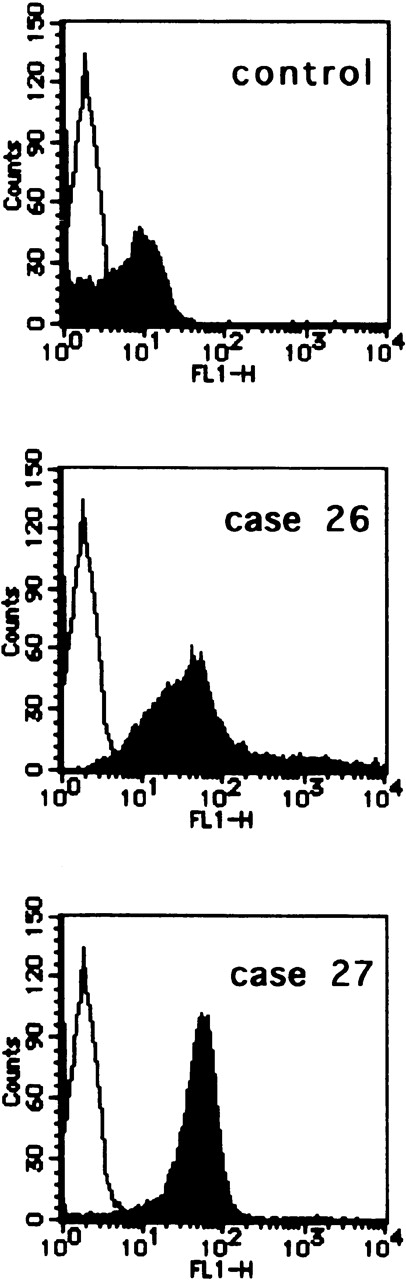
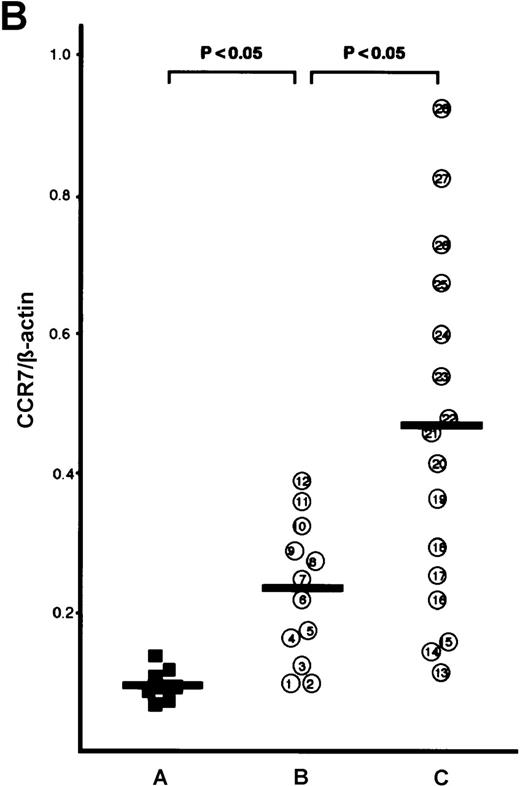
The CCR7/EBI1 expression level was evaluated with the ratio of CCR7/β actin-specific PCR product. As shown in Figure1A and 1B, the freshly isolated ATL cells expressed significantly more CCR7/EBI1 than the CD4+CD45RO+ T cells. Furthermore, the CCR7/EBI1 expression level of ATL cells from patients with lymphoid organ involvement was significantly higher than that of ATL cells from patients without involvement of lymphoid organs. Recently, we generated an anti-CCR7 mAb, designated CCR7.6B3. From flow cytometric analysis using this mAb, CCR7/EBI1-positive cells were 55% ± 14% of CD4+CD45RO+ T cells from 8 healthy volunteers, but more than 90% of ATL cells (data not shown). As shown in Figure1C, the data obtained from flow cytometric analysis were consistent with those from RT-PCR analysis. We also examined the expression level of CXCR4, the receptor for SDF-1α, by calculating MFI after staining with anti-CXCR4 mAb (Figure 1D). CXCR4-positive cells were 76% ± 18% of CD4+CD45RO+ T cells from 8 healthy volunteers (data not shown). The CXCR4 expression levels of ATL cells were much higher than those of normal CXCR4-positive CD4+CD45RO+ T cells, but there were no significant differences between ATL cells from patients with and without lymphoid organ involvement.
Analyses for CCR7/EBI1 expression.
(A) Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis for CCR7/EBI1 expression. PCR amplification of the CCR7/EBI1 and β-actin–specific products in control CD4+CD45RO+ T cells (lane 1), freshly isolated adult T-cell leukemia (ATL) cells without (lanes 2, 3, and 4; patients 7, 10, and 6, respectively) and with lymphoid organ involvement (lanes 5, 6, 7, and 8; patients 27, 26, 24, and 23, respectively) was performed for 28 PCR cycles. (B) RT-PCR analysis for CCR7/EBI1 expression in freshly isolated ATL cells from patients with and without lymphoid organ involvement. CCR7/EBI1 expression was evaluated with the ratio of CCR7/β-actin–specific product for control CD4+CD45RO+ T cells obtained from the peripheral blood of 8 healthy volunteers (A, closed squares), ATL cells from 12 patients without lymphoid organ involvement (B, open circles; patients 1 through 12), and ATL cells from 16 patients with lymphoid organ involvement (C, open circles; patients 13 through 28). RT-PCR analyses were performed independently in triplicate. (C) Flow cytometric analysis for CCR7/EBI1 expression on freshly isolated ATL cells from patients with and without lymphoid organ involvement. Flow cytometric analysis of control CD4+CD45RO+ T cells and freshly isolated ATL cells was performed with anti-CCR7 mAb, CCR7.6B3. Each point shows the mean fluorescence intensity (MFI) for CCR7/EBI1 staining, which was corrected by substrating MFI obtained with the nonbinding control IgG1 mouse mAb. Each point of group A indicates the MFI for CCR7/EBI1 staining of CCR7/EBI1-positive CD4+CD45RO+ T cells because CCR7/EBI1-positive cells were 55% ± 14% of control CD4+CD45RO+ T cells isolated from 8 healthy volunteers. The bars indicate the mean value of each group; the statistical analysis was performed using Student t test. (D) Flow cytometric analysis for CXCR4 expression on freshly isolated ATL cells from patients with and without lymphoid organ involvement. Flow cytometric analysis of control CD4+CD45RO+ T cells and freshly isolated ATL cells was performed with anti-CXCR4 mAb. Each point shows the MFI for CXCR4 staining, which was corrected by substrating MFI obtained with the nonbinding control IgG2amouse mAb. Each point of group A indicates the MFI for CXCR4 staining of CXCR4-positive CD4+CD45RO+ T cells because CXCR4-positive cells were 76% ± 18% of control CD4+CD45RO+ T cells isolated from 8 healthy volunteers. The bars indicate the mean value of each group; the statistical analysis was performed using Student t test.
Analyses for CCR7/EBI1 expression.
(A) Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis for CCR7/EBI1 expression. PCR amplification of the CCR7/EBI1 and β-actin–specific products in control CD4+CD45RO+ T cells (lane 1), freshly isolated adult T-cell leukemia (ATL) cells without (lanes 2, 3, and 4; patients 7, 10, and 6, respectively) and with lymphoid organ involvement (lanes 5, 6, 7, and 8; patients 27, 26, 24, and 23, respectively) was performed for 28 PCR cycles. (B) RT-PCR analysis for CCR7/EBI1 expression in freshly isolated ATL cells from patients with and without lymphoid organ involvement. CCR7/EBI1 expression was evaluated with the ratio of CCR7/β-actin–specific product for control CD4+CD45RO+ T cells obtained from the peripheral blood of 8 healthy volunteers (A, closed squares), ATL cells from 12 patients without lymphoid organ involvement (B, open circles; patients 1 through 12), and ATL cells from 16 patients with lymphoid organ involvement (C, open circles; patients 13 through 28). RT-PCR analyses were performed independently in triplicate. (C) Flow cytometric analysis for CCR7/EBI1 expression on freshly isolated ATL cells from patients with and without lymphoid organ involvement. Flow cytometric analysis of control CD4+CD45RO+ T cells and freshly isolated ATL cells was performed with anti-CCR7 mAb, CCR7.6B3. Each point shows the mean fluorescence intensity (MFI) for CCR7/EBI1 staining, which was corrected by substrating MFI obtained with the nonbinding control IgG1 mouse mAb. Each point of group A indicates the MFI for CCR7/EBI1 staining of CCR7/EBI1-positive CD4+CD45RO+ T cells because CCR7/EBI1-positive cells were 55% ± 14% of control CD4+CD45RO+ T cells isolated from 8 healthy volunteers. The bars indicate the mean value of each group; the statistical analysis was performed using Student t test. (D) Flow cytometric analysis for CXCR4 expression on freshly isolated ATL cells from patients with and without lymphoid organ involvement. Flow cytometric analysis of control CD4+CD45RO+ T cells and freshly isolated ATL cells was performed with anti-CXCR4 mAb. Each point shows the MFI for CXCR4 staining, which was corrected by substrating MFI obtained with the nonbinding control IgG2amouse mAb. Each point of group A indicates the MFI for CXCR4 staining of CXCR4-positive CD4+CD45RO+ T cells because CXCR4-positive cells were 76% ± 18% of control CD4+CD45RO+ T cells isolated from 8 healthy volunteers. The bars indicate the mean value of each group; the statistical analysis was performed using Student t test.
Enhancement by SLC and ELC of ATL cell adhesion to HUVECs and ICAM-1 under static conditions
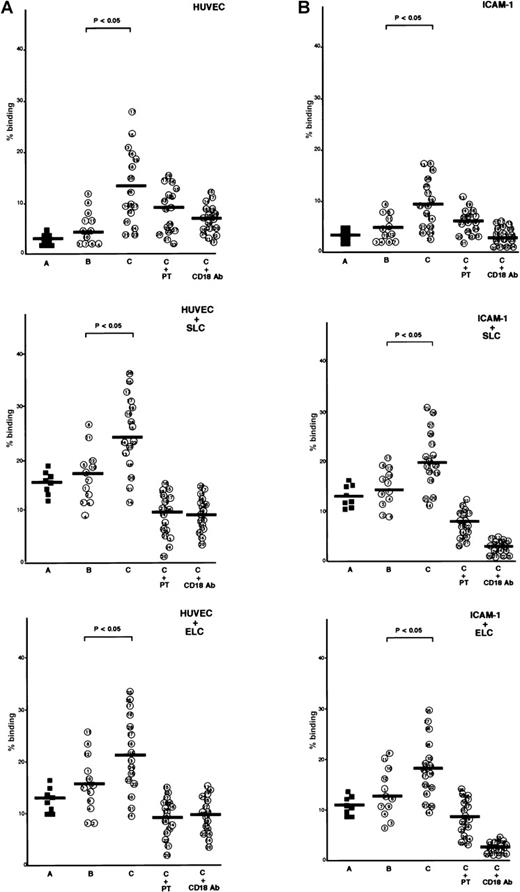
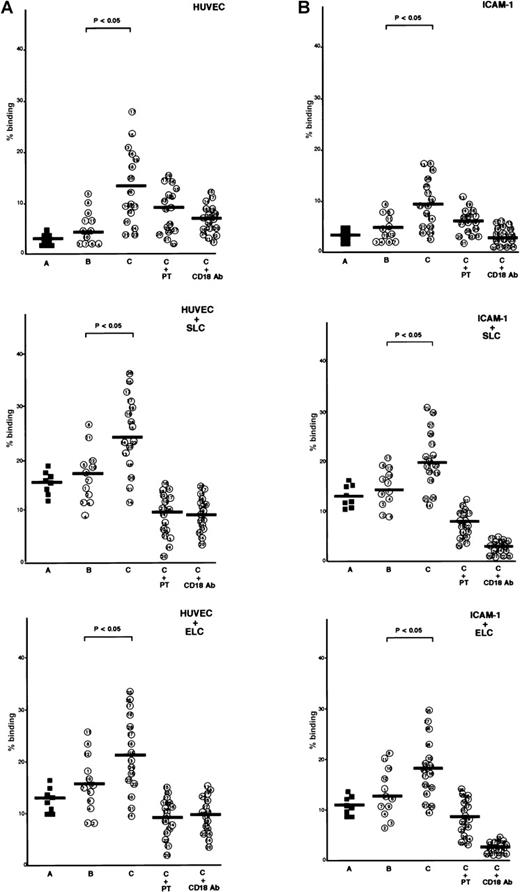
ATL cells from patients with lymphoid organ involvement adhered spontaneously and efficiently to IL-1β-activated HUVECs and immobilized recombinant ICAM-1 protein, whereas normal CD4+CD45RO+ T cells and ATL cells from patients without lymphoid organ involvement adhered poorly to IL-1β-activated HUVECs or ICAM-1 (Figure 2A and 2B). The addition of SLC or ELC to the adhesion assay buffer increased adhesion of ATL cells to IL-1β-activated HUVECs and ICAM-1. Among ATL cells from 16 patients with lymphoid organ involvement, ATL cells from patients with higher CCR7/EBI1 expression, such as patients 21 through 28, showed higher increases (more than 2-fold) in the rates of adhesion evoked by adding SLC or ELC than those from other patients, such as patients 13 through 19 (less than 2-fold). These increased adhesions were inhibited by anti-CD18 mAb or PTX, indicating that SLC- and ELC-induced adhesion to both IL-1β-activated HUVECs and ICAM-1 was PTX-sensitive and mediated by integrin β2.
Comparison of secondary lymphoid-tissue chemokine (SLC)- and EBI1-ligand chemokine (ELC)-induced adhesion of adult T-cell leukemia (ATL) cells under static conditions.
(A) Comparison of SLC- and ELC-induced adhesion of ATL cells from patients with and without lymphoid organ involvement to human umbilical vein-derived endothelial cells (HUVECs) under static conditions. Cells (2 × 105/well), which had been labeled with 51Cr, were incubated with SLC or ELC at a concentration of 30 ng/ml at 37°C for 30 minutes and were added to the well containing IL-1β–activated HUVECs. The plates were incubated at 37°C for 30 minutes and then gently washed, and radioactivity of the adherent cells in each well was counted. The cells are as follows: control CD4+CD45RO+ T cells (A, closed squares) and ATL cells from patients without (B, patients 1 through 12) and with lymphoid organ involvement (C, patients 13 through 28). ATL cells from patients with lymphoid organ involvement were blocked with 100 ng/ml pertussis toxin (PTX) for 1.5 hours at 37°C (C+PT) or with anti-CD18 mAb, 7E4 (20 μg/ml) for 20 minutes at room temperature (C+CD18 Ab) before adding of chemokines. The data are expressed as the mean percentage of the binding of indicated cells from triplicate experiments. The statistical analysis was performed using Student t test. (B) Comparison of SLC- and ELC-induced adhesion of ATL cells from patients with and without lymphoid organ involvement to ICAM-1 under static conditions. The 96-well culture plates were coated with ICAM-1 protein (25 ng/well).
Comparison of secondary lymphoid-tissue chemokine (SLC)- and EBI1-ligand chemokine (ELC)-induced adhesion of adult T-cell leukemia (ATL) cells under static conditions.
(A) Comparison of SLC- and ELC-induced adhesion of ATL cells from patients with and without lymphoid organ involvement to human umbilical vein-derived endothelial cells (HUVECs) under static conditions. Cells (2 × 105/well), which had been labeled with 51Cr, were incubated with SLC or ELC at a concentration of 30 ng/ml at 37°C for 30 minutes and were added to the well containing IL-1β–activated HUVECs. The plates were incubated at 37°C for 30 minutes and then gently washed, and radioactivity of the adherent cells in each well was counted. The cells are as follows: control CD4+CD45RO+ T cells (A, closed squares) and ATL cells from patients without (B, patients 1 through 12) and with lymphoid organ involvement (C, patients 13 through 28). ATL cells from patients with lymphoid organ involvement were blocked with 100 ng/ml pertussis toxin (PTX) for 1.5 hours at 37°C (C+PT) or with anti-CD18 mAb, 7E4 (20 μg/ml) for 20 minutes at room temperature (C+CD18 Ab) before adding of chemokines. The data are expressed as the mean percentage of the binding of indicated cells from triplicate experiments. The statistical analysis was performed using Student t test. (B) Comparison of SLC- and ELC-induced adhesion of ATL cells from patients with and without lymphoid organ involvement to ICAM-1 under static conditions. The 96-well culture plates were coated with ICAM-1 protein (25 ng/well).
Comparison of SLC- and ELC-induced adhesion of ATL cells from patients with and without lymphoid organ involvement to ICAM-1 under flow conditions
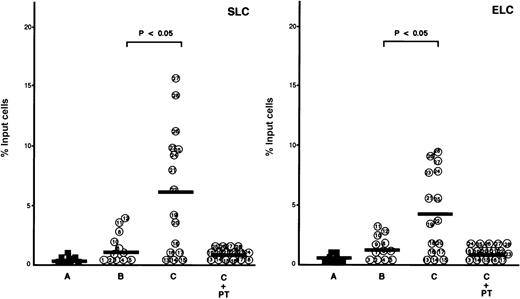
Next, we analyzed SLC-induced adhesion of CD4+CD45RO+ T cells and ATL cells from patients with and without lymphoid organ involvement to ICAM-1 under flow conditions. Adhesion was measured by counting the arrested cells for the first 4 minutes after perfusion. As shown in Figure3, CD4+CD45RO+ T cells and ATL cells adhered poorly to surfaces coated with ICAM-1 alone, whereas they adhered efficiently to surfaces coated with ICAM-1 and SLC. Furthermore, ATL cells isolated from patients with lymphoid organ involvement, such as patients 21 through 28, which had greater CCR7/EBI1 expression, increased adhesion significantly to surfaces coated with ICAM-1 and SLC, as compared to CD4+CD45RO+ T cells and ATL cells from patients without lymphoid organ involvement. These increased adhesions were also inhibited by PTX or anti-CD18 mAb. Similar results were obtained when the adhesion assay was done using surfaces coated with ICAM-1 and ELC. These findings indicate that the increased expression of CCR7/EBI1 by ATL cells enhanced their ability to adhere to surfaces coated with ICAM-1 and SLC or ELC.
Comparison of secondary lymphoid-tissue chemokines (SLC)- and EBI1-ligand chemokine (ELC)-induced adhesion of adult T-cell leukemia (ATL) cells from patients with and without lymphoid organ involvement to ICAM-1 under flow conditions.
Cells (106/ml) were perfused onto the coated dish with substrates consisting of ICAM-1 (10 μg/ml) alone or also with SLC (10 μg/ml) or ELC (10 μg/ml) at 1 dyne/cm2 for the first 4 minutes. The number of arrested cells is shown by the total number accumulated as arrested cells for the first 4 minutes in a single field of view (4X objective). The cells are as follows: control CD4+CD45RO+ T cells (A, closed squares) and ATL cells from patients without (B, patients 1 through 12) and with lymphoid organ involvement (C, patients 13 through 28). ATL cells from patients with lymphoid organ involvement were blocked with 100 ng/ml pertussis toxin (PTX) for 1.5 hours at 37°C (C+PT) or with anti-CD18 mAb, 7E4 (20 μg/ml) for 20 minutes at room temperature (C+CD18 Ab) before binding to ICAM-1 with SLC or ELC. Experiments were performed in triplicate. The bars indicate the mean value of each group; the statistical analysis was performed using Student ttest.
Comparison of secondary lymphoid-tissue chemokines (SLC)- and EBI1-ligand chemokine (ELC)-induced adhesion of adult T-cell leukemia (ATL) cells from patients with and without lymphoid organ involvement to ICAM-1 under flow conditions.
Cells (106/ml) were perfused onto the coated dish with substrates consisting of ICAM-1 (10 μg/ml) alone or also with SLC (10 μg/ml) or ELC (10 μg/ml) at 1 dyne/cm2 for the first 4 minutes. The number of arrested cells is shown by the total number accumulated as arrested cells for the first 4 minutes in a single field of view (4X objective). The cells are as follows: control CD4+CD45RO+ T cells (A, closed squares) and ATL cells from patients without (B, patients 1 through 12) and with lymphoid organ involvement (C, patients 13 through 28). ATL cells from patients with lymphoid organ involvement were blocked with 100 ng/ml pertussis toxin (PTX) for 1.5 hours at 37°C (C+PT) or with anti-CD18 mAb, 7E4 (20 μg/ml) for 20 minutes at room temperature (C+CD18 Ab) before binding to ICAM-1 with SLC or ELC. Experiments were performed in triplicate. The bars indicate the mean value of each group; the statistical analysis was performed using Student ttest.
Comparison of SLC- and ELC-evoked chemotaxis of ATL cells from patients with and without lymphoid organ involvement
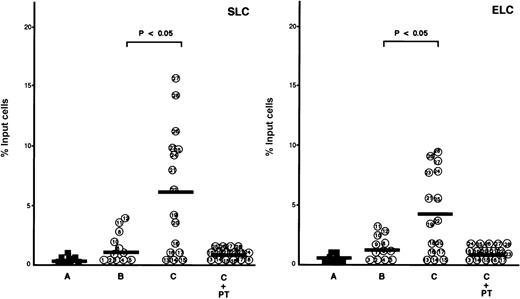
We compared the chemotactic response to SLC and ELC of ATL cells from patients with and without lymphoid organ involvement. There was no significant difference between these ATL cells in the chemotactic responses to more than 100 ng/ml of these chemokines, whereas different chemotactic responses were observed at 20 to 50 ng/ml (data not shown). Therefore, the chemotactic assays of ATL cells were performed at a concentration of 30 ng/ml of SLC and ELC. As shown in Figure4, ATL cells isolated from patients with lymphoid organ involvement migrated toward SLC and ELC, whereas normal CD4+CD45RO+ T cells and ATL cells from patients without lymphoid organ involvement showed no significant migration toward SLC and ELC at a concentration of 30 ng/ml.
Comparison of secondary lymphoid-tissue chemokine (SLC)- and EBI1-ligand chemokine (ELC)-evoked chemotaxis of adult T-cell leukemia (ATL) cells from patients with and without lymphoid organ involvement.
Chemotactic assays were performed in the presence of SLC or ELC at a low concentration (30 ng/ml) as described in Materials and Methods. The cells are as follows: control CD4+CD45RO+ T cells (A, closed squares) and ATL cells from patients without (B, patients 1 through 12) and with lymphoid organ involvement (C, patients 13 through 28). ATL cells from patients with lymphoid organ involvement were blocked with 100 ng/ml pertussis toxin (PTX) for 1.5 hours at 37°C (C+PT) before chemotactic assays. The data are expressed as the mean percentage of migrated cells toward SLC or ELC from triplicate experiments. The bars indicate the mean value of each group; the statistical analysis was performed using Student t test.
Comparison of secondary lymphoid-tissue chemokine (SLC)- and EBI1-ligand chemokine (ELC)-evoked chemotaxis of adult T-cell leukemia (ATL) cells from patients with and without lymphoid organ involvement.
Chemotactic assays were performed in the presence of SLC or ELC at a low concentration (30 ng/ml) as described in Materials and Methods. The cells are as follows: control CD4+CD45RO+ T cells (A, closed squares) and ATL cells from patients without (B, patients 1 through 12) and with lymphoid organ involvement (C, patients 13 through 28). ATL cells from patients with lymphoid organ involvement were blocked with 100 ng/ml pertussis toxin (PTX) for 1.5 hours at 37°C (C+PT) before chemotactic assays. The data are expressed as the mean percentage of migrated cells toward SLC or ELC from triplicate experiments. The bars indicate the mean value of each group; the statistical analysis was performed using Student t test.
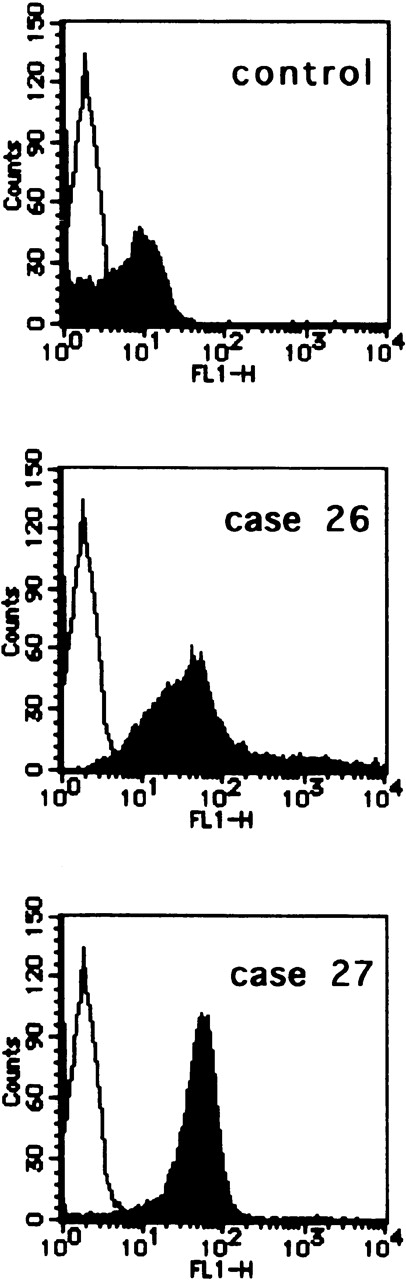
Flow cytometric analysis of the infiltrating ATL cells in the lymph nodes
To see whether infiltrating ATL cells in lymph nodes express high levels of CCR7/EBI1, we examined by flow cytometric analysis, using CCR7.6B3, the CCR7/EBI1 expression of infiltrating ATL cells prepared from lymph nodes of 2 representative patients, patients 26 and 27. We also examined, by the same method, the CCR7/EBI1 expression of control CD4+CD45RO+ T cells from reactive lymph nodes of HTLV-1-seronegative individuals who had undergone abdominal surgery. Infiltrating ATL cells and control CD4+CD45RO+ T cells were purified by the negative immunoselection technique. As shown in Figure5, infiltrating ATL cells of patients 26 and 27 expressed CCR7/EBI1 at higher levels than control CCR7/EBI1-positive CD4+CD45RO+ T cells. This further supports that increased CCR7/EBI1 expression enhances lymphoid infiltration by ATL cells.
Flow cytometric analysis of the infiltrating adult T-cell leukemia (ATL) cells in lymph nodes.
CCR7/EBI1 expression levels of infiltrating ATL cells prepared from lymph nodes of 2 representative patients, patients 26 and 27, and control CD4+CD45RO+ T cells from reactive lymph nodes of HTLV-1-seronegative individuals were compared by flow cytometric analysis using an anti-CCR7 mAb, CCR7.6B3. The infiltrating ATL cells and control CD4+CD45RO+ T cells were purified by the negative immunoselection technique as described in Materials and Methods.
Flow cytometric analysis of the infiltrating adult T-cell leukemia (ATL) cells in lymph nodes.
CCR7/EBI1 expression levels of infiltrating ATL cells prepared from lymph nodes of 2 representative patients, patients 26 and 27, and control CD4+CD45RO+ T cells from reactive lymph nodes of HTLV-1-seronegative individuals were compared by flow cytometric analysis using an anti-CCR7 mAb, CCR7.6B3. The infiltrating ATL cells and control CD4+CD45RO+ T cells were purified by the negative immunoselection technique as described in Materials and Methods.
Discussion
This is the first report to demonstrate a specific association between expression of the chemokine receptor CCR7/EBI1 and infiltration of lymphoid organs by ATL cells and to support that increased CCR7/EBI1 expression enhances the infiltration of lymphoid organs by ATL cells. Our findings are as follows: 1) RT-PCR and flow cytometric analyses showed that CCR7/EBI1 expression by ATL cells from patients with lymphoid organ involvement was significantly higher than that by ATL cells from patients without lymphoid organ involvement; 2) significantly more ATL cells from patients with lymphoid organ involvement adhered to surfaces coated with ICAM-1 and SLC or ELC under static and flow conditions than did those from patients without lymphoid organ involvement; and 3) significantly more ATL cells from patients with lymphoid organ involvement migrated toward SLC or ELC, even at a low chemokine concentration, than did those from patients without lymphoid organ involvement.
Lymphocyte homing into lymphoid organs involves a coordinated multistep process: an initial tethering and rolling step mediated by L-selectin; chemokine-mediated signaling; and firm arrest mediated by LFA-1 binding to ICAM-1 or ICAM-2 on HEV cells. To investigate the infiltration of lymphoid organs by ATL cells, we focused on the interaction between chemokines and chemokine receptors on ATL cells during this process. The physiologically relevant chemokines on HEV cells that mediate integrin activation have not been definitively elucidated, but recent studies demonstrated that SLC, ELC, and SDF-1α could each promote integrin-mediated arrest of human lymphocytes under flow conditions.16,20,21,39 The highest SLC mRNA expression levels were detected in HEV cells of lymph nodes and Peyer's patches, and lower levels were detected in stromal cells in the T-cell zones of the spleen, lymph nodes, and Peyer's patches.16Furthermore, dendritic cells in the parafollicular and inner cortex regions, the so-called T-cell zones of lymph nodes, demonstrated strong ELC mRNA expression.40,41 The high-level expression of SLC, together with the lack of detectable ELC expression by HEV cells, suggests that SLC plays a more prominent role in triggering lymphocyte adhesion to HEV cells. Because ELC is expressed at high levels by the dendritic cells of the T-cell zone, ELC may instead play an important role in attracting T cells away from HEVs into the T-cell zone and promoting T-cell–dendritic-cell encounters. The chemotactic profiles of SLC and ELC are strikingly similar: both chemokines efficiently attract naive T cells, memory T cells, and B cells.16-18,40-43 However, SDF-1α exerts chemotactic effects on T and B cells, monocytes,44 CD34+progenitor cells,45 and pre-B and pro-B cell lines.46 SDF-1α is expressed in stromal cells in lymph nodes and in a layer of cells surrounding the tonsiller germinal centers, but whether it is expressed by HEV cells has not been demonstrated definitively.47
The chemokine receptor CCR7/EBI1, the functional receptor for SLC and ELC, is expressed in CD4+ and CD8+ T cells as well as in B cells, but not in natural killer cells, monocytes, or neutrophils in peripheral blood.23,24,41CCR7/EBI1 expression in CD34+ cells, colony-forming unit granulocyte macrophage, and mature dendritic cells also has been reported.26-28,48,49CCR7/EBI1 is expressed strongly in the tissues of various lymphoid organs, such as the lymph nodes, spleen, thymus, and appendix, and weakly in the small intestine, colon, placenta, bone marrow, and fetal liver.23,24,41 In lymph nodes, CCR7/EBI1 is expressed in the parafollicular and inner cortical regions in which mature dendritic cells reside and to which antigen-loaded dendritic cells home. The expression of CCR7/EBI1 has been reported to be up-regulated in B cells infected by EB virus, CD4+ T cells infected by HHV-6 or HHV-7, and T cells treated with IL-2 alone or with phytohemmaglutinin,23,25,41 whereas CCR7/EBI1 expression was not up-regulated by Tax using JP×9 cells50(unpublished data). Although the mechanism responsible for increasing CCR7/EBI1 expression on ATL cells is unknown, the expression of CCR7/EBI1 on ATL cells was significantly higher than that on resting CD4+CD45RO+ T cells. Furthermore, ATL cells from patients with lymphoid organ involvement expressed CCR7/EBI1 at significantly higher levels and adhered more efficiently to surfaces coated with ICAM-1 and SLC under flow conditions than normal CD4+CD45RO+ T cells or ATL cells from patients without lymphoid organ involvement. Our findings thus suggest that the up-regulation of CCR7/EBI1 in ATL cells promotes their responses to SLC expressed by HEV cells in induction of integrin-mediated adhesion to ICAM-1, thereby promoting the infiltration of lymphoid organs by ATL cells. SDF-1α is the only ligand for CXCR4 to be identified so far,31-33 and CXCR4 expression on cells has been reported to be down-regulated in the presence of SDF-1α.51 52 In the present study, we showed no correlation between levels of CXCR4 expression and infiltration of lymphoid organs by ATL cells.
Several studies on the localization and infiltration of ATL cells have been published.11,35,53-57 Other workers and we showed that increased expression of CD151, α4β1 integrin, and α5β1 integrin on ATL cells contributed to the lymphoma formation through adhesion of the extracellular matrix and abnormal proliferation and localization.35,53,54 E-selectin, α4β1/VCAM-1, and 0×40/gp34 pathways have been shown to be involved in cell adhesion to endothelial cells and eventually in organ infiltration by ATL cells.55,56 Three pairs of adhesion molecules, L-selectin/peripheral lymph node addressin, cutaneous lymphocyte-associated antigen/E-selectin, and α4β7 integrin/mucosal VCAM-1 also have been reported to be associated with preferential recirculation of ATL cells to the peripheral lymph nodes, skin, and gastrointestinal mucosa, respectively.57 The circulating ATL cells from patients with lymph node, skin, and gut involvement showed higher expression levels of L-selectin, cutaneous lymphocyte-associated antigen, and α4β7 integrin, respectively, than those from patients without involvement of these organs. During chemokine-mediated activation, MIP-1α and MIP-1β produced by ATL cells activated LFA-1, resulting in increased adhesion of ATL cells to HUVEC and ICAM-1 under static conditions, although whether these chemokines exist and function efficiently on HEV cells is unknown.11 These reports and the fact that ATL cells adhere spontaneously to endothelial cells and ICAM-1 suggest that multiple mechanisms are involved in ATL cell infiltration of lymphoid organs. The results of our study support that increased CCR7/EBI1 expression on ATL cells plays a role in the infiltration of lymphoid organs by these cells.
Acknowledgment
We are grateful to Kenji Kameda for his excellent technical assistance.
Supported in part by a grant-in-aid from the Ministry of Education, Science, and Culture of Japan.
Reprints:Hitoshi Hasegawa, First Department of Internal Medicine, Ehime University, School of Medicine, Shigenobu, Ehime 791-0295, Japan; e-mail: hitoshih@m.ehime-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.