Abstract
The human β-globin locus control region (LCR) contains chromatin opening and transcriptional enhancement activities that are important to include in β-globin gene therapy vectors. We previously used single-copy transgenic mice to map chromatin opening activity to the 5′HS3 LCR element. Here, we test novel hybrid globin genes to identify β-globin gene sequences that functionally interact with 5′HS3. First, we show that an 850-base pair (bp) 5′HS3 element activates high-level β-globin gene expression in fetal livers of 17 of 17 transgenic mice, including 3 single-copy animals, but fails to reproducibly activate Aγ-globin transgenes. To identify the β-globin gene sequences required for LCR activity by 5′HS3, we linked the 815-bp β-globin promoter to Aγ-globin coding sequences (BGT34), together with either the β-globin intron 2 (BGT35), the β-globin 3′ enhancer (BGT54), or both intron 2 and the 3′ enhancer (BGT50). Of these transgenes, only BGT50 reproducibly expresses Aγ-globin RNA (including 7 of 7 single-copy animals, averaging 71% per copy). Modifications to BGT50 show that LCR activity is detected after replacing the β-globin promoter with the 700-bp Aγ-globin promoter, but is abrogated when an AT-rich region is deleted from β-globin intron 2. We conclude that LCR activity by 5′HS3 on globin promoters requires the simultaneous presence of β-globin intron 2 sequences and the 260-bp 3′ β-globin enhancer. The BGT50 construct extends the utility of the 5′HS3 element to include erythroid expression of nonadult β-globin coding sequences in transgenic animals and its ability to express antisickling γ-globin coding sequences at single copy are ideal characteristics for a gene therapy cassette.
One difficult aspect of gene therapy is in reproducibly obtaining high-level, tissue-specific, and long-term expression from genes transferred into stem cells.1-3 Because commonly used retrovirus and adeno-associated virus (AAV) vectors integrate at single copy, their transduced genes should be regulated by tissue-specific elements that function at single copy. Locus control regions (LCRs) are well suited for this task, as they direct reproducible expression from all integration sites and transgene copy numbers. For example, the human β-globin LCR directs high-level β-globin transgene expression in erythroid cells of transgenic mice, regardless of the integration site, in general.4 LCRs have also been identified in other tissue-specific loci such as the chicken lysozyme locus5and immunoglobulin mu locus.6 However, simple addition of minimal LCR elements to a transgene does not necessarily result in reproducible expression at all sites and copy numbers. In fact, recent findings show that LCR activity requires linked β-globin gene sequences, and suggest that the utility of the LCR is limited to the expression of β-globin. For example, the full β-globin LCR cannot confer reproducible transgene expression in mice on other gene sequences, such as the LacZ marker gene,7,8 γ-globin genes,9-11 or even β-globin genes that lack a 3′ element.12 13 To successfully use LCR elements in gene therapy vectors that integrate at single copy, it will be crucial to characterize the gene proximalcis-acting sequences that functionally interact with the LCR to make any given expression cassette LCR-responsive.
LCR activity is operationally defined as the ability to direct high-level position-independent transgene expression in mice and requires 2 functions: chromatin opening activity that directs transgene expression at all integration sites and transcriptional enhancement activity that boosts levels of transgene expression.14-16In the human β-globin locus, DNA elements that confer LCR activity are located 6 to 18 kilobase (kb) upstream of the ε-globin gene and comprise at least 4 DNaseI hypersensitive sites (HSs) that are developmentally stable and erythroid specific.4,17,18Analysis of the human β-globin LCR using linked cosmid or yeast artificial chromosome (YAC) transgenes in mice, in which individual HSs have been deleted or replaced with other HS elements, indicates that each HS contributes to LCR activity and may synergize via DNA looping to form a holocomplex.19-25 In contrast to the transgenic approaches described above, deletion of HSs by gene targeting suggests that the LCR is not required for chromatin opening of the endogenous murine or human β-globin loci.26,27 These findings have prompted some researchers to look for additional regulatory elements upstream of the known murine and human LCRs28 and to propose alternative binary/linking models for LCR function.14 29
The ability of small LCR elements to open chromatin at ectopic sites and direct high-level transgene expression has great promise for gene therapy. Our studies have revealed that chromatin opening by the β-globin LCR does not require all 4 HSs. We have used single-copy transgenic mouse lines to map chromatin opening activity to a 1.9-kb 5′HS3 element when it is linked to the β-globin gene,30 but expression levels are reduced to about 26% per copy. DNaseI digestion experiments demonstrated that 5′HS3, but not 5′HS2, could open chromatin at all integration sites tested.30 However, this study did not indicate whether 5′HS3 was sufficient for reproducible single-copy transgene expression or whether this element requires a functional interaction with β-globin gene sequences.
There are several candidate regulatory elements within the β-globin gene that might conceivably interact with 5′HS3 to confer LCR responsiveness. These include the β-globin promoter element,31 the intragenic enhancer,31,32 and matrix attachment region (MAR)33 in intron 2, and the 3′ enhancer.31,32,34,35 The β-globin promoter and enhancers have been extensively analyzed, but their role in LCR activation has not been systematically evaluated in single-copy transgenic mice. The minimal β-globin promoter maps to a 103-base pair (bp) fragment that is inducible by the LCR in stable transfection studies and that has been fully footprinted for transcription factor binding sites.36 We previously noted that the 265-bp β-globin promoter commonly used in gene therapy vectors does not direct reproducible expression in single-copy transgenic mice regulated by a 3.0-kb (5′HS2-4) LCR cassette.37 In contrast, the same LCR cassette directed single-copy expression from the 815-bp β-promoter. Similarly, LCR activation of multicopy γ-globin transgenes is dependent on the length of the γ-globin promoter.38 These findings suggest that cis-acting sequences in proximal globin promoters may have a role in LCR activation and should be included in gene therapy cassettes.
With regard to the β-globin enhancers, we have recently shown that reproducible expression from single-copy β-globin transgenes regulated by a 4.0-kb (5′HS1-4) LCR cassette requires 1.65 kb of 3′ sequences that include the known 260-bp β-globin 3′ enhancer.12 In addition, deletion of the β-globin 3′ enhancer in YAC transgenic mice causes a reduction in β-globin gene expression, indicating that the β-globin 3′ enhancer influences globin switching.13 These in vivo data suggest that 3′ sequences may have a role in LCR activation by 5′HS3 and should also be included in gene therapy vectors. This conclusion differs from earlier studies showing that the β-globin gene enhancers have no role in LCR-mediated induction of the promoter in stable transfection studies36,39,40 and that led to their omission from most β-globin gene therapy vectors.40-43 The importance of β-globin intron 2 sequences (that include the intragenic enhancer and MAR) for LCR activity by 5′HS3 has yet to be determined in transgenic mice.
In this study, we show that an 850-bp 5′HS3 element confers reproducible expression on a linked β-globin transgene in mice. However, 5′HS3 is incapable of similarly activating a linked Aγ-globin transgene, demonstrating a requirement for β-globin sequences in the reproducible activation of transgenes by this element. To identify the critical β-globin gene sequences, we replaced Aγ-globin sequences with various combinations of candidate β-globin regulatory elements. These hybrid transgenes all retain the Aγ coding sequences and are linked to the 850-bp 5′HS3 element. We show here that the β-globin intron 2 and 260-bp 3′ enhancer are involved in LCR activity mediated by the 5′HS3 element, and are required for reproducible single-copy transgene expression. In addition, we have extended the utility of the β-globin LCR to include expression of the Aγ-globin gene. Such a β/γ-globin hybrid gene with single-copy expression characteristics is ideally suited for gene therapy of sickle cell anemia because the γ-globin protein has better antisickling properties than β-globin.44
Materials and methods
Plasmid construction
Transgene constructs were derived from the plasmids pGSE1758,45 pBGT14,37 p141,43 and pAγ-globin (provided by S. Philipsen). pGSE1758 contains a polylinker 5′ of the 4.2-kb HpaI-EcoRV β-globin gene fragment regulated by the 815-bp promoter. pBGT14 contains a 3.0-kb LCR cassette and the 4.2-kb HpaI-EcoRV β-globin gene fragment regulated by the 815-bp promoter. The 3.0-kb LCR contains the 1.15-kb StuI-SpeI fragment of 5′HS4, the 0.85-kbSacI-PvuII fragment of 5′HS3, and the 0.95-kbSmaI-StuI fragment of 5′HS2. p141 contains aSnaBI-PstI β-globin gene fragment that includes a 372-bp RsaI-RsaI deletion in intron 2.
BGT9 was constructed by inserting the 850-bp SacI-PvuII fragment of 5′HS3 into the XhoI site of pGSE1758 usingXhoI linkers. The 5.0-kb transgene was purified as anEcoRV fragment.
BGT26 replaces all the β-globin gene sequences in BGT9 between theSalI-XbaI sites with the 4.3-kb BspHI fragment of Aγ-globin by blunt end ligation. The 5.2-kb transgene was purified as an EcoRV fragment.
BGT34 inserts the 1.9-kb NcoI–HindIII fragment from Aγ-globin into the NcoI-EcoRV sites of BGT9 using anNheI linker at the incompatible HindIII andEcoRV overhangs. The Aγ-globin sequences extend from the ATG translation start site located at the NcoI site used for subcloning, to 375-bp 3′ of exon 3, including the polyA site but not the Aγ-globin 3′ enhancer. The 3.7-kb transgene was purified as an EcoRV-NheI fragment.
BGT35 inserts the β-globin intron 2 sequences as aBamHI–EcoRI fragment into the compatibleBamHI–EcoRI sites of BGT34. These changes also replace 4 Aγ-globin codons (101-104) with β-globin exon 2 sequences, and 16 Aγ-globin codons (105-119) with β-globin exon 3 sequences. Of these 20 β-globin codons, 17 encode the same amino-acid as Aγ-globin. The 3 altered codons are described in the text. The 3.7-kb transgene was purified as an EcoRV-NheI fragment.
BGT50 contains a polylinker at the NheI site of BGT35 that addsEcoRV, AgeI, and ClaI sites 3′ of the hybrid globin gene. The 260-bp PstI fragment containing the β-globin 3′ enhancer was cloned into the NheI site using linkers. The 3.9-kb transgene was purified as a ClaI fragment.
BGT54 contains the 3.0-kb ClaI-EcoRI fragment of BGT34 linked to the 850-bp EcoRI-ClaI fragment of BGT50. The 3.9-kb transgene was purified as a ClaI fragment.
BGT76 contains the 2.1-kb ClaI-BamHI fragment of BGT26 linked to the 1.7-kb BamHI-ClaI fragment of BGT50. The 3.8-kb transgene was purified as a ClaI fragment.
BGT64 contains the 2.2-kb ClaI-BamHI fragment and the 820-bp EcoRI-ClaI fragment of BGT50 linked by the 540-bp BamHI-EcoRI β-globin intron 2 fragment of p141. The 3.5-kb transgene was purified as a ClaI fragment.
Generation of transgenic mice
Transgene DNA was prepared using Plasmid Maxi Kits (Qiagen, Santa Clara, CA). Transgene fragments were liberated from their plasmid backbones by digestion with the stated restriction enzymes. DNA fragments were recovered from 0.7% Tris-Acetate-EDTA (TAE) agarose gel slices using GeneClean II or GeneClean Spin Column Kits (Bio101, Vista, CA) and Elutip-d columns (Schleicher and Schuell, Keene, NH), and resuspended in injection buffer (10 mmol/L Tris-HCl pH 7.5, 0.2 mmol/L ethylenediamine tetraacetic acid [EDTA]). DNA concentration was determined by comparison with DNA standards run on agarose gels, and the injection fragment was diluted to 0.5-1 ng/μL in injection buffer. The diluted DNA was prespun for 20 minutes and aliquots removed for microinjection into fertilized FVB mouse eggs. Injected eggs were transferred into recipient CD1 female animals. Fetal mice were dissected 15.5 days after injection and DNA extracted from head tissue while the fetal livers were saved frozen in halves for future analysis. Head DNA was extracted by Proteinase K digestion overnight, a single phenol/chloroform extraction and isopropanol precipitation. Transgenic fetuses were identified by slot blot hybridization with the 5′HS3 probe, using standard procedures.
DNA analysis
Southern transfer and hybridization were by standard procedures. Copy-number determination was performed using a Molecular Dynamics PhosphorImager (Sunnyvale, CA). Single-copy animals showed a single random-sized end-fragment in BamHI and EcoRI digests, hybridized with the 5′HS3, β-globin intron 2, or Aγ-globin 3′ probes (Figure 1; Fragments A-C). With multicopy animals, the intensity of the end-fragment was defined as 1 transgene copy and was used to calculate the copy number of the multicopy junction-fragment in the same lane. The intactness of the transgene in the DNA sample was verified by Southern blot analysis using 2 sets of digests, 1 for 5′ intactness and 1 for 3′ intactness, appropriate for each construct. The resulting fragments were hybridized to either 5′HS3, β-globin intron 2, or Aγ-globin 3′ probes. Nonintact transgenes were not included in the calculation of copy number. Mice that were mosaic for the transgene were excluded from the study after demonstration of insignificant transgene contribution to fetal liver DNA by Southern blot analysis.
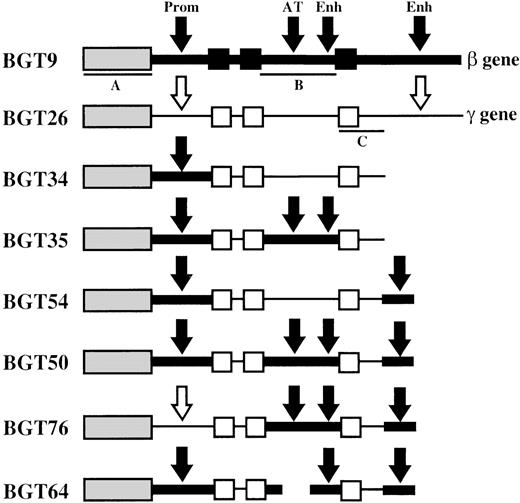
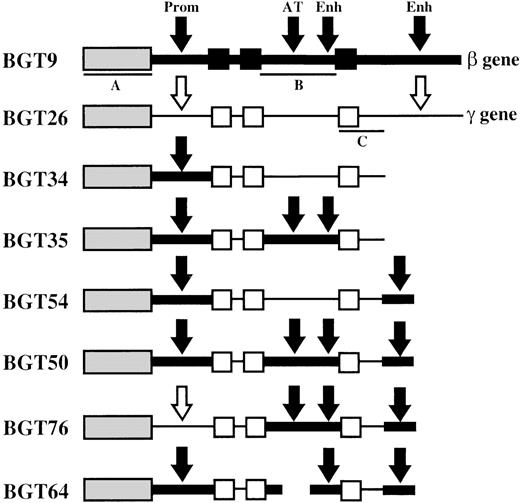
Map of transgene constructs designed to determine the importance of specific human β-globin gene sequences in LCR activity conferred by the 5′HS3 element.
The sequences used in each construct are outlined in “Materials and methods.” The β-globin gene is indicated as a thick line with exons as black boxes. β-globin gene regulatory elements are indicated by black arrows and include the 815-bp β-globin promoter (Prom), the AT-rich region (AT) that coincides with a known MAR and intragenic enhancer (Enh) located in intron 2, and the 260-bp 3′ enhancer (Enh) located downstream of the gene. Aγ-globin gene sequences are represented as thin lines, and unfilled boxes (exons) or arrows (regulatory elements). Southern probe fragments correspond toXhoI-XhoI fragment of 5′HS3 in BGT9 (A),BamHI-EcoRI fragment of β-globin intron 2 in BGT9 (B), and EcoRI-EcoRI fragment of Aγ-globin 3′ sequences in BGT26 (C).
Map of transgene constructs designed to determine the importance of specific human β-globin gene sequences in LCR activity conferred by the 5′HS3 element.
The sequences used in each construct are outlined in “Materials and methods.” The β-globin gene is indicated as a thick line with exons as black boxes. β-globin gene regulatory elements are indicated by black arrows and include the 815-bp β-globin promoter (Prom), the AT-rich region (AT) that coincides with a known MAR and intragenic enhancer (Enh) located in intron 2, and the 260-bp 3′ enhancer (Enh) located downstream of the gene. Aγ-globin gene sequences are represented as thin lines, and unfilled boxes (exons) or arrows (regulatory elements). Southern probe fragments correspond toXhoI-XhoI fragment of 5′HS3 in BGT9 (A),BamHI-EcoRI fragment of β-globin intron 2 in BGT9 (B), and EcoRI-EcoRI fragment of Aγ-globin 3′ sequences in BGT26 (C).
RNA analysis
Fetal liver (embryonic day 15.5) RNA was extracted using Trizol Reagent (Gibco BRL, Gaithersburg, MD), 1 μg was hybridized to [γ-32P]ATP-labeled double-stranded 5′DNA probe for human β-globin detection,31 or a [α-32P]dATP-labeled double-stranded 3′ DNA probe (the EcoRI fragment containing exon 3) for detection of human Aγ-globin.46 A [γ-32P]ATP-labeled double-stranded 5′DNA probe was used for mouse β-globin major detection as a loading control.31 RNA/DNA hybrids were subsequently digested with 75 units S1 nuclease (Roche Diagnostics, Laval, PQ, Canada) and run on a 6% sequencing gel as described.31 Probe excess was demonstrated by including a sample that contains 3 μg fetal liver RNA. Specific activities of human β-globin (Hβ) or human Aγ-globin (Hγ) relative to the mouse β major (Mβ) probe is described for each S1 nuclease experiment in the corresponding figure legend. The protected 170 nucleotide (nt) Hγ, 160 nt Hβ, and 95 nt Mβ bands were quantified on a Molecular Dynamics PhosphorImager and the percentage expression levels calculated according to the formula (Hβ or γ/ Mβ) × 100% and corrected for specific activity differences between the probe preparations. Expression per transgene copy was calculated as (2 Mβ genes/number Hβ or γ transgenes) × (% expression) / (% transgenicity) × 100%.
Results
To determine whether the 850-bp 5′HS3 element can direct high-level position-independent transgene expression when linked to the β-globin gene, we created the BGT9 construct (Figure 1). The β-globin gene sequences in BGT9 include the 815-bp β-globin promoter, the entire β-globin coding sequences, including both introns, and 1.65 kb of 3′ sequences, including the 260-bp 3′ β-globin enhancer. To determine whether the 850-bp 5′HS3 element requires β-globin gene sequences for such LCR activity, we also linked it to the Aγ-globin gene (BGT26 construct). BGT26 includes the 700-bp Aγ-globin promoter, the entire Aγ-globin coding sequences, including both introns, and 2.0 kb of 3′ sequences, including the 3′ Aγ-globin enhancer.
Generation of transgenic mice
These DNA constructs were purified as linear fragments and microinjected into fertilized FVB mouse eggs to create transgenic mice. The fetuses derived from these eggs were dissected at embryonic day 15.5 and genomic DNA extracted from head tissue, while the fetal livers were frozen in halves for future analyses. Positive transient transgenic founder animals were identified by slot blot hybridization, and transgene copy number subsequently deduced by genomic Southern blots after digestion with EcoRI and BamHI, which we have previously shown can unambiguously identify junction fragments that define single-copy transgenic mice.37 All founder animals were characterized to determine whether they harbored intact transgenes by Southern blot analysis with multiple diagnostic restriction enzymes (Southern data not shown). Finally, the proportion of transgenic cells in the fetal liver was compared with a bred line control by Southern blot analysis. DNA derived from half of the frozen fetal livers was digested with AccI (BGT9) or PstI (Aγ-globin transgenes) and the transgene detected by the β-globin intron 2 or 5′HS3 probes, respectively (data not shown). Only animals containing at least 1 intact transgene were analyzed and highly mosaic animals (less than 10% transgenic cells in the fetal liver) were excluded from this study.
Requirement for β-globin gene sequences
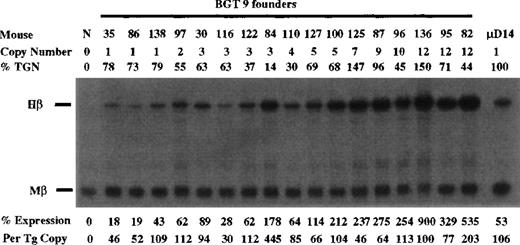
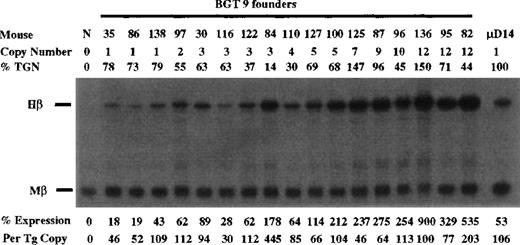
Expression from BGT9 and 26 was assayed in transgenic mice by S1 nuclease protection analysis in the fetal liver of 15.5-day transient transgenic mice. For the BGT9 construct, expression was analyzed using Hβ and Mβ probes (Figure 2). As a standard sample for quantitation of Hβ RNA levels, we included fetal liver RNA from μD14 transgenic mice that express to approximately 100% levels on a per copy basis.30 The BGT9 construct expresses significant levels of human β-globin mRNA in 17/17 transgenic mice. Expression from 3 of 3 single-copy BGT9 mice ranges from 46% to 109% per copy of the Mβ RNA, demonstrating that the 850-bp 5′HS3 element directs reproducible single-copy transgene expression when linked to the entire β-globin gene. In addition, the BGT9 construct appears to express to a higher level at single copy than the previously described 26% levels per copy from the 1.9-kb 5′HS3 element.30
Expression of human β-globin mRNA in transgenic mice containing the BGT9 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder BGT9 transgenic mice showing that BGT9 is expressed in all 17 animals, including 3 single-copy mice. These data show that the 850-bp 5′HS3 element can express reproducible levels of β-globin transcripts. Relative specific activity of Hβ/Mβ probes is 1:1. Hβ, human β-globin protected probe fragment; Mβ, mouse β major protected probe fragment; N, nontransgenic; μD, 1 copy μD14 microlocus line (discussed in text).
Expression of human β-globin mRNA in transgenic mice containing the BGT9 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder BGT9 transgenic mice showing that BGT9 is expressed in all 17 animals, including 3 single-copy mice. These data show that the 850-bp 5′HS3 element can express reproducible levels of β-globin transcripts. Relative specific activity of Hβ/Mβ probes is 1:1. Hβ, human β-globin protected probe fragment; Mβ, mouse β major protected probe fragment; N, nontransgenic; μD, 1 copy μD14 microlocus line (discussed in text).
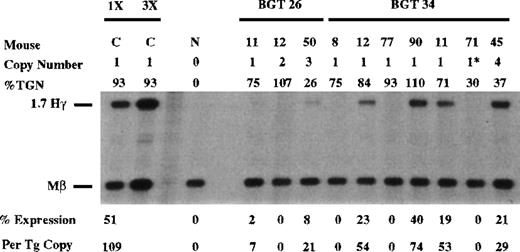
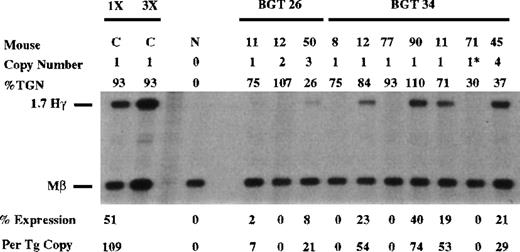
Similar expression analysis was performed on the BGT26 transgene using the Hγ and Mβ probes (Figure 3). As a standard RNA sample for all the Hγ S1 nuclease experiments, we included BGT50-48 RNA (labeled C in all figures). Expression by BGT50-48 is equivalent to the highest expressing single-copy BGT9 mouse, in that it contains 1 intact copy of the BGT50 construct (described later) and expresses γ-globin at 109% per copy of the level of Mβ (mean value from 8 experiments). S1 analysis of fetal liver RNA from BGT26 transgenic mice shows that low to moderate level expression is obtained in 2 of 3 animals with an undetectable level in the third animal. These data demonstrate that 5′HS3 cannot direct position independent transgene expression on a linked Aγ-globin gene, and suggest that LCR activity by 5′HS3 requires a functional interaction with β-globin gene sequences.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT26 and BGT34 constructs.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT26 is expressed to low or undetectable levels and that BGT34 is expressed at significant levels in 4 of the 7 transgenic mice. These data show that β-globin gene sequences are required for reproducible single-copy transgene activation by 5′HS3 and that the β-globin promoter element is not sufficient for this activity. Relative specific activities of Hγ/Mβ probes is shown. Hγ, human Aγ-globin protected probe fragment; Mβ, mouse β major protected probe fragment; N, nontransgenic; C, 50-48, the highest expressing BGT50 single-copy transgenic mouse (discussed in text; see Figure 6); 3 × , probe excess control. Copy # = 1*, 1 intact copy plus a partial copy of the transgene.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT26 and BGT34 constructs.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT26 is expressed to low or undetectable levels and that BGT34 is expressed at significant levels in 4 of the 7 transgenic mice. These data show that β-globin gene sequences are required for reproducible single-copy transgene activation by 5′HS3 and that the β-globin promoter element is not sufficient for this activity. Relative specific activities of Hγ/Mβ probes is shown. Hγ, human Aγ-globin protected probe fragment; Mβ, mouse β major protected probe fragment; N, nontransgenic; C, 50-48, the highest expressing BGT50 single-copy transgenic mouse (discussed in text; see Figure 6); 3 × , probe excess control. Copy # = 1*, 1 intact copy plus a partial copy of the transgene.
Design of novel 5′HS3 β/γ-globin hybrid transgenes
To identify the β-globin gene sequences required for LCR activity by 5′HS3, we created several novel hybrid globin genes (Figure1). BGT34 contains 5′HS3 linked to the β-globin 815-bp promoter and the Aγ-globin coding sequences terminating 375-bp downstream of exon 3. This construct does not contain the Aγ-globin 3′ enhancer, and the Aγ-globin intron 2 has no known enhancer activity. The remaining constructs are modifications of BGT34 that all retain the β-globin promoter. BGT35 has a replacement of the Aγ-globin intron 2 sequences with β-globin intron 2. This adds the β-globin intron 2 enhancer and MAR, but also alters 3 amino acids in the Aγ-globin coding sequences to their equivalents in the β-globin gene (K104R, T112K, I116H). These changes do not alter amino acids known to be important for antisickling effects.44 BGT54 is essentially the same as BGT34 but with the addition of the 260-bp β-globin 3′ enhancer 375-bp downstream of the Aγ-globin coding sequences. Finally, BGT50 contains both the β-globin intron 2 sequences and the 260-bp β-globin 3′ enhancer inserted 375-bp downstream of the Aγ-globin exon 3. BGT50 also contains the 3 amino acid alteration in the Aγ-globin coding sequences.
Expression of 5′HS3 β/γ-globin hybrid transgenes
Expression from each of the 5′HS3 β/γ-globin constructs was assayed in transgenic mice with S1 nuclease protection analysis in the fetal liver of 15.5-day transient transgenic mice as above. Human Aγ-globin messenger RNA (mRNA) was detected in 4 of 7 BGT34 transgenic mice (Figure 3). These included 5 animals containing an intact single-copy BGT34 transgene, and 1 animal (34-71) that contained an intact copy and a partial transgene (indicated by the asterisk). Only 3 of these 6 single-copy BGT34 animals expressed detectable levels, with a range of 0% to 74% per copy. This finding demonstrates that the β-globin promoter is not sufficient to rescue LCR activity by 5′HS3.
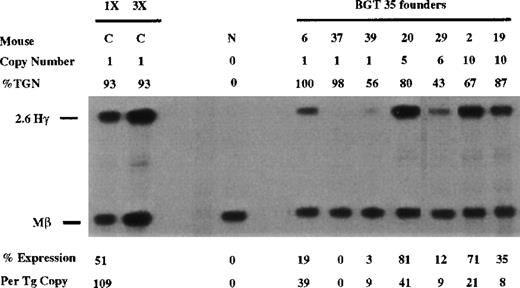
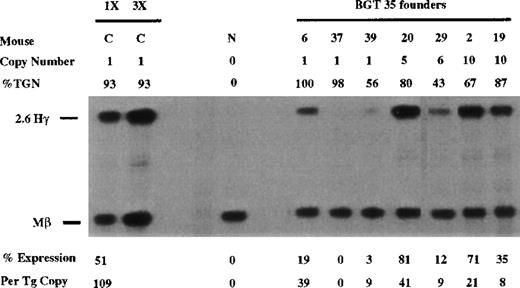
Other candidate β-globin sequences that may functionally interact with 5′HS3 include the elements in β-globin intron 2 and 3′ of the gene. Therefore, we analyzed 5′HS3 γ-globin transgenes containing the 815-bp β-globin promoter and either β-globin intron 2 (BGT 35) or the 260-bp β-globin 3′ enhancer (BGT 54) or both (BGT 50). A total of 6 of 7 BGT35 animals expressed the transgene, including 2 of 3 single-copy animals that ranged from 0% to 39% per copy (Figure 4). These data show that the β-globin promoter and β-globin intron 2 are not sufficient to rescue LCR activity directed by 5′HS3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT35 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing BGT35 is expressed at significant levels in 5 of the 7 transgenic mice. These data show that the β-globin promoter element and intron 2 sequence are not sufficient for reproducible single-copy transgene expression. Abbreviations as described in Figure 3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT35 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing BGT35 is expressed at significant levels in 5 of the 7 transgenic mice. These data show that the β-globin promoter element and intron 2 sequence are not sufficient for reproducible single-copy transgene expression. Abbreviations as described in Figure 3.
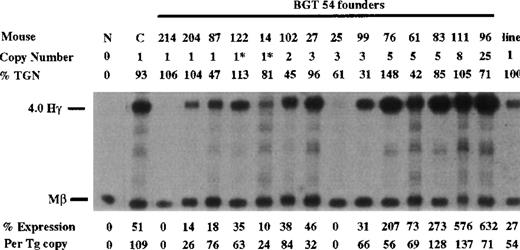
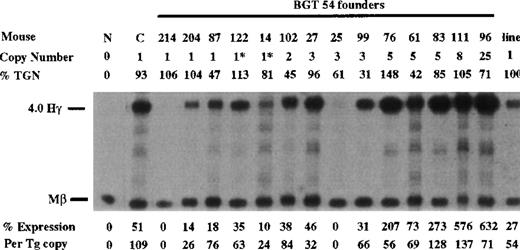
Similarly, BGT54 transgenes express significant levels of Aγ-globin mRNA in 5 of 6 single-copy animals (range 0%-76% per copy) and in 8 of 9 multicopy animals (Figure 5). Of the 6 single-copy BGT54 animals, 2 contained an intact transgene and a partial transgene (indicated by asterisks), and 1 sample represents a single-copy bred line in which RNA expression was assayed in the adult blood (indicated as the “line”). However, 1 single-copy and a 3-copy animal express undetectable levels of Aγ-globin mRNA. This finding demonstrates that the β-globin promoter and β-globin 3′ enhancer are not sufficient to rescue LCR activity by 5′HS3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT54 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT54 is expressed at significant levels in 13 of the 15 transgenic mice. These data show that the β-globin promoter element and 3′ enhancer sequence are not sufficient for reproducible single-copy transgene expression. Abbreviations as described in Figure 3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT54 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT54 is expressed at significant levels in 13 of the 15 transgenic mice. These data show that the β-globin promoter element and 3′ enhancer sequence are not sufficient for reproducible single-copy transgene expression. Abbreviations as described in Figure 3.
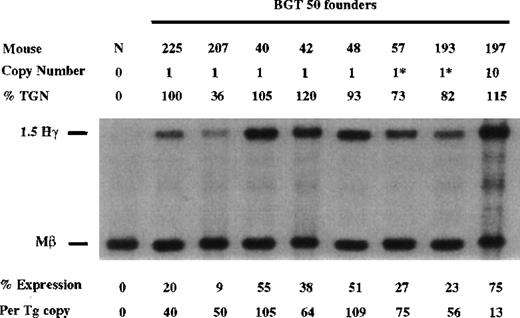
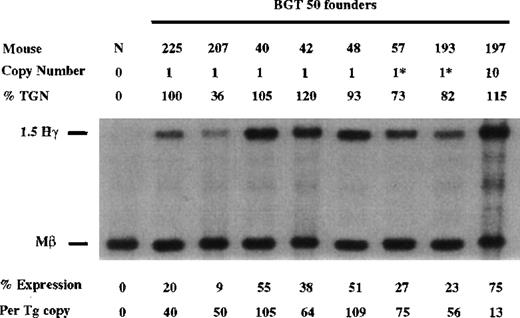
Finally, the BGT50 construct was tested in transgenic mice, and significant expression was detected in all 8 transgenic mice (Figure6). Seven single-copy animals were generated, 2 of which contained an intact and a partial transgene (indicated by asterisks). One animal (50-225) is a single-copy bred line sample in which RNA was assayed in the fetal liver. The average expression of single-copy BGT50 transgenes is 71% per copy of Mβ levels and ranges from 40% to 109% per copy. Because only BGT50 was reproducibly expressed in all transgenic mice, including all 7 single-copy animals, we conclude that LCR activity by 5′HS3 on the β-globin promoter requires a functional interaction with both β-globin intron 2 and the 260-bp β-globin 3′ enhancer.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT50 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT50 is expressed in all 8 animals, including 7 single-copy mice. These data show that the β-globin intron 2 and 3′ enhancer elements are sufficient for reproducible transgene expression when linked to the 5′HS3 element and the β-globin promoter. Abbreviations as described in Figure 3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT50 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT50 is expressed in all 8 animals, including 7 single-copy mice. These data show that the β-globin intron 2 and 3′ enhancer elements are sufficient for reproducible transgene expression when linked to the 5′HS3 element and the β-globin promoter. Abbreviations as described in Figure 3.
Expression from the Aγ-globin promoter
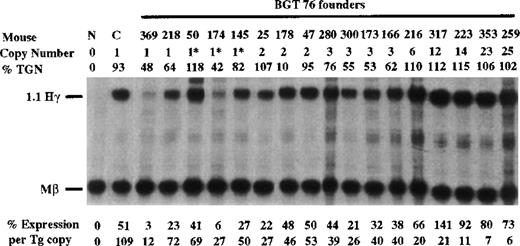
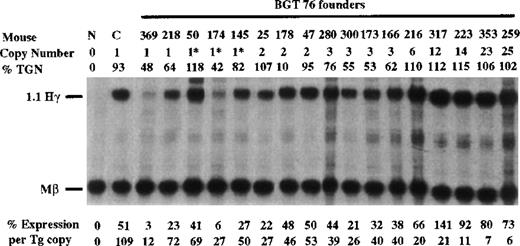
To determine whether the β-globin promoter is required, or that β-globin intron 2 sequences and the β-globin 3′ enhancer are themselves sufficient for LCR activity by 5′HS3, we created the BGT76 construct (Figure 1). BGT76 is essentially the same as BGT50 with the replacement of the 815-bp β-globin promoter with the 700-bp Aγ-globin promoter. Expression from BGT76 was assayed in transgenic mice by S1 nuclease protection analysis in the fetal liver of 15.5-day transient transgenic mice as above. All 17 BGT76 transgenic mice expressed detectable levels of human Aγ-globin mRNA (Figure7). Five single-copy animals were generated, 3 of which also contained a partial transgene (indicated by asterisks). These single-copy transgenic mice expressed a mean 46% Mβ levels ranging from 12% to 72%. This finding demonstrates that the LCR activity by 5′HS3 is not dependent on the presence of the β-globin promoter. We conclude that the Aγ-globin promoter can also be activated by a functional interaction between 5′HS3, β-globin intron 2, and the 260-bp β-globin 3′ enhancer.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT76 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT76 is expressed in all 17 animals, including 5 single-copy mice. These data show that activation of single-copy transgenes by a functional interaction between 5′HS3, the β-globin intron 2, and 3′ enhancer elements is not specific for the β-globin promoter. Abbreviations as described in Figure3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT76 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that BGT76 is expressed in all 17 animals, including 5 single-copy mice. These data show that activation of single-copy transgenes by a functional interaction between 5′HS3, the β-globin intron 2, and 3′ enhancer elements is not specific for the β-globin promoter. Abbreviations as described in Figure3.
Requirement for an AT-rich region within β-globin intron 2
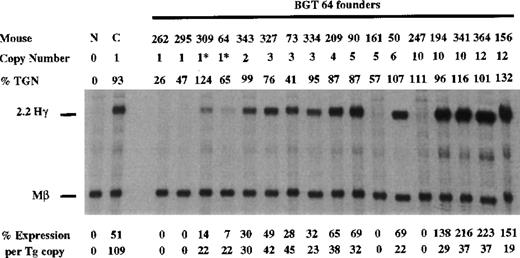
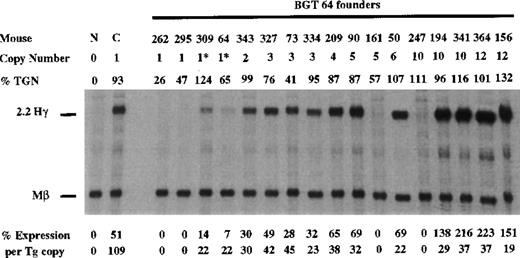
To determine whether the AT-rich region in β-globin intron 2 (located within a known MAR element) is required for LCR activity by 5′HS3, we created a derivative of BGT50 called BGT64 (Figure 1). BGT64 contains a 372-bp deletion in β-globin intron 2. The deleted sequence is deleterious to the retrovirus life-cycle when included in β-globin inserts as part of recombinant retrovirus vectors.41 43 Expression from BGT64 was assayed in transgenic mice by S1 nuclease protection analysis in the fetal liver of 15.5-day transient transgenic mice as described above. Thirteen of 17 BGT64 transgenic mice expressed detectable levels of Hγ mRNA (Figure 8), including 2 of 4 single-copy animals (range 0%-22% per copy). The 2 expressing single-copy animals contained an intact transgene and a partial transgene (indicated by asterisks). Of the 4 mice that did not express detectable levels of transcript, 2 animals contained a single intact copy of the transgene and 2 animals contained multiple copies of the transgene. These data show that the 372-bp AT-rich segment deleted from β-globin intron 2 in BGT64 is important for LCR activity by 5′HS3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT 64 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that the BGT64 is expressed at significant levels in 13 of the 17 transgenic mice. These data show that the AT-rich region in β-globin intron 2 is necessary for the single-copy expression characteristics of BGT50. Abbreviations as described in Figure 3.
Expression of human Aγ-globin mRNA in transgenic mice containing the BGT 64 construct.
S1 nuclease analysis on fetal liver RNA of 15.5-day founder transgenic mice showing that the BGT64 is expressed at significant levels in 13 of the 17 transgenic mice. These data show that the AT-rich region in β-globin intron 2 is necessary for the single-copy expression characteristics of BGT50. Abbreviations as described in Figure 3.
Discussion
Definition of the β-globin gene regulatory elements capable of conferring responsiveness to the LCR 5′HS3 element in transgenic mice serves the dual purpose of (1) examining the functional and cooperative interactions of these elements, as well as (2) creating a transgene cassette in which the expression levels are well suited for gene therapy purposes. In this study, we define the minimal combination of β-globin gene sequences capable of directing position-independent transgene expression when linked to 5′HS3, and we place special emphasis on data obtained from single-copy transgenes. Our results demonstrate that LCR activity directed by 5′HS3 is only obtained in the presence of both β-globin intron 2 and 3′ enhancer elements. The BGT50 hybrid globin transgene is ideal for gene therapy of hemoglobinopathies because it expresses reproducibly at single-copy and expresses the Aγ-globin gene under β-globin gene regulation. γ-globin protein has better antisickling properties than β-globin,44 and low-level expression of γ-globin is known to ameliorate the symptoms of both sickle cell anemia and β-thalassemia.
5′HS3 locus control region activity requires linked β-globin gene sequences
The BGT9 construct demonstrates that the 850-bp fragment of 5′HS3 contains LCR activity on both multicopy and single-copy β-globin transgenes. This fragment is considerably smaller than the 1.9-kb 5′HS3 element previously shown to possess chromatin opening activity on single-copy transgenes. In addition, the 850-bp 5′HS3 element directs approximately 70% levels of β-globin gene expression at single copy. As reproducible single-copy transgene expression is not obtained from a 125-bp minimal 5′HS3 core element,37 our data demonstrate that sequences flanking the minimal core in the 850-bp 5′HS3 element are important for LCR function. Flanking sequences extending beyond the 850-bp element may actually reduce single-copy β-globin transgene expression to approximately 26% per copy as observed with the 1.9-kb 5′HS3 fragment.30 However, results from the BGT26 transgenic mice establish that the 850-bp 5′HS3 element cannot reproducibly express the Aγ-globin gene. These data agree with published findings,11 47 and demonstrate that 5′HS3 LCR activity requires linked β-globin gene sequences.
β-globin intron 2 and 3′ enhancer synergize to confer 5′HS3 responsiveness.
A series of β/γ-globin hybrid genes were constructed to define the minimal combination of β-globin gene sequences required for LCR activity directed by 5′HS3. The hybrid transgenes and their expression in transgenic mice are summarized in Table1. From our analysis, it is clear that 5′HS3 responsiveness is not dictated by the promoter alone. For example, BGT34 expresses in only 3 of the 6 single-copy mice. In the additional presence of either the β-globin intron 2 or the 3′ enhancer, expression improves to 2 of 3 (BGT35) and 4 of 5 (BGT54) single-copy animals, respectively. Both of these constructs provide more evidence that 5′HS3 responsiveness is not an inevitable consequence of the β-globin promoter, but rather suggest that the likelihood of expression at any given integration site is increased by the presence of intragenic or downstream elements.
In contrast to the effect of the β-globin promoter, position-independent transgene expression directed by 5′HS3 was conferred by the simultaneous presence of the β-globin intron 2 and 3′ enhancer in the BGT50 and BGT76 constructs. These constructs expressed in all 7 and all 5 single-copy animals, respectively, and at high levels ranging from 40% to 109% per copy for BGT50 and 12% to 72% per copy for BGT76. The range of single-copy transgene expression from BGT50 is equivalent to that from BGT9 (46%-109%) that contains the entire β-globin gene, showing that no other β-globin gene sequences are required for full 5′HS3 responsiveness. Although the β-globin promoter is not required for LCR activity as seen by the reproducible expression in all 17 BGT76 mice that contain the Aγ-globin promoter, the β-globin promoter may account for the slightly higher levels of expression detected in the BGT50 and BGT9 mice. The Aγ-globin promoter is not autonomously silenced by 15.5 days in BGT76 mice, but the artificial configuration of β-globin intron 2 and 3′ enhancer sequences in this construct provides no information on sequences required for Aγ-globin silencing.
Together, these results show that a functional interaction between 5′HS3, the β-globin intron 2, and 3′ enhancer elements is absolutely required for single-copy transgene activation from globin promoters. These findings are the first to support a role for β-globin intron 2 in LCR activity in transgenic mice, and agree with reports that LCR activity requires sequences that are 3′ of the β-globin gene. For example, we have previously demonstrated that single-copy β-globin transgene expression from a 4.0-kb (5′HS1-4) LCR requires a fragment that includes 1.65 kb of 3′ β-globin sequences.12 Here, we refine the mapping of the required 3′ sequences to the minimal 260-bp 3′ β-globin enhancer.
5′HS3 responsiveness requires AT-rich intron 2 sequences
To more finely map the sequences within the β-globin intron 2 that are required for LCR activity by 5′HS3, we deleted a 372-bp AT-rich region in the BGT64 construct (Table 1). The results show expression in only 13 of the 17 BGT64 mice, including 2 of the 4 single-copy animals. These findings demonstrate that a combination of the intragenic enhancer and the 3′ enhancer is not sufficient to direct LCR activity from 5′HS3, even in the presence of the β-globin promoter. Our conclusions therefore differ from previous reports that AT-rich deletions do not disturb β-globin expression in murine erythroleukemia (MEL) cells.41,43 The AT-rich deletion lies outside of sequences that are required for splicing or polyadenylation of β-globin transcripts.48 As the AT-rich region is completely embedded within a 540-bpBamHI-DraI fragment that has MAR activity,33 our results may suggest that a MAR element is required for reproducible single-copy transgene expression in conjunction with the presence of the 3′ β-globin enhancer. Similar functional interactions between MARs/facilitators and enhancers have been described for the immunoglobulin mu49 and ADA LCRs.50
The precise mechanism of these functional interactions among LCR elements such as 5′HS3, MARs, and enhancers has not been elucidated to date. We note that the Holocomplex model of LCR function does not predict a requirement for gene-specific elements other than for promoters. Our finding that β-globin intron 2 and the 3′ enhancer sequences are required for LCR activity by 5′HS3 at ectopic sites suggests that either these elements are involved in chromatin opening or participate in additional DNA looping events with the LCR or promoter. Alternatively, binding by transacting factors to elements spread throughout the β-globin gene is compatible with binary or linking models of LCR function. The Holocomplex and binary/linking models are not necessarily mutually exclusive as a physical interaction between LCR elements and promoters would not interfere with a functional requirement for factors bound through the rest of the gene.
The function of the AT-rich region of the β-globin intron 2 in LCR activity remains elusive. An MAR activity is located in this region and may be important in vivo for the transgene DNA to interact with the nuclear matrix. However, the Aγ-globin 3′ enhancer fragment also contains an MAR51 but is not sufficient to rescue LCR activity in the BGT26 construct, suggesting that the AT-rich region in β-globin intron 2 may have other important functions. One possibility to consider is that AT-rich regions play roles in both transcription and the initiation of DNA replication.52,53 As the β-globin intron 2 and 3′ enhancer sequences are located within important core and auxiliary elements of the human β-globin origin of DNA replication,54 we suggest that LCR activity directed by 5′HS3 may, in fact, require a linked origin of DNA replication that is itself LCR-responsive. This latter hypothesis is testable.
Novel 5′HS3 β/γ-globin transgenes used for gene therapy
The ability of our novel hybrid globin transgenes to express to high levels at all integration sites and at single copy can be applied to both erythroid-specific transgene expression cassettes in animals and for gene therapy. For example, BGT50 is the first description of Aγ-globin coding sequences controlled by the β-globin regulatory elements, and is well suited for DNA- or viral-mediated gene therapy of both sickle cell anemia and β-thalassemia. Given that BGT50 hybrid transgenes express reproducibly in the fetal livers of transgenic mice, we predict that they will also function when transferred directly into stem cells from cord blood or adult bone marrow. Its 3.9-kb size is small enough for insertion into AAV and retrovirus gene therapy vectors. However, the AT-rich region in BGT50 has been reported to be deleterious to retrovirus replication,41 43 and, in fact, we have been unable to generate high titer stable retrovirus vectors containing the hybrid transgenes (J.R. and P.L., unpublished data). Deletion of the AT-rich region compromises expression at single copy as observed with the BGT64 transgene construct. We suggest that viral-mediated transfer of BGT50 hybrid genes for gene therapy might be best accomplished with alternative vectors such as Semliki Forest Virus (P.L. manuscript in preparation).
Finally, the BGT50 and BGT76 constructs extend the utility of the β-globin LCR in transgenic mice to include reproducible expression of Aγ-globin transgenes. We suggest that any gene could be expressed to high levels in erythroid cells by inserting its cDNA or genomic exon/intron sequences (from the ATG site to 3′ untranslated sequences) between the NcoI and BamHI sites of BGT50. In this manner, the β-globin intron 2 would function as part of a 3′ untranslated region, and the β-globin polyadenylation sites would be used for transcription termination. Such an expression cassette may be extremely useful for directing high-level erythroid expression of nonglobin transgenes in animals.
Supported by a grant from the Medical Research Council (MRC) of Canada to J.E., a postgraduate scholarship from FCAR (Quebec) to J.E.R., and supported in part by NIH grant HL55435 to P.L.
Reprints:James Ellis, Developmental Biology Program, Hospital for Sick Children, 555 University Ave, Toronto, Ontario, Canada M5G1X8; e-mail: jellis@sickkids.on.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.