Abstract
Development of nontoxic and nonmyeloablative regimens for allogeneic hematopoietic stem-cell transplantation will decrease transplantation-related mortality caused by regimen-related toxic effects. In pursuit of this goal, a dog model of stable mixed hematopoietic chimerism was established in which leukocyte-antigen–identical litter mates are given sublethal total-body irradiation (2 Gy) before stem-cell transplantation and immunosuppression with mycophenolate mofetil and cyclosporine afterward. In the current study, we examined whether donor lymphocyte infusion (DLI) could be used as adoptive immunotherapy to convert mixed to complete donor chimerism. First, 8 mixed chimeras were given unmodified DLI between day 36 and day 414 after stem-cell transplantation. After a 10- to 47-week follow-up period, there were no significant changes in the percentage of donor engraftment. Next, we immunized the donor to the minor histocompatibility antigens (mHA) of the recipient by means of repeated skin grafting. Lymphocytes from the mHA-sensitized donor were infused between day 201 and day 651 after transplantation. All 8 recipients of mHA-sensitized DLI had conversion to greater than 98% donor chimerism within 2 to 12 weeks of the infusion. Complications from mHA-sensitized DLI included graft-versus-host disease in 2 dogs and marrow aplasia in 1. These results showed that the low-dose transplant regimen establishes immune tolerance, and mHA-sensitized DLI is required to break tolerance, thereby converting mixed to complete donor chimerism. We propose that mixed chimerism established after nonmyeloablative allogeneic stem-cell transplantation provides a platform for adoptive immunotherapy that has clinical potential in the treatment of patients with malignant diseases.
Stable mixed hematopoietic chimerism has been reliably established in dog leukocyte-antigen (DLA)–identical littermates given low-dose (2 Gy) nonmyeloablative total-body irradiation (TBI) before stem-cell transplantation and immunosuppression therapy with mycophenolate mofetil (MMF) and cyclosporine (CSP) until days 28 and 35, respectively, afterward.1 Preliminary results showed that mixed donor-host chimerism can be established successfully in humans with the same regimen used in the dog model.2Although mixed chimerism may be appropriate for treatment of certain genetic hematologic diseases,3 it may be necessary to convert mixed to complete donor chimerism in patients with hematologic malignancies.
Donor T lymphocytes mediate both a graft-versus-host (GVH) effect and a graft-versus-leukemia (GVL) effect after allogeneic stem-cell transplantation.4 Donor lymphocyte infusion (DLI) has been used to treat leukemic relapse or Epstein-Barr virus–induced lymphoproliferative disorders occurring after conventional allogeneic marrow transplantation.5,6 The largest experience with DLI has been in patients with relapsed chronic myeloid leukemia (CML), in whom complete cytogenetic responses were observed in 50% to 80% of patients given DLI, findings consistent with a potent GVL activity.7-10
We hypothesized that DLI could be used to convert stable mixed chimeras to complete donor chimeras among dogs given the novel nonmyeloablative transplant regimen. We first tested this hypothesis by giving unmodified DLI to mixed chimeras. When this failed to result in complete donor chimerism, we administered lymphocytes from donors sensitized to recipient minor histocompatibility antigens (mHA) by skin grafts from recipients. Conversion to complete donor hematopoietic chimerism was observed in all cases.
Materials and methods
Study design
Ten stable mixed chimeric dogs were used in this study and received 1 to 3 DLIs. First, 8 dogs received an unmodified DLI and 4 of them subsequently received a second unmodified DLI. Chimerism status was assessed for 10 to 30 weeks after each DLI. There was no significant change in the percentage of donor chimerism after each unmodified DLI. Next, 8 mixed chimeric dogs received mHA-sensitized DLI. Six of these 8 mixed chimeras had previously received unmodified DLI. Two additional mixed chimeras (dogs E272 and E400) received mHA-sensitized DLI only.
Laboratory animals
Litters of random-bred dogs were raised at the Fred Hutchinson Cancer Research Center or obtained from commercial kennels licensed by the US Department of Agriculture. The dogs weighed from 8.2 to 14.7 kg (median, 11.2 kg) and were 7 to 11 months old (median, 8 months). Dogs were observed for indications of disease for at least 60 days before entry into the transplant regimen. All were immunized against leptospirosis, distemper, hepatitis, parvovirus, and papillomavirus. Research was performed according to the principles outlined in the Guide for Laboratory Animal Facilities and Care of the National Academy of Sciences, National Research Council. All dogs were housed in kennels certified by the American Association for Accreditation of Laboratory Animal Care. All dogs were examined at least twice daily.
Transplantation
The stable mixed chimeras in this study were obtained from different experiments that used slightly different conditioning doses of TBI and either marrow or peripheral blood as stem-cell sources. Donor marrow was collected as described previously13 and was infused intravenously at doses of 2.2 to 4.5 × 108 cells/kg of body weight (median, 4.0 × 108) within 4 hours of TBI. Canine peripheral blood stem cells (PBSC) mobilized by recombinant canine granulocyte colony-stimulating factor (G-CSF; 10 μg/kg per day given subcutaneously for 5 days) were obtained by leukapheresis.14 Infused doses of PBSC ranged from 7.0 to 10.0 × 109 cells/kg. Six recipient dogs were given 2-Gy TBI followed by marrow infusion,1 and 2 recipients (E272 and E367) were given 1-Gy TBI followed by PBSC infusion.15 TBI was delivered at 0.07 Gy/minute from 2 opposing sources of cobalt 60.13 Two recipients (E399 and E400) were given 4.5-Gy irradiation delivered at 2 Gy/minute from a 6-MeV linear accelerator to cervical, thoracic, and upper abdominal lymph nodes instead of 2-Gy TBI; this lymph node irradiation (LNI) was followed by marrow infusion.16
Posttransplantation immunosuppression
All recipients were given 10 mg/kg of MMF twice a day subcutaneously or orally the day of transplantation until day 27 after transplantation. In addition, dogs conditioned with 2-Gy TBI were given 15 mg/kg of CSP twice a day orally the day before transplantation until day 35 after transplantation.1 PBSC and LNI recipients were given 10 mg/kg of CSP the day before transplantation until day 35 after transplantation. Subsequently, they received 7.5 mg/kg of CSP on days 36 to 50, 5 mg/kg on days 51 to 75, and 3 mg/kg on days 76 to 100.15 16
Assessment of engraftment and chimerism
Hematopoietic engraftment was determined by means of sustained recoveries of granulocyte and platelet counts in serial complete blood counts after the postirradiation nadir and by documentation of donor (GAAA)n repeat polymorphism in cells from the peripheral blood and bone marrow.17 Peripheral blood was obtained every 1 or 2 weeks, and granulocyte and mononuclear cell (PBMC) fractions were separated by using Ficoll-Hypaque–gradient methods. Genomic DNA was extracted, and a polymerase chain reaction (PCR)–based assay was performed by using primers specific for informative microsatellite markers.18,19 The PCR conditions for the informative primer pairs used were described previously.18 Mixed hematopoietic chimerism was quantified by estimating the proportion of donor-specific DNA among host DNA with use of the storage phosphorimaging technique (Molecular Dynamics, Sunnyvale, CA).1 20 The accuracy of each donor-recipient chimerism analysis was confirmed by either a repeat PCR, a second informative primer, or standardized serial dilution of donor and host cells. The assay is sufficiently sensitive to reliably detect donor or host cells to a level of 2% of the total cell population.
Conversion to complete donor chimerism was defined as the complete disappearance of host-specific alleles from granulocyte, PBMC, and bone marrow fractions of serial samples collected for up to 48 weeks after DLI. On completion of studies or if clinically appropriate, dogs were humanely killed and underwent complete necropsy, including a histopathological assessment of organ systems for GVH disease (GVHD).
DLI
DLI was given at various times after transplantation. Cells for DLI were obtained by leukapheresis of donor peripheral blood.13To obtain increased numbers of T cells for the second unmodified DLI, the donors underwent Cobe-apheresis collection of mononuclear cells.14 Before infusion, the CD3+ content of the leukapheresis product was analyzed by fluorescence-activated cell sorter scanning (Becton Dickinson, San Jose, CA) using anti-CD3ε fluorescein-conjugated monoclonal antibody 17.6F921(provided by Dr Peter F. Moore, University of California Davis). Fresh leukapheresis product was given intravenously to recipients.
Donors were sensitized to recipient mHA by subcutaneous placement of 2 full-thickness recipient skin grafts (2 × 2 cm each) into bilateral flanks of the respective marrow donors. The skin grafting was done each week for 4 weeks. As described previously,22 all of the skin grafts were rejected. One week after skin grafting, sensitized donors underwent leukapheresis. In this report, infusion of lymphocytes obtained from nonsensitized donors is referred to as unmodified DLI. In contrast, mHA-sensitized DLI was obtained from donors after multiple skin grafts from the respective marrow recipients were rejected. Because the marrow donor and the mixed chimeric recipient were DLA identical, the skin grafts served to immunize the donor against mHA present in the recipient and absent in the donor.
Immune reconstitution
In vitro immune functions of mixed and complete donor chimeras were measured by standard assays of lymphocyte proliferation, including mixed lymphocyte reaction (MLR), concanavalin A (1 μg/mL) evaluation, and phytohemagglutinin (1 μg/mL) stimulation.23 Four or 6 days after triplicate plating of 1 × 105 responding PBMC/well, cells were pulsed with 0.037 MBq/well of tritium thymidine for 18 hours and harvested (Packard, Meriden, CT). Counts per minute were measured with a β-scintillation counter (Packard) and results presented as mean counts per minute ± 1 SEM or, for MLR, as the stimulation index (mean experimental counts per minute divided by mean autologous control counts per minute). In addition, peripheral blood CD4 and CD8 phenotypes were determined by fluorescence cytometric analysis with fluorescein-conjugated CA13.1E4 and streptavidin-phycoerythrin staining of biotinylated CA9.JD3, respectively.24 Marrow stroma was cultured as described previously.25
Statistical analysis
Two tests for statistical significance were used. The first involved comparison of the outcome in 10 dogs receiving either unmodified or mHA-sensitized DLI for the first time, since 2 different treatments in the same recipient were not considered independent events. Statistical significance was determined by using Monte Carlo approximation based on 5000 simulations.26 McNemar's test for paired data was used to compare the outcome achieved with unmodified DLI with that achieved with mHA-sensitized DLI in the same recipients.27
Results
Effect of unmodified DLI on hematopoietic chimerism
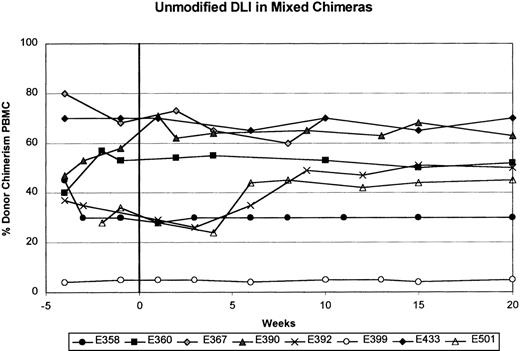
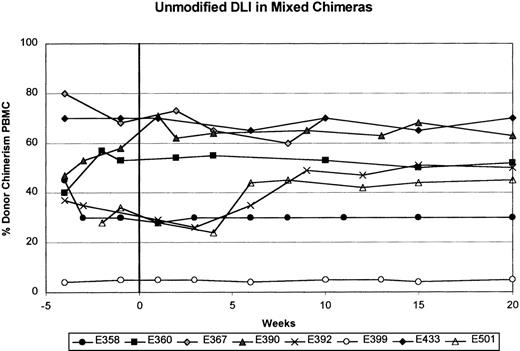
Table 1 shows results of a single unmodified DLI with 1.5 to 20.0 × 107CD3+ cells/kg (mean, 8.5 ± 2.9 × 107) given between 36 and 414 days (median, 99 days) after stem-cell transplantation in 8 mixed chimeric dogs. Four dogs (E358, E390, E392, and E433) received a second unmodified DLI with 10.0 to 26.0 × 107CD3+ cells/kg (mean, 18.6 ± 2.9 × 107) between 213 and 286 days (median, 215 days) after transplantation. Chimerism status was assessed for 10 to 30 weeks (median, 20 weeks) after each DLI. Figure1 and Table 1 show that all 8 recipients given unmodified DLI remained stable mixed chimeras, with donor engraftment ranging from 5% to 90% in both myeloid and lymphoid cells. There were no significant changes in the percentage of donor engraftment in the recipients after each unmodified DLI. In all cases, the percentage of donor engraftment remained greater in the granulocyte fraction than in the PBMC fraction.
Summary of results from the first unmodified donor lymphocyte infusion (DLI) in 8 stable mixed chimeric recipients.
The percentage of donor chimerism in the peripheral blood mononuclear cell (PBMC) fraction is shown from 4 weeks before to 20 weeks after unmodified DLI. Week 0 is the time of unmodified DLI.
Summary of results from the first unmodified donor lymphocyte infusion (DLI) in 8 stable mixed chimeric recipients.
The percentage of donor chimerism in the peripheral blood mononuclear cell (PBMC) fraction is shown from 4 weeks before to 20 weeks after unmodified DLI. Week 0 is the time of unmodified DLI.
Effect of mHA-sensitized DLI on hematopoietic chimerism
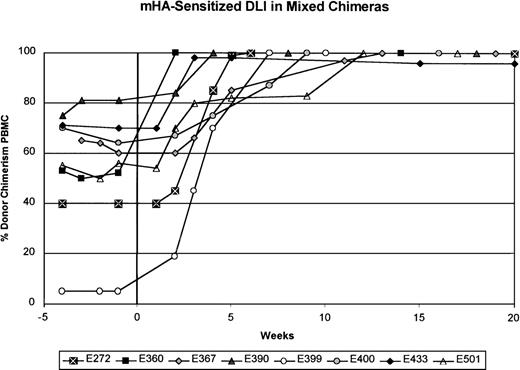
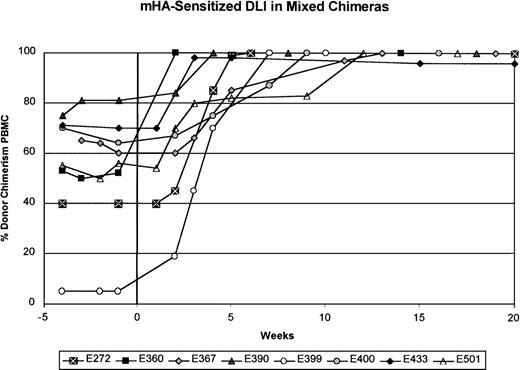
In contrast to the results obtained with unmodified DLI, mHA-sensitized DLI into 8 mixed chimeras produced conversion to at least 98% donor chimerism in all 8 recipients (Figure2 and Table 2). Except in dog E399, serial complete blood counts done before and after mHA-sensitized DLI showed no changes during the conversion to complete donor chimerism. Six of the 8 dogs had been given either a first or second unmodified DLI 10 to 30 weeks (median, 22 weeks) before mHA-sensitized DLI. The mHA-sensitized DLIs with 2.4 to 15.0 × 107 CD3+ cells/kg (mean, 7.8 ± 4.0 × 107) were given 201 to 651 days (median, 389 days) after stem-cell transplantation. Within 2 to 12 weeks after mHA-sensitized DLI, no host-specific bands were detected in granulocytes or PBMC, except in samples from dog E433, which showed 98% donor and 2% host chimerism in the PBMC fraction. In each dog studied, the granulocyte fraction converted to complete donor chimerism 1 to 3 weeks before conversion of the PBMC fraction. Complete donor chimerism was confirmed in all 8 recipients by assessment of DNA from marrow aspirates (data not shown). With exclusion of dog E433, complete chimerism was stable for the observation period of 10 to 48 weeks (median, 40 weeks) after mHA-sensitized DLI. Figure3A shows an example of conversion to complete donor chimerism (dog E390). Because a 2% residual host hematopoiesis was observed in dog E433 8 weeks after mHA-sensitized DLI, this animal was assessed for an additional 36 weeks. During this time, the percentage of donor hematopoiesis decreased to 85% in both the granulocyte and the PBMC fraction (Figure 3B). Reemergence of host hematopoiesis was not observed in the other dogs.
Summary of results from minor histocompatibility antigen (mHA)–sensitized DLI in 8 mixed chimeric recipients.
The donors were sensitized to recipient mHA after the rejection of recipient skin grafts placed into the donor dog. The percentage of donor chimerism in the PBMC fraction is shown over time. Week 0 is the time of mHA-sensitized DLI. The interval between the most recent unmodified DLI and mHA-sensitized DLI was at least 20 weeks, except for dog E367, for which it was 10 weeks.
Summary of results from minor histocompatibility antigen (mHA)–sensitized DLI in 8 mixed chimeric recipients.
The donors were sensitized to recipient mHA after the rejection of recipient skin grafts placed into the donor dog. The percentage of donor chimerism in the PBMC fraction is shown over time. Week 0 is the time of mHA-sensitized DLI. The interval between the most recent unmodified DLI and mHA-sensitized DLI was at least 20 weeks, except for dog E367, for which it was 10 weeks.
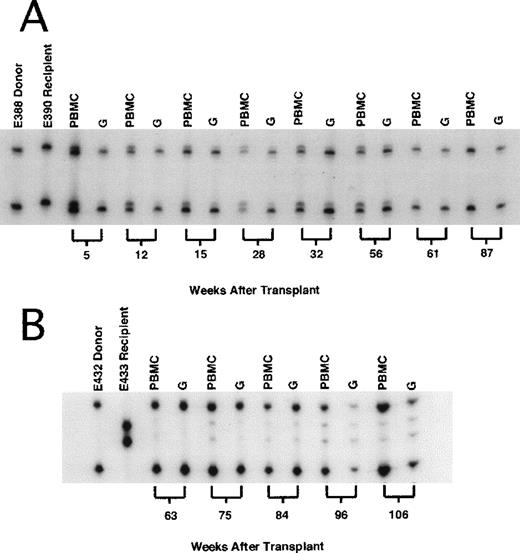
: Representative microsatellite marker studies in recipient dogs at selected times.
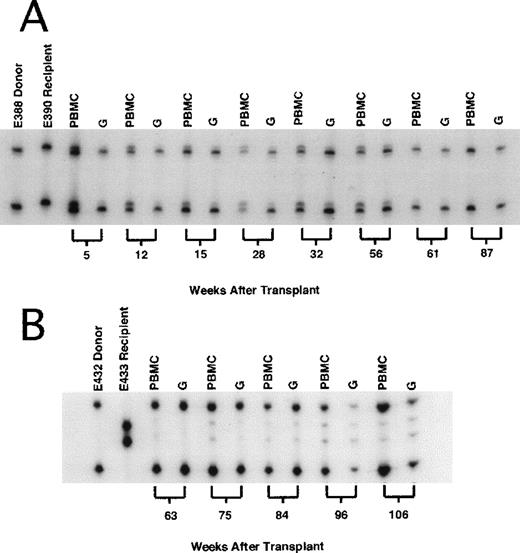
(A) Dog E390 received 2-Gy total body irradiation (TBI) and dog leukocyte antigen (DLA)–identical marrow from a litter mate and was treated after grafting with mycophenolate mofetil and cyclosporine for 4 and 5 weeks, respectively. The first unmodified DLI (8.0 × 107 CD3+ cells/kg of body weight) was given 14 weeks after transplantation and the second (10.8 × 107 CD3+ cells/kg) was given 30 weeks after transplantation, without producing a significant change in mixed-chimerism status. In contrast, mHA-sensitized DLI (7.1 × 107 CD3+ cells/kg) given 59 weeks after grafting resulted in rapid conversion to complete donor chimerism, which was sustained beyond 87 weeks after transplantation. (B) Dog E433 received the same transplant regimen, including DLA-identical litter-mate marrow, as dog E390 (above). Unmodified DLI was given twice without producing a change in mixed-chimerism status (data not shown here). Sixty weeks after allografting, mHA-sensitized DLI (8.5 × 107 CD3+/kg) was given, resulting in a rapid increase in donor chimerism to 98%. Host chimerism persisted, and by 105 weeks after allografting, had increased to 15% in both the PBMC and granulocyte fractions.
: Representative microsatellite marker studies in recipient dogs at selected times.
(A) Dog E390 received 2-Gy total body irradiation (TBI) and dog leukocyte antigen (DLA)–identical marrow from a litter mate and was treated after grafting with mycophenolate mofetil and cyclosporine for 4 and 5 weeks, respectively. The first unmodified DLI (8.0 × 107 CD3+ cells/kg of body weight) was given 14 weeks after transplantation and the second (10.8 × 107 CD3+ cells/kg) was given 30 weeks after transplantation, without producing a significant change in mixed-chimerism status. In contrast, mHA-sensitized DLI (7.1 × 107 CD3+ cells/kg) given 59 weeks after grafting resulted in rapid conversion to complete donor chimerism, which was sustained beyond 87 weeks after transplantation. (B) Dog E433 received the same transplant regimen, including DLA-identical litter-mate marrow, as dog E390 (above). Unmodified DLI was given twice without producing a change in mixed-chimerism status (data not shown here). Sixty weeks after allografting, mHA-sensitized DLI (8.5 × 107 CD3+/kg) was given, resulting in a rapid increase in donor chimerism to 98%. Host chimerism persisted, and by 105 weeks after allografting, had increased to 15% in both the PBMC and granulocyte fractions.
Comparison of unmodified DLI to mHA-sensitized DLI
With establishment of complete donor chimerism as a study end point, there was a significant difference between unmodified DLI and mHA-sensitized DLI (P = .007). Using Monte Carlo approximation, we compared the outcome of the first DLI in the 10 mixed chimeric recipients. Eight dogs received unmodified DLI, and all 8 remained mixed chimeras, whereas 2 dogs that received mHA-sensitized DLI had conversion to complete donor chimerism. This observation is strengthened by the finding that of the 8 dogs that remained mixed chimeras after unmodified DLI, 6 subsequently received mHA-sensitized DLI, and all 6 had conversion to complete donor chimerism. McNemar's test for paired data showed that the difference in outcome with unmodified DLI compared with mHA-sensitized DLI in these 6 dogs was significant (P = .03).
GVHD
Complications from mHA-sensitized DLI were observed in 3 of the 8 recipients. In dog E360, grade II GVHD of the skin and mucous membranes, confirmed by skin biopsy and histopathological examination, developed 20 days after mHA-sensitized DLI. The dog was treated with CSP (15 mg/kg per day) for 25 weeks to control GVHD. Twenty-eight weeks after mHA-sensitized DLI, necropsy and histopathological evaluation confirmed extensive chronic GVHD of the skin and liver. In contrast to the typical appearance of the thymus in dogs with active GVHD after myeloablative conditioning, the thymus in dog E360 was normocellular, with lymphoid hyperplasia in the germinal centers. In dog E390, grade II skin GVHD, confirmed by histopathological assessment, developed 4 weeks after mHA-sensitized DLI. The GVHD resolved spontaneously within 6 weeks of the onset of the rash. E390 was followed for an additional 36 weeks, during which there was no recurrence of GVHD. Histopathological evaluation done at necropsy found no GVHD.
Marrow aplasia: graft-versus-stroma effect
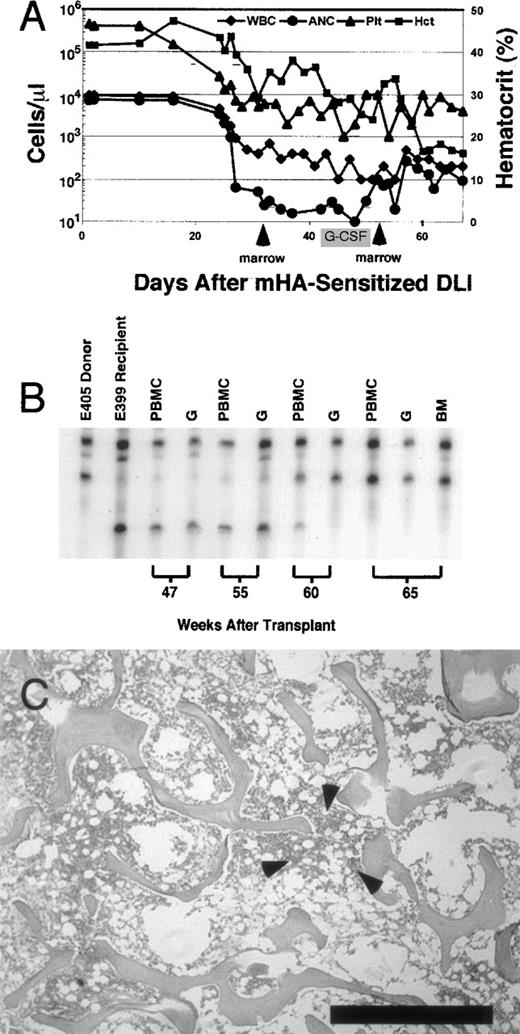
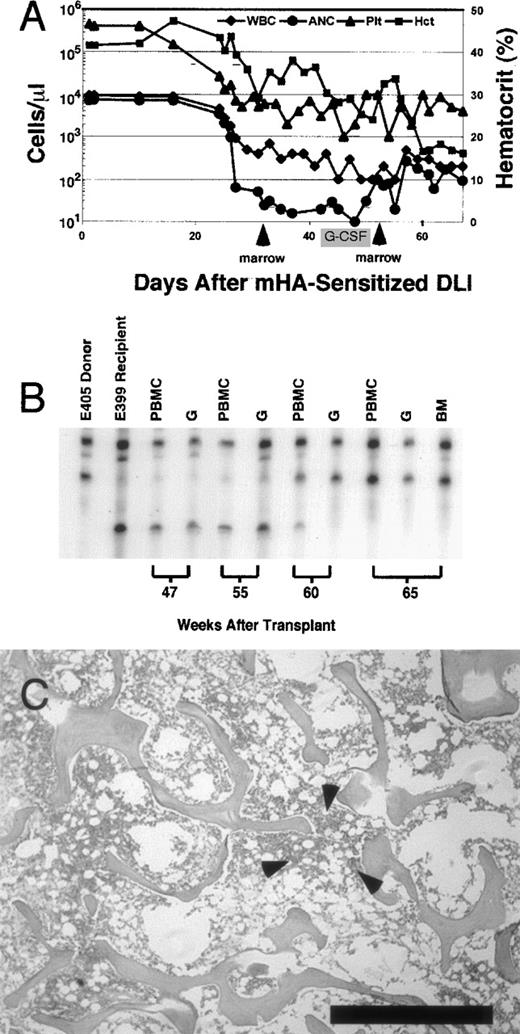
In 1 recipient, dog E399, complete marrow aplasia with pancytopenia developed 35 days after mHA-sensitized DLI. Figure4 shows a summary of peripheral blood counts over time, chimerism status, and results of bone marrow biopsies in this dog. Before mHA-sensitized DLI, peripheral blood cell counts were normal. After the onset of aplasia, despite the low numbers of circulating leukocytes, chimerism analyses consistently showed complete donor hematopoiesis. Donor marrow was infused 6 days after the onset of aplasia in an attempt to restore hematopoiesis. There was no normalization of blood counts, despite a subsequent course of G-CSF (10 μg/kg per day given subcutaneously). Nineteen days after the first salvage marrow infusion, a second stem-cell infusion with G-CSF–primed donor marrow also failed to rescue hematopoiesis. Throughout the period aplasia was present, the absolute neutrophil count did not exceed 260 cells/μL. The animal was given systemic antibiotics and a total of 12 transfusions of irradiated blood products.28 Because pneumonia developed, dog E399 was humanely killed 40 days after the onset of aplasia. Necropsy and complete histopathological examination showed no GVHD, and the marrow was extremely hypocellular, with myelofibrosis and rare foci of hematopoiesis. A marrow aspirate obtained before death generated very delayed growth of stroma in long-term marrow-culture conditions, consistent with profound stromal damage associated with a graft-versus-stroma effect.29
Summary of hematologic data from dog E399, which developed marrow aplasia after mHA-sensitized DLI.
(A) Peripheral blood counts recorded from the time of mHA-sensitized DLI. Total white blood cell count (μL), absolute neutrophil count (μL), and platelet count (μL) are plotted on the left y axis (log scale). Hematocrit is plotted on the right y axis. Arrows indicate infusion of donor marrow in an attempt to restore hematopoiesis. There was no marked recovery of absolute neutrophil count after marrow infusions. Recombinant granulocyte colony-stimulating factor was administered for the period shown. Dog E399 was humanely killed because of pneumonia 67 days after mHA-sensitized DLI. (B) Representative microsatellite marker studies at selected times. Dog E399 received 4.5-Gy lymph node irradiation, DLA-identical littermate marrow, and postgrafting therapy with mycophenolate mofetil and cyclosporine for 4 weeks and 5 weeks, respectively. Low-level donor chimerism (4%-5%) was unchanged after DLI given 25 weeks after grafting (data not shown). Skin-graft–sensitized mHA-sensitized DLI was given in week 56. After the onset of pancytopenia in week 59, complete donor chimerism was observed in PBMC, granulocyte, and bone marrow fractions. (C) Photomicrograph of biopsy specimen obtained at necropsy from anterior rib marrow of dog E399 showing hypocellular (5%) marrow. Also shown is a rare focus of primarily lymphoid hematopoiesis observed in 2 of 10 fields (arrows). The remaining marrow space contains predominantly red blood cells and few fibroblasts. Biopsy specimens (not shown) from the posterior iliac crest showed complete absence of hematopoiesis. Bar indicates 1 mm. (Stain, hematoxylin & eosin; magnification × 25.)
Summary of hematologic data from dog E399, which developed marrow aplasia after mHA-sensitized DLI.
(A) Peripheral blood counts recorded from the time of mHA-sensitized DLI. Total white blood cell count (μL), absolute neutrophil count (μL), and platelet count (μL) are plotted on the left y axis (log scale). Hematocrit is plotted on the right y axis. Arrows indicate infusion of donor marrow in an attempt to restore hematopoiesis. There was no marked recovery of absolute neutrophil count after marrow infusions. Recombinant granulocyte colony-stimulating factor was administered for the period shown. Dog E399 was humanely killed because of pneumonia 67 days after mHA-sensitized DLI. (B) Representative microsatellite marker studies at selected times. Dog E399 received 4.5-Gy lymph node irradiation, DLA-identical littermate marrow, and postgrafting therapy with mycophenolate mofetil and cyclosporine for 4 weeks and 5 weeks, respectively. Low-level donor chimerism (4%-5%) was unchanged after DLI given 25 weeks after grafting (data not shown). Skin-graft–sensitized mHA-sensitized DLI was given in week 56. After the onset of pancytopenia in week 59, complete donor chimerism was observed in PBMC, granulocyte, and bone marrow fractions. (C) Photomicrograph of biopsy specimen obtained at necropsy from anterior rib marrow of dog E399 showing hypocellular (5%) marrow. Also shown is a rare focus of primarily lymphoid hematopoiesis observed in 2 of 10 fields (arrows). The remaining marrow space contains predominantly red blood cells and few fibroblasts. Biopsy specimens (not shown) from the posterior iliac crest showed complete absence of hematopoiesis. Bar indicates 1 mm. (Stain, hematoxylin & eosin; magnification × 25.)
Attempts were made to characterize the tissue specificity of mHA-sensitized donor lymphocytes by using the chromium 51–release cytotoxicity assay,25 but these studies did not reveal marked specific lysis of marrow stroma or PBMC and are therefore not described here. Previously, cell-inhibition assays found specific cytotoxicity to recipient fibroblasts in 11 of 12 dogs sensitized with skin grafts from DLA-identical littermates.22
Immune reconstitution
As reported previously,1 measurements of immune status in stable mixed chimeras 2 to 4 months after transplantation showed no differences compared with normal control dogs in antibody production to sheep red blood cells, ratios of CD4 to CD8, and PBMC proliferative response to mitogens and MLR assays. In vitro assays of immune status 2 to 3 months after conversion to complete donor chimerism were completed. All 5 recipients without complications from mHA-sensitized DLI had PBMC proliferative responses to mitogens (20 803 ± 1094 to 76 886 ± 10 722 cpm) and MLR stimulation indexes (2.9 to 51.0) that were similar to concurrently obtained values in PBMC from normal control dogs. Ratios of CD4 to CD8 in peripheral blood from complete chimeras ranged from 2:1 to 5:1, similar to normal dogs. PBMC obtained from dog E360 (the animal with active GVHD) had decreased proliferative responses to mitogens and alloantigens compared with PBMC from other chimeric dogs (12 810 ± 1365 cpm and stimulation indexes of 0.9 to 2.3) and an increased ratio of CD4 to CD8 (7:1).
Discussion
Mixed chimerism established with sublethal irradiation of the recipient followed by DLA-identical marrow and postgrafting MMF and CSP involves all cell lineages and is stable for years after postgrafting immunosuppression is discontinued.1 The stability of mixed chimerism confirms the presence of mutual host-donor T-cell tolerance. Tolerance has also been demonstrated in mixed chimeras that had permanent acceptance of skin grafts from marrow donors but rejection of third-party grafts within 14 days (Storb R, unpublished data).
Possible mechanisms of tolerance in mixed chimeras involve either central tolerance, with thymic deletion of alloreactive T cells,30-32 or peripheral tolerance that relies on induction of peripheral clonal deletion,33 clonal anergy,34 or continuous suppression35 of the preexisting nontolerant T-cell repertoire. Studies of murine models of marrow transplantation that achieved substantial mixed chimerism and did not rely on recipient T-cell depletion showed that tolerance after myeloablative conditioning involved both peripheral and central deletion of donor reactive T cells.36 37
In the current study, we showed that once stable mixed chimerism was established, breaking tolerance and conversion to complete donor chimerism occurred only after mHA-sensitized DLI. We hypothesize that lymphocytes from immunized donors included a population of alloreactive T cells that were specific for mHA and mediated conversion to complete donor chimerism. After mHA-sensitized DLI, tolerance was reestablished at a new set point, with complete donor chimerism and without GVHD in the majority of dogs studied. In contrast, unmodified DLI did not break tolerance and failed to increase donor chimerism. These findings were observed consistently in all stable mixed chimeras studied, regardless of the initial nonmyeloablative radiation dose or stem-cell source. Although 6 of the dogs that had conversion to complete donor chimerism had received unmodified DLI previously, the time between infusions was sufficiently long to rule out the possibility of a delayed effect of unmodified DLI.
The current observations are consistent with the hypothesis that an active suppressor-cell mechanism maintains tolerance in mixed chimeras. Donor-specific suppressor T cells were previously described in humans,38 DLA-haploidentical radiation chimeras,39,40 and MHC-mismatched chimeric rats.41 We hypothesize thatsuppressor cells in the mixed chimeras prevented primary allorecognition and subsequent sensitization of T cells from the unmodified DLI. However, T cells from mHA-sensitized DLI bypassed or overwhelmed the suppressor-cell mechanism, probably because alloantigen recognition and sensitization had already occurred in the marrow donors that were given skin grafts. Sensitization to skin grafts likely expanded alloreactive T cells specific for recipient mHA and may have induced costimulatory molecules that eliminated the need for primary antigen presentation after infusion into mixed chimeras. Although additional studies are needed to determine how tolerance is attained in this model, it is likely to involve several pathways. Examples of components critical for tolerance induction are molecules such as CTLA4,42 T cells that secrete cytokines such as interleukin 10 and suppress antigen-specific responses,43 and lymphoid dendritic cells (DC2) that block naı̈ve T-cell allorecognition.44
In other clinical and experimental settings, unmodified DLI has been found to have the potential to eliminate residual host hematopoiesis after myeloablative allografting. Reports from several transplant groups indicated that 50% to 80% of patients with relapsed CML and persistence of donor lymphoid chimerism after conventional allografting had complete cytogenetic remission after unmodified DLI.7-9Kolb et al45 found that dogs given a myeloablative regimen of 10-Gy TBI and T-cell–depleted DLA-identical marrow had a safe conversion from mixed to complete donor chimerism after unmodified DLI was given 60 days after stem-cell transplantation. Pelot et al46 studied mixed chimeric mice established with a nonmyeloablative regimen of 3-Gy TBI, 7-Gy thymic irradiation, and recipient T-cell depletion followed by transplantation with T-cell–depleted MHC-mismatched marrow. They found that DLI given 5 weeks after transplantation generated a substantial increase in donor chimerism without GVHD. Slavin et al47 reported on the outcome of 25 HLA-identical allogeneic PBSC transplantations done after a moderately extensive regimen of fludarabine, busulfan, and antithymocyte globulin. Four patients with disease relapse were treated with unmodified DLI with or without chemotherapy, and 2 had induction of remission. The potent GVH effect of unmodified DLI in these studies appears to contrast with the results of our current study. The difference was likely due to the nonmyeloablative regimen we used to establish our DLA-identical mixed chimeras. This regimen did not eliminate host T cells and resulted in a complete repertoire of immunocompetent but tolerant donor and host T cells.
Although the nonmyeloablative regimens we used to establish mixed hematopoietic chimerism maintained a complete T-cell repertoire, this may have produced an increased efficacy or number of suppressor cells compared with results of a myeloablative regimen. Accordingly, van Bekkum and Kinwel-Bohre48 suggested that the efficacy of unmodified DLI in patients with relapse after conventional allografting was due to a decrease in donor suppressor cells. Patients with CML who had relapse after allogeneic transplantation of unmanipulated grafts were complete donor T-cell chimeras but appeared to lack the repertoire of allospecific T cells necessary to recognize and eliminate malignant host hematopoiesis.49 After a myeloablative transplant regimen and T-cell–depleted grafting, mixed hematopoietic chimeras had incomplete T-cell reconstitution.50 With an incomplete alloreactive T-cell repertoire, the corresponding specific suppressor cells might have been absent or present in low amounts, permitting alloreactive T cells in unmodified DLIs to mediate a GVH effect.
In an earlier study,22 mHA-sensitized DLI broke tolerance and caused GVHD in 66% of long-term complete radiation chimeras, whereas unmodified DLI did not break tolerance and failed to generate GVHD. The current study revealed a low rate (25%) of GVHD after mHA-sensitized DLI, although an alloreactive response was observed in all dogs. This suggests that in stable mixed chimeras, hematopoietic cells, compared with other tissues, were the preferred targets of the GVH effect of alloreactive T cells, even though mHA not specific to hematopoietic cells were presented to donor cells in the sensitization process. Despite the GVH activity of the mHA-sensitized DLI, tolerance was reestablished after conversion to complete donor chimerism in most dogs, a finding consistent with reemergence of a suppressor-cell population. Alternatively, “split tolerance” against nonhematopoietic host tissue was maintained in the animals in which GVHD did not develop.
The episode of marrow aplasia after mHA-sensitized DLI occurred in recipient dog E399, which had 5% donor engraftment. Marrow aplasia has been reported in up to 30% of patients after DLI, with most, but not all, patients subsequently having recovery of blood counts.7,10 Keil et al51 suggested that patients with less than 5% donor CD34+ marrow hematopoiesis despite complete donor T-cell chimerism were at particularly high risk of prolonged pancytopenia after DLI. The marrow aplasia observed in this study was most likely due to a graft-versus-stroma effect in which the mHA-sensitized DLI destroyed stromal cells because of mHA differences. Supporting evidence includes the abnormal and delayed in vitro growth of host stroma after the onset of aplasia and the 2 attempts to rescue hematopoiesis with donor stem-cell infusions that were unsuccessful even though the T cells were entirely of donor origin. This observation might represent an approach to developing a large-animal model that could be used to identify the mHA responsible for graft-versus-stroma effects.
Our clinical aim is to decrease the toxicity of allogeneic transplantation by establishing mixed chimerism with use of nonmyeloablative TBI combined with the synergistic immunosuppression provided by CSP and MMF. Preliminary studies in humans showed that mixed chimerism could be successfully established between HLA-identical siblings with the same conditioning regimen used in the dog model.2 Furthermore, the toxicity of allogeneic transplantation was decreased substantially and inpatient hospital admission was not required. The nonmyeloablative approach to hematopoietic stem-cell transplantation represents a fundamental shift from myeloablative chemoradiotherapy preparative regimens previously used for allogeneic transplantation. This new approach relies on the allogeneic effect to achieve engraftment and disease eradication.
The findings from the current study may be relevant for clinical studies in which conversion to complete donor chimerism is necessary for treatment of malignant disease. It is not known whether unmodified DLI will also fail to convert stable mixed to complete donor chimerism in patients treated with this nonmyeloablative regimen. If unmodified DLI is not clinically effective, strategies to increase the GVH effect would be needed to produce conversion to complete donor chimerism in patients. In this study, the mHA-sensitized lymphocytes were generated after skin grafts from the mixed chimeric recipient were placed into the donor. Although this approach is not directly feasible clinically, ex vivo generation of mHA-specific T cells has been successful and clinical studies of this technique are ongoing.52,53 Future studies will attempt to generate and expand mHA-specific T cells ex vivo and test their in vivo activity by using this dog model. To improve the safety of mHA-specific immunotherapy, a “suicide-gene” strategy could be used; this would involve donor T-cell retroviral transduction with the herpes simplex virus thymidine kinase gene to confer specific sensitivity to ganciclovir.21
In summary, mixed chimerism provides a platform for adoptive immunotherapy in which mHA-sensitized DLI can exert a potent GVH effect. In this setting, unmodified DLI did not change chimerism status, whereas all 8 canine recipients of mHA-sensitized DLI promptly had converted to at least 98% donor chimerism. In recipients without GVHD or marrow aplasia, immune reconstitution was rapid. Conversion from mixed to complete donor chimerism is a powerful indicator of in vivo GVH activity and establishes a large-animal model for GVL activity.
Acknowledgments
We are grateful to the technicians of the shared Canine Resource and Hematology and Transplantation Biology Laboratories. Barbara Johnston, DVM, provided veterinary support. We are indebted to Dr George Sale for thorough review of pathology specimens and to Gretchen Johnson for technical assistance. We are grateful to Bonnie Larson, Helen Crawford, Lori Ausburn, and Sue Carbonneau for outstanding secretarial support; to Sabine Hadulco, Roche Bioscience, Nutley, NJ, for the gift of MMF; to Dr Elizabeth C. Squires, Sangstadt Medical Corp, Menlo Park, CA, for the gift of CSP; and to Amgen for the gift of recombinant canine G-CSF.
Sponsored in part by grants DK42716, CA15704, and CA78902 from the National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, MD, and a grant from Amgen Inc, Thousand Oaks, CA. G.E.G. received support from NIH grant DK09718. R.S. also received support from the Laura Landro Salomon Endowment Fund and a prize awarded by the Joseph Steiner Krebsstiftung, Bern, Switzerland.
Corresponding author:George E. Georges, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: ggeorges@fhcrc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.