Abstract
We have analyzed the properties of anti-factor VIII (FVIII) immunoglobulin (Ig) G recovered by affinity chromatography on FVIII-Sepharose from the IgG fraction of the plasma of healthy individuals and nonresponder patients with hemophilia A. Affinity-purified anti-FVIII antibodies were found to neutralize FVIII activity and to bind to FVIII with an affinity similar to that of anti-FVIII IgG that had been affinity-purified from the plasma of inhibitor-positive hemophilia patients and of patients with anti-FVIII autoimmune disease. The antibodies also exhibited patterns of reactivity with thrombin-digested FVIII similar to those of FVIII inhibitors and preferentially recognized epitopes located in the light chain of FVIII. These observations suggest that FVIII inhibitors occurring in hemophilia A and in patients with anti-FVIII autoimmune disease originate from the expansion of preexisting natural anti-FVIII clones that exhibit FVIII-neutralizing properties.
Factor VIII (FVIII) inhibitors are immunoglobulin (Ig) G antibodies that neutralize FVIII procoagulant activity in plasma. The inhibitors arise as alloantibodies in approximately 25% of patients with severe hemophilia A and 5% to 15% of patients with hemophilia of mild or moderate severity who are treated with plasma-derived FVIII concentrates or recombinant FVIII.1 In patients with severe hemophilia, major deletions, a common inversion, nonsense mutations in the FVIII gene, and early initiation of substitutive therapy have been shown to increase the risk of inhibitor development.2-4 In rare instances, FVIII inhibitors arise as autoantibodies in patients with no hemostatic defect, often in the context of autoimmune disease or during the postpartum period.5 The presence of natural autoantibodies to FVIII that exhibit FVIII-neutralizing properties has also been reported in healthy individuals.6
The inhibitory activity to FVIII of normal IgG is best detected following purification of anti-FVIII IgG on an affinity column of Sepharose-conjugated FVIII, because the IgG fraction of normal human plasma contains both anti-FVIII antibodies and anti-idiotypic antibodies directed against the anti-FVIII antibodies.7 In addition to antibodies that neutralize FVIII, the IgG of hemophilia patients with inhibitors, patients with autoantibodies to FVIII, and healthy individuals also contain antibodies that bind to FVIII but do not exhibit inhibitory activity.8
Immunoblotting assays with inhibitor plasmas have shown that most anti-FVIII antibodies with FVIII-neutralizing activity bind to epitopes located on the 44-kd heavy chain–derived or the 72-kd light chain–derived fragment of FVIII.9 Immunoprecipitation assays have demonstrated that inhibitors selectively bind the light chain of FVIII, with predominant binding to the C2 domain.10 In the present study, we demonstrate that anti-FVIII antibodies that share functional properties of FVIII inhibitors are present in low amounts in the IgG fraction of the plasma of nonresponder hemophiliacs and in normal human IgG. These observations suggest that FVIII inhibitors in patients with hemophilia A represent an expansion of preexisting natural anti-FVIII B cell clones rather than a de novo immune response to a foreign antigen.
Patients and methods
Patient samples
Plasma was obtained from 4 high-responder patients with severe hemophilia A (ie, inhibitory titer in plasma above 10 Bethesda units/mL), 3 patients with severe hemophilia A but with no detectable inhibitors (ie, nonresponder patients), 2 patients with spontaneous inhibitors to FVIII (autoantibodies to FVIII), and 3 healthy blood donors. Blood was collected in 0.13-mol/L sodium citrate (1:9 vol/vol) and immediately centrifuged at 2500g for 15 minutes at room temperature. Plasma was aliquoted and stored at −20°C. IgG was isolated from all plasma samples by ammonium sulfate precipitation followed by affinity-chromatography on protein G-Sepharose (Pharmacia, Uppsala, Sweden). Pooled normal human IgG (intravenous immunoglobulin, IVIg; Sandoglobulin) was used as a source of normal IgG. F(ab′)2 fragments were prepared from IVIg by pepsin digestion using 2% pepsin (wt/wt) (Sigma, St. Louis, MO) in 0.2 mol/L sodium acetate (pH 4.1) for 18 hours at 37°C. Chromatography on protein G-Sepharose (Pharmacia) was used to separate F(ab′)2 fragments from remaining intact IgG and Fc fragments as assessed by enzyme-linked immunosorbent assay (ELISA) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The concentrations of IgG and F(ab′)2 fragments were determined spectrophotometrically at 280 nM.
Purification of anti-FVIII antibodies by affinity chromatography
For affinity purification of anti-FVIII antibodies, IgG diluted in phosphate-buffered saline (PBS), pH 7.4, containing 0.1% NaN3 was loaded onto an affinity column of 10 mL of CNBr-activated Sepharose (Pharmacia) to which 25 000 IU of immunopurified plasma-derived FVIII (LFB, Les Ulis, France) had been coupled, and the IgG recirculated through the column overnight at 4°C. The column was washed with PBS, and effluents were collected. The IgG was then eluted with 0.2 mol/L glycine HCl, pH 2.8. The eluate was dialyzed against PBS and concentrated using Centriprep (Amicon, Beverly, MA).
ELISA and radioimmunoassays for anti-FVIII antibodies
ELISA plates (Nunc, Roskilde, Denmark) or flat-bottom radioimmunoassay (RIA) plates (Falcon 3912; Becton Dickinson, Oxnard, CA) were coated with highly purified FVIII (Hemophil M; Baxter Hyland, Glendale, CA) at 10 IU/mL in PBS, pH 7.4, overnight at 4°C. Plates were saturated with 1% fish gelatin in PBS for 60 minutes at 37°C. In the case of ELISA, after washing with PBS, the plates were incubated with increasing concentrations of IgG for 2 hours at room temperature before extensive washing with PBS and addition of goat antihuman IgG antibodies coupled to alkaline phosphatase (Sigma). Optical densities recorded in the presence of secondary antibody alone were used as background for calculating specific binding. In the case of RIA, [125I]-labeled anti-FVIII IgG was preincubated at a concentration of 3 μg/mL with increasing concentrations of competing unlabeled IgG for 4 hours at 37°C. Samples were then transferred to the FVIII-coated plates and incubated for an additional hour at 37°C. Plates were washed, and bound radioactivity was assessed using a gamma counter.
Quantitation of FVIII inhibitory activity
FVIII-neutralizing activity was measured in plasma and in purified IgG preparations using the method of Kasper et al11 and expressed in Bethesda units (BU) per milligram of IgG. Plasma was heated 1 hour at 56°C prior to testing. Heated plasma and affinity-purified anti-FVIII IgG were incubated with an equal volume of pooled citrated human plasma (Dade-Behring, Marburg, Germany) for 2 hours at 37°C. FVIII activity was measured in a 1-stage clotting assay by a manual activated partial thromboplastin reagent (APTT) method after mixing serial dilutions of the test samples with clotting FVIII-deficient plasma and Pathromtin (Dade-Behring) as activators, as described.11
Immunoblotting
Recombinant human FVIII (Baxter Hyland) was dialyzed against 50 mM Tris, 0.15 mol/L NaCl, and 2 mM CaCl2, pH 7.4, before digestion with human thrombin (Sigma) (0.10 U/70 μg FVIII) for 1 hour at room temperature. The reaction was stopped by the addition of 10−5 mol/L D-phenylalanyl-L-prolyl-L-arginine chloromethylketone in 2% glycerol, 2% SDS, 62.5 mM Tris (pH 6.8), 5% β-mercaptoethanol, and 0.02% bromophenol blue. Activation of FVIII with thrombin generated a 44-kd protein band, which was not detected when nonactivated FVIII was used in the immunoblotting experiments. Digested FVIII was then subjected to electrophoresis on 10% SDS-PAGE prior to transfer onto nitrocellulose for 60 minutes at 0.8 mA/cm2 using a semidry electroblotter. The membranes were blocked with PBS containing 0.2% Tween 20. Antibodies to be tested (50 μg/mL in PBS-0.2% Tween) were then incubated with the membranes following the addition of 1 sample per slot in a Cassette Miniblot System (Immunetics, Cambridge, MA) overnight at 4°C as described.12 The membranes were washed and incubated with goat antihuman Fcγ antibody coupled to alkaline phosphatase (Sigma) followed by incubation with nitroblue tetrazolium/bromo-chloro-indolyl-phosphate substrate (Promega, Madison, WI). Colorimetric quantitation was performed by densitometry in a reflective mode using a high-resolution CCD camera system (Masterscan, Scanalytics, Billerica, MA). Blotted proteins were then stained using colloidal gold (Protogold, Biocell, Cardiff, Wales) and subjected to a second densitometric analysis to score the protein profile and to quantitate transferred proteins. Data were analyzed using a Macintosh computer and the IGOR software (Wavemetrics, Lake Oswego, OR). Densitometric profiles of immunoreactivity were compared with the corresponding protein profiles, following correction of the migration defects by superimposition of protein peaks.
Immunoprecipitation assay
Immunoprecipitation assays were performed using purified A1 and A2 domains, recombinant C2 domain, purified light chain, and FVIII.13 FVIII and its polypeptide components were radiolabeled with Na-125I. Serial dilutions of samples of affinity-purified anti-FVIII IgG were mixed with the radiolabeled domains or FVIII for 18 hours prior to binding the immune complexes on protein G-Sepharose for 4 hours. After washing to remove unbound [125I]-proteins, the bound [125I]-protein was measured in a gamma counter. Immunoprecipitation units were defined as bound/total [125I]-protein per milligram of IgG. Four negative controls in the absence of IgG were averaged and subtracted from the bound/total values.
Biosensor measurement of antibody binding to FVIII
Real-time analysis of antigen-antibody complex formation between anti-FVIII IgG and the C2 and A2 domains, the light chain, and plasma-derived FVIII was performed using a BIAcore 2000 instrument (Biacore, Uppsala, Sweden). Affinity-purified anti-FVIII IgG was dialyzed against 0.1 mol/L NaHCO3, pH 8.3, and allowed to react with biotin for 3 hours at 4°C. Biotinylated IgG was immobilized on an SA streptavidin-coated sensor chip in 10 mM HEPES, 150 mM NaCl, 0.005% Tween 20, 5 mM CaCl2, pH 7.4, (HBS-CaCl2) according to the procedure described by the manufacturer. All procedures were performed at 25°C. Thirty microliters of HBS-CaCl2 containing 6.25-, 12.5-, 25-, 50-, or 100-nM plasma-derived FVIII or FVIII fragments to be tested were injected at a continuous flow rate of 5 μL/min, allowing a contact time with the antibodies of 7 minutes. Dissociation was monitored over a period of 4 minutes following the injection of HBS-CaCl2 alone. After each analysis, regeneration of the chip surface was achieved by 2 injections of 10 μL of 100-mM HCl. Background association and dissociation curves were scored under similar experimental conditions by injecting FVIII and FVIII fragments onto an uncoated sensor chip. Background curves were subtracted from test curves prior to calculation of association (ka) and dissociation (kd) constants using the BIAevaluation 3.0 Software (Pharmacia). The values of equilibrium dissociation constants (Kd) were calculated as kd/ka. We verified that soluble anti-FVIII IgG antibodies inhibit the binding of C2 to immobilized anti-FVIII IgG in a dose-dependent manner, thus validating the use of surface plasmon resonance (SPR) for the study of antigen-antibody complex formation in the case of polyclonal preparations of affinity-purified anti-FVIII IgG and domains of FVIII.
Results
Immunoaffinity purification of anti-FVIII antibodies
IgG antibodies were isolated by affinity chromatography on an FVIII column from purified IgG from the plasma of 4 high-responder patients (ie, inhibitory titer in plasma above 10 BU/mL) with severe hemophilia A, 3 hemophiliacs without detectable inhibitor (ie, nonresponders), 2 patients with autoantibodies to FVIII, and 3 healthy blood donors and from IVIg. FVIII-reactive antibodies were recovered from the chromatography column in all cases. A mean of 1.6% (range, 0.40%-2.8%) and 3.1% (range, 2.4%-3.7%) of the total amount of loaded IgG was retained on the FVIII column in the case of the hemophilia patients with inhibitor and of patients with autoantibodies to FVIII, respectively (Table 1). The amount of IgG retained was 0.6% (0.4%-0.8%) for IgG of nonresponder hemophilia patients, 0.02% (0.01%-0.03%) for IgG of healthy donors, and 0.26% in that of IVIg. These results on the recovery of anti-FVIII IgG are in agreement with previous observations (ie, 0.06%-1.75%).7 8
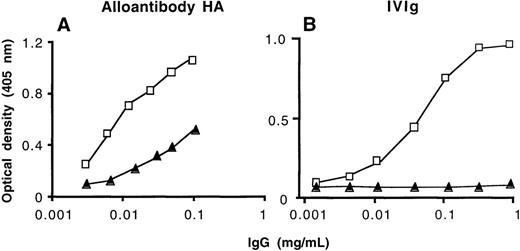
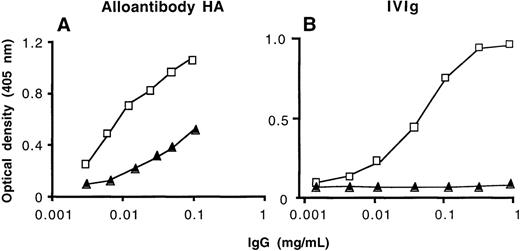
We assessed the ability of affinity-purified anti-FVIII antibodies to bind to FVIII and to neutralize FVIII procoagulant activity. These antibodies bound to insolubilized FVIII as assessed by ELISA (Figure1). There was no binding to FVIII of IgG in the effluents of the affinity columns (data not shown). Soluble human FVIII inhibited the binding of [125I]-labeled natural anti-FVIII IgG to immobilized FVIII in a dose-dependent manner, as assessed by RIA (data not shown). The neutralizing activity of anti-FVIII antibodies toward FVIII was increased 1.9- to 57-fold in immunopurified anti-FVIII IgG as compared with unfractionated IgG of the 4 severe hemophilia A patients with inhibitors (Table2). Inhibition of FVIII procoagulant activity could not be determined in the case of anti-FVIII IgG from nonresponder hemophiliacs unless IgG of the 3 donors was pooled (Table2). There was no relationship between the relative increase in anti-FVIII–neutralizing activity achieved by affinity purification and the relative amount of anti-FVIII IgG antibodies that was retained on the FVIII affinity column. No inhibitory activity to FVIII was detected in anti-FVIII IgG that had been affinity-purified from the plasma of healthy donors under the experimental conditions used. However, a neutralizing activity of 15 BU/mg of IgG (15 ± 1.4 BU/mg of IgG in 3 separate experiments) was recovered after immunoaffinity purification of IVIg, despite the lack of detectable inhibitory activity to FVIII in unfractionated IVIg. Taken together, these data indicate that IgG of nonresponder hemophiliacs and normal polyclonal human IgG contain antibodies with FVIII binding capability in amounts that are approximately 10 to 100 times lower than in the IgG fraction of high-responder severe hemophilia A patients and patients with spontaneous inhibitor to FVIII. Our results further demonstrate that anti-FVIII IgG with neutralizing properties to FVIII is present in the IgG fraction of plasma of nonresponder hemophilia A patients and in normal polyclonal human IgG.
Binding of affinity-purified anti-FVIII IgG to FVIII by ELISA.
Unfractionated IgG (closed triangles) and the corresponding affinity-purified anti-FVIII IgG (open squares) were tested at similar concentrations for their ability to bind to insolubilized FVIII. (A) High-responder patient with hemophilia A (HA); (B) intravenous immunoglobulin (IVIg).
Binding of affinity-purified anti-FVIII IgG to FVIII by ELISA.
Unfractionated IgG (closed triangles) and the corresponding affinity-purified anti-FVIII IgG (open squares) were tested at similar concentrations for their ability to bind to insolubilized FVIII. (A) High-responder patient with hemophilia A (HA); (B) intravenous immunoglobulin (IVIg).
Immunoblotting of affinity-purified anti-FVIII antibodies
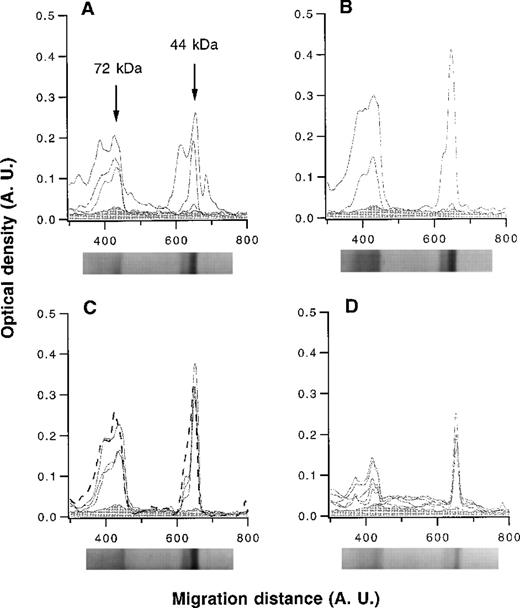
The patterns of reactivity of affinity-purified anti-FVIII IgG with thrombin-cleaved FVIII were analyzed by semiquantitative immunoblotting. Anti-FVIII IgG of hemophilia patients with inhibitor recognized epitopes in either or both of the 72-kd and 44-kd fragments of FVIII (Figure 2). Anti-FVIII antibodies of patients with spontaneous inhibitor, nonresponder hemophilia patients, healthy blood donors, and IVIg bound to both the 72-kd and 44-kd fragments with similar intensities of binding (Figure 2). Thus, immunoblotting did not allow us to distinguish between disease-associated and natural anti-FVIII IgG antibodies.
Densitometric profiles of the reactivity of anti-FVIII IgG.
Anti-FVIII IgG was affinity-purified from the plasma of 4 hemophilia patients with inhibitor (A), 2 patients with autoantibodies to FVIII (B), 3 healthy blood donors and pooled normal human IgG (IVIg) (C), and 5 nonresponder hemophilia patients (D). IgG was tested at a concentration of 50 μg/mL. The densitometric pattern of reactivities of IgG of each individual is depicted as a full-line curve; that of anti-FVIII IgG from IVIg is depicted as a broken-line curve in panel C. Gray areas depict the densitometric pattern observed in the presence of secondary antibody alone. Migration distances and optical densities are expressed as arbitrary units (AU). Arrows in panel A indicate the FVIII digestion fragments of 44 and 72 kd. For each group of individuals, a representative immunoblot is shown below the X axis.
Densitometric profiles of the reactivity of anti-FVIII IgG.
Anti-FVIII IgG was affinity-purified from the plasma of 4 hemophilia patients with inhibitor (A), 2 patients with autoantibodies to FVIII (B), 3 healthy blood donors and pooled normal human IgG (IVIg) (C), and 5 nonresponder hemophilia patients (D). IgG was tested at a concentration of 50 μg/mL. The densitometric pattern of reactivities of IgG of each individual is depicted as a full-line curve; that of anti-FVIII IgG from IVIg is depicted as a broken-line curve in panel C. Gray areas depict the densitometric pattern observed in the presence of secondary antibody alone. Migration distances and optical densities are expressed as arbitrary units (AU). Arrows in panel A indicate the FVIII digestion fragments of 44 and 72 kd. For each group of individuals, a representative immunoblot is shown below the X axis.
Immunoprecipitation
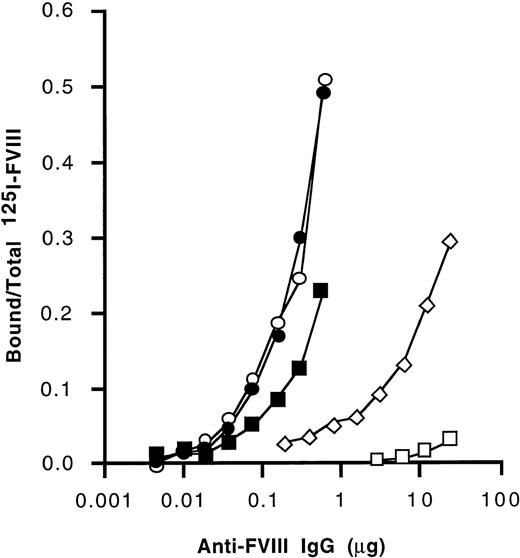
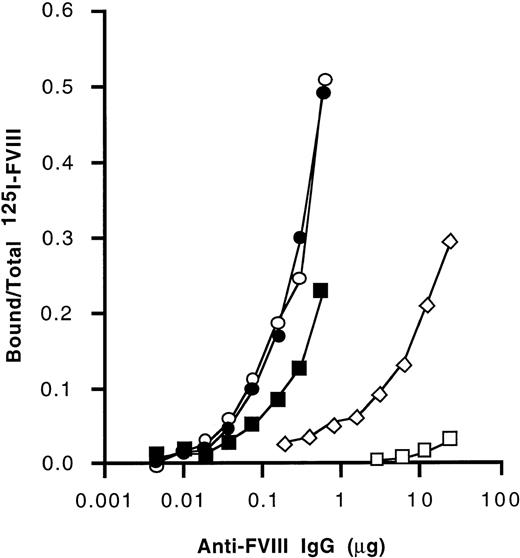
We further investigated the domain specificity of anti-FVIII antibodies by immunoprecipitation using radiolabeled FVIII, FVIII light chain, and the A1, A2, and C2 domains of FVIII. As shown in Figure3, anti-FVIII IgG that had been affinity-purified from IVIg exhibited a higher titer of immunoprecipitating antibodies to the light chain of FVIII and the C2 domain than to intact FVIII and to the A2 domain of FVIII. Binding to the A1 domain was barely detectable. Affinity-purified IgG from the 2 nonresponder hemophilia patients and from a healthy individual also immunoprecipitated the light chain of FVIII at higher titers than intact FVIII (Table 3). A similar pattern of immunoprecipitation was observed with the autoantibody. For anti-FVIII IgG from a high-responder hemophilia patient, the immunoprecipitation titer was lower with intact FVIII than with the A2 domain. The anti-FVIII and anti–light chain titers were similar. A2 domain was immunoprecipitated at a higher titer than the C2 domain. Because the anti-C2 titers were lower than those of light chain, there is a probably significant antibody binding to the A3 or C1 domains as well. A3 and C1 binding can be suggested for all IgG tested except IVIg. Thus, immunoprecipitation patterns did not allow us to discriminate between the various sources of affinity-purified anti-FVIII antibodies that we tested.
Immunoprecipitation of FVIII and FVIII domains by anti-FVIII IgG.
Anti-FVIII antibodies affinity-purified from IVIg were coincubated with radiolabeled purified A1 and A2 domains, recombinant C2 domain, purified FVIII light chain, and FVIII. Following immunoprecipitation, ratios of bound radioactivity to total radioactivity were calculated. Open squares: A1 domain; open diamonds: A2 domain; open circles: C2 domain; closed circles: FVIII light chain; closed squares: plasma-derived human FVIII.
Immunoprecipitation of FVIII and FVIII domains by anti-FVIII IgG.
Anti-FVIII antibodies affinity-purified from IVIg were coincubated with radiolabeled purified A1 and A2 domains, recombinant C2 domain, purified FVIII light chain, and FVIII. Following immunoprecipitation, ratios of bound radioactivity to total radioactivity were calculated. Open squares: A1 domain; open diamonds: A2 domain; open circles: C2 domain; closed circles: FVIII light chain; closed squares: plasma-derived human FVIII.
Kinetics of binding of immunopurified anti-FVIII IgG to FVIII
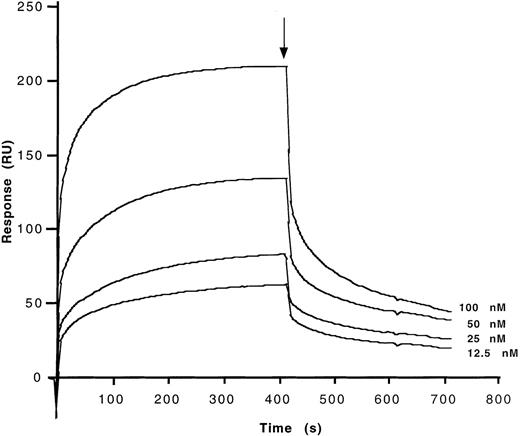
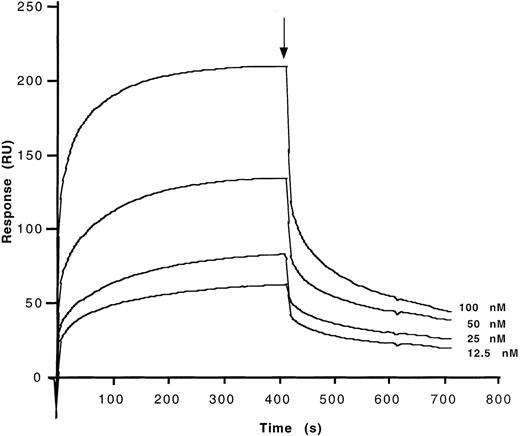
Binding characteristics of anti-FVIII antibodies to the different domains of FVIII were studied by means of surface-plasmon resonance (BIAcore). The C2 and A2 domains, the light chain of FVIII, and plasma-derived FVIII were allowed to interact with affinity-purified anti-FVIII IgG antibodies that had been covalently coupled to a sensor chip. Second-order association rate constants (ka) and first-order dissociation rate constants (kd) were calculated from the association and dissociation curves after correction of the curves for background binding of FVIII and FVIII fragments to an uncoated chip (Table 4). Calculated dissociation constants (Kd) for the interaction of affinity-purified anti-FVIII IgG of a high-responder hemophiliac, of a patient with autoantibody to FVIII, of a nonresponder hemophiliac, of a healthy donor, and of IVIg with the C2 and A2 domains of FVIII, FVIII light chain, and plasma-derived FVIII were in the same orders of magnitude. Binding of the A2 domain of FVIII to anti-FVIII IgG from all sources was 10- to 100-fold lower than that of the other fragments (Table 4). Representative binding curves are shown in Figure4, in the case of C2 binding to anti-FVIII IgG from IVIg. The use of F(ab′)2fragments in this experiment indicates that binding of FVIII and its domains to the antibodies is dependent on the variable regions of the antibodies.
Determination of kinetic parameters of the binding of affinity-purified anti-FVIII IgG from IVIg to the C2 fragment of FVIII.
F(ab′)2 fragments of anti-FVIII IgG were covalently coupled to the sensor chip. Real-time binding was studied for C2 in HBS-CaCl2 at the concentrations indicated. At the time indicated by the arrow, the ligand solution was replaced by HBS-CaCl2 alone, and dissociation curves were recorded. Ordinate represents the binding response as measured by resonance units (RU).
Determination of kinetic parameters of the binding of affinity-purified anti-FVIII IgG from IVIg to the C2 fragment of FVIII.
F(ab′)2 fragments of anti-FVIII IgG were covalently coupled to the sensor chip. Real-time binding was studied for C2 in HBS-CaCl2 at the concentrations indicated. At the time indicated by the arrow, the ligand solution was replaced by HBS-CaCl2 alone, and dissociation curves were recorded. Ordinate represents the binding response as measured by resonance units (RU).
Competitive binding of affinity-purified anti-FVIII antibodies to FVIII
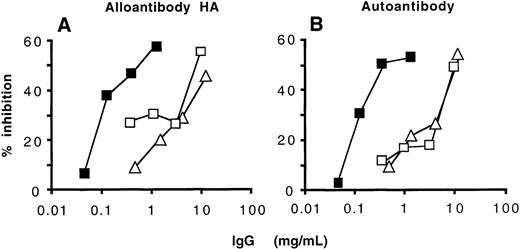
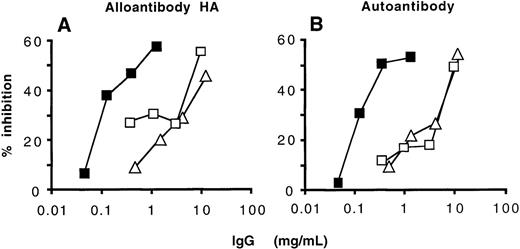
Because natural and disease-associated anti-FVIII antibodies (ie, that of healthy donors and nonresponder hemophiliacs and that of autoimmune patients and high-responder hemophiliacs, respectively) could not be distinguished based on their specificity toward domains of FVIII, the fine epitopic specificity of anti-FVIII antibodies that had been affinity-purified from IVIg was compared in competitive binding experiments with that of radiolabeled affinity-purified anti-FVIII IgG of 1 high-responder patient with hemophilia A and 1 patient with autoantibodies to FVIII. Anti-FVIII IgG of the 2 patients were iodinated to a specific activity of 3.5 × 106cpm/μg using the iodogen method, and RIAs were performed as described in “Patients and methods.” Anti-FVIII antibodies purified from IVIg inhibited the binding of labeled anti-FVIII inhibitors (Figure5). The concentration of unlabeled anti-FVIII antibodies purified from IVIg necessary to achieve 50% inhibition of binding of radiolabeled inhibitors was 66- to 133-fold greater than those of radiolabeled inhibitors. Unfractionated IVIg and IVIg depleted of anti-FVIII IgG used as negative controls also expressed an inhibitory capacity. However, the amount of these antibodies required to inhibit 50% of binding of radiolabeled anti-FVIII inhibitors was more than 300-fold greater than that of radiolabeled antibodies (Figure 5). Thus, results of both the functional assay and competitive binding by RIA indicate that natural and disease-associated anti-FVIII IgG recognize common epitopes on the FVIII molecule.
Competitive inhibition of the binding to FVIII between natural anti-FVIII IgG from IVIg and FVIII inhibitors from patients.
Radiolabeled anti-FVIII IgG alloantibody of a high-responder hemophiliac (A) and anti-FVIII IgG autoantibody of an autoimmune patient (B) (3 μg/mL) were incubated with increasing concentrations of anti-FVIII IgG from IVIg (closed squares), unfractionated IVIg (open squares), and IVIg depleted of anti-FVIII antibodies (open triangles) for 4 hours at 37°C. The percentage of inhibition was calculated as described in “Patients and methods.”
Competitive inhibition of the binding to FVIII between natural anti-FVIII IgG from IVIg and FVIII inhibitors from patients.
Radiolabeled anti-FVIII IgG alloantibody of a high-responder hemophiliac (A) and anti-FVIII IgG autoantibody of an autoimmune patient (B) (3 μg/mL) were incubated with increasing concentrations of anti-FVIII IgG from IVIg (closed squares), unfractionated IVIg (open squares), and IVIg depleted of anti-FVIII antibodies (open triangles) for 4 hours at 37°C. The percentage of inhibition was calculated as described in “Patients and methods.”
Discussion
In the present study, we have analyzed the properties of anti-FVIII IgG recovered by affinity chromatography on FVIII-Sepharose from IgG of healthy individuals and nonresponder hemophilia A patients. Anti-FVIII antibodies were found to neutralize FVIII function as an enzymatic cofactor and to bind to FVIII with an affinity similar to that of anti-FVIII IgG affinity-purified from the plasma samples of hemophilia patients with inhibitor and individuals with anti-FVIII autoantibodies. These observations suggest that FVIII inhibitors occurring in severe hemophilia and in patients with anti-FVIII autoimmune disease originate from the expansion of preexisting natural anti-FVIII clones with neutralizing properties.
Natural antibodies of the IgG isotype directed against self antigens are present in normal human serum.12,14-18 A large body of data substantiates the hypothesis that naturally occurring antibodies do not result from a break of tolerance but are rather generated by positively selected autoreactive B cells.19,20 Some of these antibodies bind to FVIII.6,7 The isotype distribution of natural anti-FVIII IgG closely follows that of IgG subclasses in normal serum.7 A subset of natural anti-FVIII antibodies exhibits inhibitory activity toward FVIII, whereas other subpopulations of antibodies bind to FVIII without exhibiting neutralizing activity, as in the case of anti-FVIII alloantibodies of inhibitor-positive hemophilia patients.8 We have isolated anti-FVIII IgG from pooled normal human IgG (IVIg) by affinity purification. Approximately 0.26% of IgG specific for FVIII was recovered, which is in the range of yields of purification of natural anti-FVIII antibodies reported in previous observations (ie, 0.06%-0.17%).7 Anti-FVIII antibodies in the eluate contained 15 ± 1 BU/mg of FVIII-neutralizing activity, whereas no such activity was detected in unfractionated IVIg under the conditions of the assay that were used. The results of neutralizing activity of anti-FVIII IgG isolated from IVIg are in line with those previously described for natural anti-FVIII antibodies by Gilles et al.7 The average equilibrium dissociation constant (Kd) of the affinity-purified natural anti-FVIII autoantibodies toward FVIII was 3.76 ± 1.81 nM (mean ± SD), a value that is consistent with previously reported affinities of natural autoreactive IgG toward self antigens.18 21-27
By immunoblotting, natural anti-FVIII IgG recognized the A2 domain and the light chain of FVIII. By using immunoprecipitation assays with whole FVIII, its light chain, and the A2 and C2 domains, we observed that natural anti-FVIII antibodies isolated from IVIg preferentially bound to epitopes within the recombinant C2 domain and, at a 60-fold–lower level, to the A2 domain. Anti-C2 antibodies interfere with FVIII function because the C2 domain of FVIII interacts with von Willebrand factor and with phospholipids.28-31
Anti-FVIII antibodies isolated from the plasma of nonresponder hemophiliacs and from IVIg exhibited higher titers of immunoprecipitation of the light chain than of whole FVIII, suggesting that epitopes masked in the case of intact FVIII are accessible in that of the isolated light chain. Immunoprecipitation patterns were diverse among individuals of different origin and did not allow us to clearly delineate one source of antibody from the others. In this regard, it is of interest that affinity-purified natural anti-FVIII antibodies were able to compete with affinity-purified anti-FVIII inhibitors of hemophilia patients and autoimmune patients for binding to the FVIII molecule. Furthermore, the calculated dissociation constant (kd) of the interaction of affinity-purified anti-FVIII antibodies of various sources with FVIII, FVIII light chain, and domains of FVIII were found to be in the same order of magnitude. Results of binding experiments using the BIAcore correlated with those of the immunoprecipitation assays. Thus, neither the broad specificity of anti-FVIII antibodies toward domains of FVIII nor their finer epitopic characterization by means of RIA allowed us to differentiate between natural anti-FVIII IgG and anti-FVIII inhibitor from patients with hemophilia or patients with anti-FVIII autoimmune disease.
Several factors may account for the fact that plasma levels of FVIII remain normal under physiologic conditions despite the presence of natural anti-FVIII antibodies: the low frequency of natural anti-FVIII antibodies, which we estimated to be 100-fold lower than in inhibitor-positive hemophilia patients; a lower fraction of antibodies with inhibitor properties among the population of natural anti-FVIII antibodies as compared with IgG alloantibodies of hemophiliacs; complex formation with circulating FVIII,32 and peripheral control of inhibitory antibodies by anti-idiotypic antibodies. The latter were reported to be present in the plasma of healthy blood donors,7 in pooled normal human IgG,33 and in the plasma of hemophilic patients after successful desensitization to FVIII.34 The ability of natural anti-idiotypes to effectively control anti-FVIII antibody activity was shown in patients with anti-FVIII auto- and alloantibodies who were successfully treated with IVIg.32,35-37 Based on the accumulated knowledge about the immunomodulatory properties of therapeutic immunoglobulin in autoimmune diseases, we propose that neutralizing and nonneutralizing anti-idiotypic antibodies interact with the appropriate anti-FVIII membrane immunoglobulin molecule on the B cell and modulate the proliferation/differentiation of FVIII-specific B-cell clones.32,33,38 39
The finding of a lower inhibitory activity of natural anti-FVIII IgG (0-15 BU/mg of IgG) as compared with inhibitor anti-FVIII IgG of hemophilia patients and patients with anti-FVIII autoimmune disease (46.2 ± 21.6 BU/mg of IgG) suggests that the relative distribution of inhibitory and noninhibitory IgG among anti-FVIII antibodies is different under physiologic and pathological conditions, because the affinity of natural antibodies to FVIII was similar to that of autoimmune and alloimmune FVIII antibodies.
Anti-FVIII IgG of nonresponder patients with severe hemophilia but without evidence of inhibitory activity in unfractionated plasma exhibited properties similar to that of natural anti-FVIII IgG isolated from IVIg and anti-FVIII IgG affinity-purified from the plasma of high-responder patients. Thus, anti-FVIII antibodies of nonresponder hemophiliacs bind to FVIII with an affinity similar to that of inhibitors, predominantly recognize the C2 and A2 domains of FVIII, and may neutralize FVIII activity. Earlier evidence has shown that nonresponder hemophiliacs may produce anti-FVIII antibodies that are masked as they become complexed in the circulation.40 Thus, nonresponder hemophiliacs are able to mount an anti-FVIII response. They are, however, capable of efficiently controlling the anti-FVIII response despite their being exposed to repeated challenges with FVIII. In this regard, nonresponder hemophilia patients would behave similarly to desensitized hemophilia patients.
Taken together, our observations indicate that FVIII inhibitors occurring in patients with severe hemophilia and in patients with anti-FVIII autoimmune disease originate from the expansion of preexisting natural anti-FVIII clones with neutralizing properties. Because natural anti-FVIII clones may be controlled with specific anti-idiotypes from normal IgG, the present findings may have important therapeutic implications. It is thus possible to extrapolate that boosting of naturally existing anti-idiotypic antibodies that control FVIII inhibitor, by immunization using appropriate molecules that carry shared idiotypic determinants, may help in vaccinating or treating hemophilia A patients. Alternatively, it is possible to conceive strategies involving passive administration of selected anti-idiotypic antibodies that neutralize FVIII inhibitor and suppress clones producing inhibitor.
Acknowledgments
The authors thank Dr S. Raut and T. Croughs for helpful suggestions and M. F. Bloch, S. Rose, and E. Bonnin for technical assistance. FVIII was a kind gift from Baxter Hyland. Sandoglobulin was a gift from the Central Laboratory of the Swiss Red Cross, Bern, Switzerland. The FVIII matrix was a gift from the Laboratoire Français des Biotechnologies (LFB), Les Ulis, France.
Supported by Institut National de la Santé et de la Recherche Médicale (INSERM) and Centre National de la Recherche Scientifique (CNRS), France; Assistance publique-Hôpitaux de Paris (grant AOM96 124); and by the Bayer Pharma, France. S.L.-D. is a recipient of a grant from Bayer-Pharma. A.M. is a recipient of a fellowship from the Ministère de la Recherche et de la Technologie, France.
A.M. and S.L.-D. contributed equally to this work.
Reprints:Michel D. Kazatchkine, INSERM U430, Hôpital Broussais, 96, rue Didot, F-75014 Paris, France; e-mail:kaveri@hbroussais.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.