Abstract
Crystal formation by monoclonal immunoglobulins is a well-known but rare complication of B-cell neoplasia. We have designed an in vivo model of cryocrystalglobulinemia by grafting to mice hybridoma clones producing a pathogenic monoclonal immunogloblulin (Ig) G3κ. One clone, 8A4, secreted a singular IgG3 that formed crystals both in the proliferating plasma cells and as mesangial and subendothelial deposits in the kidney glomeruli. Morphologic analysis of kidneys revealed neutrophil infiltration and endocapillary hyperplasia, while the morphology of deposits was reminiscent of those in cryocrystalglobulinemia patients. A variant clone that only differed from 8A4 by a 3–amino acid deletion in the Vκ CDR1 increased its secretion level by 7-fold and produced an abundant bona fide serum monoclonal cryoglobulin in mice, without crystal formation within tumoral cells; it yielded no subendothelial deposits but only amorphous precipitates in capillary lumens of kidney glomeruli, reminiscent of those seen in the human hyperviscosity syndrome, without other glomerular lesions. A limited variation in the Vκdomain thus proved able to increase secretion, to abrogate crystallization, and to modify patterns of glomerular lesions and deposits. Both the crystallizing and noncrystallizing IgG3κ sequences were related to previously reported murine cryoglobulins, all including a γ3 chain and canonical VH sequences. Two additional variants of 8A4 with identical VH and VL domains but having switched to IgG1 also lost crystal formation, further showing this feature of 8A4 to result from a unique 3-dimensional conformation of the complete immunoglobulin, relying on V and C domain primary structures of both chains.
Cryocrystalglobulinemia is characterized by crystallization of a monoclonal immunoglobulin (Ig) within plasma cells and as crystal deposits affecting various tissues, especially the kidneys. It occurs in humans as a rare complication of B-cell immunoproliferative disorders, first described by Von Bonsdorff in association with myeloma1 but also associated with other B-cell neoplasms, including monoclonal gammopathies of undetermined significance.2-5 By contrast, cryocrystalglobulinemia has never been reported in mice, although a number of murine monoclonal cryoglobulins, all of the IgG3 isotype, have been thoroughly studied.6-10 The exclusive presence of IgG3 within monoclonal murine cryoglobulins and, also, the enrichment of the IgG3 subclass within mixed cryoglobulins have been ascribed to a strong tendency of this isotype to self-aggregate due to a more positively charged CH2 domain.6,10 Monoclonal IgG3 with a wide range of antigen specificities has been found to cryoprecipitate. Although the precise mechanisms of cryoprecipitation are not understood, convincing data exist supporting an electrostatic model: γ3 constant regions would promote electrostatic interactions and self-aggregation of Ig independently of their antigen-binding site. Besides the role of constant regions, not all monoclonal IgG3s behave as cryoglobulins and not all cryoglobulins induce kidney lesions; it is thus clear that variable region sequences also play a role in self-aggregation, precipitation, and nephritogenicity, noticeably by providing additional positively charged residues. More specifically, residues 6 and 23 of the heavy (H) chain variable domain have been postulated to play a role in precipitation in a study comparing 6 IgG3 cryoglobulins with several noncryoprecipitating monoclonal IgG3s.10 Glutamine and lysine were found at positions 6 and 23, respectively, in cryoglobulins and were more positively charged than their counterparts in noncryoglobulins.10
To study the molecular basis of cryocrystallization, we used a singular hybridoma clone, 8A4, producing a monoclonal IgG3 antibody against a hydrogenase from Wolinella succinogenes and spontaneously forming intracellular crystals. Complete sequences of Ig chains were determined at the complementary DNA (cDNA) level, looking for unusual V region sequences that may have been responsible for impaired trafficking and crystallization within plasma cells and in tissue deposits. We assayed the in vivo nephrotoxicity of the 8A4 IgG3 and developed an experimental model of cryocrystalglobulinemia by grafting 8A4 hybridoma cells to mice. Pathologic alterations of mice kidneys were thoroughly examined. This model also allowed us to show that limited sequence variation of the Vκ region can convert an Ig responsible for organized glomerular deposits typical of cryocrystalglobulinemia with glomerulonephritis into a more classical serum cryoglobulin, leading to isolated thrombi in vascular lumens like those seen in the hyperviscosity syndrome, without an inflammatory reaction.
Material and methods
Production of hybridomas
Female Balb/c mice (Harlan, Borchen, Germany) were immunized by intraperitoneal injection of 100 μg of purified membrane-bound hydrogenase from Wolinella succinogenes (kindly provided by Dr Achim Kröger, Department of Microbiology, University of Frankfurt) mixed with the same volume (100 μL) of adjuvant (ABM-2; Pan-Systems, Aidenbach, Germany). Blood sera showed positive signals specific against the antigen in an enzyme-linked immunosorbent assay (ELISA). Boosting was performed 4 times at 4-week intervals using 50 μg of antigen each time.
After the final boosting, the mice were killed and the spleen was removed aseptically. The spleen cells were fused with P3/NSI/1-Ag4-1 (DSM ACC 145) myeloma cells by using 50% polyethylene glycol 1500 (Roche Diagnostics, Mannheim, Germany). Hybridoma cell selection was performed with hypoxanthine and azaserine. The cells were cultured and cloned according to standard protocols.11 12
Standard ELISA
For the standard ELISA, 96-well polystyrene plates (Greiner, Frickenhausen, Germany) were coated with hydrogenase (1.5 μg/mL) or antimouse Ig Hybridoma Screening Reagent (Roche Diagnostics) diluted 1:500 in 0.2 mol/L Na2CO3, pH 9.5, for 1 hour at room temperature. Washing steps were performed using 0.9% NaCl and 0.1% Tween 20. Blocking was done with 1% bovine serum albumin (BSA) in 50-mM Tris-HCl, pH 7.5; 500 mM NaCl; and 0.1% Tween 20. A total of 50 μL of blood serum, hybridoma supernatant, or purified monoclonal antibody diluted in 50-mM Tris-HCl, pH 7.5; 500 mM NaCl; and 0.1% BSA were added to each well and incubated for 1 hour. Bound antibody was detected with a secondary antimouse IgG antibody (whole molecule) conjugated to alkaline phosphatase (Sigma, Deisenhofen, Germany), diluted 1:1000 in blocking buffer. The color reaction was developed with p-nitrophenylphosphate (1 mg/mL) in 0.5 mM MgCl2, 10% (vol/vol) diethanolamine, pH 9.8. After 30 or 60 minutes of incubation at room temperature, signal intensity was determined with an ELISA reader at 405 nm, using 450 nm as the reference wavelength.
Immunochemical studies
The classes, subclasses, and light (L) chain type of the secreted antibodies were determined using an immunologic subtyping kit (Roche Diagnostics) according to the manufacturer's instructions.
Selected clones were grown in standardized conditions at 1 × 106 cells/mL for 24 hours, and Ig production was measured by ELISA (mouse IgG ELISA; Roche Diagnostics) following the manufacturer's instructions.
Purification of antibodies was done by applying 50 mL of hybridoma supernatants to pre-equilibrated protein G affinity columns. Eluted antibodies were concentrated by ultrafiltration (Centricon, 50 kd cutoff; Amicon, Beverly, MA) and stored at 4°C.
Purification of crystals from cultured cells was done by lysing cells in 1% NP40, 0.5% sodium dodecyl sulfate (SDS), 5 mM ethylenediaminetetraacetic acid, 150-mM NaCl, and 50-mM Tris-HCl, pH 8.0, and submitting cell debris to proteinase K (0.75 U/mL for 15 minutes). Centrifugation yielded a pellet only consisting in crystals, which were washed and resuspended in 0.06 mol/L Tris-HCl, pH 6.8, and 2% SDS.
SDS–polyacrylamide gel electrophoresis (PAGE) was done according to standard procedures.13 Samples were denatured in sample buffer for 3 minutes at 95°C and subsequently analyzed on a 14% gel. Proteins were transferred onto a polyvinylidine difluoride membrane (Millipore, Bedford, MA). Detection of Ig H and L chains was done with alkaline phosphatase–conjugated antimouse IgG (whole molecule; Sigma), diluted 1:1000 in blocking buffer, for 1 hour at room temperature.
Purification of the 8A4-NP1 cryoglobulin was done by incubating sera from 8A4-NP1–grafted mice at 4°C for 1 week; the precipitate was then washed in cold phosphate-buffered saline (PBS) and redissolved at 37°C in PBS. Agar zone electrophoresis of sera obtained at sacrifice was performed using a Hydrasis apparatus (Sebia, Issy-les-Moulineaux, France).
Immunofluorescence and microscopy studies on cultured cells
For immunofluorescent intracellular staining, cultured cells were fixed and permeabilized for 2 minutes in 50% acetone, 50% ethanol and were air-dried. Staining was carried out with antimouse IgG antiserum conjugated to fluorescein isothiocyanate (FITC; Sigma). Cells were examined at a ×400 magnification by epifluorescence.
For confocal fluorescence microscopy, hybridoma cells were plated onto collagen I–coated glass coverslips. Fixation was performed in 3.7% (wt/vol) paraformaldehyde. For blocking and permeabilization of the cells, the coverslips were treated in PBS containing 0.2% (wt/vol) BSA and 0.2% (vol/vol) Triton X-100. The fluorochrome-labeled secondary antibody was diluted in blocking buffer at a concentration of 3 μg/mL for Cy3-labeled antimouse IgG (whole molecule; Sigma, L'Isle d'Abeau, France) or 15 μg/mL for FITC-labeled antimouse γ chain and antimouse κ chain antibodies (Sigma). Confocal microscopy on a Sarastro 2000 microscope (Molecular Dynamics, Uppsala, Sweden) was carried out as described.14
For electron microscopy and immunogold labeling, hybridoma cells were fixed with 4% paraformaldehyde plus 1% glutaraldehyde. Monoclonal Ig produced by the cells was labeled with a gold particle–coupled antibody goat antimouse (Amersham, Braunschweig, Germany; 1:5 diluted with PBS). After washing off unbound antibody (PBS, 4× for 5 minutes each) the material was refixed with 1% glutaraldehyde. To enlarge the gold particles, the silver amplification method was applied. Thin sections of resin-embedded cells were cut with the Ultracut S (Reichert, Vienna, Austria), double-contrasted with uranyl acetate and lead citrate, and viewed in a Philips 208S electron microscope.
Immunomorphologic analysis in BALB/c mice
Various organ and tumor samples were collected from mice carrying tumors. Light microscopic examination was performed on multiple slides stained either with hematoxilin/eosine, Schiff periodic acid, light green trichrome, silver methenamine, or toluidine blue. Frozen blocks of all major organs (liver, kidney, spleen, heart) and of tumor obtained at sacrifice were cut in 4-μm–thick slices. Organs were studied for the presence of deposited mouse Ig by immunofluorescence with fluorescein-conjugated polyclonal rabbit antisera against mouse Ig (Zymed, San Francisco, CA). For ultrastructural studies, small samples of kidney were fixed in 2.5% glutaraldehyde. Sections were then stained with uranyl acetate and examined using a Jeol 100 CX electron microscope.
Nucleic acids studies, RACE, sequence analysis, and computing of molecular models
Total RNA was prepared from the crystal-forming 8A4 clone and its variants using the RNeasy Kit (Qiagen, Hilden, Germany). Two micrograms of total RNA were used as templates for the synthesis of single-stranded cDNA by reverse transcriptase (Superscript II RT, Gibco BRL, Gaithersburg, MD) using an oligo-dT15 primer (Roche Diagnostics) or a γ3-subclass–specific primer. The cDNA was used as a template for amplification of H and L chain sequences by polymerase chain reaction (PCR)15 using Thermus aquaticus (Taq) polymerase (Promega, Madison, WI). The native N-terminal and C-terminal ends of the cDNA were amplified by 5′- and 3′-RACE (rapid amplification of cDNA ends) PCR using the 5′/3′ RACE kit (Roche Diagnostics). NP91 and NP92 H chain variable region cDNA were amplified using primers specific for the 5′ untranslated region of the VH558 germline gene segment and for the γ1 constant region gene segment. The Vκ region cDNA was amplified using primers matching the 5′ untranslated region of the Vκ21G and the Cκ gene segment. PCR products were cloned into pBluescript II KS+ (Stratagene, La Jolla, CA) and sequenced by the dideoxynucleotide method.16 At least 3 independently obtained PCR products were analyzed by sequencing both strands. Searches in the EMBL European Molecular Biology Laboratory database were performed using the FASTA program.17 The assignment of V(D)J gene segments was done with the help of the DNAPLOT program at the IMGT (ImMunoGeneTics) server (http://www.genetik.uni-koeln.de/dnaplot/). Amino acid–based search, alignment with the Kabat numbering scheme, and canonic class assignment in the Kabat database18 were done using the KabatMan software (version 2.19;http://www.biochem.ucl.ac.uk/∼martin/abs/).19 Molecular modeling of the 8A4 κ chain and its variant from the 8A4-NP1 clone by comparison to known Ig sequences was carried out using ProMod software.20
Results
Immunoglobulin production in hybridoma cells
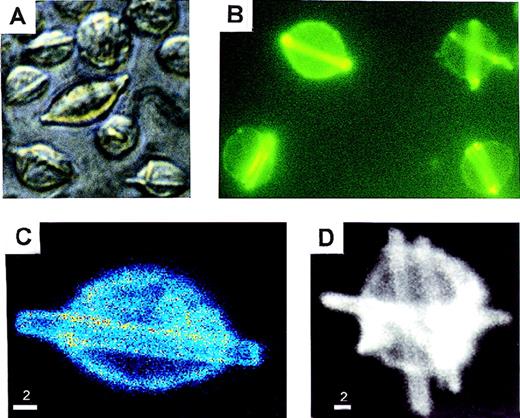
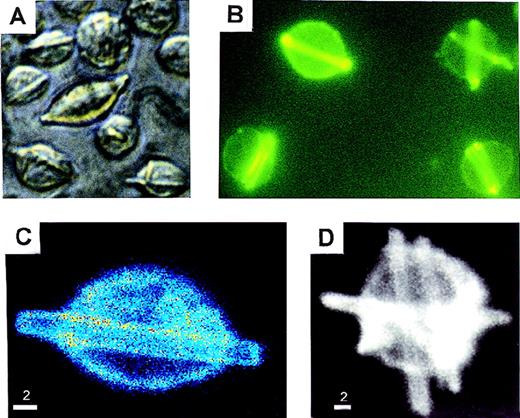
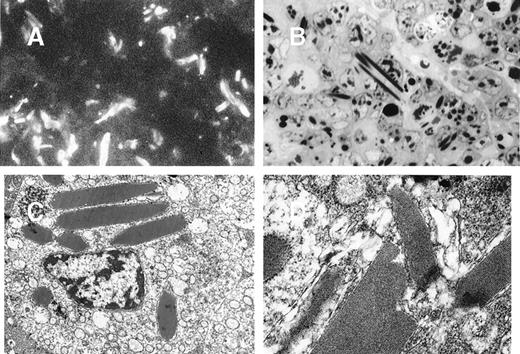
We isolated and studied the 8A4 hybridoma clone producing an IgG3κ antibody against the membrane-bound hydrogenase from Wolinella succinogenes. A low secretion rate of mouse IgG3 was estimated by ELISA on cell culture supernatants of 8A4 (2.3 ± 0.06 μg/106 cells per 24 hours). By contrast, light microscopy showed that cells cultured at 37°C readily developed abundant and large rod-shape crystals, which accumulated in living cells (from 1 to 10 crystals per cell) and progressively extended in length, occasionally protruding beyond the cell surface; crystals also accumulated in the liquid culture media (Figure1A). By immunofluorescence or confocal microscopy, these crystals appeared to include the secreted antibody and strongly stained with antimouse IgG antisera (Figure 1B, 1C, 1D). The crystals could also be specifically labeled by immuno–electron microscopy with a gold particle– (10 nm) coupled antibody goat antimouse IgG (not shown). Crystals purified from cultured 8A4 cells were analyzed by SDS-PAGE; Western blotting revealed that they included the monoclonal Ig H and L chain (not shown), and Coomassie blue staining revealed that the monoclonal Ig was the sole component of crystals (Figure 2A).
In vitro crystal formation in 8A4 cells.
(A) Light microscopy of living cells (original magnification ×400). (B) Immunofluorescence on acetone/ethanol-fixed cells stained with fluorescein-labeled anti-IgG (original magnification ×400). (C and D) Confocal fluorescence microscopy of 8A4 cells: hybridoma cell stained with antimouse IgG-Cy3 conjugate. Rod-like intracellular crystals display strong signals. Intensities are coded by pseudocolors (C) or brightness (D). Scale bar: 2 μm.
In vitro crystal formation in 8A4 cells.
(A) Light microscopy of living cells (original magnification ×400). (B) Immunofluorescence on acetone/ethanol-fixed cells stained with fluorescein-labeled anti-IgG (original magnification ×400). (C and D) Confocal fluorescence microscopy of 8A4 cells: hybridoma cell stained with antimouse IgG-Cy3 conjugate. Rod-like intracellular crystals display strong signals. Intensities are coded by pseudocolors (C) or brightness (D). Scale bar: 2 μm.
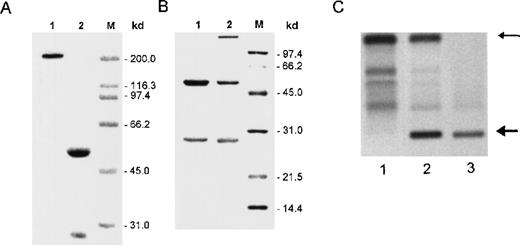
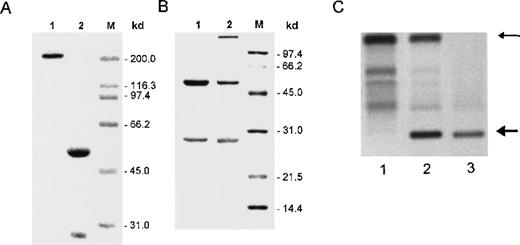
Immunochemical analysis of cell supernatants and crystals.
(A) SDS-PAGE analysis of crystals formed in vitro by 8A4 cells and analyzed under nonreducing (lane 1) or reducing (lane 2) conditions; only polypeptides corresponding to immunoglobulin H and L chains were detected by Coomassie blue staining. (B) SDS-PAGE analysis under reducing conditions of antibodies purified from 8A4 (lane 1) and 8A4-NP1 (lane 2); Coomassie blue staining; molecular weight markers are indicated in kilodaltons. (C) Agar zone electrophoresis of serum from an 8A4 mouse (lane 1), an 8A4-NP1 mouse (lane 2), and the solubilized cryoglobulin precipitate obtained from 8A4-NP1 serum (lane 3); the upper arrow indicates serum albumin, and the bottom arrow marks the monoclonal cryoglobulin peak.
Immunochemical analysis of cell supernatants and crystals.
(A) SDS-PAGE analysis of crystals formed in vitro by 8A4 cells and analyzed under nonreducing (lane 1) or reducing (lane 2) conditions; only polypeptides corresponding to immunoglobulin H and L chains were detected by Coomassie blue staining. (B) SDS-PAGE analysis under reducing conditions of antibodies purified from 8A4 (lane 1) and 8A4-NP1 (lane 2); Coomassie blue staining; molecular weight markers are indicated in kilodaltons. (C) Agar zone electrophoresis of serum from an 8A4 mouse (lane 1), an 8A4-NP1 mouse (lane 2), and the solubilized cryoglobulin precipitate obtained from 8A4-NP1 serum (lane 3); the upper arrow indicates serum albumin, and the bottom arrow marks the monoclonal cryoglobulin peak.
The mutated IgG3 8A4-NP1 was obtained as a spontaneous variant of 8A4 that lost production of intracellular crystals but kept secreting an IgG3κ antibody that bound hydrogenase. In this particular variant, Ig secretion raised 7 times to 15.9 ± 1.3 μg/106 cells per 24 hours. When antibodies secreted by 8A4 and 8A4-NP1 cells were purified and analyzed by SDS-PAGE, they appeared to include normal-size Ig chains with similar migrations for the H chains and a slightly faster migration for the 8A4-NP1 L chain (Figure 2B). Two further subclones without crystal production (NP91 and NP92) had preserved identical H and L chain variable domains but had performed a class switch, now both secreting IgG1κ.
In vivo pathogenic properties of 8A4 and 8A4-NP1 in mice
Monoclonal IgG3 was produced in vivo in mice injected with the 8A4 or 8A4-NP1 hybridoma (Table 1). Although no detectable monoclonal peak was observed in 8A4 mice, the 8A4-NP1 IgG3 manifested as an abundant monoclonal peak in sera of 8A4-NP1 mice, representing more than 50% of total serum protein; this monoclonal component readily precipitated when sera were incubated in the cold and thus behaves as a type I monoclonal cryoglobulin (Figure 2C). There was no clinical evidence of cutaneous lesions or arthritis in mice.
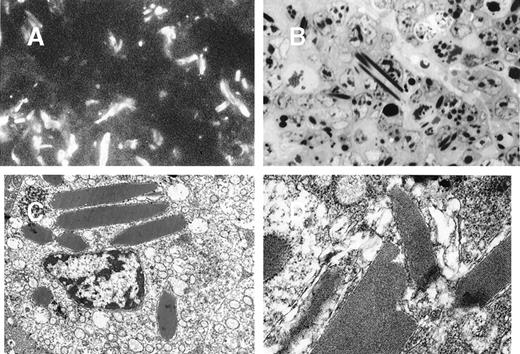
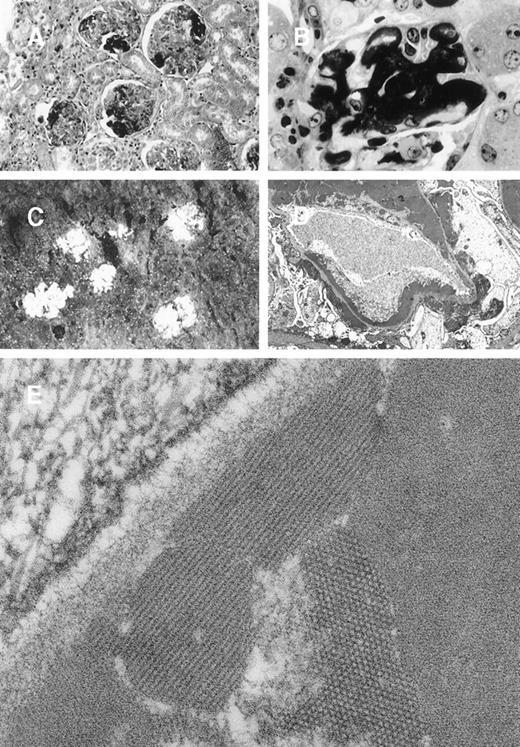
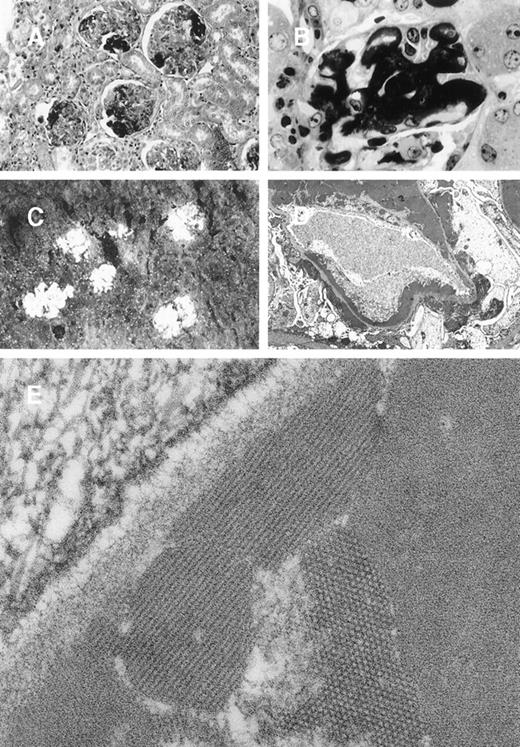
In 8A4 mice, light microscopic examination of tumor biopsies showed abundant crystal inclusions similar to Ig crystals observed in cultured cells (Figure 3). Contrasting with the absence of a monoclonal peak, kidney samples showed marked glomerular lesions with abundant wire-loop deposits along the glomerular basement membrane, predominating in mesangium on the endothelial aspect of the basement membrane and often resulting in pseudo-thrombi (Figure4A and 4B); to a lesser extent, deposits were also found on the epithelial side and occasionally invaded the urinary space. In some glomeruli, Ig deposits were associated with endocapillary proliferation and polymorphonuclear leukocyte infiltration. By immunofluorescence, deposits strongly stained with an antimouse IgG and were present within all glomeruli (Figure 4C). No deposits were found in other locations of the kidney or in liver or spleen. Electron microscopy also confirmed the crystalline nature of extracellular renal deposits by demonstrating their regular striation of 12- to 14-nm periodicity (Figure 4D and 4E). The crystals observed in vitro in cultured cells (Figure 1) and in vivo in tumoral cells (Figure 3), either by immunofluorescence staining or by electron microscopy, were identical to those formed in animals as kidney deposits (Figure 4).
Tumors induced by 8A4 cells grafted to mice.
Immunofluorescence microscopy with antimouse Ig conjugate (A, original magnification ×1000) and light microscopy (B, semi-thin section, toluidine blue staining, original magnification ×1000) showed numerous polymorphic crystals located in tumor cell cytoplasms. (C and D) Electron microscopy showed osmiophilic and highly organized crystalline inclusions in rough ergastoplasm cisternae with a periodicity of 12 nm (C, original magnification ×6000; D, ×25 000).
Tumors induced by 8A4 cells grafted to mice.
Immunofluorescence microscopy with antimouse Ig conjugate (A, original magnification ×1000) and light microscopy (B, semi-thin section, toluidine blue staining, original magnification ×1000) showed numerous polymorphic crystals located in tumor cell cytoplasms. (C and D) Electron microscopy showed osmiophilic and highly organized crystalline inclusions in rough ergastoplasm cisternae with a periodicity of 12 nm (C, original magnification ×6000; D, ×25 000).
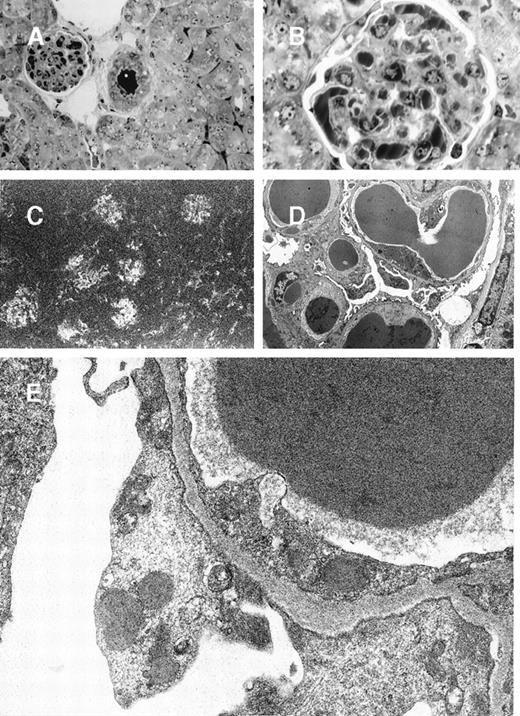
Glomerular lesions in 8A4 mice kidneys.
(A and B) Light microscopy showing the proliferative and exudative changes (A, original magnification ×200, light green trichromic staining) and the voluminous depositions of material predominantly in mesangial and subendothelial areas (B, original magnification ×1000, semi-thin section, toluidine blue staining). (C) Immunofluorescence microscopy with antimouse Ig conjugate (original magnification ×200) showed intense staining of deposits strictly limited to glomeruli without any staining of peritubular capillary lumen. (D and E) Electron microscopy showed osmiophilic deposits in mesangial and subendothelial spaces (wire-loop lesion) with few subendothelial deposits. The glomerular capillary lumens were free of deposits (D, original magnification ×5000). Note the highly organized crystalline glomerular deposits formed by densely packed microtubules (external diameter = 12 nm) in transversal section and parallel arrays in longitudinal section with a 12-nm striation periodicity (E, original magnification ×60 000).
Glomerular lesions in 8A4 mice kidneys.
(A and B) Light microscopy showing the proliferative and exudative changes (A, original magnification ×200, light green trichromic staining) and the voluminous depositions of material predominantly in mesangial and subendothelial areas (B, original magnification ×1000, semi-thin section, toluidine blue staining). (C) Immunofluorescence microscopy with antimouse Ig conjugate (original magnification ×200) showed intense staining of deposits strictly limited to glomeruli without any staining of peritubular capillary lumen. (D and E) Electron microscopy showed osmiophilic deposits in mesangial and subendothelial spaces (wire-loop lesion) with few subendothelial deposits. The glomerular capillary lumens were free of deposits (D, original magnification ×5000). Note the highly organized crystalline glomerular deposits formed by densely packed microtubules (external diameter = 12 nm) in transversal section and parallel arrays in longitudinal section with a 12-nm striation periodicity (E, original magnification ×60 000).
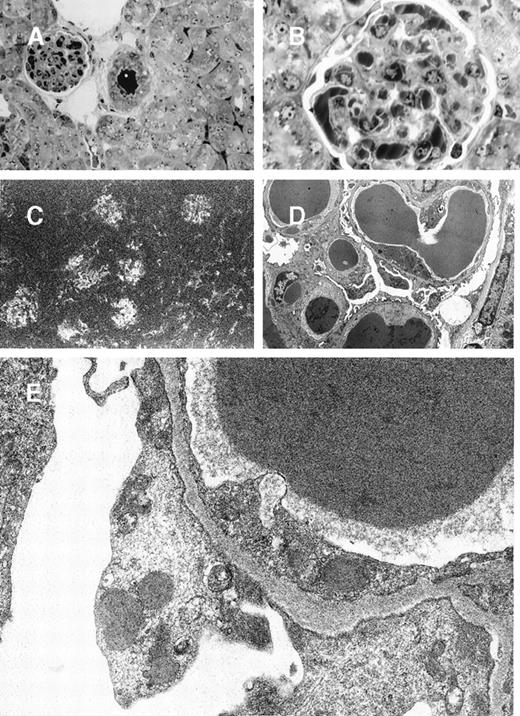
In mice grafted with the 8A4-NP1 hybridoma cells producing noncrystallizing IgG3, tumor cells did not contain any intracytoplasmic crystalline inclusion (data not shown); neither could crystalline organized deposits be found in glomeruli. Only abundant amorphous Ig precipitates predominating in glomerular capillary lumens but also in peritubular capillaries and arteriolar lumens were found without hypercellularity or polymorphonuclear leukocyte afflux (Figure5A and 5B). Thrombi (intracapillary precipitates) were amorphous, without any crystalloid, fibrillar, or microtubular organization (Figure 5C, 5D, 5E). Numerous cytoplasmic dense bodies without any ultrastructural organization were found in glomerular endothelial and epithelial cells and in proximal tubular cells (Figure 5E).
8A4-NP1 cryoglobulin, renal lesions observed in mice kidneys.
(A and B) Light microscopy showing intravascular precipitates not solely in glomerular capillary lumens but also in peritubular capillary and arteriolar lumens. Note the absence of inflammatory reaction. Semi-thin sections, toluidine blue staining (A, original magnification ×400; B, ×1000). (C) Immunofluorescence microscopy with antimouse Ig conjugate (original magnification ×200) showed staining of intravascular cryoglobulin aggregates. (D and E) Electron microscopy showed osmiophilic amorphous precipitates in capillary lumens without crystalline substructure and without any deposits in subendothelials spaces. Note also the amorphous dense bodies in epithelial and endothelial cell cytoplasms (D, original magnification ×2500; E, ×12 000).
8A4-NP1 cryoglobulin, renal lesions observed in mice kidneys.
(A and B) Light microscopy showing intravascular precipitates not solely in glomerular capillary lumens but also in peritubular capillary and arteriolar lumens. Note the absence of inflammatory reaction. Semi-thin sections, toluidine blue staining (A, original magnification ×400; B, ×1000). (C) Immunofluorescence microscopy with antimouse Ig conjugate (original magnification ×200) showed staining of intravascular cryoglobulin aggregates. (D and E) Electron microscopy showed osmiophilic amorphous precipitates in capillary lumens without crystalline substructure and without any deposits in subendothelials spaces. Note also the amorphous dense bodies in epithelial and endothelial cell cytoplasms (D, original magnification ×2500; E, ×12 000).
Immunoglobulin cDNA sequencing
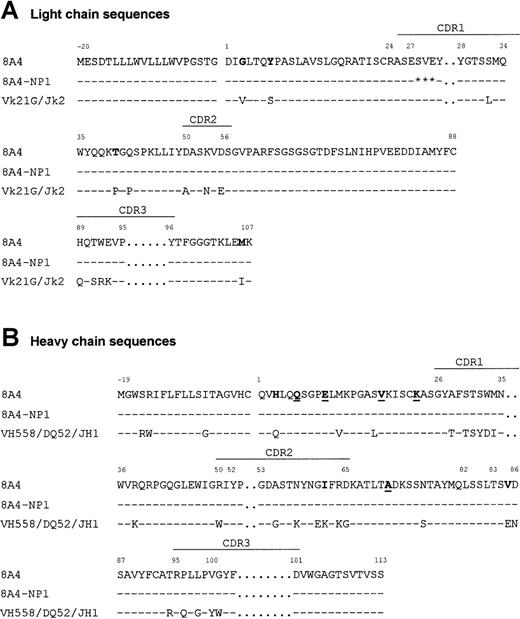
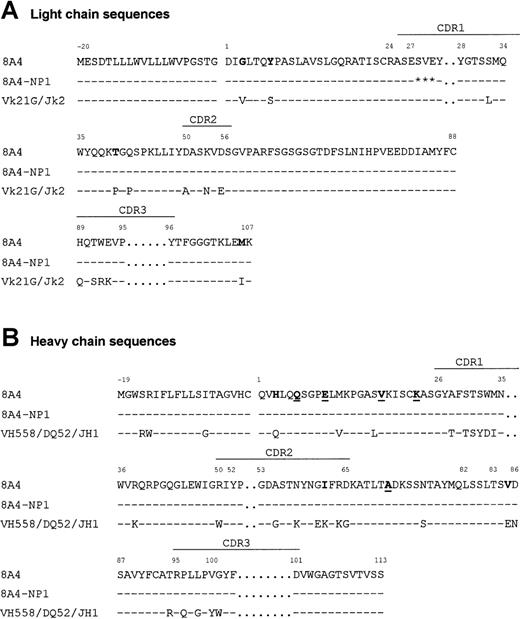
Sequence of the 8A4 Ig chain cDNAs showed that the VHDJH and VκJκ regions had an overall normal structure (Figure 6). The VH region was most closely related (87% identical) to the VH558 germline gene21 rearranged to the DQ52 and JH1 segments, while the Vκ region was related (95% identical) to Vκ21G22 and rearranged to Jκ2. Several unusual substitutions were found, including Gly(+3), Tyr(+7), Thr(+40), and Met(+106) in the VκJκ region and His(+3), Ile(+62), and Val(+85) in the VH region. The VH sequence also included Gln(+6) and Lys(+23), previously reported as canonic in murine monoclonal cryoglobulins.10 Ig mRNA sequencing revealed that 8A4-NP1 only differed from the original 8A4 by a 3-codon deletion in the Vκ region CDR1 (Figure 6).
Variable region sequences.
Alignment of the primary sequences of 8A4 and 8A4-NP1 proteins with the germinally encoded VH558/DQ52/JH1 and Vκ21G/Jκ2 sequences. Dashes indicate identities. Residues potentially involved in cryoprecipitation or crystal formation are in bold (unusual residues typical of 8A4) or underlined (residues shared with other cryoglobulins). Stars indicate deleted residues. Amino acids are numbered according to Kabat.18
Variable region sequences.
Alignment of the primary sequences of 8A4 and 8A4-NP1 proteins with the germinally encoded VH558/DQ52/JH1 and Vκ21G/Jκ2 sequences. Dashes indicate identities. Residues potentially involved in cryoprecipitation or crystal formation are in bold (unusual residues typical of 8A4) or underlined (residues shared with other cryoglobulins). Stars indicate deleted residues. Amino acids are numbered according to Kabat.18
Molecular models of the 8A4 and 8A4-NP1 Vκ domains showed that residues exposed to the solvent differed around the 3–amino acid deletion, so that Ser(+26) and Glu(+27) preceding the deletion and Tyr(+28), Gly(+29), and Thr(+30) following the deletion had lateral chains exposed to the solvent in 8A4 and not in 8A4-NP1. The cDNA sequences of the constant regions of L and H chain showed no deviation from the published sequences.23 24
Variable region sequences were also determined from both 8A4 variants, which had undergone switching to the IgG1 isotype and had simultaneously lost intracellular crystal formation: For both variants, VH and Vκ sequences fell in complete identity with those of 8A4.
Discussion
Crystal formation by monoclonal Ig has been reported in various conditions featuring kidney alterations. Monoclonal L chain crystallization typical of human myeloma-associated Fanconi syndrome has been thoroughly documented at the molecular level: It most often involves free κ L chain, and all 4 cases studied at the molecular level were related to the same VκI subgroup gene, O2-O12.25-27 By contrast, molecular analysis has never been carried out in cases featuring spontaneous crystallization of complete Ig, and there was no experimental model of this condition. In human beings, organized aggregates made up of complete IgG are observed in 2 conditions: myeloma-associated cryocrystalglobulinemias and glomerulopathies with organized microtubular monoclonal immunoglobulin deposits (GOMMID), a complication of chronic lymphocytic leukemia, with monoclonal IgG forming crystal-like microtubular inclusions in both the leukemic cells and the kidneys.28 GOMMID can also be observed as a complication of monoclonal gammopathies without detectable serum cryoglobulin.
To set up a model of murine cryocrystalglobulinemia, we took advantage of a hybridoma clone spontaneously forming intracellular IgG3 crystals when cultured at 37°C. When hybridoma cells were grafted into mice, they yielded tumors that kept producing intracellular crystals in vivo. Similarly to the situation in human cryocrystalglobulinemia, these mice readily developed typical crystalline kidney deposits along the inner aspect of the glomerular basement membrane. Crystalline organized deposits also led to a strong inflammatory reaction featuring endocapillary cell proliferation and afflux of neutrophils. Although considerable amounts of the monoclonal Ig were secreted by the tumor and apparently circulated through the kidneys to give abundant deposits, no monoclonal peak was detectable in serum by electrophoresis, thus indicating a rapid deposition process and clearance of the protein in its soluble form, even at 37°C in body fluids. In human cryoglobulinemias also, cryoglobulins that precipitate at 37°C may be undetectable in serum, and it is well-known that there is no correlation between the amount of detectable monoclonal Ig in serum and their ability to yield renal deposits.28 29
By contrast, when mice were injected with tumors secreting the 8A4-NP1 variant, which has lost the production of intracellular crystals, a strong signal for a monoclonal peak migrating among gammaglobulins appeared by serum electrophoresis in freshly collected sera from tumoral mice. This monoclonal Ig disappeared when the serum was kept for several days at room temperature and behaved as a type I cryoglobulin, giving an abundant precipitate exclusively made up of the monoclonal Ig. In those mice, no crystalline deposits were observed in the kidney either; rather, amorphous Ig precipitates developed in vascular lumens of kidneys.
The 8A4 cryocrystalglobulin sequence was strongly related to that of previously reported murine cryoglobulins, because it belonged to the IgG3 class and also carried neutral and positively charged glutamine and lysine residues at positions 6 and 23, respectively.10Because the 8A4-NP1 Ig only differed from 8A4 by a 3-codon deletion in the κ chain CDR1, it also carried the canonic H chain structures previously reported for murine monoclonal cryoglobulins, with a γ3 constant region and glutamine 6 and lysine 23.10 Other unusual features shared by 8A4, 8A4-NP1, and all 6 previously sequenced murine cryoglobulins were the presence of glutamate at position 10, valine at position 18, and alanine at position 71. In fact, the short κ CDR1 deletion in 8A4-NP1 not only suppressed crystal formation but also strongly enhanced in vitro and in vivo Ig secretion (resulting in a high amount of the monoclonal IgG3κ in mice sera) and modified nephritogenicity of the monoclonal Ig, which only yielded unorganized intravascular thrombi with no inflammatory reaction. According to modeling data, the main difference between both monoclonal Ig might be the loss of some charged amino acids at the surface of the Vκ domain in 8A4-NP1, which might impede electrostatic interactions involved both in crystal formation and in affinity of Ig precipitates for the subentholial space of glomeruli.
Some previously reported murine cryoglobulins were encoded by germline V genes, and it was suggested that certain pathogenic autoantibodies may be germinally encoded.30 By contrast, the 8A4 IgG3κ carries several mutations and binds a defined bacterial antigen. It is also clear that the pathogenicity of 8A4 relies on the global structure of the complete Ig rather than on its antigen specificity. Indeed, crystal formation is lost in switch variants carrying the very same V regions linked to a γ1 H chain constant region. Strikingly in the 8A4-NP1 variant clone, mutation of the Vκ region resulted in loss of crystal formation in the plasma cells without suppressing the antigen specificity for Wollinella hydrogenase.
Various patterns of glomerular lesions have previously been reported to be induced by murine cryoglobulins and to be independent from their antigen binding activity.7,9,30,31 In the case of a cryoglobulin rheumatoid factor, replacement of the κ LC with a λ1 LC resulted in preservation of cryoglobulin activity and of glomerulonephritis despite a loss of antigen specificity.30Neutrophils may play a special role in the development of renal lesions: Certain Ig deposits are able to provoke glomerular neutrophil infiltration and further induce wire-loop glomerular lesions; others do not promote cell infiltration and solely induce intracapillary amorphous thrombi reminiscent of those seen with 8A4-NP1.30 31
Finally, we have established a murine model of glomerulonephritis induced by highly organized crystalline monoclonal IgG3κ deposits. Crystal formation in tumoral B cells was correlated with crystalline organization of IgG3κ glomerular wire-loop deposits. Either a mutation in the L chain or a change in the H chain isotype suppressed crystal formation within monoclonal B cells. In animals with tumors, mutation of the Vκ domain resulted in a striking difference in nephritogenicity, with not solely a loss of crystalline glomerular deposits, replaced with amorphous Ig precipitates in vascular lumens, but also the absence of inflammatory reaction.
Acknowledgments
We thank Prof Joachim Kirsch for kindly having placed the confocal fluorescence microscope at our disposal, Dr Roland Pfeiffer for help with the sample preparation and microscopy, Prof Philippe Babin for providing us with access to the electron microscope in Poitiers, and Emmanuelle Magnoux, Béatrice Fernandez, Nathalie Quellard, Josette Nicaud, and Isabelle Poulidor for expert technical assistance.
Supported by grants from Ligue contre le Cancer (ComitéRégional de la Haute-Vienne), Conseil Régional du Limousin, Association pour la Recherche sur le Cancer (grant 9121), AREN Poitou-Charentes, PHRC AOM96 058, and INSERM #4R001B. J.-U.R. supported by grants from the Deutsche Forschungsgemeinschaft (SFB 472), the Max-Planck-Gesellschaft, and the Fonds der Chemischen Industrie. S.D. was a recipient from a Société Française de Néphrologie fellowship.
Reprints:Michel Cogné, Laboratoire d'Immunologie, CNRS EP118, Centre Hospitalier Universitaire de Limoges, Institut Universitaire de France, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.