Abstract
We examined the chemoprotective effects of KF41399, a novel derivative of carbazole compounds, on severe thrombocytopenia induced by nimustine (ACNU, 45 mg/kg administered for 2 consecutive days intravenously) in mice. Administration schedule studies revealed that pretreatment of mice with KF41399 was necessary to improve thrombocytopenia. Oral administration of KF41399 ameliorated thrombocytopenia induced by ACNU and accelerated the rate of platelet recovery in a dose-dependent fashion. In addition, KF41399 pretreatment improved the decrease in body weight and spleen weight and in the colony-forming activity of bone marrow mononuclear cells (MNC). Oral administration of KF41399 to normal mice induced G0/G1-phase accumulation of MNC as well as hematopoietic progenitor cells (lineage negative cells [Lin−]) and reduced the colony-forming activity of MNC. In Lin− cells derived from KF41399-treated mice, up-regulation of Bcl-2 and down-regulation of cyclin E and cyclin A proteins were observed. In the same cells, a decrease in the phosphorylated form of Rb protein and an increase in the p130 protein were observed without changes in the protein level of cell cycle-dependent kinase 2 (Cdk2), Cdk4, and Cdk6. More important, KF41399 did not affect the antitumor activity of ACNU against mouse Sarcoma180 and human lung cancer LC-6. However, 25-mg/kg KF41399 treatment reduced the antitumor activity of ACNU against human lung cancer Lu-65, and 5 mg/kg KF41399 caused a slight reduction of the antitumor activity of ACNU without inducing thrombocytopenia. These results suggest that KF41399 might be useful as a chemoprotective agent to improve chemotherapy-induced thrombocytopenia and types of other toxicity.
Hematopoiesis is a complex process in which hematopoietic stem cells are capable of self-renewal to maintain a long-term supply of progenies and are stimulated to differentiate into multiple lineages by various growth factors. It is generally assumed that the majority of hematopoietic stem cells are in the quiescent (G0) phase of the cell cycle, and only a few actively cycling hematopoietic stem cells supply all the hematopoietic cells at a given time.1 This concept is supported by evidence that quiescent hematopoietic stem cells are resistant to the cytotoxic effects of 5-fluorouracil (5-FU), whereas most of their cycling counterparts are sensitive to 5-FU, thus resulting in myelosuppression. Thrombocytopenia induced by radiotherapy and chemotherapy is a major complication in patients treated for cancer. Agents capable of enhancing host defense mechanisms or hematopoietic homeostasis could be useful in treating myelosuppression caused by radiotherapy and chemotherapy.
For decades it was thought that megakaryocytopoiesis was regulated by a lineage-specific humoral factor, thrombopoietin (TPO). TPO, first cloned as an Mpl ligand, has been shown to be a primary regulator of megakaryocytopoiesis and thrombopoesis.2-5 Furthermore, the administration of TPO improved hematopoietic recovery in radiotherapy- and/or chemotherapy-induced myelosuppression in mice.6
Induction of G0/G1 arrest of proliferative hematopoietic progenitor cells is an alternative way to reduce myelosuppression because it protects stem cells from toxicity to radiotherapy and chemotherapy. It has been reported that tetrapeptide acetyl-N-Ser-Asp-Lys-Pro (AcSDKP) inhibits the entry into DNA synthesis of human marrow progenitor cells but shows no effect on leukemic cells and that pretreatment with AcSDKP shows a protective effect against doxorubicin-induced mortality and hematotoxicity in mice.7,8 In addition, transforming growth factor (TGF)-β also induces G0/G1 arrest and suppresses the growth of proliferating hematopoietic progenitor and leukemia cells.9 10
Cell cycle progression from G0/G1- to S-phase is regulated by the G1 cyclin/Cdk complexes that phosphorylate Rb protein.11,12 Rb is a transcription repressor when it is bound to transcription factors, such as members of the E2F family, and Rb function is regulated by its phosphorylation status.13,14 In the mid-G1 phase of the cell cycle, cyclin D/Cdk4 or cyclin D/Cdk6 triggers the onset of the S-phase by phosphorylating Rb. In the G1/S boundary of the cell cycle, cyclin E/Cdk2 hyper-phosphorylates Rb, thus releasing E2F from Rb.15,16 Released E2F is involved in the transcriptional activation of genes important for DNA synthesis.17 Cyclin A/Cdk2 plays an essential role in progression through the S-phase of the cell cycle.18 The activity of the G1cyclin/Cdk complex is also negatively regulated by Cdk inhibitors (CDKI), such as p21Waf-1 and p27 Kip1, and the binding of CDKI to Cdk induces G0/G1arrest.14
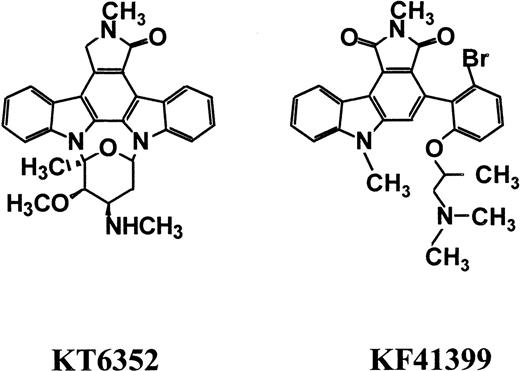
We previously reported that a derivative of indolocarbazole compounds, KT6352 6-methyl staurosporine (Figure1), increases the number of megakaryocyte colonies in the presence of interleukin-3 (IL-3).19However, KT6352, either alone or in combination with stem cell factor (SCF), did not increase the number of megakaryocyte colonies. Although intraperitoneal administration of KT6352 increased the number of peripheral platelets, colony-forming unit–megakaryocytes, and spleen weight in mice, the oral (PO) administration of KT6352 showed little thrombopoietic activity because of its poor bioavailability (Shiotsu et al, unpublished data). In the course of screening for small molecules that could improve thrombocytopenia induced by nimustine (ACNU), we found that KF41399, a derivative of carbazole compounds, elevated platelet counts, when given orally, and accelerated the rate of platelet recovery. In this report, we show the detailed pharmacologic properties of KF41399 as a chemoprotective agent. Oral administration of KF41399 reduces thrombocytopenia induced by ACNU, and the chemoprotective effect of KF41399 is schedule dependent. We also show that the oral administration of KF41399 to normal mice causes G0/G1 arrest of bone marrow progenitor cells that is accompanied by up-regulation of the Bcl-2 protein level. These effects of KF41399 could protect hematopoietic progenitor cells from the toxic effects of ACNU.
Materials and methods
Animals and tumors
BALB/c male mice, at 5 weeks old, were purchased from Nippon SLC (Shizuoka, Japan). BALB/c-nu/nu male mice and DDY male mice, at 6 weeks old, were purchased from Nippon Clea (Tokyo, Japan). All animals were maintained on commercial food, given water ad libitum, and housed at 23°C ± 5°C and 55% ± 5% relative humidity throughout the experiment. Mouse Sarcoma180 cells were provided by the National Cancer Center Research Institute (Tokyo, Japan). Human lung adenocarcinoma LC-6 and large cell carcinoma Lu-65 cell lines were obtained from Dr Ohnishi (Central Institute for Experimental Animals, Kanagawa, Japan) and were passaged by inoculation into the flanks of adult BALB/c-nu/nu male mice.
Cell culture
K562 cell line was obtained from American Type Culture Collection (Rockville, MD). K562 cells were cultured in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (GIBCO), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (GIBCO) at 37°C in a humidified atmosphere of 5% CO2 in air. Cell lysate from exponentially growing K562 cells was used as a positive control because K562 cells express high level of Cdk and cyclins (Shiotsu Y et al, manuscript in preparation).
Compounds and antibodies
KF41399 (Figure 1) was synthesized in our institute (patent number WO9809967). Structure–activity relationship studies of carbazole compounds in ACNU-induced thrombocytopenia will be described elsewhere. The compound was verified to be free of lipopolysaccharide by Limulus lysate assay using the Limulus Test (Wako Pure Chemicals, Osaka, Japan). For in vivo studies, KF41399 was dissolved in distilled water. These monoclonal antibodies (mAb) were obtained from PharMingen (San Diego, CA): fluorescein isothiocyanate (FITC)-conjugated antimouse c-Kit mAb; FITC-conjugated antimouse Gr-1 mAb; biotin-conjugated antimouse Gr-1 mAb; phycoerythrin (PE)-conjugated antimouse Mac-1 mAb; biotin-conjugated antimouse Mac-1 mAb; biotin-conjugated antimouse CD61 mAb; biotin-conjugated antimouse B220 mAb; biotin-conjugated antimouse TER117 mAb; biotin-conjugated antimouse CD4 mAb; and biotin-conjugated antimouse CD8 mAb. Anti-Bcl-2, anti-cyclin E, and anti-Rb mAbs were also obtained from PharMingen. FITC-labeled goat antimouse IgG and PE-labeled goat antimouse IgG were obtained from Vector (Burlingame, CA). Streptavidin-conjugated PE was obtained from Becton Dickinson (Bedford, MA). Anti-cyclin A mAb was obtained from Kyowa Medex (Tokyo, Japan). Anti-cyclin D1 mAb was obtained from IBL (Gunma, Japan). Anti-Bax polyclonal antibody was obtained from Oncogene Research (Cambridge, MA). Anti-Cdk4, anti-Cdk6, and anti-p130 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Cdk2 mAb was obtained from Upstate Biotechnology (Lake Placid, NY). Anti-β–actin mAb was obtained from Sigma (St Louis, MO).
Study design
A group of 5 mice was treated with KF41399, ACNU, or both. KF41399 was administered orally at a volume of 0.2 mL/animal twice a day (BID) at 8-hour intervals. Control animals were given oral administrations of distilled water instead of KF41399. ACNU was obtained from Sankyo (Tokyo, Japan) and was diluted with distilled water. The maximum tolerant dose of ACNU for mice, 45 mg/kg, was administered intravenously (IV) for 2 consecutive days. Administration dose, schedule, and frequency of KF41399 and ACNU are shown in Table1. The appropriate schedule for KF41399 treatment was examined based on the number of peripheral platelets in mice administered with KF41399 (25 mg/kg) orally before or after ACNU treatment. To examine the dose-dependent effect of KF41399 on ACNU-induced thrombocytopenia in mice, various doses of KF41399 (0.2, 1, 5, and 25 mg/kg) were administered orally from day 0 to day 2. After this 45 mg/kg ACNU was administered on day 3 and day 4 (protocol G, Table 1).
Peripheral blood cell count
Blood samples were collected by puncture of the eye vein, and complete blood cell counts were measured using the Sysmex automatic analyzer (TOA Medical Electronics, Kobe, Japan).
Preparation of bone marrow mononuclear cells and hematopoietic progenitor cells
Mice were killed by rapid cervical dislocation. Bone marrow cells were harvested by flushing 2 femur and tibia with MK medium, as described previously.20 Mononuclear cells (MNC) were isolated by Nycoprep Animal (Nycomed, Oslo, Norway) according to the manufacturer's instruction. Lin− cells were isolated as described previously.21 Briefly, MNC were incubated with biotinylated anti-lineage markers (Mac-1, Gr-1, B220, TER119, CD4, CD8, and CD61), and Lin+ cells were depleted with streptavidin-conjugated magnetic beads (PerSeptive Diagnostics, Cambridge, MA).
Cell cycle analysis
MNC and Lin− cells were analyzed for nuclear DNA content using the method previously described. Briefly, 1 × 105 cells were suspended in a solution containing 0.1% Nonidet P-40 (Nacalai Tesque, Kyoto, Japan), 50 μg/mL propidium iodide (Wako), and 50 μg/mL RNase (Sigma). The cell cycle distribution was determined by the analysis of DNA content using the FACScan and CellFIT systems according to the manufacturer's protocol (Becton Dickinson).
Phenotype analysis of MNC
We compared the phenotype of MNC derived from ACNU-treated mice and pre-treated mice with KF41399 using the method previously described.22 Briefly, 1 × 105 MNC were incubated at 4°C for 30 minutes with 100 μL (1.0 μg/mL) FITC-conjugated antimouse c-Kit mAb, FITC-conjugated antimouse Gr-1 mAb, PE-conjugated antimouse Mac-1 mAb, or biotin-conjugated antimouse CD61 mAb. MNC were washed twice with ice-cold phosphate-buffered saline (PBS) to remove unbound antibody, resuspended in 1.0 μg /mL streptavidin-conjugated PE and incubated at 4°C for 30 minutes in the dark. MNC were washed twice with ice-cold PBS and resuspended in 1% paraformaldehyde in PBS, pH 7.4. Mouse IgG1 (Vector) was used as an isotype-matched negative control for each sample. Ten thousand events were analyzed for each sample by FACScan.
Cell lysis and immunoblotting
K562 cells and Lin− cells were lysed for 10 minutes by the addition of 50 μL ice-cold lysis buffer (50 mmol/L HEPES–NaOH [pH 7.4], 250 mmol/L NaCl, 1 mmol/L EDTA, 1% Nonidet P-40, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride [Sigma], 5 μg/mL leupeptin [Sigma], 2 mmol/L Na3VO4 [Wako], 1 mmol/L NaF [Wako], 10 mmol/L β-glycerophosphate [Wako]) on ice, and the cell lysate was clarified by centrifugation. Ten micrograms cell lysate of each sample was electrophoresed on 12.5% or 7.5% SDS–PAGE (Dai-ichi Pure Chemicals, Tokyo, Japan), transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA), and immunoblotted with the appropriate antibodies. For detection, the blots were incubated with the appropriate ECF antibody (alkaline–phosphatase-conjugated anti-IgG antibody; Bio-Rad, Hercules, CA) and developed using the ECF detection system (Molecular Dynamics, Sunnyvale, CA), according to the manufacturer's instructions. Membranes were scanned into Fluor Imager (Molecular Dynamics) and were analyzed by using Image Quant software (Molecular Dynamics).
Colony-forming cell assay
Serum-free culture was grown in 35-mm Petri dishes (Nunc, Copenhagen, Denmark) as previously described.23 The culture consisted of 1 × 104/mL MNC, IMDM, 0.3% agar (Difco, Detroit, MI), 1% deionized bovine serum albumin (fraction V; Sigma), 0.6 mg/mL fully iron-saturated transferrin (Boehringer Mannheim, Marburg, Germany), and 0.1 mg/mL cholesterol (Wako). Cytokines added to serum-free culture system were as follows: 100 U/mL recombinant mouse IL-3 (rmIL-3; Genzyme, Cambridge, MA), 10 ng/mL recombinant human SCF (rhSCF; Genzyme), 10 ng/mL recombinant human TPO (rhTPO; Pepro Tech, Rocky Hill, NJ), 1 U/mL recombinant human erythropoietin (rhEPO; Chugai Pharmaceutical, Tokyo, Japan), and 10 ng/mL of recombinant human granulocyte colony-stimulating factor (rhG-CSF; Kyowa Hakko Kogyo, Tokyo, Japan). Dishes were incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. After 7 days' culture, colony-forming cells (CFC) were identified after they were stained with Mayer's hematoxylin (Wako). CFC included all hematopoietic colonies of more than 50 cells. The number of CFC was counted using an inverted microscope.
KF41399 effects on antitumor activity of ACNU
Mouse Sarcoma180 was inoculated in the flanks of DDY mice. Human lung adenocarcinoma LC-6, or Lu-65, was inoculated in the flanks of nude mice. KF41399 was administered orally twice daily for 2 or 3 days. This was followed by ACNU administration at a dose of 45 mg/kg for 2 consecutive days. For the evaluation of antitumor activity of ACNU, tumor volume was calculated using the following formula: Tumor Volume (mm3) = (Length × Width2)/2. This calculation was performed according to the method of the National Cancer Institute, by measuring the length (mm) and the width (mm) of tumors.24
Drug efficacy was expressed as the ratio of mean V/V0 value against that of a control group (mean V of treated group/mean V of control group; T/C), where V is the tumor volume on the day of evaluation and the V0 is the tumor volume on the day of initial treatment with the drug. At the same time, the peripheral platelet count of each animal was measured using the Sysmex (TOA Medical Electronics) automatic analyzer.
Statistical analysis
To evaluate the effects of KF41399 on thrombocytopenia, spleen weight loss, body weight loss, MNC decrease, reduction of CFC activity, and antitumor activity caused by ACNU, statistical significance was determined using the Mann-Whitney U test. P < .05 was considered statistically significant.
Results
Hematologic effects of KF41399
We first examined the thrombopoietic effects of KF41399 alone in mice. When a variety of doses of KF41399 (0.2, 1.0, 5.0 and 25 mg/kg) was administered orally twice daily for 3 days (protocol B, Table 1) to normal mice, few effects on peripheral platelet counts were observed (data not shown). The maximum tolerant dose of ACNU treatment for 2 consecutive days (protocol I, Table 1) induced severe thrombocytopenia with the nadir of platelet counts at 9.0 × 104/μL on day 11 in mice (Table2). Using this thrombocytopenia model, the schedule-dependent effects of KF41399 were examined. KF41399 (25 mg/kg BID ×3 PO) was administered to mice 3 days before, 1 day before, or 1 day after ACNU treatment (protocols D, E, and F, respectively, in Table1). As shown in Table 2, pretreatment of mice with KF41399 improved the nadir of platelet counts and shortened the duration of thrombocytopenia and the time required for recovery to a normal platelet level. The efficacy was more pronounced in the group in which KF41399 was administered 3 days before treatment (protocol D) than the group in which it was administered 1 day before treatment (protocol E). The former regimen (protocol D) almost completely prevented ACNU-induced thrombocytopenia. However, post-treatment with KF41399 (protocol F) showed no improvement in thrombocytopenia induced by ACNU. These results indicated that only pretreatment with KF41399 was effective in ameliorating thrombocytopenia.
Next, the dose-dependent effect of KF41399 on ACNU-induced thrombocytopenia was examined. Various doses of KF41399 (0.2, 1, 5, and 25 mg/kg) were administered orally from day 0 to day 2, followed by 45 mg/kg ACNU treatment on day 3 and day 4 (protocol G, Table 1). The mean platelet nadir was slightly increased to 1.4 × 105/μL in mice treated with 1.0 mg/kg KF41 399 and 5.0 mg/kg KF41 399 treatment increased the nadir of platelet counts to 3.9 × 105/μL (Table3). In mice treated with 25 mg/kg KF41399, significant improvement in thrombocytopenia (5.7 × 105/μL) was observed. KF41399 pretreatment also slightly improved the neutropenia induced by ACNU (data not shown).
KF41399 reduced the decrease in mononuclear cells, spleen weight, and body weight
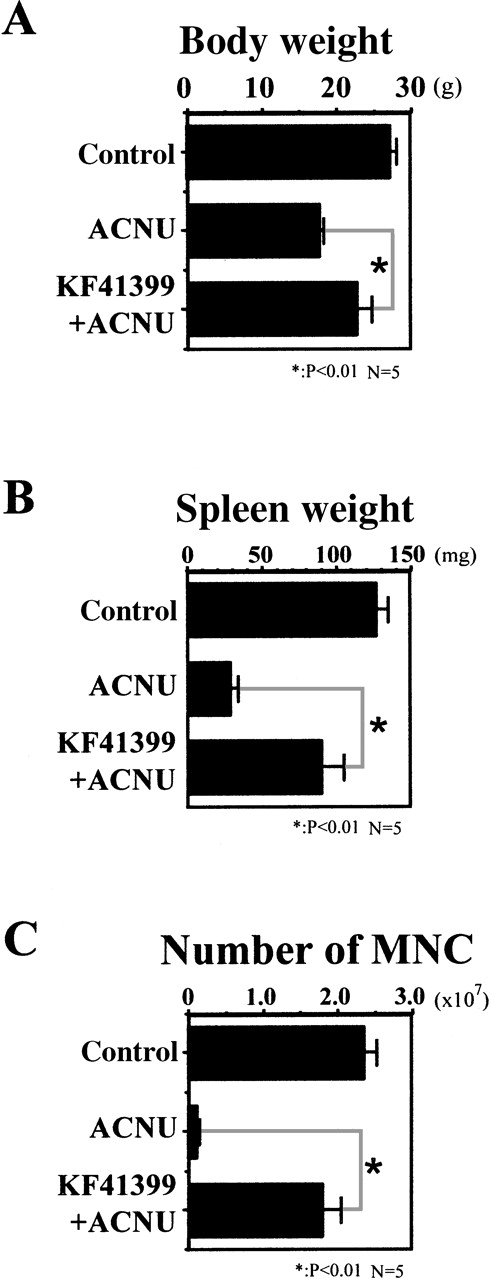
Mice were administered orally with or without KF41399 (25 mg/kg) for 3 days, followed by ACNU treatment as described in Table1 (protocols D, I). Although control mice had 2.4 ± 0.16 × 106 MNC per femur and tibia, the maximum tolerant dose of ACNU administration resulted in a marked decrease of MNC counts to as low as 1.1 ± 0.33 × 105 per femur and tibia on day 7 (Figure 2C). Oral administration of KF41399 (25 mg/kg) significantly ameliorated the decrease of MNC to as high as 1.8 ± 0.24 × 106per femur and tibia on day 7 (Figure 2C) and increased the number of MNC above the baseline level on day 14 (data not shown). Furthermore, pretreatment with KF41399 significantly overcame the decreased body and spleen weights caused by the maximum tolerant dose of ACNU treatment (Figures 2A and 2B).
Oral administration of KF41399 reduced the toxicity caused by ACNU.
In the ACNU-treated group, mice were administered 45 mg/kg ACNU IV for 2 consecutive days on day 3 and day 4 (protocol I, Table 1). In the KF41399 pretreated group, mice were treated with 25 mg/kg orally KF41399 twice daily for 3 days, from day 0 to day 2. This was followed by the IV administration of 45 mg/kg ACNU for 2 consecutive days on day 3 and day 4 (protocol D, Table 1). Control animals were administered distilled water (protocol A, Table 1). Body weight on day 14 (A), spleen weight on day 14 (B), and number of bone marrow-derived MNC per femur and tibia on day 7 (C) were measured as described in “Materials and methods.” Results are expressed as the mean ± SD. n = 5 in each group.
Oral administration of KF41399 reduced the toxicity caused by ACNU.
In the ACNU-treated group, mice were administered 45 mg/kg ACNU IV for 2 consecutive days on day 3 and day 4 (protocol I, Table 1). In the KF41399 pretreated group, mice were treated with 25 mg/kg orally KF41399 twice daily for 3 days, from day 0 to day 2. This was followed by the IV administration of 45 mg/kg ACNU for 2 consecutive days on day 3 and day 4 (protocol D, Table 1). Control animals were administered distilled water (protocol A, Table 1). Body weight on day 14 (A), spleen weight on day 14 (B), and number of bone marrow-derived MNC per femur and tibia on day 7 (C) were measured as described in “Materials and methods.” Results are expressed as the mean ± SD. n = 5 in each group.
Chemoprotective effects of KF41399 on colony-forming cells
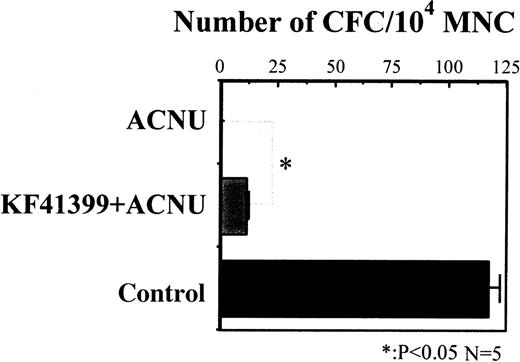
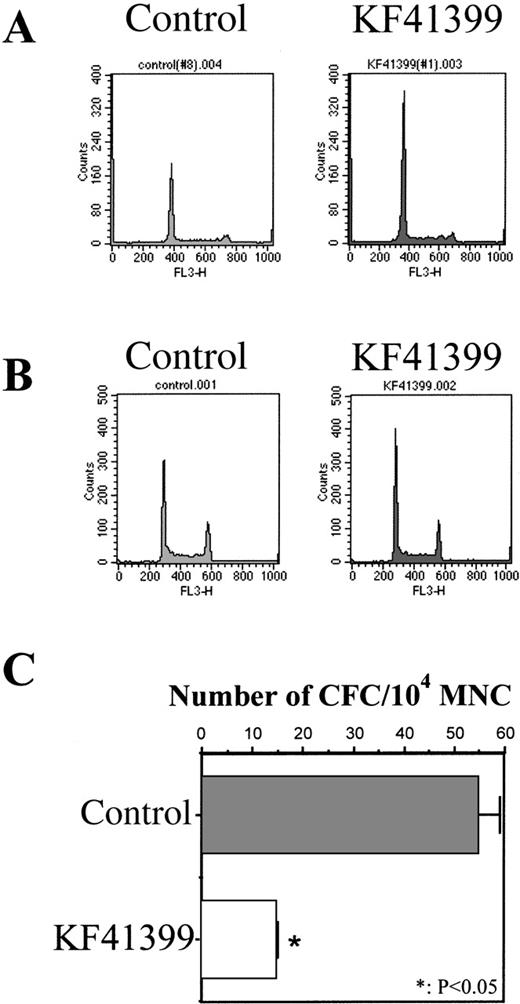
We compared the colony-forming activity of MNC derived from mice treated with ACNU alone and mice pretreated with KF41399 followed by ACNU administration (protocols H, I in Table 1). MNC were prepared from both groups of mice on day 7, and colony-formation assays were performed in the presence of rmIL-3, rhSCF, rhTPO, rhEPO, and rhG-CSF. As shown in Figure 3, 117 ± 7 of CFC/104 MNC were detected in control mice; however, no CFC were detected in ACNU-treated mice. In KF41399 pretreated mice, 18 ± 2 CFC/104 MNC were detected.
Oral administration of KF41399 improved the reduction of CFC activity caused by ACNU.
In the ACNU-treated group, mice were administered 45 mg/kg ACNU IV for 2 consecutive days on day 3 and day 4 (protocol I, Table 1). In the KF41399 pretreated group, mice were treated with 25 mg/kg KF41399 orally twice daily for 10 days from day 0 to day 9. This was followed by the IV administration of 45 mg/kg ACNU for 2 consecutive days on day 3 and day 4 (protocol H, Table 1). Control animals were administered distilled water (protocol A, Table 1). On day 7, MNC was prepared and 104 MNC were seeded in 0.3% of soft agar medium, as described in “Materials and methods.” n = 5 in each group.
Oral administration of KF41399 improved the reduction of CFC activity caused by ACNU.
In the ACNU-treated group, mice were administered 45 mg/kg ACNU IV for 2 consecutive days on day 3 and day 4 (protocol I, Table 1). In the KF41399 pretreated group, mice were treated with 25 mg/kg KF41399 orally twice daily for 10 days from day 0 to day 9. This was followed by the IV administration of 45 mg/kg ACNU for 2 consecutive days on day 3 and day 4 (protocol H, Table 1). Control animals were administered distilled water (protocol A, Table 1). On day 7, MNC was prepared and 104 MNC were seeded in 0.3% of soft agar medium, as described in “Materials and methods.” n = 5 in each group.
Phenotype analysis of mononuclear cells
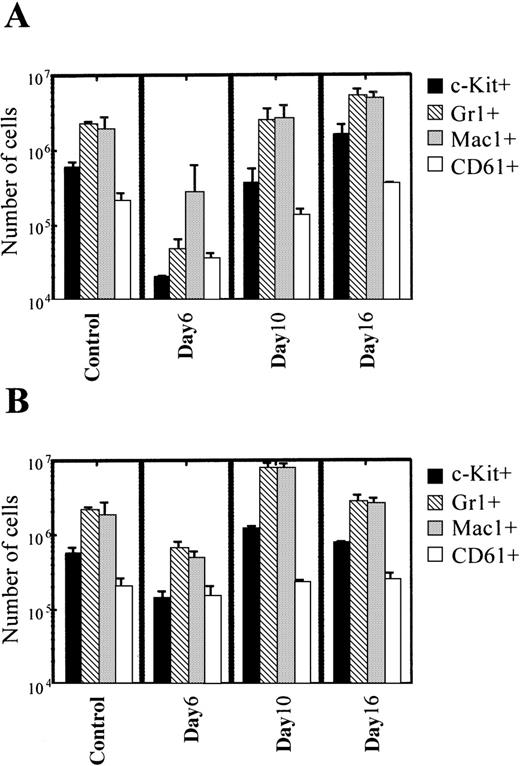
We analyzed the phenotype of MNC derived from ACNU-treated mice and KF41399 pretreated mice by FACScan. Mice were administered orally with or without KF41399 (25 mg/kg) followed by ACNU treatment, as described in Table 1 (protocols H, I). Figure4A indicates the numbers of each lineage of MNC in ACNU-treated mice. Compared with control mice, a decrease in each lineage of MNC was observed on day 6. The percentage control for each lineage in ACNU-treated mice decreased as follows: c-Kit+, 3.4%; Gr-1+, 2.2%; Mac-1+, 14.5%; CD61+, 17.2%. On day 10, the percentage control for each lineage was as follows: c-Kit+, 60%; Gr-1+, 115%; Mac-1+, 141%; CD61+, 65%. On day 16, the percentage control for each lineage in ACNU-treated mice increased as follows: c-Kit+, 272%; Gr-1+, 248%; Mac-1+, 265%; CD61+, 166%.
Pretreatment with KF41399 suppressed the decrease and accelerated the recovery of each lineage of MNC.
(A) Time course of the phenotype of MNC derived from ACNU-treated mice. Mice were administered 45 mg/kg ACNU IV for 2 consecutive days, on day 3 and day 4 (protocol I, Table 1). Control animals were administered distilled water (protocol A, Table 1). MNC was prepared on indicated days, and the phenotype of MNC was analyzed by FACScan as described in “Materials and methods.” (B) Time course of the phenotype of MNC derived from the KF41399 pretreated mice. Mice were treated with 25 mg/kg KF41399 orally twice daily for 10 days, from day 0 to day 9, followed by the IV administration of 45 mg/kg ACNU for 2 consecutive days, on day 3 and day 4 (protocol H, Table 1). Results are expressed as the mean ± SD. n = 5 in each group.
Pretreatment with KF41399 suppressed the decrease and accelerated the recovery of each lineage of MNC.
(A) Time course of the phenotype of MNC derived from ACNU-treated mice. Mice were administered 45 mg/kg ACNU IV for 2 consecutive days, on day 3 and day 4 (protocol I, Table 1). Control animals were administered distilled water (protocol A, Table 1). MNC was prepared on indicated days, and the phenotype of MNC was analyzed by FACScan as described in “Materials and methods.” (B) Time course of the phenotype of MNC derived from the KF41399 pretreated mice. Mice were treated with 25 mg/kg KF41399 orally twice daily for 10 days, from day 0 to day 9, followed by the IV administration of 45 mg/kg ACNU for 2 consecutive days, on day 3 and day 4 (protocol H, Table 1). Results are expressed as the mean ± SD. n = 5 in each group.
Figure 4B indicates the number of each lineage of MNC derived from mice pretreated with KF41399 followed by ACNU treatment. KF41399 preadministration suppressed the decrease in MNC number and accelerated the recovery of each lineage. The percentage of control for each lineage on day 6 was as follows: c-Kit+, 25%; Gr-1+, 31%; Mac-1+, 26%; and CD61+, 73%. On day 10, a clear increase in each cell lineage was observed, and the percentage of control for each lineage was as follows: Gr-1+, 363%; Mac-1+, 422%; and CD61+, 112%. Furthermore, the phenotype of MNC was similar to that in control mice on day 16.
Effects of KF41399 on cell cycle and colony-forming activity of MNC and Lin− cells
To elucidate the mechanism of chemoprotection by KF41399, we analyzed the cell cycle distribution of MNC and Lin−cells derived from mice treated with KF41399 alone. After 25 mg/kg KF41399 was administered orally to normal BALB/c mice twice daily for 3 days (protocol C, Table 1), bone marrow MNC and Lin− cells were isolated, and their cell cycle distributions were analyzed by FACScan, as described in “Materials and methods.” Compared with untreated mice, G0/G1-phase accumulation was observed in MNC (Figure 5A) and Lin−cells (Figure 5B) in KF41399-treated mice. When we compared the colony-forming activity of MNC derived from KF41399-treated mice and untreated mice, a marked decrease in the ratio of colony-forming cells/104 MNC was observed in the KF41399-treated group (Figure 5C).
Oral administration of KF41399 induced G0/G1 arrest of MNC and Lin−cells in mice.
Mice were treated with 25 mg/kg KF41399 orally twice daily for 3 days (protocol B, Table 1). Control animals were administered distilled water instead of KF41399. MNC and Lin− cells were prepared on day 3, cell cycle distribution was analyzed by FACScan, and CFC activity was analyzed as described in “Materials and methods.” KF41399 induced G0/G1 arrest of MNC (A) and Lin− cells (B). Data shown are representative of at least 3 independent experiments. KF41399 effects on CFC activity of MNC (C). Results are expressed as the mean ± SD. n = 5 in each group.
Oral administration of KF41399 induced G0/G1 arrest of MNC and Lin−cells in mice.
Mice were treated with 25 mg/kg KF41399 orally twice daily for 3 days (protocol B, Table 1). Control animals were administered distilled water instead of KF41399. MNC and Lin− cells were prepared on day 3, cell cycle distribution was analyzed by FACScan, and CFC activity was analyzed as described in “Materials and methods.” KF41399 induced G0/G1 arrest of MNC (A) and Lin− cells (B). Data shown are representative of at least 3 independent experiments. KF41399 effects on CFC activity of MNC (C). Results are expressed as the mean ± SD. n = 5 in each group.
Effects of KF41399 on the level of cell cycle regulating proteins in Lin− cells
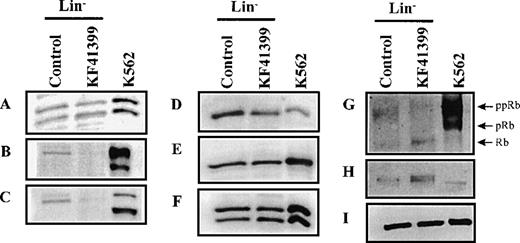
To further elucidate the arresting points of the cell cycle, the expression levels of cell cycle regulating proteins were examined by immunoblotting. As a positive control, cell lysate fromexponentially growing K562 cells was used. Lin− cells were prepared from mice treated with KF41399 twice daily for 3 days (protocol C, Table 1) or from untreated mice. When we compared the levels of G1 cyclins and Cdk in Lin− cells derived from KF41399-treated mice and untreated mice, a marked decrease in cyclin E and cyclin A proteins was observed (Figures6B, 6C) in the KF41399 group, whereas no significant change in the level of cyclin D1, Cdk2, Cdk4, and Cdk6 proteins was observed in the untreated group (Figures 6A, 6F, 6D, 6E respectively). Because the phosphorylation status of Rb is a critical step for G1/S transition, we investigated the effect of KF41399 on the expression and mobility of Rb proteins by immunoblotting. As a positive control, exponentially growing K562 cells were analyzed in parallel, and they showed mainly slower-migrating phosphorylated Rb proteins, which consisted of multiple bands with a molecular mass range of 112 to 114 kd (Figure 6G). Compared with Lin− cells derived from untreated mice, Lin− cells derived from KF41399 pretreated mice had lower levels of and faster migrating Rb proteins (Figure 6G). In addition, up-regulation of p130 was observed in Lin−cells derived from KF41399-treated mice (Figure 6H). No significant changes in β-actin levels were observed among the different conditions examined (Figure 6I).
KF41399 administration modulated the expression level of cell cycle regulating proteins in Lin− cells.
Mice were treated with 25 mg/kg KF41399 orally twice daily for 3 days (protocol B, Table 1). Control animals were administered distilled water instead of KF41399. Lin− cells were purified on day 3, and cell lysate was prepared. The expression level of each protein was detected as described in “Materials and methods.” Cyclin D1 (A), cyclin E (B), cyclin A (C), Cdk4 (D), Cdk6 (E), Cdk2 (F), Rb (G), p130 (H), and β-actin (I). Phosphorylation status of Rb was shown as ppRb (hyper-phosphorylated Rb), pRb (hypo-phosphorylated Rb), and Rb (unphosphorylated Rb).
KF41399 administration modulated the expression level of cell cycle regulating proteins in Lin− cells.
Mice were treated with 25 mg/kg KF41399 orally twice daily for 3 days (protocol B, Table 1). Control animals were administered distilled water instead of KF41399. Lin− cells were purified on day 3, and cell lysate was prepared. The expression level of each protein was detected as described in “Materials and methods.” Cyclin D1 (A), cyclin E (B), cyclin A (C), Cdk4 (D), Cdk6 (E), Cdk2 (F), Rb (G), p130 (H), and β-actin (I). Phosphorylation status of Rb was shown as ppRb (hyper-phosphorylated Rb), pRb (hypo-phosphorylated Rb), and Rb (unphosphorylated Rb).
Effect of KF41399 on the levels of apoptosis regulating proteins in Lin− cells
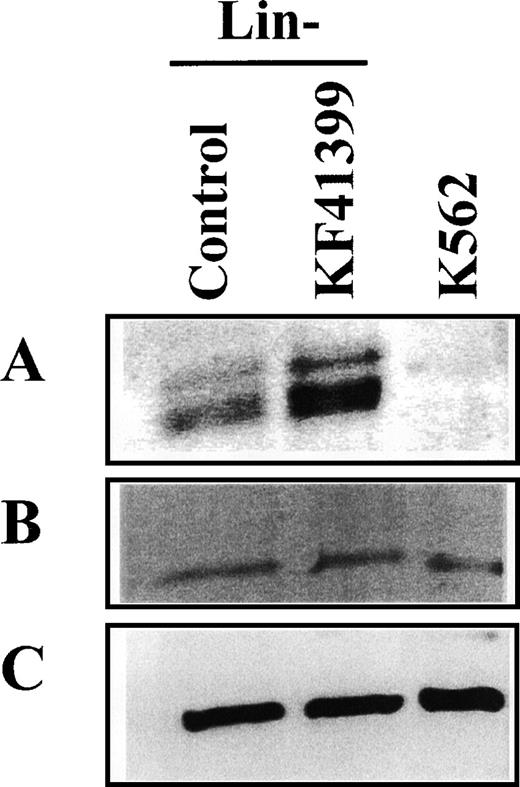
Next, we compared the levels of apoptosis regulating proteins in Lin− cells derived from KF41399-treated mice and untreated mice. In KF41399 treated mice, a marked increase in the anti-apoptotic Bcl-2 protein level and a slower migrating form of Bcl-2 protein was observed (Figure7A), whereas no significant changes in pro-apoptotic Bax (Figure 7B) or β-actin (Figure 7C) levels were observed.
KF41399 administration increased the expression level of Bcl-2 protein in Lin− cells.
Mice were treated with 25 mg/kg KF41399 orally twice daily for 3 days (protocol B, Table 1). Control animals were administered distilled water instead of KF41399. Lin− cells were purified on day 3, and cell lysate was prepared. The expression level of each protein was detected, as described in “Materials and methods.” Bcl-2 (A), bax (B), and β-actin (C).
KF41399 administration increased the expression level of Bcl-2 protein in Lin− cells.
Mice were treated with 25 mg/kg KF41399 orally twice daily for 3 days (protocol B, Table 1). Control animals were administered distilled water instead of KF41399. Lin− cells were purified on day 3, and cell lysate was prepared. The expression level of each protein was detected, as described in “Materials and methods.” Bcl-2 (A), bax (B), and β-actin (C).
Effect of KF41399 on the antitumor activity of ACNU
To rule out the possibility that KF41399 might affect the antitumor activity of ACNU in vivo, we used a mouse syngeneic tumor model and 2 human tumor xenograft models. In mouse Sarcoma180 cells inoculated into DDY mice, ACNU (45 mg/kg administered for 2 consecutive days intravenously) resulted in a marked growth inhibitory activity and a T/C value of 24%. Pretreatment with KF41399 (25 mg/kg twice a day ×3 PO) did not affect this growth inhibitory activity (Table4). In this tumor-bearing mouse model, KF41399 also improved the thrombocytopenia and body weight loss induced by ACNU. Oral administration of KF41399 alone had no effect on tumor growth or platelet counts (Table 4).
The administration of ACNU (45 mg/kg ×2) intravenously caused a remission in human lung carcinoma LC-6 in nude mice (T/C, 0%). However 1 of 5 mice died in ACNU-treated group (Table5). Pretreatment with 25 mg/kg KF41399 (twice a day orally) did not affect the tumor regressive activity of ACNU (T/C, 0%), and all the mice survived. In this xenograft model, ACNU also induced thrombocytopenia in nude mice, and pretreatment with KF41399 improved thrombocytopenia and body weight loss induced by ACNU. In addition, no antitumor activity against LC-6, thrombopoietic activity, or toxicity in nude mice was observed when KF41399 was administered orally as a single agent.
ACNU (45 mg/kg administered for 2 consecutive days) intravenously resulted in marked antitumor activity (T/C, 17%) against another human large cell lung carcinoma, Lu-65 (Table6). In this xenograft model, pretreatment with 5 mg/kg KF41399 (twice a day for 3 days orally) resulted in a slight reduction of the antitumor activity of ACNU (T/C, 27%), and pretreatment with 25 mg/kg KF41399 (twice a day for 3 days orally) resulted in a marked reduction in the antitumor activity of ACNU (T/C, 54%) against Lu-65 (P < .05). However, pretreatment with KF41399 (5 mg/kg and 25 mg/kg) still prevented thrombocytopenia and reduced body weight loss induced by ACNU. Oral administration of KF41399 alone did not result in any antitumor activity against Lu-65, thrombopoietic activity, or toxicity in nude mice.
Discussion
For decades many investigators have made efforts to search for agents that prevent thrombocytopenia induced by chemotherapy and radiotherapy. Recently, we reported that KT6352, a derivative of indolocarbazole compounds, stimulates bone marrow cells to increase the number of megakaryocyte colonies in the presence of IL-3, but not with SCF or without IL-3. Intraperitoneal administration of KT6352 resulted in significant thrombopoietic activity in mice.19Furthermore, KT6352 improved thrombocytopenia induced by ACNU, mitomycin C, and radiation. However, the oral administration of KT6352 had little effect on platelet counts (Shiotsu et al, unpublished data). In the course of screening for KT6352 derivatives that could treat thrombocytopenia induced by ACNU, mitomycin C, or radiation, we found that a series of carbazole compounds, including KF41399, could improve thrombocytopenia by oral administration. Structure–activity relationship studies of carbazole compounds on thrombocytopenia will be described elsewhere. When KF41399 was added to a megakaryocyte colony assay system containing IL-3, no significant increase of megakaryocyte colony number was observed, and the oral administration of KF41399 to normal mice had no effect on peripheral platelet counts (data not shown). These results indicate that KF41399 has little, if any, thrombopoietic activity itself, and it seems that the molecular targets of KF41399 might be different from those of KT6352.
Administration schedule studies showed that pretreatment with KF41399 improved thrombocytopenia induced by ACNU but that posttreatment was ineffective. In addition, we observed a more pronounced effect when KF41399 was administrated 3 days before ACNU treatment rather than 1 day before ACNU treatment (Table 2). In this study, we show that the preadministration of KF41399 for 2 or 3 days improved thrombocytopenia induced by ACNU. When we examined the effective duration of KF41399 treatment, twice daily administration of KF41399 1 day before ACNU treatment was somewhat effective, 1-day pretreatment with KF41399 was less effective than 3-day pretreatment at suppressing thrombocytopenia (data not shown). These results indicate the possibility that pretreatment with KF41399 may protect hematopoietic progenitor cells or megakaryocyte progenitor cells from cytotoxicity caused by ACNU.
We also compared the cellularity, colony-forming activity, and phenotypes of MNC derived from ACNU-treated mice and those derived from mice pretreated with KF41399 followed by ACNU treatment. Although ACNU treatment significantly decreased the number of MNC in bone marrow and, at the same time, reduced the colony-forming activity of CFC, pretreatment with KF41399 increased MNC numbers and the colony-forming activity of CFC (Figures 2, 3). Phenotype analysis showed that ACNU treatment decreased the number of all lineages of bone marrow MNC, whereas pretreatment with KF41399 ameliorated the decrease of c-Kit+, Gr-1+, Mac-1+, and CD61+ cells (Figure 4). These observations could explain the improvement in the colony-forming activity of CFC and the protective effects of KF41399 on hematopoietic progenitor cells against cytotoxicity by ACNU. The protective effects of KF41399 on c-Kit+ and CD61+ cells might cause a rapid recovery in peripheral platelet counts. Furthermore, KF41399 diminished the ACNU-induced effects on spleen and body weight (Figure 2). These data indicate that KF41399 pretreatment promotes the rapid recovery of each lineage of MNC from ACNU-induced damage.
It has been established that the regulation of the cell death pathway involves several key molecules.25,26 Bcl-2 and its homologue Bcl-xL, which prolong cell survival by inhibiting apoptosis, are highly expressed in hematopoietic progenitor cells, whereas terminally differentiated myeloid cells lack Bcl-2 expression.27,28 Our data also support these observations. Although mouse progenitor (Lin−) cells expressed a high level of Bcl-2 protein, the human differentiated leukemia K562 cell line showed a decrease in the expression of Bcl-2 proteins (Figure7). Another Bcl-2 homologue, Bax, which binds to Bcl-2 protein, is a death promoter. The ratio of Bcl-2 to Bax modulates apoptosis.29 Deng et al30,31 reported that IL-3 or bryostatin-1, a macrocyclic lactone natural product, induces phosphorylation of Bcl-2 and suppresses apoptosis in murine myeloid cells. Our data showed that the oral administration of KF41399 to normal mice increased the expression of Bcl-2 protein in Lin− cells and had no effect on Bax protein level (Figure 7). Furthermore, in Lin− cells derived from KF41399-treated mice, slower and faster migrating forms of Bcl-2 were observed, which is indicative of changes in the phosphorylation status of Bcl-2 protein. It has been reported that Bcl-2 is phosphorylated by protein kinase C, but the precise mechanism of the up-regulation or phosphorylation of Bcl-2 is still unknown.29 Taken together with these studies, our results suggest that KF41399 helps hematopoietic progenitor cells escape from anticancer, drug-induced apoptosis by the up-regulation or phosphorylation of Bcl-2, or both.
Oral administration of KF41399 to normal mice induced the G0/G1 arrest of MNC and Lin−cells and reduced the activity of CFC (Figure 5). When we analyzed the levels of G1 cyclins and Cdk in Lin−cells, a clear decrease in cyclin E and cyclin A was observed in Lin− cells derived from KF41399-treated mice, whereas no changes in cyclin D1, Cdk2, Cdk4, or Cdk6 were observed (Figure 6). These results suggest the possibility that cyclin/Cdk2 activity might be reduced because of the down-regulation of cyclin E and cyclin A. We have previously reported that UCN-01 (7-hydroxy staurosporine), a derivative of indolocarbazole compounds, inhibits Cdk2 activity and induces G1 arrest in many cell types.32,33However, KF41399 had no direct inhibitory effect against purified Cdk2 (Shiotsu et al, unpublished data), suggesting the possibility that KF41399 modulates some other upstream molecules that regulate the expression of cyclin E or cyclin A. When human epidermoid carcinoma A431 cells are treated with UCN-01, an induction of G1 arrest and the up-regulation of p21Waf-1 and p27Kip1 were observed.32 In Lin− cells, little expression of p21Waf-1and p27Kip1 was observed (data not shown). This finding is consistent with the observation that p21Waf-1 and p27Kip1 are highly expressed in differentiated myeloid cells, whereas hematopoietic progenitor cells express low levels of CDKI.34
It has been reported that the unphosphorylated form of Rb protein is present in the G0 phase, the phosphorylated form of Rb protein (pRb) is present early in the G1 phase, and hyper-phosphorylated forms of Rb protein (ppRb) are present late in the G1, S, G2, and M phases of the cell cycle.35,36 We investigated the effects of KF41399 on the expression and phosphorylation status of Rb protein by immunoblotting. Cell lysate from K562 cells was used as a positive control because it has been shown that exponentially growing K562 cells express high levels of pRb and ppRb proteins.37 As reported previously, K562 cells showed mainly phosphorylated Rb proteins (pRb and ppRb). Down-regulation of pRb and ppRb was observed in Lin−cells derived from KF41399-treated mice compared with control mice (Figure 6G).
Furthermore, we examined the effects of KF41399 on the protein expression level of p130, a member of the Rb family, because previous studies showed that the E2F-4/p130 complex was unique to quiescent cells, that it repressed the transcription of E2F promoter regulating genes, and that the E2F-4/p130 complex maintained CD34+cells in a quiescent state.38,39 Recently, it has been reported that the cyclin E/Cdk2 complex was inactivated by p130 in MEF cells lacking p27Kip1 and p21Waf-1, suggesting that p130 could substitute for CDKI.40 Our data showed that the expression level of p130 in Lin− cells was up-regulated by KF41399 administration (Figure 6H), and they were consistent with the findings that KF41399 treatment could induce G0/G1 accumulation in Lin−cells.
Accumulation of theG0/G1 phase in Lin− cells might result from indirect effects, such as the induction of TGF-β or α-interferon. Furukawa et al8 reported that TGF-β induces cell cycle arrest at late in the G1 phase of the human monocytic leukemia cell line JOSK-1, and this G1 arrest is associated with an accumulation of the unphosphorylated form of Rb protein. TGF-β has also been shown to inhibit the clonal growth of murine Lin−Sca-1+ bone marrow progenitors, and it increased the number of Lin−Sca-1+cells in the G1 phase of the cell cycle.7Further investigation is needed to clarify whether KF41399 can stimulate the production of any humoral factor, such as TGF-β or α-interferon, that could induce G0/G1 arrest.
To rule out the possibility that G1-phase accumulation by KF41399 could affect the growth of a tumor or reduce the activity of antitumor agents, we investigated the effects of KF41399 on the antitumor activity of ACNU against mouse Sarcoma180, human lung carcinoma LC-6, and Lu-65, which were inoculated into mice. Pretreatment with KF41399 (25 mg/kg twice a day for 2 days orally) improved thrombocytopenia and body weight loss induced by ACNU without affecting antitumor activity against mouse Sarcoma180 (Table 4). In nude mice inoculated with LC-6 human lung adenocarcinoma, pretreatment with KF41399 (25 mg/kg twice a day for 2 days orally) ameliorated thrombocytopenia but did not alter the antitumor activity of ACNU (Table 5). Furthermore, KF41399 pretreatment improved body weight loss and death caused by ACNU in this model. As mentioned previously,pretreatment with KF41399 for 1 day ameliorated thrombocytopenia caused by ACNU in BALB/c mice, and the oral administration of KF41399 (25 mg/kg BID) for 2 days improved thrombocytopenia in these 2 models. In another xenograft model, human lung adenocarcinoma Lu-65, the oral administration of KF41399 (25 mg/kg twice a day) for 3 days reduced the antitumor activity of ACNU without inducing thrombocytopenia (Table 6). On the other hand, a lower dose of KF41399 pretreatment (5 mg/kg twice a day for 3 days orally) slightly reduced the antitumor activity of ACNU against Lu-65 without inducing thrombocytopenia. From these results, it is apparent that the optimum dose and duration of KF41399 pretreatment could ameliorate thrombocytopenia with minimal effect on the antitumor activity of ACNU. Our data suggest that KF41399 may improve the quality of life for patients receiving chemotherapy.
The reason for this contradictory result is unknown. However, it is presumed that the status of apoptosis regulatory and cell cycle regulatory molecules, such as Bcl-2, G1 cyclin, Rb family proteins, might be different among Sarcoma180, LC-6, and Lu-65. These results also suggest the possibility that KF41399 might affect Bcl-2 protein expression and G0/G1 check point function, which are operative in normal hematopoietic progenitor cells and in some tumor cells, such as Lu-65, but further studies are needed. When the K562 cell line or the human lung carcinoma A549 cell line was cultured with KF41399 (up to 2 μmol/L), no induction of G1-phase accumulation was observed (Shiotsu et al, unpublished data). We are now examining the effect of KF41399 on cell cycle distribution and on Bcl-2 protein level in different tumor cell lines. The precise mechanism of chemoprotection by KF41399 is essential to evaluate its potential usefulness as a therapeutic agent. Continued studies to search for the molecular targets of KF41399 are in progress.
In conclusion, our studies have shown that in vivo pretreatment with KF41399 protects hematopoietic progenitor cells from ACNU toxicity, improves thrombocytopenia, and reduces spleen weight loss and body weight loss. These chemoprotective effects of KF41399 on hematopoietic progenitor cells could result from the up-regulation of Bcl-2 or the down-regulation of cyclin E and cyclin A, resulting in G0/G1-phase accumulation in the cell cycle. Although KF41399 may have potential as a clinical chemoprotective drug of a new chemotype, further studies are needed to identify its molecular target in bone marrow progenitor cells and tumor cells.
Acknowledgment
We thank Dr S. Sharma (Tokyo Research Laboratories, Kyowa Hakko Kogyo, Tokyo, Japan) for critically reading this manuscript.
Reprints:Shiro Akinaga, Kyowa Hakko Kogyo Pharmaceutical Laboratories, 1188 Shimotogari Nagaizumi-cho, Sunto-gun, Shizuoka 411-8731, Japan; e-mail:shiro.akinaga@kyowa.co.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.